Abstract
Cellular oxidative stress plays an important role in retinal pigment epithelial (RPE) cell death during aging and the development of age-related macular degeneration. Early reports indicate that during phagocytosis of rod outer segments, there is an increase of RPE oxidative stress and an upregulation of PPARγ mRNA in these cells. These studies suggest that activation of PPARγ may modulate cellular oxidative stress. This paper presents a brief review of recent studies that investigate RPE oxidative stress under various experimental conditions. This is followed by a detailed review on those reports that examine the protective effect of the natural PPARγ ligand, 15d-PGJ2, against RPE oxidative stress. This agent can upregulate glutathione and prevent oxidant-induced intracellular reactive oxygen species accumulation, mitochondrial depolarization, and apoptosis. The cytoprotective effect of this agent, however, is not shared by other PPARγ agonists. Nonetheless, this property of 15d-PGJ2 may be useful in future development of pharmacological tools against retinal diseases caused by oxidative stress.
1. AGE-RELATED MACULAR DEGENERATION: POSSIBLE INVOLVEMENT OF RPE
Age-related macular degeneration (AMD) is the leading cause of legal blindness in individuals 50 years of age or older in the United States and developed countries. AMD can be divided into two major forms as follows: (i) nonneovascular form, also known as “dry” or “nonexudative” form; as clinical findings of this form include drusen and abnormalities of the retinal pigment epithelium (RPE) and (ii) neovascular form, also known as “wet” or “exudative” form, which is defined by the appearance of choroidal neovascularization with subsequent subretinal fibrosis or disciform scarring. Patients with drusen larger than 63 μm in diameter (termed “soft drusen”) have a high risk of developing choroidal neovascularization [1].
There is evidence that pathological alterations of RPE around macula area may be partially responsible for the development of AMD [2, 3]. Clinical abnormalities of RPE in AMD include clumping and atrophy of these cells. RPE is involved in the ingestion of photoreceptor outer segments and the general health of photoreceptors. As a result, pathological changes of RPE can lead to photoreceptor cell death and visual impairment. Study with human cadaver eyes indicates that there is an age-dependent RPE apoptosis as evidenced by TUNEL staining [4]. A separate study further indicates that eye specimens from patients with AMD show statistically more macular RPE apoptosis than those without AMD [5].
2. POSSIBLE ROLES OF OXIDATIVE STRESS IN AMD
Retina is exposed to a combination of sunlight, high concentrations of polyunsaturated fatty acids, and high oxygen environment. It is proposed that reactive oxygen species (such as hydrogen peroxide, superoxide anion, hydroxyl radicals, and singlet oxygen) are constantly generated in this environment. As a result, oxidative stress is believed to have an important role in RPE apoptosis and in the development of AMD [2, 3].
An increase of oxidative stress in RPE is associated with an increase of cellular catalase, metallothionein [6], and glutathione S-transferase [7], which should serve as a protective mechanism to decrease the cytotoxicity caused by H2O2 and other reactive oxygen species. This protective mechanism declines with age. For example, a study analyzing metallothionein levels in RPE of macular region showed a significant (68%) decrease in aged donors (mean age = 80-year-old) as compared to those from younger donors (mean age = 58-year-old) [8]. A separate report also concluded that there was an age-dependent decrease of catalase activity in RPE [9]. These studies suggest that RPE cells in the elderly are more susceptible to oxidative stress-induced damage.
3. STUDIES OF OXIDATIVE STRESS ON RPE: PREVENTION BY PHARMACOLOGICAL AGENTS
Given the observations that RPE might be the prime targets for oxidative stress, a number of studies are conducted to study this issue. A majority of research use direct oxidative agents, such as hydrogen peroxide (H2O2) or t-butylhydroperoxide (tBH), to initiate cellular oxidative stress, as further discussed below. Other conditions of experimental oxidative stress include: intense light [10–12], iron [13], and oxidative metabolites that are toxic to cells, such as A2E [14, 15], acrolein [16], and oxysterols [17–19].
By using H2O2 or tBH as the direct source of oxidative stress on RPE, a number of studies focus on strategies to build up cellular defense mechanisms against the insult. Several reports explore the importance of cellular antioxidative enzymes, such as catalase [20], glutathione-S-transferase [21, 22], superoxide dismutase [23], and methionine sulfoxide reductase [24]. Growth factors including lens epithelium-derived growth factor [25], keratinocyte growth factor [26], and pigment epithelium-derived factor [27] are also protective against oxidative stress. Other proteins that can enhance RPE antioxidative mechanism against H2O2 include bcl-2 [28], alpha B-crystallin [29], melatonin [30], and poly(ADP-ribose) polymerase [31].
In addition to those protein factors discussed above, many investigators seek the use of small-molecule pharmacological agents to prevent RPE damage caused by H2O2 or tBH. Examples of these pharmacological agents include: (R)-alpha-lipoic acid [32], 17-beta-estradiol [33], flavonoids [34], and L-carnitine [35]. The endogenous PPARγ ligand, 15-deoxy-delta-12,14-prostaglandin J2(15d-PGJ2), is also very effective in preventing RPE oxidative stress, as further discussed below.
4. PREVENTION OF OXIDATIVE STRESS-INDUCED RPE DEATH BY 15D-PGJ2
15d-PGJ2, a prostaglandin derivative, is normally present in tissues at low levels (<1 nM), but can reach high concentrations during infection and inflammation [36]. Under in vitro conditions, it can be induced by chemical [37] or physical [38] stress. It has a very potent anti-inflammatory effect [39]. For example, it is a potent inhibitor of macrophage [40–42] and microglia [43–45] activation.
During RPE ingestion of rod outer segments, there is a generation of H2O2 [6, 46] and a 10-fold upregulation of PPARγ mRNA [47]. Based on these observations, it is likely that PPARγ is involved in RPE cellular responses toward H2O2. One can hypothesize that PPARγ agonists should modulate cellular defense against oxidative stress.
We reported earlier that the PPARγ agonist,15d-PGJ2, protected H2O2-induced RPE cell death [48]. With primary human RPE cells, pretreatment of cells overnight with 15d-PGJ2 dose-dependently prevented H2O2-induced cytotoxicity, such that the viability raised from ∼25% (H2O2 only) to ∼80% of control. Maximal protection was observed at ∼2 μM 15d-PGJ2. Similar protection was made in the human ARPE-19 cell line. While H2O2 caused significant nuclear condensation, a sign of apoptosis; this was largely prevented by 1 μM 15d-PGJ2 (see Figure 1). However, it should be mentioned that the protective effect by 15d-PGJ2 was not shared by other PPARγ agonists, such as ciglitazone, azelaoyl PAF, or LY171883. These results raised the possibility that the protective effect by 15d-PGJ2 was not mediated through PPARγ activation. This idea was supported by other investigators, as further discussed below.
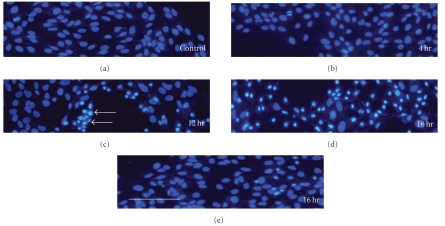
Figure 1.
Prevention of H2O2-induced nuclear condensation by 15d-PGJ2. The human RPE cell line ARPE-19 cells were treated with 1.5 mM H2O2 for various periods of time, and then processed for nuclear staining by bisbenzimide (Hoechst 33258) to identify apoptotic cells [48]; (a): untreated cells; (b): 4 hours; (c): 12 hours; (d): 16 hours after treatment. Arrows in (c) point to representative cells with condensed nuclei, an indication of apoptosis. (e): Cells were pretreated with 1 μM 15d-PGJ2 overnight, followed by 1.5 mM H2O2 for 16 hours (without 15d-PGJ2). The number of apoptotic cells was greatly reduced by 15d-PGJ2. Scale bar: 100 μm.
The cytoprotective effect of 15d-PGJ2 on H2O2-treated RPE was further studied by Qin et al. [49]. These investigators confirmed that 1 μM 15d-PGJ2 effectively prevented H2O2-induced cell death. Other PPARγ agonists, such as AGN195037 or Roziglitazone, had no protective effects. Importantly, reduction of PPARγ by siRNA did not block the protective effect of 15d-PGJ2. This set of experiments together with those described above strongly suggests that 15d-PGJ2 protect RPE cells through a PPARγ-independent mechanism. Some properties of 15d-PGJ2 are independent of PPARγ activation, as reviewed by Straus and Glass [39].
Subsequent studies by Qin et al. [49] indicated that 15d-PGJ2 could upregulate glutamylcyteine synthetase, the rate-limiting enzyme that regulates glutathione (GSH) synthesis. These investigators reported that 15d-PGJ2 at 1-2 μM induced GSH levels to ∼300% of control. With 1 μM 15d-PGJ2, the maximal induction occurred at 18–24 hours after treatment. This GSH induction appeared to depend on JNK and p38 pathways because inhibitors of these pathways greatly reduced GSH induction by 15d-PGJ2. Induction of GSH by 15d-PGJ2 is also observed in other cell types [37, 50, 51]. Since intracellular GSH is very important in cellular defense against oxidative stress, the induction of GSH should have an important role in the protective effect caused by 15d-PGJ2 treatment. Even though induction of heme oxygenase-1 (HO-1) was associated with cytoprotective effects of 15d-PGJ2 in other studies [52], this enzyme had no roles in the protection observed in this experimental system.
If 15d-PGJ2 greatly induced intracellular GSH, one would expect that this agent should reduce oxidant-induced intracellular reactive oxygen species generation. Indeed, we reported earlier that 15d-PGJ2 could reduce H2O2- and tBH-induced reactive oxygen species in human ARPE-19 cells [53]. For example, pretreatment of cells with 1 μM 15d-PGJ2 reduced 1 mM H2O2-generated reactive oxygen species to ∼80% of untreated cells challenged with H2O2. Similar reduction was observed in cells challenged with tBH. This reduction apparently was enough to keep free radical levels under a critical threshold, thus rendering cells survive an otherwise detrimental oxidant insult.
Our study further indicated that 15d-PGJ2 helped RPE cells to maintain mitochondrial integrity [53]. This is significant because mitochondria are intimately involved in apoptosis. Oxidative stress can induce mitochondria dysfunction, which is a critical event that leads to cytochrome c release and subsequent activation of caspases, a group of enzymes that executes apoptosis [54, 55]. An important event associated with mitochondrial dysfunction is a drop of mitochondrial membrane potential (ΔΨm), that is, mitochondrial depolarization. This event initiated by oxidative stress was largely prevented by 1 μM 15d-PGJ2 (see Figure 2). This is likely to prevent cytochrome c release and subsequent activation of the apoptosis pathway.
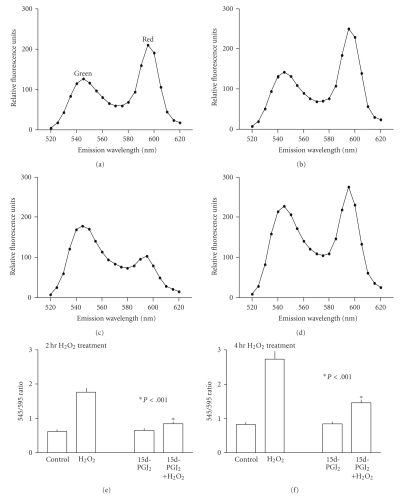
Figure 2.
Prevention of H2O2-induced mitochondrial membrane depolarization by 15d-PGJ2. Binding of the JC-1 dyes to mitochondria leads to the appearance of two peaks. The green peak (at ∼545 nm) represents JC-1 monomers of this dye. The red peak (at ∼595 nm) represents JC-1 aggregates, which is caused by the negative charge of mitochondrial membrane. Depolarization of mitochondrial membrane causes a shift in the emission spectrum from red to green color, which can be quantified by a fluorescence plate reader. The relative intensity of these two peaks is a measurement of relative mitochondrial potential such that a higher ratio represents more mitochondrial membrane depolarization. (a)–(d): The JC-1 emission spectra between 520 nm to 620 nm were determined for cells under various conditions [53]; (a): untreated cells; (B): cells treated with 1 μM 15d-PGJ2 overnight; (c): cells treated with 1.5 mM H2O2 for 2 hours; (d): Cells treated with 1 μM 15d-PGJ2 overnight, then with 1.5 mM H2O2 (without 15d-PGJ2) for 2 hours. Note H2O2 caused a shift of the relative intensity of the peaks, and 15d-PGJ2 pretreatment restored membrane potential to a condition closer to untreated cells. (e)-(f): Cells were pretreated with 1 μM 15d-PGJ2 overnight, then with 1.5 mM H2O2 (without 15d-PGJ2) for 2 hours (e) or 4 hours (f); then the 545/595 emission intensity ratios were determined. Note in either 2-hour or 4-hour treatment, H2O2 caused an increase of the 545/595 emission intensity ratio, indicating mitochondrial depolarization. 15d-PGJ2 pretreatment restored the ratio to that similar to control value (P < .001 between H2O2-treated and 15d-PGJ2+H2O2-treated cells in (e) and (f)).
5. CYTOPROTECTIVE VERSUS CYTOTOXIC EFFECTS OF 15D-PGJ2
In addition to those studies described above regarding the protective effect of 15d-PGJ2 against oxidative stress on RPE, this agent is cytoprotective toward other retinal cells. For example, Aoun et al. [56] reported that glutamate could induce oxidative stress and cell death in the rat retinal ganglion cell line, RGC-5 cells. This cell death was prevented by 1–5 μM 15d-PGJ2. Outside of retina, 15d-PGJ2 was effective in preventing glutamate-induced cell death of primary cortical neurons [51]. Both groups attributed the protective effect through the antioxidative property of 15d-PGJ2. In this respect, it should be noted that this agent can also prevent cell death caused by toxic metabolites of oxidative stress. For example, we reported earlier that 15d-PGJ2 prevented cytotoxicity of oxysterols, toxic cholesterol metabolites generated under oxidative stress [57]. The cytoprotective effect of 15d-PGJ2 in other experimental systems were also described in reports by Kawamoto et al. [58] and Itoh et al. [59].
It is clear now that 15d-PGJ2 can induce intracellular oxidative stress [60, 61]. It is likely that this agent at low concentrations (1–5 μM) can cause low levels of oxidative stress, thus inducing the build up of cellular defense mechanisms against oxidative stress. However, at high concentrations, this agent can cause severe oxidative stress and cell death [60, 61]. Induction of apoptosis by this agent was reported in several cell types [62–64]. This interesting bifunctional property of 15d-PGJ2 has been reported [50], and is a subject of review by Na and Surh [65]. This also prompts a recent microarray study analyzing the regulation of prosurvival and prodeath genes by this agent [66].
6. CONCLUDING REMARKS
Oxidative stress is believed to play an important role in RPE cell death during aging and the development of age-related macular degeneration. During phagocytosis of rod outer segments, there is an upregulation of PPARγ in RPE cells. The natural PPARγ ligand 15d-PGJ2 has a potent protective effect for RPE under oxidative stress. This agent can upregulate GSH and prevent oxidant-induced intracellular reactive oxygen species accumulation, mitochondrial depolarization, and apoptosis (see Figure 3). There is also evidence that 15d-PGJ2 can prevent glutamate-induced death of cultured retinal ganglion cells. Current data suggests that this cytoprotection is not mediated through the activation of PPARγ. The antioxidative property of 15d-PGJ2 may be useful in future development of pharmacological tools against retinal diseases caused by oxidative stress.
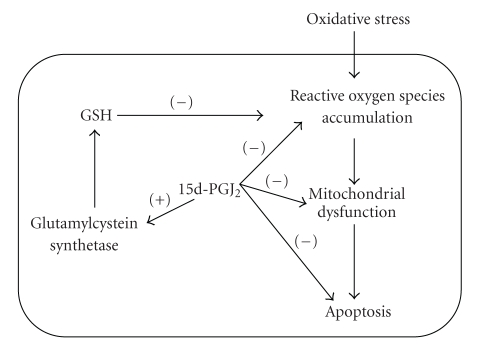
Figure 3.
Protective effects of 15d-PGJ2 against oxidative stress. Oxidative stress on RPE cells can lead to intracellular accumulation of reactive oxygen species. This can result in mitochondrial dysfunction, which in turn causes activation of the apoptosis pathway. Current data suggests that 15d-PGJ2 can block each of these events. One mechanism that causes this protection is through upregulation of GSH synthesis by activation of the glutamylcystein synthetase. There is a possibility that other cytoprotective mechanisms are also activated that lead to prevention of apoptosis. This remains to be studied.
Finally, based on anti-inflammatory effects of 15d-PGJ2, we would like to speculate that this agent might be effective in the treatment of other ocular diseases such as idiopathic autoimmune anterior uveitis. To confirm our hypothesis, we intend to explore the effect of 15d-PGJ2 on experimental autoimmune anterior uveitis (EAAU) which serves as an animal model of idiopathic human autoimmune anterior uveitis [67, 68].
ACKNOWLEDGMENT
This work was supported by funds from Research to Prevent Blindness (NY, USA).
References
- 1.O'Connell SR, Bressler NM. Age-related macular degeneration. In: Regillo CD, Brown GC, Flynn HW Jr., editors. Vitreoretinal Disease: The Essentials. New York, NY, USA: Thieme Medical Publishers; 1999. pp. 213–240. [Google Scholar]
- 2.Cai J, Nelson KC, Wu M, Sternberg P, Jr., Jones DP. Oxidative damage and protection of the RPE. Progress in Retinal and Eye Research. 2000;19(2):205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 3.Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Molecular Vision. 1999;5:32–42. [PMC free article] [PubMed] [Google Scholar]
- 4.Del Priore LV, Kuo Y-H, Tezel TH. Age-related changes in human RPE cell density and apoptosis proportion in situ. Investigative Ophthalmology & Visual Science. 2002;43(10):3312–3318. [PubMed] [Google Scholar]
- 5.Dunaief JL, Dentchev T, Ying G-S, Milam AH. The role of apoptosis in age-related macular degeneration. Archives of Ophthalmology. 2002;120(11):1435–1442. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- 6.Tate DJ, Jr., Miceli MV, Newsome DA. Phagocytosis and H2O2 induce catalase and metallothionein gene expression in human retinal pigment epithelial cells. Investigative Ophthalmology & Visual Science. 1995;36(7):1271–1279. [PubMed] [Google Scholar]
- 7.Singhal SS, Godley BF, Chandra A, et al. Induction of glutathione S-transferase hGST 5.8 is an early response to oxidative stress in RPE cells. Investigative Ophthalmology & Visual Science. 1999;40(11):2652–2659. [PubMed] [Google Scholar]
- 8.Tate DJ, Newsome DA, Oliver PD. Metallothionein shows an age-related decrease in human macular retinal pigment epithelium. Investigative Ophthalmology & Visual Science. 1993;34(7):2348–2351. [PubMed] [Google Scholar]
- 9.Liles MR, Newsome DA, Oliver PD. Antioxidant enzymes in the aging human retinal pigment epithelium. Archives of Ophthalmology. 1991;109(9):1285–1288. doi: 10.1001/archopht.1991.01080090111033. [DOI] [PubMed] [Google Scholar]
- 10.Gao X, Talalay P. Induction of phase 2 genes by sulforaphane protects, retinal pigment epithelial cells against photooxidative damage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(28):10446–10451. doi: 10.1073/pnas.0403886101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanito M, Kwon Y-W, Kondo N, et al. Cytoprotective effects of geranylgeranylacetone against retinal photooxidative damage. Journal of Neuroscience. 2005;25(9):2396–2404. doi: 10.1523/JNEUROSCI.4866-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zareba M, Szewczyk G, Sarna T, et al. Effects of photodegradation on the physical and antioxidant properties of melanosomes isolated from retinal pigment epithelium. Photochemistry and Photobiology. 2006;82(4):1024–1029. doi: 10.1562/2006-03-08-ra-836. [DOI] [PubMed] [Google Scholar]
- 13.Voloboueva LA, Killilea DW, Atamna H, Ames BN. N-tert hydroxylamine, a mitochondrial antioxidant, protects human retinal pigment epithelial cells from iron overload: relevance to macular degeneration. The FASEB Journal. 2007;21(14) doi: 10.1096/fj.07-8396com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suter M, Remé C, Grimm C, et al. Age-related macular degeneration. The lipofusion component N-retinyl- N-retinylidene ethanolamine detaches proapoptotic proteins from mitochondria and induces apoptosis in mammalian retinal pigment epithelial cells. Journal of Biological Chemistry. 2000;275(50):39625–39630. doi: 10.1074/jbc.M007049200. [DOI] [PubMed] [Google Scholar]
- 15.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Investigative Ophthalmology & Visual Science. 2000;41(7):1981–1989. [PubMed] [Google Scholar]
- 16.Jia L, Liu Z, Sun L, et al. Acrolein, a toxicant in cigarette smoke, causes oxidative damage and mitochondrial dysfunction in RPE cells: protection by (R)-α-lipoic acid. Investigative Ophthalmology & Visual Science. 2007;48(1):339–348. doi: 10.1167/iovs.06-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joffre C, Leclère L, Buteau B, et al. Oxysterols induced inflammation and oxidation in primary porcine retinal pigment epithelial cells. Current Eye Research. 2007;32(3):271–280. doi: 10.1080/02713680601187951. [DOI] [PubMed] [Google Scholar]
- 18.Ong JM, Aoki AM, Seigel GM, et al. Oxysterol-induced toxicity in R28 and ARPE-19 cells. Neurochemical Research. 2003;28(6):883–891. doi: 10.1023/a:1023223409798. [DOI] [PubMed] [Google Scholar]
- 19.Chang JY, Liu L-Z. Toxicity of cholesterol oxides on cultured neuroretinal cells. Current Eye Research. 1998;17(1):95–103. doi: 10.1076/ceyr.17.1.95.5252. [DOI] [PubMed] [Google Scholar]
- 20.Rex TS, Tsui I, Hahn P, et al. Adenovirus-mediated delivery of catalase to retinal pigment epithelial cells protects neighboring photoreceptors from photo-oxidative stress. Human Gene Therapy. 2004;15(10):960–967. doi: 10.1089/hum.2004.15.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda A, Crabb JW, Palczewski K. Microsomal glutathione S-transferase 1 in the retinal pigment epithelium: protection against oxidative stress and a potential role in aging. Biochemistry. 2005;44(2):480–489. doi: 10.1021/bi048016f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang F-Q, Alssadi R, Morehead P, Awasthi YC, Godley BF. Enhanced expression of glutathione- S-transferase A1-1 protects against oxidative stress in human retinal pigment epithelial cells. Experimental Eye Research. 2005;80(1):113–119. doi: 10.1016/j.exer.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Kasahara E, Lin L-R, Ho Y-S, Reddy VN. SOD2 protects against oxidation-induced apoptosis in mouse retinal pigment epithelium: implications for age-related macular degeneration. Investigative Ophthalmology & Visual Science. 2005;46(9):3426–3434. doi: 10.1167/iovs.05-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sreekumar PG, Kannan R, Yaung J, Spee CK, Ryan SJ, Hinton DR. Protection from oxidative stress by methionine sulfoxide reductases in RPE cells. Biochemical and Biophysical Research Communications. 2005;334(1):245–253. doi: 10.1016/j.bbrc.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 25.Matsui H, Lin L-R, Singh DP, Shinohara T, Reddy VN. Lens epithelium-derived growth factor: increased survival and decreased DNA breakage of human RPE cells induced by oxidative stress. Investigative Ophthalmology & Visual Science. 2001;42(12):2935–2941. [PubMed] [Google Scholar]
- 26.Geiger RC, Waters CM, Kamp DW, Glucksberg MR. KGF prevents oxygen-mediated damage in ARPE-19 cells. Investigative Ophthalmology & Visual Science. 2005;46(9):3435–3442. doi: 10.1167/iovs.04-1487. [DOI] [PubMed] [Google Scholar]
- 27.Tsao Y-P, Ho T-C, Chen S-L, Cheng H-C. Pigment epithelium-derived factor inhibits oxidative stress-induced cell death by activation of extracellular signal-regulated kinases in cultured retinal pigment epithelial cells. Life Sciences. 2006;79(6):545–550. doi: 10.1016/j.lfs.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 28.Godley BF, Jin GF, Guo YS, Hurst JS. Bcl-2 overexpression increases survival in human retinal pigment epithelial cells exposed to H2O2 . Experimental Eye Research. 2002;74(6):663–669. doi: 10.1006/exer.2001.1146. [DOI] [PubMed] [Google Scholar]
- 29.Alge CS, Priglinger SG, Neubauer AS, et al. Retinal pigment epithelium is protected against apoptosis by αB-crystallin. Investigative Ophthalmology & Visual Science. 2002;43(11):3575–3582. [PubMed] [Google Scholar]
- 30.Liang FQ, Green L, Wang C, Alssadi R, Godley BF. Melatonin protects human retinal pigment epithelial (RPE) cells against oxidative stress. Experimental Eye Research. 2004;78(6):1069–1075. doi: 10.1016/j.exer.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Jarrett SG, Boulton ME. Poly(ADP-ribose) polymerase offers protection against oxidative and alkylation damage to the nuclear and mitochondrial genomes of the retinal pigment epithelium. Ophthalmic Research. 2007;39(4):213–223. doi: 10.1159/000104683. [DOI] [PubMed] [Google Scholar]
- 32.Voloboueva LA, Liu J, Suh JH, Ames BN, Miller SS. (R-α-lipoic acid protects retinal pigment epithelial cells from oxidative damage. Investigative Ophthalmology & Visual Science. 2005;46(11):4302–4310. doi: 10.1167/iovs.04-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu X, Tang Y, Li F, et al. Protection against hydrogen peroxide-induced cell death in cultured human retinal pigment epithelial cells by 17β-estradiol: a differential gene expression profile. Mechanisms of Ageing and Development. 2005;126(11):1135–1145. doi: 10.1016/j.mad.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Hanneken A, Lin FF, Johnson J, Maher P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Investigative Ophthalmology & Visual Science. 2006;47(7):3164–3177. doi: 10.1167/iovs.04-1369. [DOI] [PubMed] [Google Scholar]
- 35.Shamsi FA, Chaudhry IA, Boulton ME, Al-Rajhi AA. L-carnitine protects human retinal pigment epithelial cells from oxidative damage. Current Eye Research. 2007;32(6):575–584. doi: 10.1080/02713680701363833. [DOI] [PubMed] [Google Scholar]
- 36.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nature Medicine. 1999;5(6):698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 37.Lim SY, Jang JH, Na HK, Lu SC, Rahman I, Surh YJ. 15-Deoxy-Δ12,14-prostaglandin J2 protects against nitrosative PC12 cell death through up-regulation of intracellular glutathione synthesis. Journal of Biological Chemistry. 2004;279(44):46263–46270. doi: 10.1074/jbc.M406555200. [DOI] [PubMed] [Google Scholar]
- 38.Taba Y, Sasaguri T, Miyagi M, et al. Fluid shear stress induces lipocalin-type prostaglandin D2 synthase expression in vascular endothelial cells. Circulation Research. 2000;86(9):967–973. doi: 10.1161/01.res.86.9.967. [DOI] [PubMed] [Google Scholar]
- 39.Straus DS, Glass CK. Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Medicinal Research Reviews. 2001;21(3):185–210. doi: 10.1002/med.1006. [DOI] [PubMed] [Google Scholar]
- 40.Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 41.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 42.Castrillo A, Diaz-Guerra MJ, Hortelano S, Martin-Sanz P, Bosca L. Inhibition of IκB kinase and IκB phosphorylation by 15-deoxy-Δ12,14-prostaglandin J2 in activated murine macrophages. Molecular and Cellular Biology. 2000;20(5):1692–1698. doi: 10.1128/mcb.20.5.1692-1698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrova TV, Akama KT, Van Eldik LJ. Cyclopentenone prostaglandins suppress activation of microglia: down-regulation of inducible nitric-oxide synthase by 15-deoxy-Δ12,14- prostaglandin J2 . Proceedings of the National Academy of Sciences of the United States of America. 1999;96(8):4668–4673. doi: 10.1073/pnas.96.8.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernardo A, Levi G, Minghetti L. Role of the peroxisome proliferator-activated receptor-γ (PPAR-γ) and its natural ligand 15-deoxy-Δ12,14;-prostaglandin J2 in the regulation of microglial functions. The European Journal of Neuroscience. 2000;12(7):2215–2223. doi: 10.1046/j.1460-9568.2000.00110.x. [DOI] [PubMed] [Google Scholar]
- 45.Koppal T, Petrova TV, Van Eldik LJ. Cyclopentenone prostaglandin 15-deoxy-Δ12,14;-prostaglandin J2 acts as a general inhibitor of inflammatory responses in activated BV-2 microglial cells. Brain Research. 2000;867(1-2):115–121. doi: 10.1016/s0006-8993(00)02270-8. [DOI] [PubMed] [Google Scholar]
- 46.Miceli MV, Liles MR, Newsome DA. Evaluation of oxidative processes in human pigment epithelial cells associated with retinal outer segment phagocytosis. Experimental Cell Research. 1994;214(1):242–249. doi: 10.1006/excr.1994.1254. [DOI] [PubMed] [Google Scholar]
- 47.Ershov AV, Bazan NG. Photoreceptor phagocytosis selectively activates PPARγ expression in retinal pigment epithelial cells. Journal of Neuroscience Research. 2000;60(3):328–337. doi: 10.1002/(SICI)1097-4547(20000501)60:3<328::AID-JNR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 48.Garg TK, Chang JY. Oxidative stress causes ERK phosphorylation and cell death in cultured retinal pigment epithelium: prevention of cell death by AG126 and 15-deoxy-Δ12,14-PGJ2 . BMC Ophthalmology. 2003;3:5. doi: 10.1186/1471-2415-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin S, McLaughlin AP, De Vries GW. Protection of RPE cells from oxidative injury by 15-deoxy-Δ12,14-prostaglandin J2 by augmenting GSH and activating MAPK. Investigative Ophthalmology & Visual Science. 2006;47(11):5098–5105. doi: 10.1167/iovs.06-0318. [DOI] [PubMed] [Google Scholar]
- 50.Levonen AL, Dickinson DA, Moellering DR, Mulcahy RT, Forman HJ, Darley-Usmar VM. Biphasic effects of 15-deoxy-Δ12,14-prostaglandin J2 on glutathione induction and apoptosis in human endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21(11):1846–1851. doi: 10.1161/hq1101.098488. [DOI] [PubMed] [Google Scholar]
- 51.Saito Y, Nishio K, Numakawa Y, et al. Protective effects of 15-deoxy-Δ12,14-prostaglandin J2 against glutamate-induced cell death in primary cortical neuron cultures: induction of adaptive response and enhancement of cell tolerance primarily through up-regulation of cellular glutathione. Journal of Neurochemistry. 2007;102(5):1625–1634. doi: 10.1111/j.1471-4159.2007.04701.x. [DOI] [PubMed] [Google Scholar]
- 52.Satoh T, Baba M, Nakatsuka D, et al. Role of heme oxygenase-1 protein in the neuroprotective effects of cyclopentenone prostaglandin derivatives under oxidative stress. European Journal of Neuroscience. 2003;17(11):2249–2255. doi: 10.1046/j.1460-9568.2003.02688.x. [DOI] [PubMed] [Google Scholar]
- 53.Garg TK, Chang JY. 15-deoxy-Δ12,14-Prostaglandin J2 prevents reactive oxygen species generation and mitochondrial membrane depolarization induced by oxidative stress. BMC Pharmacology. 2004;4:6. doi: 10.1186/1471-2210-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 55.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 56.Aoun P, Simpkins JW, Agarwal N. Role of PPAR-γ ligands in neuroprotection against glutamate-induced cytotoxicity in retinal ganglion cells. Investigative Ophthalmology & Visual Science. 2003;44(7):2999–3004. doi: 10.1167/iovs.02-1060. [DOI] [PubMed] [Google Scholar]
- 57.Chang JY, Liu L. Peroxisome proliferator-activated receptor agoinsts prevent 25-OH-cholesterol induced c-jun activation and cell death. BMC Pharmacology. 2001;1:10. doi: 10.1186/1471-2210-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawamoto Y, Nakamura Y, Naito Y, et al. Cyclopentenone prostaglandins as potential inducers of phase II detoxification enzymes. 15-deoxy-Δ12,14-prostaglandin J2-induced expression of glutathione S-transferases. Journal of Biological Chemistry. 2000;275(15):11291–11299. doi: 10.1074/jbc.275.15.11291. [DOI] [PubMed] [Google Scholar]
- 59.Itoh K, Mochizuki M, Ishii Y, et al. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Δ12,14-prostaglandin J2 . Molecular and Cellular Biology. 2004;24(1):36–45. doi: 10.1128/MCB.24.1.36-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kondo M, Oya-Ito T, Kumagai T, Osawa T, Uchida K. Cyclopentenone prostaglandins as potential inducers of intracellular oxidative stress. Journal of Biological Chemistry. 2001;276(15):12076–12083. doi: 10.1074/jbc.M009630200. [DOI] [PubMed] [Google Scholar]
- 61.Li L, Tao J, Davaille J, et al. 15-deoxy-Δ12,14-prostaglandin J2 induces apoptosis of human hepatic myofibroblasts. A pathway involving oxidative stress independently of peroxisome-proliferator-activated receptors. Journal of Biological Chemistry. 2001;276(41):38152–38158. doi: 10.1074/jbc.M101980200. [DOI] [PubMed] [Google Scholar]
- 62.Zander T, Kraus JA, Grommes C, et al. Induction of apoptosis in human and rat glioma by agonists of the nuclear receptor PPARγ . Journal of Neurochemistry. 2002;81(5):1052–1060. doi: 10.1046/j.1471-4159.2002.00899.x. [DOI] [PubMed] [Google Scholar]
- 63.Harris SG, Phipps RP. Prostaglandin D2, its metabolite 15-d-PGJ2, and peroxisome proliferator activated receptor-γ agonists induce apoptosis in transformed, but not normal, human T lineage cells. Journal of Immunology. 2002;105(1):23–34. doi: 10.1046/j.0019-2805.2001.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Padilla J, Kaur K, Cao HJ, Smith TJ, Phipps RP. Peroxisome proliferator activator receptor-γ agonists and 15-deoxy-Δ12,14(12,14)-PGJ2 induce apoptosis in normal and malignant B-lineage cells. Journal of Immunology. 2000;165(12):6941–6948. doi: 10.4049/jimmunol.165.12.6941. [DOI] [PubMed] [Google Scholar]
- 65.Na HK, Surh YJ. Peroxisome proliferator-activated receptor γ (PPARγ) ligands as bifunctional regulators of cell proliferation. Biochemical Pharmacology. 2003;66(8):1381–1391. doi: 10.1016/s0006-2952(03)00488-x. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z, Aris VM, Ogburn KD, Soteropoulos P, Figueiredo-Pereira ME. Prostaglandin J2 alters pro-survival and pro-death gene expression patterns and 26 S proteasome assembly in human neuroblastoma cells. Journal of Biological Chemistry. 2006;281(30):21377–21386. doi: 10.1074/jbc.M601201200. [DOI] [PubMed] [Google Scholar]
- 67.Jha P, Sohn J-H, Xu Q, et al. The complement system plays a critical role in the development of experimental autoimmune anterior uveitis. Investigative Ophthalmology & Visual Science. 2006;47(3):1030–1038. doi: 10.1167/iovs.05-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jha P, Sohn JH, Xu Q, et al. Suppression of complement regulatory proteins (CRPs) exacerbates experimental autoimmune anterior uveitis (EAAU) Journal of Immunology. 2006;176(12):7221–7231. doi: 10.4049/jimmunol.176.12.7221. [DOI] [PubMed] [Google Scholar]