Abstract
Objective
Hypoxia induces coronary artery dilation, but the responsible mechanism is largely unknown. Many stimuli induce arterial smooth muscle relaxation by reducing ser19-myosin regulatory light chain (MLC) phosphorylation. Other stimuli can induce smooth muscle relaxation without reductions in ser19-MLC phosphorylation. This form of relaxation has been termed force suppression and appears to be associated with heat shock protein 20 (HSP20) phosphorylation on ser16. We investigated whether hypoxia-induced sustained dilation in swine coronary arteries was promoted without ser19-MLC dephosphorylation and associated with ser16-HSP20 phosphorylation. Nitroglycerin vasodilation served as control.
Methods
In a pressure myograph, the tunica media of intact pre-contracted (PGF2α;10-5 m) porcine coronary artery segments were cannulated using a microdialysis catheter. Diameter responses and interstitial lactate/pyruvate ratios were studied during 90 min hypoxia, hypoxia + reoxygenation (60 min), nitroglycerin (100 μm, 90 min), and nitroglycerin + wash-out (60 min). The arterial segments were snap-frozen and analysed for ser16-HSP20 phosphorylation and ser19-MLC phosphorylation.
Results
The normalized diameter responses to hypoxia (6.1 ± 4.3%) and nitroglycerin (12.6 ± 1.6%) were both significantly greater than normoxic control arteries (-10.5 ± 1.8%, anova, P < 0.05). Ser16-HSP20 phosphorylation was increased with hypoxia and nitroglycerin treatment and ser16-HSP20 phosphorylation correlated with changes in diameters (n = 29, r2 = 0.64, P < 0.001). Ser19-MLC phosphorylation was not significantly altered by hypoxia. The lactate/pyruvate ratio was significantly increased in hypoxic arteries but did not correlate with diameters or ser16-HSP20 phosphorylation.
Conclusion
Ser16-HSP20 phosphorylation is a potential regulator of hypoxia-induced dilation in coronary arteries.
Keywords: hypoxia, metabolism, microdialysis, signal transduction, smooth muscle, vasodilation
Vasodilation is a relevant response to decreased tissue oxygen tension in order to increase blood flow and oxygen supply to an endangered region. Hypoxia-induced vasodilation in systemic conduit arteries differs in nature from other types of vasodilation and a single responsible mechanism has not been identified (Taggart & Wray 1998).
Correlation between vascular reactivity and interstitial lactate suggests a role for lactate in hypoxic vasodilation in porcine coronary arteries (Frøbert et al. 2002) and an energy limitation mechanism has not been ruled out in hypoxic vasodilation.
In activated vascular smooth muscle, increased [Ca2+]i combines with calmodulin and activates myosin light chain kinase. This enzyme phosphorylates myosin regulatory light chains (MLC) on serine-19 (ser19), which promotes cross-bridge cycling with the thin filament and results in a sustained contractile response (Opie 1998). In many cases, smooth muscle relaxation proceeds via a reversal of this contraction process: reduction of [Ca2+]i, inactivation of MLC kinase, and dephosphorylation of MLC (Rembold et al. 2000a). However, smooth muscle relaxation induced by increases in cAMP and cGMP are more complex and may induce relaxation without MLC dephosphorylation and this has also been found in hypoxia-induced smooth muscle relaxation (Obara et al. 1997, Taggart et al. 1997). When tissues are maximally stimulated with contractile agonists the sustained phase of cAMP and cGMP-dependent relaxation has been shown to be associated with phosphorylation of heat shock protein 20 (HSP20) on serine 16 (ser16) which has been hypothesized to alter the conformation of thin filaments and thereby inhibit attachment of phosphorylated cross bridges (Rembold et al. 2000a). The exact mechanism of force uncoupling by HSP20 is not known but could be because of interference of phosphorylated HSP20 with the myosin binding to actin (Meeks et al. 2005). The amino acid residues 110-121 of HSP20, which have a high degree of sequence homology with a region of cardiac troponin I, are highly conserved among the other heat shock proteins and could represent the specific part of HSP20 that regulates contractile force (Rembold et al. 2000a). Others have shown (Flynn et al. 2003) that transduction of HSP20 phosphopeptide analogues into porcine coronary artery dose-dependently relaxes smooth muscle and that transduction of a scrambled sequence of the same amino acids does not. Furthermore, HSP20 has also been co-localized with actin in myocardium and phosphorylated HSP20 increases myocyte shortening suggesting a role also in cardiac myocyte function (Pipkin et al. 2003).
In the present study, we hypothesized that hypoxia-induced sustained dilation in swine coronary arteries was promoted without MLC dephosphorylation and associated with phosphorylation of HSP20 on ser16. Thus, we expected an association between coronary artery diameter increase and ser16 phosphorylation and we anticipated a correspondence between interstitial lactate concentration and ser16 phosphorylation during hypoxia. We previously found that nitroglycerin induces vascular smooth muscle relaxation and significantly increases HSP20 phosphorylation (Rembold & O’Connor 2000b) and we therefore used nitroglycerin vasodilation as a positive control.
Material and methods
Material
Hearts from 70 to 90 kg hogs were obtained shortly after slaughter (animals were killed in the abattoir in accordance with internationally approved standards). The aorta was cannulated and the coronary circulation perfused with 100 mL of a buffer solution (PSS) bubbled with 5% CO2-95% O2. The hearts were bathed in PSS at 5 °C for approximately 2 h until the start of the experiment. The left anterior descending coronary artery (LAD) was carefully dissected and the proximal 3-4 cm of the artery left intact. The arterial segments usually have one to three branches in the proximal part. The branches were ligated with 5-0 silk sutures during dissection in cold PSS. The anterior surface of the LAD was marked in both ends with Indian ink and the in situ length of the segment was recorded.
Pressure myograph
Cylindrical arterial segments (1.5-2 cm) were mounted at both ends on stainless steel cannulae and fastened with sutures in a 20 mL vessel chamber. The arterial segments were bathed in PSS continuously bubbled with 5% CO2-95% O2 (PO2 > 650 mmHg; ISO2-D oxygen analyser, WPI, Sarasota, FL, USA) and the temperature was gradually raised to 37 °C. The internal pressure was controlled by adjustment of two reservoirs containing PSS mounted on a pressure column and connected to the cannulae. Pressure transducers close to the ‘arterial’ end of each cannula continuously measured the internal pressure. The segments were stretched to the in situ length by operating a micrometer device on one of the cannulae and a pressure of 40 mmHg was applied to the vessel during a stabilizing period of 1 h. The external diameter of the arterial segment was automatically determined by video imaging at a frequency of 20 Hz. Internal arterial pressure, the outer diameter, and a video image of the arterial segment were continuously monitored and stored on computer (Vessel View software, version 1.2; JP Trading, Aarhus, Denmark). Before the start of the experiments the video dimension analyser was calibrated with a 3000 × 3000 μm phantom image in the horizontal and vertical directions. At the end of the experiments the calibration was verified by repeated measurements of the phantom.
Microdialysis and calculations
Microdialysis catheters (CMA/11 microdialysis probe; CMA, Stockholm, Sweden) were placed in the interstitium of the coronary artery mounted in the pressure myograph. The microdialysis catheters have a 6 kDa molecular cut-off, an outer diameter of 0.24 mm and a membrane length of 4 mm. Perfusion of the catheter was started immediately at a rate of 0.3 μl min-1 with isotonic saline. The catheters were perfused for 60 min prior to the experiments. The effluent from the microdialysis catheters (dialysate) was collected in consecutive 30 min fractions (9 μl). There was a delay of 12 min between the passage of the perfusate through the microdialysis catheter and collection in vials. This time delay was considered in the calculations and all data are presented in real time.
Glucose, lactate and pyruvate concentrations in the dialysate were measured by an automated spectrophotometric kinetic enzymatic analyzer (CMA 600; CMA). The analyser enzymatically converts lactate, pyruvate, and glucose to H2O2. Peroxidase catalyses a reaction between H2O2 and other substrates to form a red-violet-coloured product, quinonediimine. The rate of formation of the quinonediimine is measured at 546 nm and is proportional to the lactate, pyruvate, and glucose concentrations.
Procedures
During a stabilizing period of 1-2 h, contraction of the coronary arteries was induced by addition of potassium chloride (125 mm) until a reproducible response was obtained (at least twice). After an additional stabilizing period with PSS in the organ bath a stable arterial contraction was induced with prostaglandin F2α (PGF2α; 10-5 m).
Hypoxia was induced by 5% CO2-95% N2 (PO2 approximately 35 mmHg). Simultaneously the PSS in the lumen was exchanged with deoxygenated PSS. Oxygenation was re-established after 90 min of hypoxia by washing with PSS aerated with 5% CO2-95% O2 and the arterial response during the following 60 min was recorded (reoxygenation period). pH in the organ bath and the dialysate was monitored with a small pH electrode (MI-410; Microelectrodes, Bedford, NH, USA) connected to a pH meter (PHM210; Radiometer, Copenhagen, Denmark).
Nitrovasodilation was induced with addition of 100 μm of nitroglycerin in the organ bath and in the coronary arterial lumen while continuously bubbling with 5% CO2-95% O2. The response to nitroglycerin was rapid but not prolonged and it was necessary to change the nitroglycerin-PSS three to four times in order to sustain vasorelaxation. In some experiments, after 90 min the organ bath and arterial lumen was washed with PSS aerated with 5% CO2-95% O2 and the arterial response during the following 60 min was recorded (nitroglycerin wash-out).
Freezing
Acetone and dry ice was prepared in a 50 mL test tube (about 8 cm3 dry ice per 15 mL of acetone). After the treatment protocol the sutures on the stainless steel cannulae were cut and the artery was quickly pulled off and placed in cold acetone/dry ice slurry (20 g/20 mL) at -78 °C. It was ensured that the entire artery was covered by acetone and that extra dry ice was added to the acetone. The acetone/dry ice slurry including the artery was allowed to equilibrate with room temperature for at least 2 h in order to dehydrate the tissue.
HSP20 Phosphorylation by immunostaining
Rabbit anti-HSP20 antibody was made by repeated injections of our recombinant HSP20. After confirmation of an antigenic response, serum was collected and used for immunostaining (for blot see Rembold & O’Connor 2000b). HSP20 phosphorylation was estimated in tissues homogenized in 0.5 mL of 1% SDS, 10% glycerol, 1 mm DTT [controls show that this buffer extracts as much HSP20 as the buffer described by Murad (Rapoport et al. 1982)] by means of a small glass-glass homogenizer. The sample was then vortexed, frozen in liquid nitrogen and stored at -78 °C.
Proteins were separated by isoelectric focusing (50 : 50 ampholyte mixture of pI 4-6.5 and pI 5-8), blotted to nitrocellulose, and then incubated with the above anti HSP20 antibody. After washing, incubation with secondary antibodies, and detection with enhanced chemiluminescence, the blots were quantitated by laserscanning. Quantification of the fraction of HSP20 phosphorylation was estimated from the shift in the isoelectric point observed with ser16 phosphorylation. See Figure 1 for a representative blot of HSP20 phosphorylation including identification of the bands that represent ser16 phosphorylation. Ser16 phosphorylation is reported as mol Pi/mol HSP20.
Figure 1.
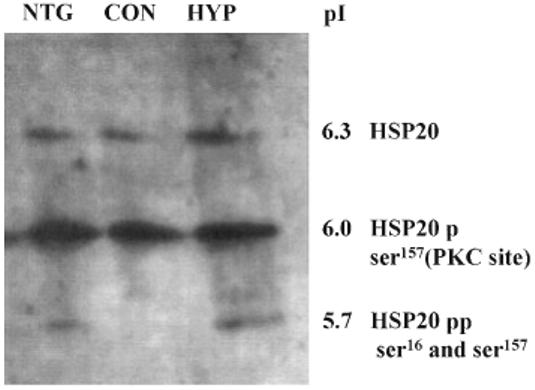
A representative Western blot of HSP20 immunore-activity. Three coronary artery segments were stimulated with PGF2α 10-5 m. One was then relaxed by addition of nitroglycerin (NTG, 100 μm) for 90 min (left), a second remained contracted (Con, centre), and the third relaxed by induction of hypoxia (Hyp) for 90 min, right). Arteries were then frozen, homogenized, proteins separated by isoelectric focusing, immunostained with rabbit anti-HSP20 antibody, and imaged with enhanced chemiluminescence. The top, faint band is unphosphorylated HSP20, the centre, major band is HSP20 phosphorylated on the PKC site, and the lower (more acidic) band is HSP20 phosphorylated at both the PKC site and serine 16.
MLC Phosphorylation
MLC phosphorylation was estimated in tissues frozen and homogenized as described above. Proteins were first separated by isoelectric focusing (ampholyte 50 : 50 mixture of pI 4-6.5 and pI 4.5-5.4), blotted to nitrocellulose, and then incubated with anti-MLC antibody. After washing incubation with secondary antibodies, and detection with enhanced chemiluminescence, the blots were quantitated by laserscanning. Prior work with two-dimensional isoelectric focusing/SDS PAGE technique resolves and quantifies five species: (i) smooth muscle specific (SM) dephosphorylated; (ii) monophosphorylated SM; (iii) diphosphorylated SM + dephosphorylated non-muscle (NM) isoform; (iv) monophosphorylated NM; and (v) diphosphorylated NM (Gaylinn et al. 1989). We measured MLC phosphorylation as the ratio of the monophosphorylated SM divided by the sum of the dephosphorylated SM and the mono-phosphorylated SM and report results as mol Pi/mol MLC. This is valid for three reasons: (i) the amount of diphosphorylation on ser19 and thr18 is small (only 10-20% of monophosphorylated form 1); (ii) diphosphorylation does not appear to produce functional effects (Haeberle et al. 1988, Gaylinn et al. 1989); iii) the fractional phosphorylation of SM- and NM-MLC isoforms is the same (Monical et al. 1993).
Solutions
The PSS had the following composition (mM): NaCl 119, NaHCO3 25, KCl 4.7, MgSO4 1.2, CaCl2 1.5, glucose 5.5 and 2-[4-(2-hydroxyethyl)-1-piperazinyl]-ethansulphonic acid (HEPES) 20. In experiments where K-PSS was used, NaCl was exchanged for KCl on an equimolar basis to a final K+-concentration of 125 mm. All solutions were made with analytical grade chemicals and twice distilled water. PGF2α (Dinoprost) was obtained from Upjohn, Germany.
Statistics
Values are presented as mean ± standard error and number of vessels (one per pig). Because the coronary artery diameter varied between pigs the steady state diameter induced by PGF2α was used as an internal standard (100%). Data were evaluated by one-way analysis of variance (anova) with Dunnett’s post hoc test for multiple comparisons. Bivariate associations were examined by least square linear regression. P < 0.05 was regarded as significant.
Results
Response to hypoxia and hypoxia-reoxygenation
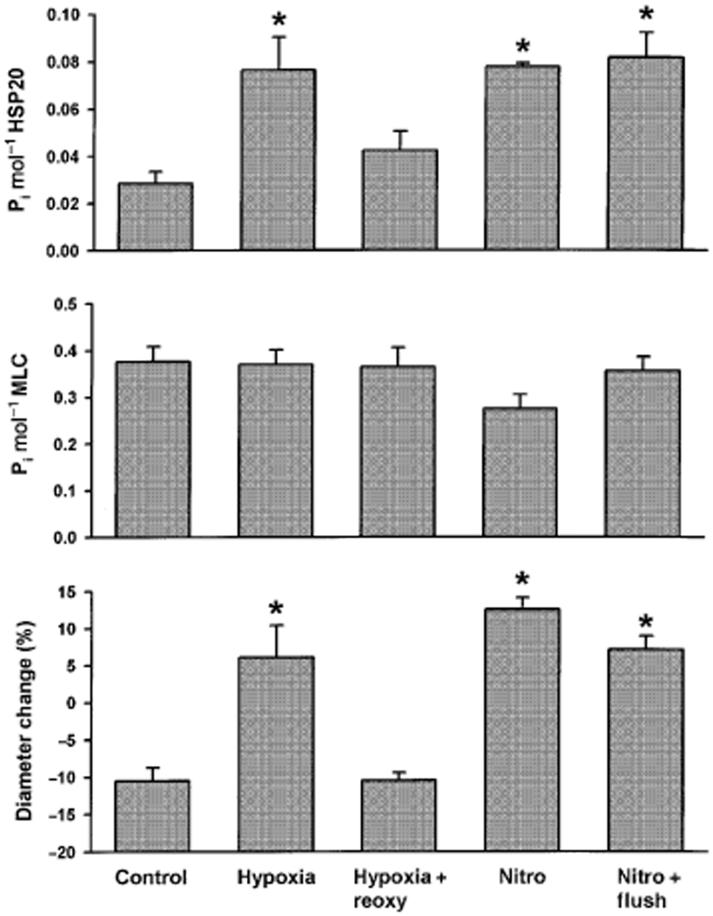
Data were obtained from 29 arteries (one per pig), all contracted by 10-5 m prostaglandin F2α (steady state contracted diameter 3.50 ± 0.11 mm). Ninety minutes of hypoxia significantly increased normalized diameter (Fig. 2) and ser16-HSP20 phosphorylation (anova, P < 0.05; Fig. 3) without significantly altering ser19-MLC phosphorylation. Sixty minutes of reoxygenation after 90 min of hypoxia was associated with diameters, ser16-HSP20 phosphorylation, and ser19-MLC phosphorylation values that did not significantly differ from control, suggesting that the hypoxia-dependent increased diameter and ser16-HSP20 phosphorylation were reversed by reoxygenation.
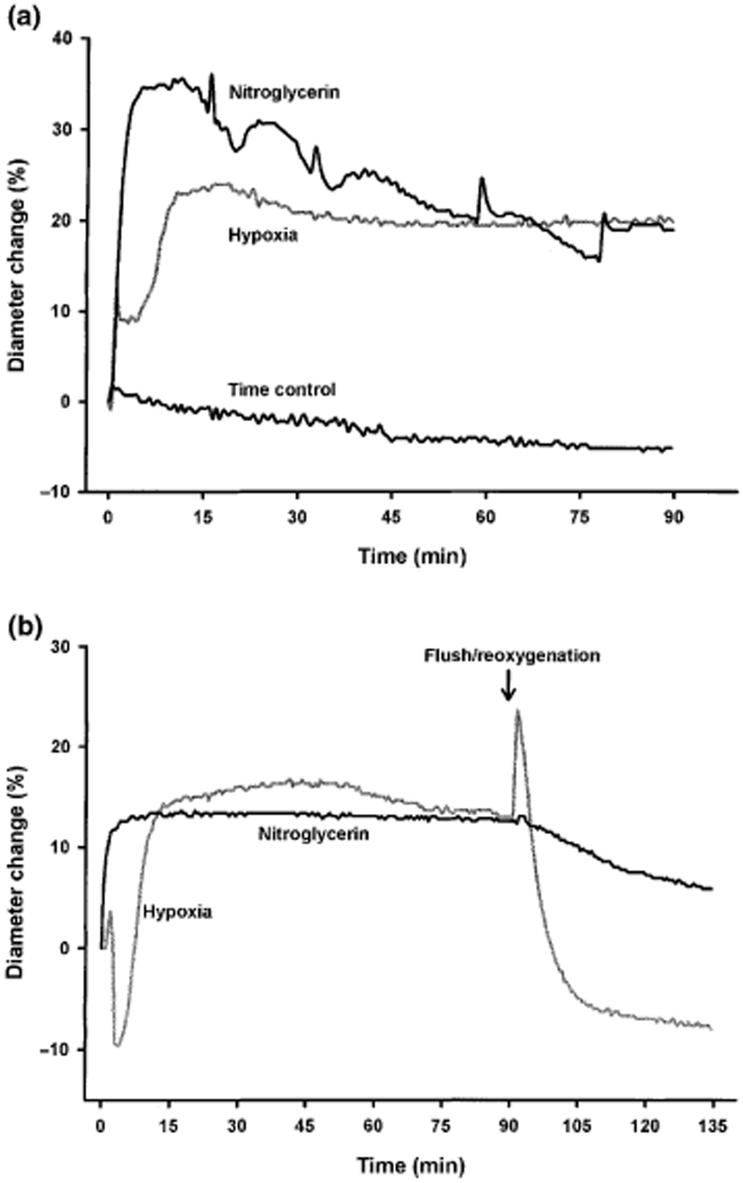
Figure 2.
(a) Representative diameter tracings (normalized to per cent change) of coronary arteries exposed to nitroglycerin, hypoxia and a time control experiment. The response to nitroglycerin was rapid but not prolonged and it was necessary to change the nitroglycerin-PSS four times in order to sustain vasorelaxation and this is the reason for the spikes in the nitroglycerin tracing. (b) Tracings of arteries exposed to hypoxia and reoxygenation and to nitroglycerin and flush.
Figure 3.
Ser16-HSP20 phosphorylation (top panel), ser19-MLC phosphorylation (second panel), and diameters (bottom panel) were measured in swine coronary arteries in a control situation (n = 6), after 90 min of hypoxia (n = 6), 90 min of nitroglycerin (Nitro) (n = 6), following hypoxia and reoxy-genation for 60 min (Hypoxia + Reoxy) (n = 6) and following nitroglycerin and 60 min wash-out (Nitro + Flush) (n = 5). *P < 0.05 compared with control, anova.
Response to nitroglycerin
Nitroglycerin significantly increased normalized diameter and ser16-HSP20 phosphorylation (anova, P < 0.05; Fig. 3). Ser19-MLC phosphorylation values appeared to be lower, but did not reach statistical significance. These data suggest that the mechanism responsible for nitroglycerin vasodilation may differ from hypoxic vasodilation. For unclear reasons, wash-out of nitroglycerin was not associated with a reduction in arterial diameter. This was associated with ser16-HSP20 phosphorylation and ser19-MLC phosphorylation values that did not differ from nitoglycerin.
Dependence of vasodilation on ser16-HSP20 phosphorylation
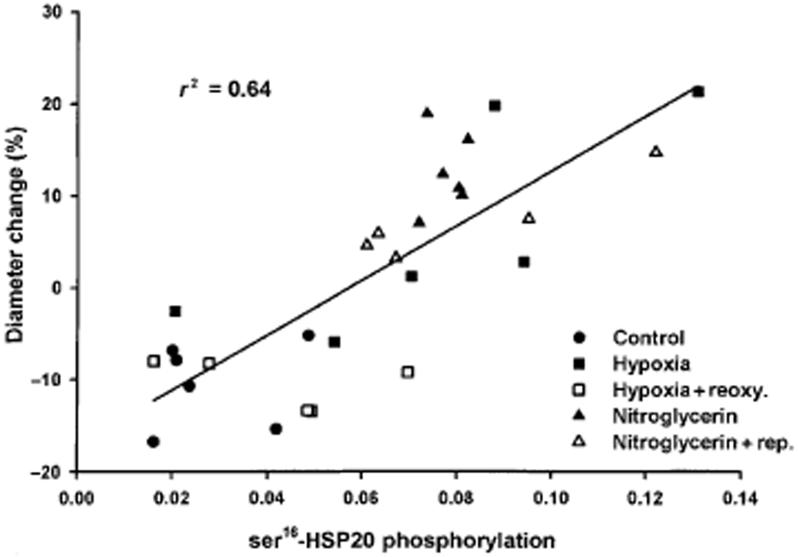
If ser16-HSP20 phosphorylation regulates the sustained inhibitory phase of relaxation, then a correlation with artery diameter could be expected. Figure 4 shows a scatter plot of changes in diameters and ser16-HSP20 phosphorylation with a highly significant correlation (r2 = 0.64; P < 0.001).
Figure 4.
Correlation between ser16-HSP20 phosphorylation and coronary artery diameter change in per cent (baseline = 0%). Each symbol represents a separate vessel.
Glucose, lactate and pyruvate
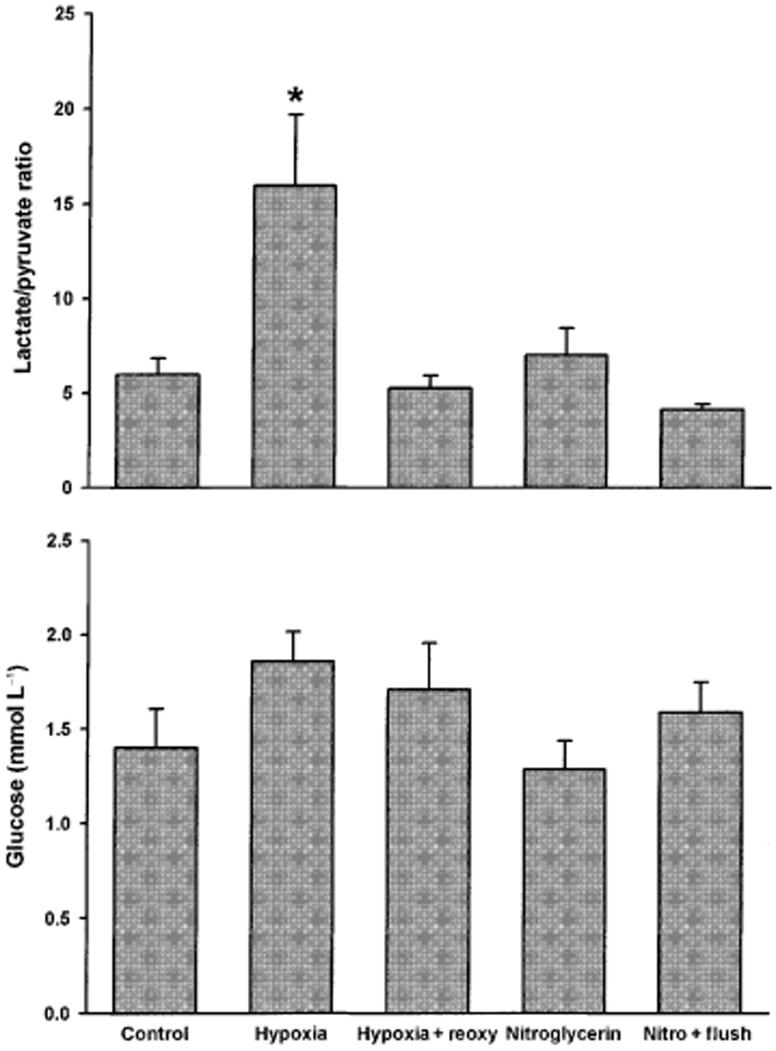
The lactate/pyruvate ratio is a sensitive measure of tissue hypoxia and this ratio was significantly increased in hypoxic arteries (Fig. 5). Pyruvate and lactate increase the phosphorylation potential in the heart (Laughlin et al. 1993) but there were no correlation between interstitial concentrations of lactate, pyruvate or glucose and phosphorylation of HSP20 on ser16.
Figure 5.
Lactate/pyruvate ratio (top panel) and glucose concentration (bottom panel) were measured in microdialysis samples. Vertical axis legends and sample sizes as in Figure 3. *P < 0.05 compared with control, anova.
Discussion
The main findings in this study of HSP20 phosphorylation and interstitial metabolites in hypoxia- and nitroglycerin-induced dilation of swine coronary arteries during hypoxic conditions were: (1) ser16-HSP20 phosphorylation increased with hypoxia and nitroglycerin and was highly significantly correlated with changes in diameters suggesting a similar mechanism of action to suppress force; (2) hypoxic vasodilation was not associated with a significant change in ser19-MLC phosphorylation, suggesting that the relaxation was ‘force suppression’; (3) the lactate/pyruvate ratio was significantly increased only in hypoxic arteries. There was no correlation between lactate/pyruvate ratio and diameters or ser16-HSP20 phosphorylation.
Several candidates have been suggested as regulators of hypoxia-induced vasodilation-lactate (Frøbert et al. 2002), adenosine (MacLean et al. 1998), endothelium-derived factors (Cable et al. 1999), opening of KATP channels (Daut et al. 1990) and decreased Ca2+ sensitivity (Shimizu et al. 2000) - but when each candidate has selectively been blocked or knocked out the hypoxic vasodilation is more or less unchanged (Taggart & Wray 1998, Frøbert et al. 2002). This has lead to the hypothesis that the processes involved in hypoxia-induced vasodilation are likely to be many, acting in concert, rather than the action of one altered parameter (Taggart & Wray 1998).
Previously, smooth muscle relaxation was hypothesized to be a single process involving reversal of the contractile process. We have demonstrated that smooth muscle relaxation can involve (1) reductions in [Ca2+]i-dependent ser19-MLC phosphorylation or (2) a sustained inhibition of force associated with ser16-HSP20 phosphorylation and not dependent on reductions in ser19-MLC phosphorylation, i.e. force suppression (Rembold et al. 2000a, Rembold et al. 2001). We have found relations between ser16-HSP20 phosphorylation and relaxation when tissues were treated with nitroglycerin (Rembold et al. 2000a), forskolin (Rembold et al. 2001), or thermal stress (O’Connor & Rembold 2002, Rembold & Kaufman 2003) in swine carotid artery. In contrast, vasorelaxation with Mg2+ did not significantly change ser16-HSP20 phosphorylation (Rembold & O’Connor 2000b). As hypoxia may result in reduction of cytosolic [ATP], and thus in a possible decline in phosphorylation potential, reduction in ser19-MLC dephosphorylation has been suggested to be involved in hypoxic vasodilation (Taggart & Wray 1998) and this has also been demonstrated in rat carotid artery (Zhao et al. 1996). However, findings by others (Obara et al. 1997, Taggart et al. 1997) and our results (Fig. 3) suggest this is not the case.
Heat shock proteins constitute an evolutionary highly conserved family of proteins and all cell types in the blood vessel wall respond to a number of cellular stresses with increased synthesis of heat shock proteins (Snoeckx et al. 2001). HSP20 is expressed in human soleus muscle, myocardium, and diaphragm (Kato et al. 1994). The question which arises from the present study is whether the increase in ser16-HSP20 phosphorylation with hypoxia is an adaptive change serving to protect the smooth muscle cells from potentially harmful stimuli or whether the increase is a way to explain the long sought mechanism of hypoxic vasodilation. In a previous study of rat aortas during moderate hypoxia, HSP90 expression was increased but the authors did not correlate force generation and HSP90 expression (Almgren & Olson 1999). The findings were hypothesized to represent a protective response designed to preserve or recover the function of cellular proteins essential for contraction (Almgren & Olson 1999). Despite the fact that the hypoxic stimulus was well controlled in the present study and induced a fast response on tension the 90-min steady state diameter demonstrated considerable variation in ser16-HSP20 phosphorylation. Nevertheless, there was a good correlation between diameter responses and ser16-HSP20 phosphorylation (Fig. 4). This might indicate that hypoxia does not merely induce a general (protective) HSP20 response but that the apparent dose-response relationship between diameter and ser16-HSP20 phosphorylation is related to a mechanism responsible for hypoxic dilation. The exact mechanism of force uncoupling by HSP20 is not known. Our current hypothesis is that phosphorylated HSP20, which has sequence homology with troponin I, is able to interfere with the myosin binding to actin and thereby causing relaxation, as seen for troponin I in striated muscle. We have recently published data in favour of this hypothesis (Meeks et al. 2005), suggesting a rejection of an alternative hypothesis that associates HSP20 phosphorylation with depolymerization and disassembly of the filaments and filament/cytoskeletal interaction.
It is the first time that we analyse HSP20 in pig coronary arteries, and the first time hypoxia is associated with HSP20 phosphorylation. The present study was not specifically designed to achieve a high phosphorylation response and baseline phosphorylation levels were slightly lower than previously reported (Rembold et al. 2000a) and these of course vary with protocols, preparations and conditions for the experiments.
The lactate/pyruvate ratio was significantly increased in hypoxic arteries but did not correlate with diameters or ser16-HSP20 phosphorylation. Lactate is a metabolic indicator of anaerobic metabolism and also a vasodilator in itself. While we previously found a correlation between vascular reactivity and interstitial lactate we were unable to document a unique role for this metabolite in hypoxic vasodilation because addition of dichloroacetate, an agent that reduces lactate generation by activating pyruvate dehydrogenase, promoted a more pronounced diameter increase compared with hypoxia (Frøbert et al. 2002). Although an energy limitation mechanism has been hypothesized to contribute to hypoxia-induced vasodilation, in a recent study we found that metabolic perturbation with insulin and propranolol was without effect on diameter change during hypoxia (Frøbert et al. 2004).
In conclusion, we found that hypoxia-induced vasodilation correlated with ser16-HSP20 phosphorylation, but not ser19-MLC phosphorylation. HSP20 is a potential regulator of hypoxia- and nitroglycerin-induced dilation in coronary arteries.
Acknowledgments
The study was supported by The Danish Heart Foundation (no. 03-1-2-12-22050), the John and Birthe Meyer Foundation, Karen Elise Jensens Fond, the Novo Nordisk Foundation, the Danish Medical Research Council, the Mid Atlantic AHA (0051087U), and the National Institutes of Health (HL71191).
References
- Almgren CM, Olson LE. Moderate hypoxia increases heat shock protein 90 expression in excised rat aorta. J Vasc Res. 1999;36:363–371. doi: 10.1159/000025675. [DOI] [PubMed] [Google Scholar]
- Cable DG, Pompili VJ, O’Brien T, Schaff HV. Recombinant gene transfer of endothelial nitric oxide synthase augments coronary artery relaxations during hypoxia. Circulation. 1999;100:II335–II339. doi: 10.1161/01.cir.100.suppl_2.ii-335. [DOI] [PubMed] [Google Scholar]
- Daut J, Maier-Rudolph W, von Beckerath N, et al. Hypoxic dilatation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990;247:1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Flynn CR, Komalavilas P, Tessier D, et al. Transduction of biologically active motifs of the small heat shock-related protein HSP20 leads to relaxation of vascular smooth muscle. FASEB J. 2003;17:1358–1360. doi: 10.1096/fj.02-1028fje. [DOI] [PubMed] [Google Scholar]
- Frøbert O, Mikkelsen EO, Bagger JP, Gravholt CH. Measurement of interstitial lactate during hypoxia-induced dilatation in isolated pressurised porcine coronary arteries. J Physiol. 2002;539:277–284. doi: 10.1113/jphysiol.2001.013180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøbert O, Bagger JP, Simonsen U, Lund S, Gravholt CH. Insulin increases glycolysis without further vasodilation in porcine coronary arteries exposed to hypoxia. Clin Sci (Lond) 2004;107:213–220. doi: 10.1042/CS20040006. [DOI] [PubMed] [Google Scholar]
- Gaylinn BD, Eddinger TJ, Martino PA, et al. Expression of nonmuscle myosin heavy and light chains in smooth muscle. Am J Physiol. 1989;257:C997–1004. doi: 10.1152/ajpcell.1989.257.5.C997. [DOI] [PubMed] [Google Scholar]
- Haeberle JR, Sutton TA, Trockman BA. Phosphorylation of two sites on smooth muscle myosin. Effects on contraction of glycerinated vascular smooth muscle. J Biol Chem. 1988;263:4424–4429. [PubMed] [Google Scholar]
- Kato K, Goto S, Inaguma Y, et al. Purification and characterization of a 20-kDa protein that is highly homologous to alpha B crystallin. J Biol Chem. 1994;269:15302–15309. [PubMed] [Google Scholar]
- Laughlin MR, Taylor J, Chesnick AS, DeGroot M, Balaban RS. Pyruvate and lactate metabolism in the in vivo dog heart. Am J Physiol. 1993;264:H2068–H2079. doi: 10.1152/ajpheart.1993.264.6.H2068. [DOI] [PubMed] [Google Scholar]
- MacLean DA, Sinoway LI, Leuenberger U. Systemic hypoxia elevates skeletal muscle interstitial adenosine levels in humans. Circulation. 1998;98:1990–1992. doi: 10.1161/01.cir.98.19.1990. [DOI] [PubMed] [Google Scholar]
- Meeks MK, Ripley ML, Jin Z, Rembold CM. Heat shock protein 20 mediated force suppression in forskolin relaxed swine carotid artery. Am J Physiol Cell Physiol. 2005;288:C633–639. doi: 10.1152/ajpcell.00269.2004. [DOI] [PubMed] [Google Scholar]
- Monical PL, Owens GK, Murphy RA. Expression of myosin regulatory light-chain isoforms and regulation of phosphorylation in smooth muscle. Am J Physiol. 1993;264:C1466–C1472. doi: 10.1152/ajpcell.1993.264.6.C1466. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Rembold CM. Heat-induced force suppression and HSP20 phosphorylation in swine carotid media. J Appl Physiol. 2002;93:484–488. doi: 10.1152/japplphysiol.00009.2002. [DOI] [PubMed] [Google Scholar]
- Obara K, Bowman PS, Ishida Y, Paul RJ. Effects of hypoxia on [Ca2+]i, pHi and myosin light chain phosphorylation in guinea-pig taenia caeci. J Physiol. 1997;503(Pt 2):427–433. doi: 10.1111/j.1469-7793.1997.427bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie LH. Physiology, from Cell to Circulation. Vol. 3. Lippincott-Raven; Philadelphia, New York: 1998. The Heart. [Google Scholar]
- Pipkin W, Johnson JA, Creazzo TL, et al. Localization, macromolecular associations, and function of the small heat shock-related protein HSP20 in rat heart. Circulation. 2003;107:469–476. doi: 10.1161/01.cir.0000044386.27444.5a. [DOI] [PubMed] [Google Scholar]
- Rapoport RM, Draznin MB, Murad F. Sodium nitroprusside-induced protein phosphorylation in intact rat aorta is mimicked by 8-bromo cyclic GMP. Proc Natl Acad Sci USA. 1982;79:6470–6474. doi: 10.1073/pnas.79.21.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold CM, Kaufman E. Heat induced HSP20 phosphorylation without increased cyclic nucleotide levels in swine carotid media. BMC Physiol. 2003;3:3. doi: 10.1186/1472-6793-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold CM, Foster DB, Strauss JD, Wingard CJ, Eyk JE. cGMP-mediated phosphorylation of heat shock protein 20 may cause smooth muscle relaxation without myosin light chain dephosphorylation in swine carotid artery. J Physiol. 2000a;524(Pt 3):865–878. doi: 10.1111/j.1469-7793.2000.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold CM, O’Connor M. Caldesmon and heat shock protein 20 phosphorylation in nitroglycerin- and magnesium-induced relaxation of swine carotid artery. Biochim Biophys Acta. 2000b;1500:257–264. doi: 10.1016/s0925-4439(99)00112-x. [DOI] [PubMed] [Google Scholar]
- Rembold CM, O’Connor M, Clarkson M, Wardle RL, Murphy RA. Selected contribution: HSP20 phosphorylation in nitroglycerin- and forskolin-induced sustained reductions in swine carotid media tone. J Appl Physiol. 2001;91:1460–1466. doi: 10.1152/jappl.2001.91.3.1460. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Bowman PS, Thorne G, III, Paul RJ. Effects of hypoxia on isometric force, intracellular Ca(2+), pH, and energetics in porcine coronary artery. Circ Res. 2000;86:862–870. doi: 10.1161/01.res.86.8.862. [DOI] [PubMed] [Google Scholar]
- Snoeckx LH, Cornelussen RN, Van Nieuwenhoven FA, Reneman RS, Van Der Vusse GJ. Heat shock proteins and cardiovascular pathophysiology. Physiol Rev. 2001;81:1461–1497. doi: 10.1152/physrev.2001.81.4.1461. [DOI] [PubMed] [Google Scholar]
- Taggart MJ, Wray S. Hypoxia and smooth muscle function: key regulatory events during metabolic stress. J Physiol. 1998;509(Pt 2):315–325. doi: 10.1111/j.1469-7793.1998.315bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart MJ, Menice CB, Morgan KG, Wray S. Effect of metabolic inhibition on intracellular Ca2+, phosphorylation of myosin regulatory light chain and force in rat smooth muscle. J Physiol. 1997;499(Pt 2):485–496. doi: 10.1113/jphysiol.1997.sp021943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Rhoades RA, Packer CS. Hypoxia-induced pulmonary arterial contraction appears to be dependent on myosin light chain phosphorylation. Am J Physiol. 1996;271:L768–L774. doi: 10.1152/ajplung.1996.271.5.L768. [DOI] [PubMed] [Google Scholar]