Abstract
Background:
Chronic alcoholics experience increased incidence and severity of infections, the mechanism of which is incompletely understood. Dendritic cells (DC) migrate from peripheral locations to lymph nodes (LN) to initiate adaptive immunity against infection. Little is known about how chronic alcohol exposure affects skin DC numbers or migration.
Methods:
Mice received 20% EtOH in the drinking water for up to 35 weeks. Baseline Langerhans cell (LC) and dermal DC (dDC) numbers were enumerated by immunofluorescence (IF). LC repopulation after inflammation was determined following congenic bone marrow (BM) transplant and ultraviolet (UV) irradiation. Net LC loss from epidermis was determined by IF following TNF-α or CpG stimulation. LC and dDC migration into LN was assessed by flow cytometry following epicutaneous FITC administration.
Results:
Chronic EtOH consumption caused a baseline reduction in LC but not dDC numbers. The deficit was not corrected following transplantation with non-EtOH-exposed BM and UV irradiation, supporting the hypothesis that the defect is intrinsic to the skin environment rather than LC precursors. Net loss of LC from epidermis following inflammation was greatly reduced in EtOH-fed mice versus controls. Ethanol consumption for at least 4 weeks led to delayed LC migration into LN, and consumption for at least 8 weeks led to delayed dDC migration into LN following epicutaneous FITC application.
Conclusions:
Chronic EtOH consumption causes decreased density of epidermal LC, which likely results in decreased epidermal immunosurveillance. It also results in altered migratory responsiveness and delayed LC and dDC migration into LN, which likely delays activation of adaptive immunity. Decreased LC density at baseline appears to be the result of an alteration in the skin environment rather than an intrinsic LC defect. These findings provide novel mechanisms to at least partially explain why chronic alcoholics are more susceptible to infections, especially those following skin penetration.
Keywords: Skin, Mouse, Migration, Langerhans Cells, Dermal Dendritic Cells
Alcohol is the most frequently abused substance in the United States. Alcoholism is associated with increased incidence and severity of bacterial infections (Cook, 1998; Happel and Nelson, 2005; MacGregor and Louria, 1997; Szabo, 1999). Alcoholics also appear to suffer from more skin infections than nonalcoholics (Harnisch et al., 1989; Smith and Fenske, 2000). However, the mechanism behind the observed increased susceptibility to infection is not well understood.
The skin plays an important role in the defense against infection. Its responsibility is not only to restrict the entry of pathogens into the body but also to alert the innate and adaptive immune systems when this barrier is breached. To do this, the skin contains a surveillance network of specialized cells, dendritic cells (DC). The DC of the epidermis are termed Langerhans cells (LC). Murine LC can be identified as CD11c+, MHC Cl II+, Langerin+, CD8− (Douillard et al., 2005; Valladeau and Saeland, 2005). Dermal DC (dDC) represent a more heterogeneous DC population present in the dermis, and can be characterized as CD11c+, MHC Cl II+, Langerin−, CD8− (Ruedl et al., 2000). LC and dDC internalize and process antigen (Ag) encountered within the skin and simultaneously survey the epidermis for danger signals in the form of Toll-like receptor ligands or inflammatory cytokines. Upon encounter with danger signals, LC and dDC change their chemokine receptor and adhesion molecule profiles to exit the skin via the dermal lymphatics and enter the draining lymph nodes (LN). Because of their proximity to dermal lymphatics, dDC have a shorter transit time to LN than LC (Kissenpfennig et al., 2005). LC and dDC migration into the LN is a rate-limiting step in the activation of naive T cells (Gunn et al., 1999; Itano and Jenkins, 2003). It is critical that these processes not be delayed or diminished to ensure timely activation of immune responses. The effect of chronic EtOH consumption on LC and dDC migratory function has not been previously investigated.
The goal of this study was to determine the effects of chronic EtOH consumption on LC and dDC migratory function using an in vivo mouse model for alcoholism (Song et al., 2002). When numbers of LC and dDC were examined in skin prior to stimulation, EtOH-fed mice demonstrated reduced LC (but not dDC), an effect that appeared to be intrinsic to the skin environment rather than the LC precursor. Net LC loss from the epidermis in response to inflammation was diminished compared to water-fed controls, and both LC and dDC from EtOH-fed mice exhibited delayed arrival in the LN compared to cells from control mice. The data presented herein indicate that EtOH-induced decreases in baseline LC numbers, as well as delays LC and dDC migratory function, may provide novel means by which EtOH-exposed DC are less able to rapidly initiate immune responses to pathological insults introduced through the skin.
MATERIALS AND METHODS
Mice and EtOH Feeding Regimen
Female C57BL/6 mice were purchased from National Cancer Institute (Frederick, MD) and housed in specific pathogen free facilities. At 8 weeks of age, mice received 10% weight/volume (w/v) EtOH in deionized water as their sole source of drinking water for 2 days, were advanced to 15% w/v EtOH for 5 days and finally onto 20% w/v on day 7. Mice were maintained on 20% EtOH for up to 35 weeks. Age-matched control mice were maintained on water from the same source as EtOH mice throughout the experiment. Both EtOH and control mice were allowed access to rodent chow ad libitum. EtOH treatment durations given in experimental protocols correspond to the number of weeks on 20% EtOH. C57Bl/6 mice on this Meadows-Cook model gain weight normally, and do not experience elevations in blood corticosterone levels (Cook et al., 2007). Congenic C57Bl/6 (CD45.1) mice were obtained from an in-house breeding colony. All animal procedures were approved by the animal care use committee at the University of Iowa.
Preparation of Epidermal Sheets and Enumeration of LC by Immunofluorescence in Situ
Ear skin was depilated with Nair (Church and Dwight Co, Princeton, NJ) and split into dorsal and ventral sheets with the aid of forceps. The dorsal sheet was incubated in 0.5 M ammonium thiocyanate for 5 to 10 minutes or 0.02 M EDTA for 30 minutes to separate the epidermis from the dermis. The epidermis was fixed in cold acetone, blocked with 10% goat serum (Pel-Freez Biologicals, Rogers, AR) and 20% anti-CD16/32 (FcγRIII/II) antibody (Ab) 2.4G2 (Unkeless, 1979), and then stained with PE anti-MHC Cl II (BD Biosciences, San Diego, CA). Multiple nonoverlapping 10× images were captured on each tissue using an Olympus BX-51 fluorescent microscope (Olympus America, Inc., Center Valley, PA), and the number of MHC Cl II+ LC/mm2 was determined.
Preparation of Dermal Tissue Sections
Dorsal skin specimens were excised, depilated, covered in OCT medium (Tissue-Tek, Torrance, CA) and frozen by submersion in liquid nitrogen. Six micron sections were prepared, fixed in cold acetone and blocked with 10% goat serum and 20% anti-CD16/32 (FcγRIII/II) Ab 2.4G2 (Unkeless, 1979). The sections were stained with unconjugated anti-CD11c (N418) (Metlay et al., 1990), followed by polyclonal Cy3-conjugated goat anti-hamster IgG (Jackson Immunoresearch, West Grove, PA). Lengths of multiple nonoverlapping segments of dermis were measured on each section and the number of dDC/100 μm was determined.
Bone Marrow Reconstitution/UV Irradiation
Ethanol-fed or control C57Bl/6 (CD45.2) mice were given a split dose (500 cGy followed by 600 cGy) of total body γ-irradiation 4 hours apart with a 81-16A J.L. Shepherd Co. (San Fernando, CA) irradiator, equipped with a 137Cs source. Bone marrow (BM) was isolated from femurs and pelvis of untreated congenic mice (CD45.1). Single cell suspensions were prepared and 1 × 106 nucleated BM cells were transferred intravenously via the retro-orbital plexus into the irradiated recipients. Ultraviolet (UV) irradiation was performed 2 weeks after transplant and consisted of 30 minutes of short wave (254 nm) irradiation with a UVGL-58 UV lamp (UVP; Fisher, Waltham, MA), 15 cm from the source. EtOH orwater feeding was continued for the respective groups throughout the course of the experiment. Mice were euthanized 2 weeks after UV irradiation, ears were harvested, and single cell suspensions of epidermis were prepared for flow cytometric analysis.
Intradermal Ear Injections
Mice received 40 μl intradermal (i.d.) injections containing either 10 μg CpG 1826 (Coley, Wellesley, MA), 0.2 μg murine TNF-α (PeproTech, Rocky Hill, NJ), or pyrogen free Dulbecco's phosphate-buffered saline (PBS; vehicle; Invitrogen, Carlsbad, CA) to the dorsal surface of each ear pinna. After 30 minutes or 1 hour, mice were euthanized, ears harvested and LC enumerated by immunofluorescence (IF) in epidermal sheets.
FITC Sensitization
FITC (Sigma-Aldrich, St Louis, MO) was dissolved in a 1:1 mixture of acetone and n-butyl phthalate to a final concentration of 0.5%. Mice received 25 μl of 0.5% FITC to the surface of the left side of the unshaved abdomen and 25 μl of vehicle to the right side. Inguinal LN were harvested at various times after FITC application. No FITC+ events were observed in the LN that received vehicle only.
Cell Preparation for Flow Cytometry
Lymph nodes were minced with a scalpel and then incubated in 25 μg/ml Liberase Blendzyme III (Roche Diagnostics, Indianapolis, IN) and 20 μg/ml DNase I (Roche Diagnostics) for 30 minutes at 37°C. Single cell suspensions were prepared by passage through 23 gauge needles. For intracellular staining, cells were permeabilized using the BD Intracellular Staining Kit (BD Biosciences) and stained simultaneously for intracellular and surface Ag.
Ears were split into dorsal and ventral surfaces. Dorsal sheets were incubated in 0.5% trypsin (Amresco, Solon, OH) in PBS for 30 minutes at 37°C. The dermis was removed and the epidermis dissociated using frosted microscope slides. The epidermal cell preparation was passed through a 70-μm filter and then subjected to Fico-Lite (Atlanta Biologicals, Norcross, GA) density gradient to remove debris and dead cells.
Antibodies used for flow cytometric staining included CD11c-Cy5.5PE (N418) purchased from eBioscience (San Diego, CA), MHC Cl II-PE (M5/114.15.2) purchased from BD Biosciences, and CCR7-biotin (4B12) purchased from Biolegend (San Diego, CA). N418, a hamster antimouse CD11c (Metlay et al., 1990); A20, a rat IgG2a antimouse CD45.1 (Shen, 1981) and 104, a rat IgG2a anti-mouse CD45.2 (Shen, 1981) were derived from hybridomas in the laboratory and conjugated to biotin, Cy5, or FITC using standard procedures. Unconjugated polyclonal rabbit anti-mouse Langerin (CD207; ImGenex, San Diego, CA) was detected using goat anti-rabbit Ig-Cy5 (Jackson ImmunoResearch).
Polyclonal purified rat IgG (Jackson ImmunoResearch) was used as an isotype control. To counteract background binding to FcγR, anti-CD16/32 (clone 2.4G2) and rat serum (Pel-Freez Biologicals) were incubated with cell samples during staining.
Flow Cytometric Analysis
Data were acquired on a BD FacsCalibur (BD Biosciences) equipped with CellQuestPro software (BD Biosciences) and analyzed using FlowJo 6.3.3 software (TreeStar Inc, Ashland, OR). Dead cells were excluded by low angle and orthogonal light scatter. Spectral overlaps between FITC and PE, PE and Cy5.5PE and Cy5 and Cy5.5PE were corrected by manual compensation on singly stained positive controls. At least 100,000 cells were collected per sample.
Statistics
The p-values were calculated from the two-tailed unpaired Student's t-test using InStat (GraphPad Software, San Diego, CA). Values of p < 0.05 were considered significant.
RESULTS
Loss of Epidermal LC in Chronic EtOH-Fed Mice
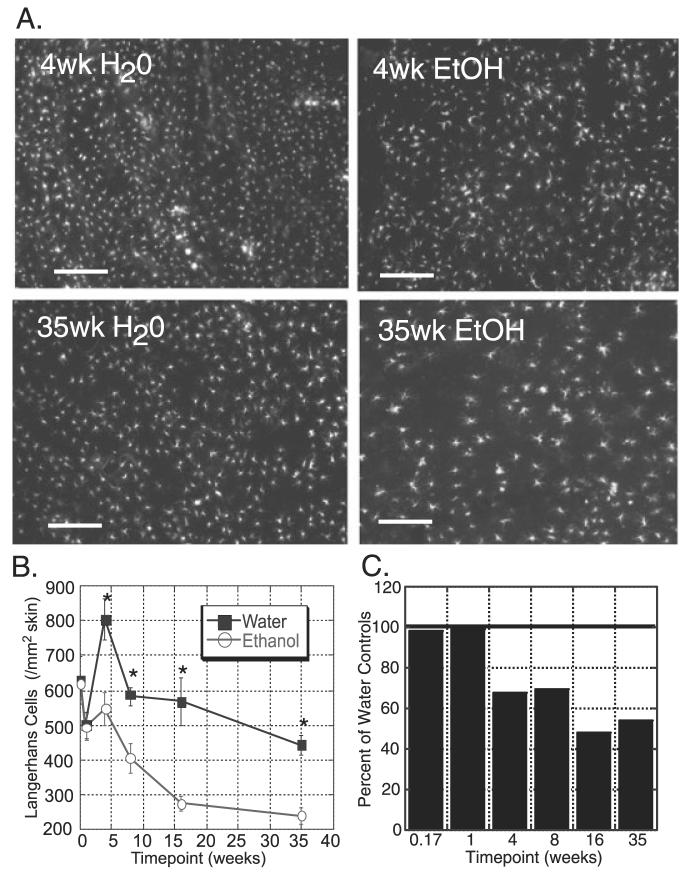
The overall goal of this study was to assess the effect of chronic EtOH feeding on LC and dDC migration from skin. However, prior to studying the effects of EtOH on LC and dDC migration, it was important to understand whether EtOH feeding altered baseline numbers of these cells. To determine LC numbers in unstimulated epidermis, epidermal sheets were prepared from ears of mice following varying lengths of EtOH feeding, from 1 week after any EtOH consumption (1 day on 20% EtOH) up to 35 weeks of chronic EtOH feeding. The sheets were stained with anti-MHC Cl II and the mean density of LC in the epidermal sheets determined. Surprisingly, the density of LC in EtOH-fed animals was significantly and dramatically reduced after as little as 4 weeks of chronic EtOH feeding (Fig. 1). This decrease was persistent and progressive for as long as 35 weeks of EtOH exposure. The observed decrease in LC density was not due to loss of MHC Cl II expression on LC as a result of EtOH feeding, as Langerin and MHC Cl II expression in epidermal cell suspensions from EtOH-fed mice was completely coincident (data not shown).
Fig. 1.
Chronic ethanol (EtOH)-fed mice have reduced baseline numbers of Langerhans cells (LC) in the epidermis. (A) Representative images of epidermal ear sheets stained for MHC Cl II after 4 weeks (top) or 35 weeks (bottom) of EtOH or control (water) feeding. Original magnification 10×, bars = 20 μm. (B) Number of LC/mm2 in the epidermis of EtOH fed (■) or age-matched water-fed (control; ○) mice. n ≥ 4 mice/time point (except 35 weeks n = 3). LC density in EtOH-fed mice was statistically [p(2) ≤ 0.05] lower than that of control mice from 4 to 35 weeks. Error bars represent standard error of the mean. (C) Number of LC/mm2 in the epidermis of EtOH-fed mice, normalized to LC numbers in control mice.
In agreement with published work (Cumberbatch et al., 2002b), LC numbers in control mice were found to decrease with age, such that by 35 weeks (approximately 10 months of age), LC density in control mice was half of that at 4 weeks (Fig. 1B). The age-related reduction in LC numbers was also noted in the EtOH-fed mice, however these mice experienced a dramatic reduction in LC in addition to this aging phenomenon. When normalized to the water-fed controls (Fig. 1C), EtOH-fed mice demonstrated approximately a 30% reduction in LC at 4 to 8 weeks of EtOH exposure, and this increased to a 50% reduction at 16 to 35 weeks. These findings indicate that chronic EtOH consumption leads to a progressive decay in baseline epidermal LC numbers.
Maintenance of dDC in EtOH-Fed Mice
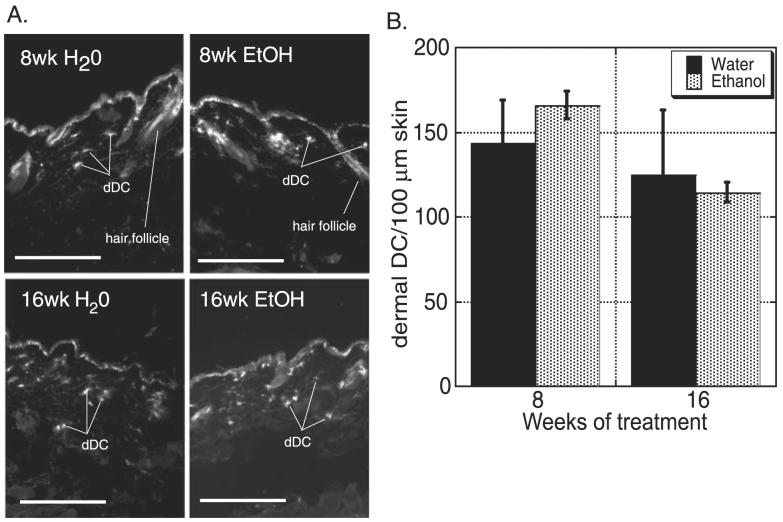
To address whether EtOH-induced LC attrition is paralleled in other DC populations of the skin, the frequency of CD11c+ dDC in cross-sections of skin from EtOH-fed and control mice was examined at 8 and 16 weeks of chronic EtOH feeding (Fig. 2). No difference in frequency between EtOH-fed and control mice was found at either time point. This finding suggests that the mechanism accounting for the decay in LC numbers does not impact dDC numbers.
Fig. 2.
Chronic ethanol (EtOH) feeding for 8 to 16 weeks has no effect on dermal dendritic cell (dDC) numbers. (A) Representative images of dorsal ear skin harvested and stained with anti-CD11c from EtOH-fed and control mice. Original magnification 20×, bars = 20 μm. (B) Number of CD11c+ cells (dDC)/100 μm of skin of EtOH fed (gray bars) or age-matched water-fed (control; black bars) mice. No differences in frequency of dDC were observed between control and EtOH-fed mice. n ≥ 3 mice/time point. Error bars represent standard error of the mean.
Reconstitution of LC in an EtOH Environment
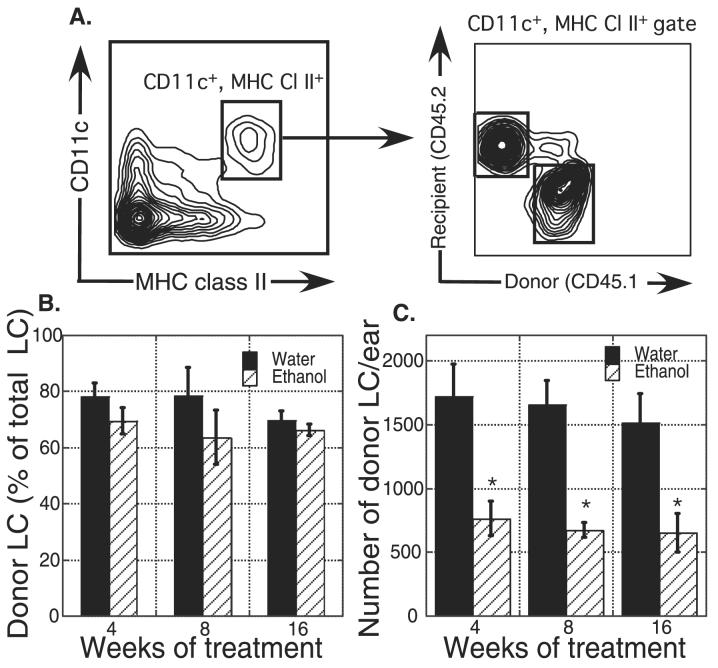
Decreased baseline LC numbers in EtOH-fed mice could be the result of alterations intrinsic to LC and/or their precursors, or due to changes in the epidermal environment in which the LC reside. Steady-state adult LC are repopulated from local precursors of unknown phenotype, but severe inflammatory stimulation of skin, such as UV irradiation, will induce BM precursors to contribute to replenishment of the LC pool (Merad et al., 2002). To determine the relative effect of EtOH feeding on LC precursors versus the epidermal environment, BM from C57Bl/6 congenic (CD45.1; donor) mice (not fed EtOH) was adoptively transferred into lethally irradiated EtOH-fed or control (CD45.2; recipient) mice and allowed to reconstitute for 2 weeks. The mice were then UV irradiated to promote LC depletion and allow donor-derived BM precursors to contribute to LC reconstitution. Two weeks later, LC chimerism in the epidermis was examined. LC were identified as CD11c+, MHC Cl II+ in epidermal cell suspensions, and were segregated into recipient or donor origin based on CD45.1/CD45.2 expression (Fig. 3A). About 70 to 80% of the LC in control mice were donor derived, and this percentage was unchanged with EtOH consumption (Fig. 3B). However, despite very similar levels of chimerism, donor LC numbers were significantly reduced in the EtOH-fed mice (Fig. 3C). This supports the hypothesis that the defect in LC numbers seen in EtOH-fed mice is independent of LC precursors and instead is intrinsic to the skin environment. Chronic EtOH consumption for as little as 4 weeks causes reduced ability to repopulate a normal number of LC into the epidermis following inflammation, which may lead to reduced recovery of skin surveillance by LC following extreme inflammation (e.g., sunburn) as well as at baseline.
Fig. 3.
Chronic ethanol (EtOH)-fed mice have reduced numbers of Langerhans cells (LC) in epidermis following congenic bone marrow (BM) transplant and ultraviolet (UV) irradiation. Untreated BM cells (CD45.1) were transplanted into lethally irradiated control or EtOH-fed mice (CD45.2). Two weeks later the mice received UV irradiation, and 2 weeks after irradiation, ears were harvested. (A) Gating strategy for LC in epidermal cell suspensions. Comparison of donor LC recovery from EtOH-fed or control mice displayed as percent of total LC (B) and total donor LC number/ear (C). Weeks of EtOH or water treatment were counted at the time of BM reconstitution. *Indicates p(2) ≤ 0.05 for control versus EtOH at the specified time point. n = 3 to 6 mice/group. Error bars represent standard error of the mean.
LC Migration out of the Epidermis
Given the basal reduction in LC frequency in the EtOH-fed mice, the original question remained as to whether LC in the epidermis of these mice could emigrate appropriately in response to stimulation. To test this, i.d. injections of CpG, TNF-α, or PBS (control) were performed and LC were counted in epidermal sheets after 0.5, 1, and 2 hours—the time frame in which epidermal LC have been reported to emigrate in response to these stimuli (Ban et al., 2000; Cumberbatch et al., 1994). The net number of LC leaving 1 mm2 of epidermis following stimulation and percent reduction within that area were calculated (Tables 1 and 2). Additional net emigration was not observed between 1 and 2 hours after injection (data not shown). Surprisingly, the injection of PBS alone caused measurable net LC migration out of the epidermis, particularly in 4 weeks EtOH-fed and control mice, presumably due to inflammation caused by mechanical trauma during the injection procedure (Dearman et al., 2004).
Table 1.
Influence of CpG on Epidermal LC Frequencies
| Net number of LC leaving epidermis/mm2 (% reduction)b |
||||
|---|---|---|---|---|
| Treatmenta | PBS 30 minutes (%) |
CpG 30 minutes (%) |
PBS 1 hour (%) |
CpG 1 hour (%) |
| 4-week water | 285 (35) | 249 (31) | 207 (25) | 335 (41) |
| 4-week EtOH | 187 (34) | 156 (28) | 48 (8) | 79 (14) |
| 16-week water | 35 (6) | 54 (9) | 2 (0) | 104 (18) |
| 16-week EtOH | −61 (−22)c | 6 (2) | −96 (−35)c | −73 (−26)c |
Data are derived from 4 to 6 mice/time point.
Net number of LC leaving epidermis = average LC density in untreated ears − average LC density in treated ears. Percent reduction = average number of LC leaving epidermis/average untreated ear LC density × 100.
Negative values represent increases in LC over untreated levels.
Table 2.
Influence of TNF-α on Epidermal LC Frequencies
| Net number of LC leaving epidermis/mm2 (% reduction)b |
||||
|---|---|---|---|---|
| Treatmenta | PBS 30 minutes (%) |
TNF-α 30 minutes (%) |
PBS 1 hour (%) |
TNF-α 1 hour (%) |
| 4-week water | 285 (35) | 301 (37) | 207 (25) | 262 (32) |
| 4-week EtOH | 187 (34) | 175 (32) | 48 (8) | 100 (18) |
| 8-week water | −19 (−3)c | 99 (17) | 66 (11) | 36 (6) |
| 8-week EtOH | 42 (10) | −31 (−7)c | −22 (−5)c | −39 (−9)c |
Data are derived from 4 to 6 mice/time point.
Net number of LC leaving epidermis = average LC density in untreated ears − average LC density in treated ears. Percent reduction = average number of LC leaving epidermis/average untreated ear LC density × 100.
Negative values represent increases in LC over untreated levels.
After 4 weeks of EtOH feeding, reduced net numbers of LC migrated out of treated epidermis. At most, net emigration was about 65% compared to control mice in response to all stimuli examined including PBS (Tables 1 and 2). The net reduction of LC numbers in control mice was statistically significant (p < 0.05) by 30 minutes following CpG administration, whereas EtOH-fed mice never achieved a statistically significant net reduction in LC numbers. In addition, 4-week EtOH-fed mice demonstrated reduced net percentages of LC migrating out of the epidermis 1 hour after CpG administration as compared to 4-week water-fed mice (Table 1).
After 16 weeks of EtOH feeding, no net reduction in LC numbers was found in epidermis following CpG treatment; instead LC began to accumulate in the treated epidermis (Table 1). This also occurred following PBS injections in EtOH-fed mice, and may be due to increased lateral migration of LC into the treated epidermis in the EtOH-fed mice (Nishibu et al., 2006) in addition to loss of LC emigration. These phenomena appear to be accentuated by age, as the 16-week control mice also showed the evidence of decreased net migration out of epidermis in response to CpG compared to 4-week control mice (although some net emigration was still present; Table 1).
Because LC from 6-month-old mice fail to emigrate in response to i.d. TNF-α (Cumberbatch et al., 2002b), 8 weeks of EtOH consumption was the latest time point examined following treatment with this stimulus. Similar to the response to CpG in 16-week EtOH-fed mice, TNF-α treatment of 8-week EtOH-fed mice no longer resulted in reduction of epidermal LC numbers; instead LC accumulated in the epidermis (Table 2). In contrast, control mice in both 4- and 8-week groups achieved statistically significant (p < 0.05) net reductions in LC numbers by 30 minutes following stimulation. Thus, chronic EtOH consumption results in impaired reduction in epidermal LC following inflammation as evidenced by both reduction in net number of LC emigrating (due in part to decreased total numbers of LC in the epidermis following EtOH feeding) and reduced net percentage of LC emigrating from the area of inflammation.
FITC+ LC Migration Into the Lymph Node
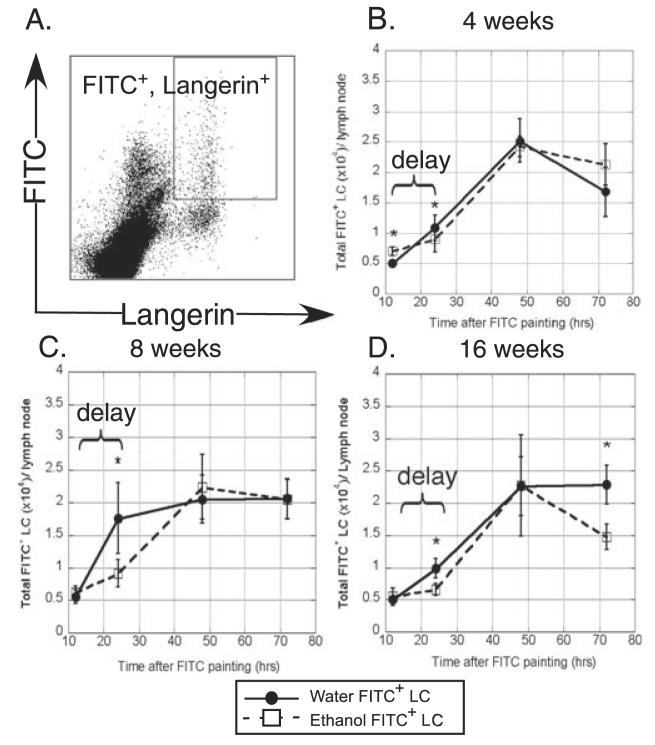
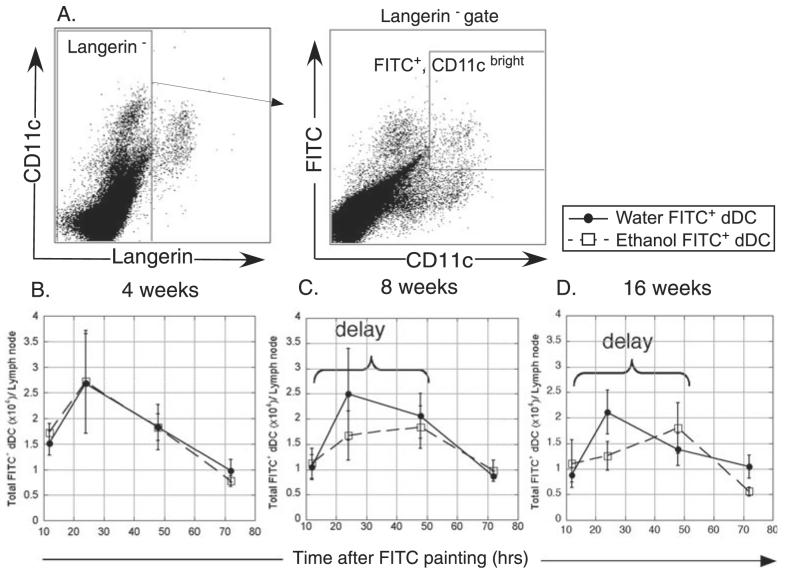
Numerous additional factors control LC migration to LN subsequent to their exodus from the epidermis (Mummert et al., 2004; Randolph et al., 2005; Sozzani, 2005). To determine to what degree LC migration into the skin draining LN is affected by EtOH consumption, FITC was applied epicutaneously to the flanks of mice fed EtOH or water for 4, 8 or 16 weeks. The accumulation of FITC bearing LC (Langerin+, FITC+) was assayed in inguinal LN after 12, 24, 48, and 72 hours (Fig. 4). Subset analyses revealed that this population represents only the CD8− epidermal-derived LC (data not shown) (Douillard et al., 2005). FITC (rather than TNF-α or CpG) was specifically chosen as the stimulus for these experiments because it allowed fluorescent distinction of recent LC (or dDC) immigrants from those that migrated prior to application of the stimulus. (FITC could not be used for LC emigration studies because its fluorescence obliterates the ability to clearly identify specific cell populations by IF.)
Fig. 4.
Delayed migration of FITC+ Langerhans cells (LC) into the draining lymph node (LN) of ethanol (EtOH)-fed mice. Abdominal skin was FITC painted and inguinal LN cells harvested for flow cytometry. (A) Gating strategy for identification of FITC+ LC in LN. (B–D) Comparison of FITC+ LC accumulation in LN of control or 4, 8 or 16 weeks EtOH-fed mice. n = 16 mice at 12 and 24 hours and n = 5 to 6 mice for remaining time points. *Indicates p(2) ≤ 0.05 for control versus EtOH at the specified time point. Error bars represent standard error of the mean.
Frequencies of FITC+ LC in LN from 4-, 8-, and 16-week EtOH-fed mice were significantly lower than that of control mice at 24 hours after FITC application (Fig. 4B-D). By 48 hours the numbers of migrated LC in LN of EtOH-fed mice were again similar to those of controls, demonstrating a delay in LC migration prior to 24 hours. At the peak of the response, both water and EtOH-fed mice achieved a similar magnitude of FITC+ LC in the LN, indicating equal degrees of mobilization despite EtOH-fed mice having 30 to 50% reduced LC numbers in untreated epidermis. At 16 weeks of EtOH exposure, recently migrated LC were significantly reduced in number 72 hours following FITC application (Fig. 4D). This may indicate a shorter lifespan of chronic EtOH-exposed LC in the LN following migration relative to control LC, resulting in less efficient stimulation of naive T cells.
FITC+ dDC Migration Into the Lymph Node
To explore whether dDC experienced a similar delay in accumulation in the LN, enumeration of FITC+ dDC in the LN was performed. Dermal DC (Langerin−, CD11c+, FITC+) were identified in inguinal LN 12 to 72 hours following epicutaneous FITC application (Fig. 5). Dermal DC from EtOH-fed and control mice demonstrated similar migration after 4 weeks of EtOH treatment (Fig. 5B). However at 8 to 16 weeks, EtOH-fed mice demonstrated altered dDC migration (Fig. 5C and 5D). Although displaying more inter-mouse variability than LC migration, in 8-week control mice, maximal migration occurred at 24 hours, whereas in EtOH-fed mice, maximal migration was delayed to 48 hours. This delay was more apparent in 16-week EtOH-fed mice. Furthermore, the 8 to 16 weeks EtOH-fed mice displayed a trend toward reduced total numbers of migrated dDC at the peak of the response compared to control mice.
Fig. 5.
Delayed FITC+ dermal dendritic cell (dDC) accumulation into draining lymph node (LN) of ethanol (EtOH)-fed mice. Abdominal skin was FITC painted and inguinal LN cells harvested for flow cytometry. (A) Gating strategy for the identification of FITC+ dDC in LN. (B–D) Comparison of FITC+ dDC accumulation in LN of control or 4, 8 or 16 weeks EtOH-fed mice. n = 5 to 8 mice/time point. Error bars represent standard error of the mean.
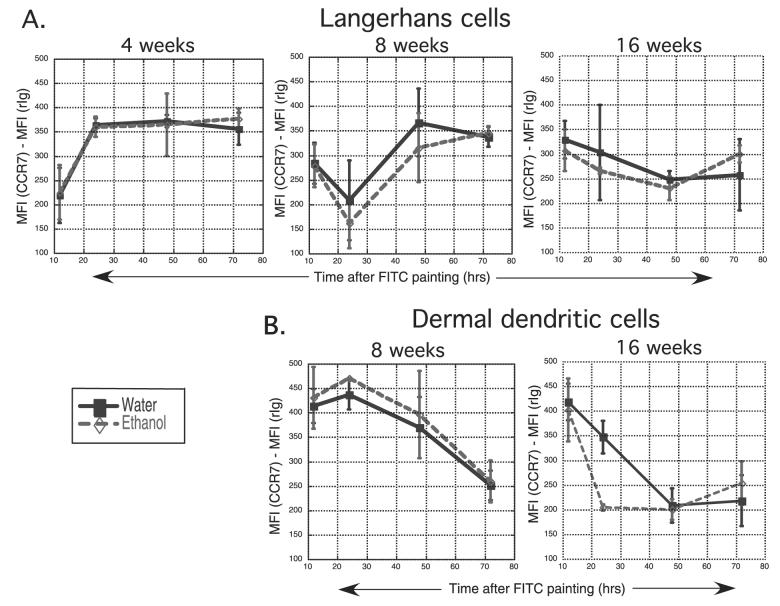
CCR7 Expression on Migrated LC and dDC
CCR7 expression is required for both dDC and LC migration into the LN (Ohl et al., 2004). Because migration of both of these cell types into LN was delayed in the presence of chronic EtOH feeding, the level of CCR7 expression on migrated LC and dDC was measured in mice where delayed migration was noted, as a potential mechanism for the delayed migration. Overall, FITC+ LC and FITC+ dDC expressed similar levels of CCR7 in the LN of water and EtOH-fed mice (Fig. 6). A trend toward lower CCR7 expression levels on EtOH-exposed dDC occurred at only one time point (24 hours after FITC painting in 16-week EtOH-fed mice, Fig. 6B) and thus was not a finding common to the many treatment groups where delayed migration was observed.
Fig. 6.
CCR7 expression is not altered on migrated Langerhans cells (LC) and dermal dendritic cells (dDC) in lymph nodes (LN) of ethanol (EtOH)-fed mice. Abdominal skin was FITC painted and inguinal LN cells harvested for flow cytometry. Mean fluorescence intensity (MFI) attributable to CCR7 expression was determined by subtracting the MFI of the isotype control (rIg) from the MFI of CCR7 staining. (A) Comparison of CCR7 MFI of LC from control or 4-, 8-, or 16-week EtOH-fed mice. (B) Comparison of CCR7 MFI of dDC from control or 8- or 16-week EtOH-fed mice. n = 3 to 9 mice/time point. Error bars represent standard error of the mean.
DISCUSSION
EtOH consumption has numerous potential negative effects on the immune system, including DC, which could contribute to the observed increase in infectious disease incidence and severity in alcoholics. As DC are responsible for initiation of adaptive immunity, EtOH-induced DC alterations would be expected to have profound effects on the ability to combat infection. However, few studies have assessed the effect of EtOH exposure on DC numbers or function. Acute EtOH exposure results in reduced allostimulatory capacity, decreased costimulatory molecule expression, decreased IL-12 and increased IL-10 production by human monocyte-derived DC (Mandrekar et al., 2004; Szabo et al., 2004). In mice, 11 days of EtOH given in a modified Lieber DeCarli diet resulted in decreased production of IL-6, IL-12, IFN-γ, and IL-17 when DC from EtOH mice were cultured with Ag-specific T cells in the presence of Ag (Heinz and Waltenbaugh, 2007). In chronic alcoholics with cirrhosis, decreased numbers of DC are present in the blood, and inflammatory cytokine production by these cells is reduced (Laso et al., 2007). Using a murine model of chronic EtOH consumption similar to ours, Lau et al. (2006) found lower levels of costimulatory molecule expression on CpG-activated murine splenic DC, and reduced ability of these DC to activate allogeneic T cells in vitro. Furthermore, the same group reported that EtOH increased the migration of hepatic but not splenic DC to draining LN following subcutaneous transfer of these DC into the footpad (Lau et al., 2007). The effect of chronic EtOH consumption on LC and dDC numbers and migratory capacity has not previously been investigated.
The purpose of this study was to determine the effect of chronic EtOH feeding on LC and dDC migratory function in vivo. The model used for EtOH feeding allows evaluation of extended periods of EtOH exposure without evidence of stress-induced effects on the immune system (Cook et al., 2007). To have a framework in which to interpret migrated LC and dDC numbers following stimulation in EtOH-fed mice, baseline numbers of these populations in the skin were first established. EtOH feeding was observed to result in the loss of 30 to 50% of LC from the epidermis with as little as 4 weeks of EtOH consumption, but persistent maintenance of the dDC population in the presence of up to 16 weeks of EtOH (Figs 1 and 2). These novel results extend previous studies in our laboratory that indicate that DC numbers are reduced in the spleens (but not LN) of EtOH-fed mice, beginning after as little as 4 weeks of EtOH treatment (A.S. and K.N., unpublished data). DC play a vital role in Ag uptake and processing in the skin, in preparation for transport to draining LN (Allan et al., 2006). The decreased baseline density of LC likely results in decreased epidermal Ag encounter/immunosurveillance. Such alterations may provide bacteria and viruses with an advantage in initiating an infection following penetration of the skin in chronic alcohol-exposed individuals.
In general, mechanisms to explain the decreased density of LC with EtOH consumption could be localized to a defect intrinsic to LC or to the environment in which they reside. To distinguish these possibilities, a model was employed that allows distinction of LC that arise from an endogenous (chronic EtOH exposed or control) source—the local LC precursor in the skin, and a nascent source—the recently BM derived LC precursor. In adults, LC are derived from a local radio-resistant precursor population (Merad et al., 2002) of unknown phenotype. BM precursors contribute to replenishment of adult LC only under severe inflammatory conditions such as UV irradiation or graft versus host disease (Merad et al., 2002, 2004).
Using this model, it was observed that reconstitution of lethally irradiated EtOH-fed or control mice with BM from untreated congenic mice followed by UV irradiation resulted in similar percentages of LC chimerism between control and EtOH-fed mice (Fig. 3). This indicated that the local precursor, regardless of whether it was chronically exposed to EtOH or not, gave rise to essentially equivalent fractions of LC in EtOH-exposed skin. However, persistently reduced total numbers of donor (and recipient) LC were observed in the EtOH-fed recipients compared to control transplant recipients. Additionally, studies in our laboratory have found no alterations in BM cellularity or impairment in the ability of BM precursors from mice fed EtOH for up to 16 weeks to differentiate into DC (A.S. and M.M., unpublished data), which supports the continued competence of EtOH-exposed BM precursors to produce DC. In aggregate, these findings suggest that LC loss in EtOH-fed mice is not the result of defective precursor proliferation or differentiation, but is intrinsic to the skin environment. Persistently reduced LC populations in EtOH-fed mouse skin following UV irradiation indicate that in addition to baseline defects in immunosurveillance, EtOH-fed mice are also unable to recover normal levels of cutaneous immunity following severe inflammation. This could provide a mechanism for the observed poor prognosis of alcoholic burn victims, who may succumb to bacterial infections because cutaneous immunity as well as skin integrity has been compromised (Brown et al., 2006; Choudhry and Chaudry, 2006).
With baseline LC and dDC numbers established in EtOH-fed and control mice, studies of the ability of inflammatory and antigenic stimuli to induce LC and dDC migration were performed. Stimulation of EtOH-fed mice with i.d. CpG or TNF-α resulted in impaired reduction of epidermal LC numbers and percentages in the 2 hours following stimulation, compared to control epidermis (Tables 1 and 2 and data not shown). While this result is partly dependent on the baseline loss of LC from EtOH-exposed epidermis, a lower net percent reduction of LC in EtOH mice indicates that additional functional changes are present in the remaining EtOH-exposed LC. It remains possible that EtOH shifts peak net LC emigration to a much later time point than examined in this study. This would not change the general conclusion that LC migratory function is altered by EtOH exposure.
Enumeration of cells remaining in the epidermis following stimulation examines the cumulative effects of lateral migration within the epidermis as well as migration out of the epidermis and, in theory, migration into the epidermis. The relative contribution of these mechanisms to the observed diminished decrease in LC density in EtOH-fed mice following inflammation has not yet been delineated, although migration of BM LC precursors into the epidermis is unlikely given the mild inflammatory stimuli utilized, relative to those shown to allow repopulation with BM precursors (Merad et al., 2002, 2004). Another theoretical possibility to explain the increased LC density seen primarily in EtOH-exposed epidermis (e.g., 30 minutes after TNF-α injection following 8-week EtOH exposure; Table 2) is proliferation of skin resident LC precursors. However, the timeframe of 30 minutes to 1 hour is not sufficient to allow skin-resident LC precursors to proliferate. Thus, the likely explanation for the increases in LC density is heightened lateral migration of LC within the epidermis (Nishibu et al., 2006). When the diminished relative decrease in LC density is combined with the lower baseline numbers of LC in EtOH-fed mice, the potential impact on prompt initiation of immune responses in the draining LN is magnified.
Phosphate-buffered saline injection alone was sufficient to induce decreases in epidermal LC numbers in 4-week EtOH-fed and control mice. Contaminating endotoxin in the PBS was ruled out as a cause for the LC migration because the levels were <0.03 EU/ml. Suction blister formation on human subjects elicits similar decreases in epidermal LC numbers in the absence of exogenous application of inflammatory stimuli, associated with increased local TNF-α, IL-6, IL-1,and CCL2 production (Dearman et al., 2004). Previous reports using PBS as the vehicle for injection of inflammatory mediators into murine epidermis did not observe this phenomenon; however, this may be due to different mouse strains and/or smaller volumes injected into the ear (Cumberbatch et al., 2002a,b). Four weeks control mice showed a much greater decrease in epidermal LC following PBS injection than did 8- or 16-week control mice, suggesting that age also impacts LC function (in addition to the previously published influence on LC numbers) (Cumberbatch et al., 2002b).
In addition to causing altered net LC migration from epidermis, chronic EtOH consumption in mice contributes to delayed LC and dDC trafficking to LN. LC migration showed a consistent delay in the EtOH-fed mice, after as little as 4 weeks of chronic EtOH consumption (Fig. 4). In contrast, dDC migration was minimally affected after 4 weeks of EtOH consumption but began to show progressive evidence of delay by 8 and 16 weeks (Fig. 5). The observed differences between LC and dDC migration could be due to their different anatomical locations, resulting in exposure to different amounts of EtOH or its metabolites. Furthermore, the migration program utilized by LC differs from that of dDC. LC must down-regulate E-cadherin to allow release from keratinocytes and simultaneously up-regulate α6-integrin to traverse the basement membrane (Price et al., 1997). These steps are not required for dDC migration. CCR7 expression is required for both dDC and LC migration into LN, and was found not to differ between EtOH-exposed and control FITC + LC and dDC in LN (Fig. 6). The lack of change in CCR7 expression on LC and dDC in LN was not entirely unexpected because only cells with sufficiently high levels of CCR7 would be likely to gain entrance into the LN (Luther et al., 2000). It does not rule out a role for delayed up-regulation of CCR7 as a mechanism for delayed entry of EtOH-exposed LC or dDC into LN (Vecchi et al., 1999). In addition, EtOH-induced delayed down-regulation of CCR1, CCR2, CCR5, or CCR6 (needed to retain both immature dDC and LC in the area of inflammation) may play a role in the observed delayed entry of these cells into LN (Cook et al., 2000; Vecchi et al., 1999).
Although the differences in migrated LC numbers between EtOH-fed and control LN are small at 24 hours after FITC application, they are statistically significant. They also may be biologically significant, resulting in delayed initiation of immunity, particularly when combined with the more rapid LC disappearance from LN with long-term (16 weeks) EtOH exposure (Fig. 4D), and if other EtOH-induced changes exist in the function of the migrating cells similar to those reported in splenic DC populations (Lau et al., 2006).
Despite the presence of approximately 40% fewer LC in the epidermis of EtOH-fed mice, almost identical numbers of LC were ultimately mobilized into the LN following FITC skin painting. It is well documented that various inflammatory stimuli are unable to mobilize more than approximately 30 to 60% of LC out of non-EtOH-exposed epidermis (Ban et al., 2000; Cumberbatch et al., 1994, 2002a,b). Clearly a large reservoir of LC remains in normal skin following inflammatory stimulation. Thus the present results could be explained if the LC that are lost in the baseline state as a result of EtOH feeding are selectively those that are normally unresponsive to stimulation. FITC skin painting might then lead to a greater fractional depletion of the remaining skin LC in EtOH-fed mice than in controls, resulting in similar numbers of LC ultimately arriving in the draining LN. In this sense, the “average” ability of a particular LC to migrate from EtOH-exposed epidermis would actually be increased relative to controls.
The hypothesis of greater depletion of epidermal LC following epicutaneous FITC application in EtOH-fed mice than in controls does not easily fit with the observation that fewer net LC were lost from the epidermis of EtOH-fed than control mice following TNF-α or CpG treatment. The reason for this apparent discrepancy could be differential migration of LC in response to different stimuli, functional differences between ear and abdominal skin LC or the epidermal environment in these locations in response to stimulation, or increased lateral migration in EtOH-exposed epidermis. It is unlikely to be attributable to slower kinetics of LC migration out of epidermis in response to inflammatory stimuli in EtOH-fed mice, because by 2 hours following TNF-α or CpG administration, additional loss of LC from EtOH-exposed (and control) epidermis ceased (data not shown).
In summary, this study demonstrates for the first time that mice chronically fed EtOH suffer from a loss of epidermal LC numbers,which is expected to result in decreased immunosurveillance of the epidermis. Furthermore, the novel findings of EtOH-induced alterations in migratory responsiveness in vivo (particularly delayed LC and dDC migration into LN following antigenic challenge) may result in delayed activation of the adaptive immune system. These data lay the groundwork for future investigations into more precise definition of the mechanisms for alterations in LC numbers, as well as LC and dDC migration deficits resulting from chronic EtOH feeding.
ACKNOWLEDGMENTS
This work is part of collaborative projects in alcohol immunology funded by NIH Interactive Research Program Grants AA-014405 (R.T. Cook), AA-014400 (T.J. Waldschmidt), AA-014406 (A.J. Schlueter), AA-014418 (Z.K. Ballas), The University of Iowa Carver College of Medicine; and AA-012450 (T.R. Jerrells), The University of Nebraska Medical Center. We thank Teresa Duling for expertise with flow cytometry.
This work was supported by National Institutes of Health Grants AA014405 and AA014406.
REFERENCES
- Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Ban E, Dupre L, Hermann E, Rohn W, Vendeville C, Quatannens B, Ricciardi-Castagnoli P, Capron A, Riveau G. CpG motifs induce Langerhans cell migration in vivo. Int Immunol. 2000;12:737–745. doi: 10.1093/intimm/12.6.737. [DOI] [PubMed] [Google Scholar]
- Brown LA, Cook RT, Jerrells TR, Kolls JK, Nagy LE, Szabo G, Wands JR, Kovacs EJ. Acute and chronic alcohol abuse modulate immunity. Alcohol Clin Exp Res. 2006;30:1624–1631. doi: 10.1111/j.1530-0277.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Chaudry IH. Alcohol intoxication and post-burn complications. Front Biosci. 2006;11:998–1005. doi: 10.2741/1857. [DOI] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system–a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, Sullivan LM, Smith SR, Greenberg HB, Narula SK, Lipp M, Lira SA. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, Nashelsky MB, Ray NB, Haugen RH, Waldschmidt TJ. Thymocytes, pre-B cells and organ changes in a mouse model of chronic ethanol ingestion. Absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol Clin Exp Res. 2007;313:1746–1758. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch M, Dearman RJ, Groves RW, Antonopoulos C, Kimber I. Differential regulation of epidermal langerhans cell migration by interleukins (IL)-1alpha and IL-1beta during irritant- and allergen-induced cutaneous immune responses. Toxicol Appl Pharmacol. 2002a;182:126–135. doi: 10.1006/taap.2002.9442. [DOI] [PubMed] [Google Scholar]
- Cumberbatch M, Dearman RJ, Kimber I. Influence of ageing on Langerhans cell migration in mice: identification of a putative deficiency of epidermal interleukin-1beta. Immunol. 2002b;105:466–477. doi: 10.1046/j.1365-2567.2002.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch M, Fielding I, Kimber I. Modulation of epidermal Langerhans' cell frequency by tumour necrosis factor-alpha. Immunol. 1994;81:395–401. [PMC free article] [PubMed] [Google Scholar]
- Dearman RJ, Bhushan M, Cumberbatch M, Kimber I, Griffiths CE. Measurement of cytokine expression and Langerhans cell migration in human skin following suction blister formation. Exp Dermatol. 2004;13:452–460. doi: 10.1111/j.0906-6705.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- Douillard P, Stoitzner P, Tripp CH, Clair-Moninot V, Ait-Yahia S, McLellan AD, Eggert A, Romani N, Saeland S. Mouse lymphoid tissue contains distinct subsets of langerin/CD207 dendritic cells, only one of which represents epidermal-derived Langerhans cells. J Invest Dermatol. 2005;125:983–994. doi: 10.1111/j.0022-202X.2005.23951.x. [DOI] [PubMed] [Google Scholar]
- Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2:428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- Harnisch JP, Tronca E, Nolan CM, Turck M, Holmes KK. Diphtheria among alcoholic urban adults. A decade of experience in Seattle. Ann Intern Med. 1989;111:71–82. doi: 10.7326/0003-4819-111-1-71. [DOI] [PubMed] [Google Scholar]
- Heinz R, Waltenbaugh C. Ethanol-consumption modifies dendritic cell antigen presentation in mice. Alcohol Clin Exp Res. 2007;31:1759–1771. doi: 10.1111/j.1530-0277.2007.00479.x. [DOI] [PubMed] [Google Scholar]
- Itano AA, Jenkins MK. Antigen presentation to naive CD4 T cells in the lymph node. Nat Immunol. 2003;4:733–739. doi: 10.1038/ni957. [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Laso FJ, Vaquero JM, Almeida J, Marcos M, Ofrao A. Chronic alcohol consumption is associated with changes in the distribution, immunophenotype, and the inflammatory cytokine secretion profile of circulating dendritic cells. Alcohol Clin Exp Res. 2007;31:846–854. doi: 10.1111/j.1530-0277.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- Lau AH, Abe M, Thomson AW. Ethanol affects the generation, cosignaling molecule expression, and function of plasmacytoid and myeloid dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2006;79:941–953. doi: 10.1189/jlb.0905517. [DOI] [PubMed] [Google Scholar]
- Lau AH, Thomson AW, Colvin BL. Chronic ethanol exposure affects in vivo migration of hepatic dendritic cells to secondary lymphoid tissue. Hum Immunol. 2007;68:577–585. doi: 10.1016/j.humimm.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA. 2000;97:12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor RR, Louria DB. Alcohol and infection. Curr Clin Top Infect Dis. 1997;17:291–315. [PubMed] [Google Scholar]
- Mandrekar P, Ctalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J Immunol. 2004;173:3398–3407. doi: 10.4049/jimmunol.173.5.3398. [DOI] [PubMed] [Google Scholar]
- Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz MG, Lira SA, Charo I, Cook DN, Weissman IL, Strober S, Engleman EG. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10:510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummert DI, Takashima A, Mummert ME. Langerhans cells in CD44-deficient mice emigrate from the epidermis but fail to reach the lymph nodes after hapten application. J Invest Dermatol. 2004;122:846–847. doi: 10.1046/j.0022-202X.2004.22127.x. [DOI] [PubMed] [Google Scholar]
- Nishibu A, Ward BR, Jester JV, Ploegh HL, Boes M, Takashima A. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J Invest Dermatol. 2006;126:787–796. doi: 10.1038/sj.jid.5700107. [DOI] [PubMed] [Google Scholar]
- Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Forster R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Price AA, Cumberbatch M, Kimber I, Ager A. Alpha 6 integrins are required for Langerhans cell migration from the epidermis. J Exp Med. 1997;186:1725–1735. doi: 10.1084/jem.186.10.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- Ruedl C, Koebel P, Bachmann M, Hess M, Karjalainen K. Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes. J Immunol. 2000;165:4910–4916. doi: 10.4049/jimmunol.165.9.4910. [DOI] [PubMed] [Google Scholar]
- Shen F-W. Monoclonal Antibodies to Mouse Lymphocyte Differentiation Alloantigens. Elsevier/North-Holland Biomedical Press; Amsterdam: 1981. [Google Scholar]
- Smith KE, Fenske NA. Cutaneous manifestations of alcohol abuse. J Am Acad Dermatol. 2000;43:1–16. doi: 10.1067/mjd.2000.104512. [DOI] [PubMed] [Google Scholar]
- Song K, Coleman RA, Zhu X, Alber C, Ballas ZK, Waldschmidt TJ, Cook RT. Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol. 2002;72:1109–1116. [PubMed] [Google Scholar]
- Sozzani S. Dendritic cell trafficking: more than just chemokines. Cytokine Growth Factor Rev. 2005;16:581–592. doi: 10.1016/j.cytogfr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- Szabo G, Dolganiuc A, Mandrekar P, White B. Inhibition of antigen-presenting cell functions by alcohol: implications for hepatitis C infection. Alcohol. 2004;33:241–249. doi: 10.1016/j.alcohol.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Vecchi A, Massimiliano L, Ramponi S, Luini W, Bernasconi S, Bonecchi R, Allavena P, Parmentier M, Mantovani A, Sozzani S. Differential responsiveness to constitutive vs. inducible chemokines of immature and mature mouse dendritic cells. J Leukoc Biol. 1999;66:489–494. doi: 10.1002/jlb.66.3.489. [DOI] [PubMed] [Google Scholar]