Abstract
Catch-up growth is defined as a linear growth rate greater than expected for age after a period of growth inhibition. We hypothesized that catch-up growth occurs because growth-inhibiting conditions conserve the limited proliferative capacity of growth plate chondrocytes, thus slowing the normal process of growth plate senescence. When the growth-inhibiting condition resolves, the growth plates are less senescent and therefore grow more rapidly than normal for age. To test this hypothesis, we administered propylthiouracil to newborn rats for 8 wk to induce hypothyroidism and then stopped the propylthiouracil to allow catch-up growth. In untreated controls, the growth plates underwent progressive, senescent changes in multiple functional and structural characteristics. We also identified genes that showed large changes in mRNA expression in growth plate and used these changes as molecular markers of senescence. In treated animals, after stopping propylthiouracil, these functional, structural, and molecular senescent changes were delayed, compared with controls. This delayed senescence included a delayed decline in longitudinal growth rate, resulting in catch-up growth. The findings demonstrate that growth inhibition due to hypothyroidism slows the developmental program of growth plate senescence, including the normal decline in the rate of longitudinal bone growth, thus accounting for catch-up growth.
IN HUMANS AND OTHER mammals, the release from growth-inhibiting conditions leads to supranormal linear growth, termed catch-up growth. Catch-up growth has been observed after transient hypothyroidism, GH deficiency, glucocorticoid excess, malnutrition, and various systemic diseases (1). Recent evidence suggests that the mechanism responsible for catch-up growth resides within the growth plates, not the central nervous system as previously supposed (2). To explain this observation, we hypothesized that catch-up growth occurs because growth-inhibiting conditions slow the normal process of growth plate senescence.
Growth plate senescence refers to a developmental program that occurs during postnatal life (3). With increasing age, the growth plate undergoes both functional and structural changes; there is a decline in growth rate, chondrocyte proliferation rate, column density, and heights of the proliferative and hypertrophic zones (4). Previous studies suggest that growth plate senescence is not a function of time per se but rather of chondrocyte proliferation (3). Thus, growth plate chondrocytes may have a limited growth capacity, which is gradually exhausted, causing growth deceleration and other senescent changes (3,5). This program of growth plate senescence that occurs at the organ level does not necessarily involve the process referred to as cellular senescence (6). In fact, the limited proliferative capacity of growth plate chondrocytes that appears to underlie growth plate senescence may not be cell autonomous but instead appears to be dependent on cell-cell and/or cell-matrix interactions within the growth plate (7).
We hypothesized that catch-up growth occurs because growth-inhibiting conditions slow growth plate chondrocyte proliferation, thus conserving the proliferative capacity of the chondrocytes and consequently slowing senescence. Thus, after a growth-inhibiting condition resolves, the growth plates are less senescent than normal and consequently grow more rapidly than normal, resulting in catch-up growth (3,5,8). Limited data in glucocorticoid-treated rabbits support that hypothesis (8). To test the model more definitively and determine whether this model may provide a general explanation for catch-up growth in mammals, we decided to study catch-up in a different species, the rat, after a different growth-inhibiting condition, hypothyroidism. Newborn rats were treated with propylthiouracil (PTU) for 8 wk to induce hypothyroidism. After the PTU was discontinued, catch-up growth occurred. During the catch-up growth, multiple functional, structural, and molecular markers were studied and compared with untreated controls to determine whether the previous period of hypothyroidism had delayed the programmed senescence of the growth plate.
To study molecular markers of growth plate senescence, we identified genes whose mRNA expression in growth plate chondrocytes changes markedly during growth plate senescence. These genes included chondroadherin, osteoprotegerin, secreted frizzled-related protein 4, reelin, and nuclear protein 1. Chondroadherin is a cartilage matrix protein that binds both collagen type II (9) and chondrocytes, through α2β1, integrin (10), suggesting that it regulates chondrocyte function (9). Osteoprotegerin is a decoy receptor for receptor activator of nuclear factor-κB ligand and therefore plays a central role in bone biology. It is reportedly expressed by growth plate chondrocytes and may negatively regulate cartilage resorption by an inhibitory effect on osteoclast recruitment to the growth plate (11). Mice lacking functional osteoprotegerin show growth plate abnormalities (12). In humans, inactivating mutations of the gene encoding osteoprotegerin result in juvenile Paget’s disease, which is primarily characterized by excessive bone resorption but also causes short stature (13). Secreted frizzled-related protein 4 inhibits action of the Wnt system (14), which is critical for normal growth plate function (15,16). Reelin is an extracellular glycoprotein that plays a critical role in brain development (17). It may also have effects outside the central nervous system (18) but does not have a known role in the growth plate. Nuclear protein 1 is a nuclear basic helix-loop-helix protein that promotes proliferation (19) but has no known function in the growth plate.
Materials and Methods
Animal study design
All animal procedures were approved by the National Institute of Child Health and Human Development Animal Care and Use Committee. Animals were housed and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (20).
Female Sprague Dawley rats (Harlan, Indianapolis, IN) were made hypothyroid by introducing PTU (1 g/liter; Sigma-Aldrich, St. Louis, MO) at 1 d of age into the drinking water of the mother (21). The PTU-containing water was changed twice per wk. PTU treatment was discontinued at 8 wk of age. The PTU-treated animals were weaned from their mother at 11 wk of age when they were mature enough to support their own nutritional needs. Untreated animals served as concurrent controls. To minimize estrogen effects on the growth plate, all rats were given depot leuprolide acetate (3 mg/kg, sc; Tap Pharmaceuticals, Deerfield, IL) every 6 wk, starting at 3 wk of age (22). PTU-treated and control animals were killed at 8, 11, 13, 16, and 21 wk of age (n = 8–16/group). Additional control animals were killed at 3 and 6 wk of age. Twelve and 2 h before all animals were killed, they received 5-bromo-2′-deoxyuridine (BrdU; 100 μg/g body weight, ip; Sigma-Aldrich).
Weights were measured weekly for all animals. For the cohort of animals killed at 16 wk of age, tail lengths were measured weekly. At the time the animals were killed, tibiae were excised and their lengths measured with digital calipers. For each animal, one proximal tibia was fixed in 10% phosphate-buffered formalin. From the other proximal tibia, the medial and lateral cortical bone near the growth plate was removed by cutting with a scalpel blade. Next, incisions were made in the cross-sectional plane through the epiphyses and metaphyses, several millimeters from the growth plate, allowing us to discard much of the bone proximal and distal to the growth plate. At that point, each proximal tibia was embedded in optimal cutting temperature compound (Electron Microscopy Sciences, Hatfield, PA) and frozen on dry ice. We did not attempt to remove all the bone that surrounds the growth plate before freezing. Instead, we removed only enough cortical bone to permit sectioning. The remaining bone was removed by microdissection of longitudinal sections (see below). Heart, liver, kidney, and uterus were weighed at the time the animals were killed. Serum was collected at the time the animals were killed from 8-wk-old animals for free T4 measurements and from 11-wk-old animals for free T4 and IGF-I measurements (Diagnostic Systems Laboratory, Inc., Webster, TX).
Quantitative histology
Formalin-fixed proximal tibiae were decalcified in 10% EDTA, and embedded in paraffin. Five-micrometer longitudinal sections were stained with Masson Trichrome and analyzed using a light microscope with a VIA-100 video measurement system (Boeckeler, Tucson, AZ). The observer was blinded to the age and treatment group. Measurements were made as previously described (23) with the following modifications: the overall growth plate height was measured from the metaphyseal margin of the terminal hypertrophic cell lacuna to the epiphyseal margin of the resting zone. For most time points, this latter margin represented the edge of the epiphyseal bone. However, for the 8-wk PTU-treated group, ossification lagged, and thus, the margin was taken as the point at which epiphyseal hypertrophic chondrocytes were noted. Resting zone height was measured parallel to the long axis of the bone from the edge of epiphyseal bone to the first cell of a proliferative column. This measurement was performed in three areas of the growth plate and averaged. The number of resting zone chondrocytes was counted as the number of cells per 0.2-mm growth plate width (measured perpendicular to the long axis of the bone). Column density was assessed as the number of hypertrophic columns per 0.5-mm growth plate width in six areas of the growth plate and averaged.
Cell proliferation
BrdU is incorporated into newly formed DNA and thus labels replicating cells. Immunohistochemical staining for BrdU was performed using a BrdU detection kit (Zymed, San Francisco, CA) according to the manufacturer’s protocol. The trypsin exposure time (10–60 min) and the 3,3′-diaminobenzidine exposure time (40 sec to 4 min) were adjusted to achieve strong staining in a discrete subset of proliferative chondrocytes without significant staining in the remaining proliferative chondrocytes or any hypertrophic chondrocytes. The observer, blinded to the age and treatment status, assessed the BrdU-labeling index, defined as the number of positively stained cell nuclei divided by the total number of nuclei, assessed within complete columns. Twenty columns in the central part of the growth plate were counted for each animal and the results averaged.
Growth plate microdissection, RNA isolation, and quantitative real-time PCR
Longitudinal, frozen sections (60 μm) of proximal tibial growth plates were thawed and immediately placed in 70% ethanol, fixed in 100% methanol, stained with eosin, dehydrated in graded alcohol baths, and microdissected in xylene using an inverted microscope. Proliferative zone, prehypertrophic area, and the proximal hypertrophic zone were isolated en bloc using straight razor blades and hypodermic needles. This area of the growth plate was chosen to avoid bone contamination. Gene expression changes were only measured up to 11 wk of age due to the technical difficulty of microdissecting growth plate cartilage in older animals. RNA was isolated as previously described (24). Quality of extracted total RNA was confirmed using an Agilent 2100 bioanalyzer (Agilent, Palo Alto, CA). Each sample was reverse transcribed to cDNA using random primers (100 ng/sample; Invitrogen Corp., Carlsbad, CA) and SuperScript II reverse transcriptase (200 U; Invitrogen) according to the manufacturer’s instructions. Real-time quantitative PCR was performed using an ABI prism 7000 sequence detection system instrument (Applied Biosystems, Foster City, CA) and the following commercial assays containing specific primers and intron-spanning FAM-labeled TacMan probes (Applied Biosystems): chondroadherin, rn00569771_m1; osteoprotegerin, rn00563499_m1; secreted frizzled-related protein 4, rn00585549_m1; reelin, rn00589609_m1; nuclear protein 1, rn00586046_m1. The relative concentration of each transcript is presented relative to the average amount found in 3-wk-old control animals and adjusted for the amount of starting cDNA (using the calibrator gene 18S rRNA) as well as the efficiency of the PCRs using the formula: relative expressioni = (Ei)ΔCTi/(Er)ΔCTr), where i represents the gene of interest, r represents 18S ribosomal RNA, E represents efficiency of the PCR, ΔCT = [(average CT in 3 wk group) − (CT of sample)], and CT represents the threshold cycle (25). Serial 10-fold dilutions of bone cDNA were used to determine the efficiencies of the PCRs (25). The quantitative PCR was performed with cDNA from six different animals, and reactions were carried out in triplicate under the following thermal cycling conditions: 50 C for 2 min and denaturation at 95 C for 10 min, followed by 45 cycles of 15 sec at 95 C and 1 min at 60 C.
Statistical analysis
Data are presented as mean ± sem. A two-way ANOVA for the effects of time and treatment was performed for all growth, histology, and cell proliferation data. Catch-up growth was considered to be present if there was a significant time-treatment interaction term during the recovery period (beginning at 11 wk). A t test was performed at 8 and 16 wk of age for tail length to assess the effect of PTU and the completeness of recovery respectively. The t test was performed at 16 wk of age and not 21 wk of age because tail lengths were measured only in the cohort killed at 16 wk of age. For other growth measurements, the t test was performed at 8 and 21 wk of age. For real-time PCR data, a one-way ANOVA for the effect of time was performed for the control group, and a t test was performed at 8 and 11 wk of age to assess the effect of hypothyroidism and growth inhibition on expression of age-regulated genes.
Results
PTU treatment induced hypothyroidism and growth failure
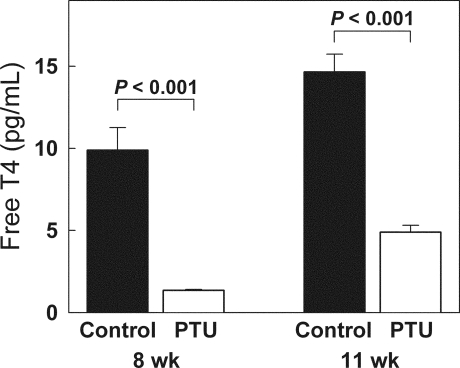
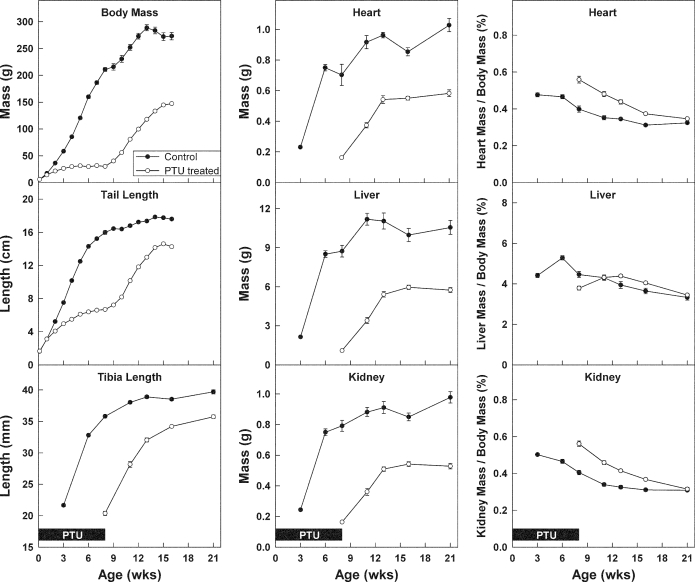
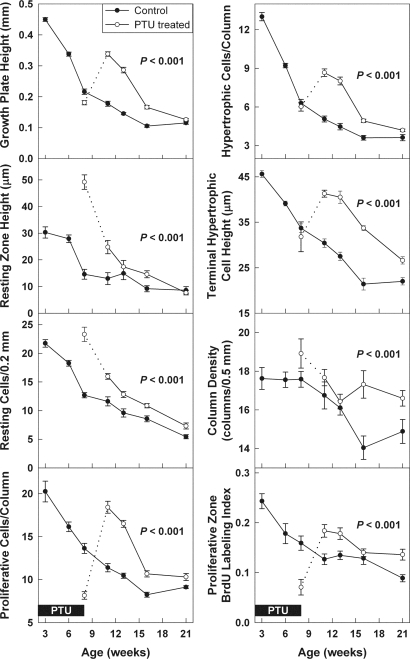
Female rats were made hypothyroid by adding PTU into the drinking water from 1 d of age until 8 wk of age. To try to minimize estrogen effects on the growth plate, all rats were given depot leuprolide acetate. As expected, PTU treatment induced hypothyroidism. At 8 wk of age, free T4 levels were approximately 7 times lower in PTU-treated animals, compared with controls (Fig. 1). Once the PTU treatment was discontinued, the animals gradually recovered. At 11 wk of age, the free T4 levels were approximately 3 times lower in the previously PTU-treated animals, compared with controls (Fig. 1). Serum IGF-I was also lower in the previously PTU-treated animals at 11 wk (440 ± 50 ng/ml), compared with controls (1370 ± 90 ng/ml, P < 0.001). PTU-induced hypothyroidism inhibited growth of body mass; tail length; tibial length; and heart, liver, and kidney mass (P < 0.001, Fig. 2). Decreased longitudinal bone growth was in part due to a lower rate of chondrocyte proliferation in the proliferative zone. At the end of the treatment period (8 wk time point), the BrdU-labeling index was approximately 2.2 times lower in the proximal tibial growth plate of PTU-treated animals (0.071 ± .016), compared with controls (0.16 ± .014, P < 0.001). Hypothyroidism also affected the structure of the growth plate. The resting zone of PTU-treated animals was remarkably wide due to delayed epiphyseal ossification. At 8 wk of age, hypothyroid (PTU treated) animals exhibited a greater number of resting zone cells (P < 0.001) and a larger resting zone height (P < 0.001) but a smaller overall growth plate height (P = 0.02) with fewer proliferative chondrocytes per column compared with control animals (P < 0.001; Fig. 3).
Figure 1.
Serum free T4 levels (mean ± sem) in rats during and after PTU treatment. Female rats (white bars) were made hypothyroid by adding PTU to the drinking water from birth until 8 wk of age. Untreated animals served as controls (black bars). At the end of the 8-wk treatment period, animals that received PTU had lower free T4 levels. Three weeks after the end of treatment, free T4 levels had increased but not normalized.
Figure 2.
Growth (mean ± sem) in body mass, tail length, tibial length, organ (heart, liver, and kidney) mass, and organ mass relative to body mass during and after PTU treatment in young rats. Female rats (open symbols) were made hypothyroid by adding PTU to the drinking water from birth until 8 wk of age. Untreated animals served as controls (closed symbols). All rats received depot leuprolide acetate every 6 wk, starting at 3 wk of age. During the PTU treatment period (solid boxes, pertains to all graphs), growth was inhibited in treated animals (P < 0.001 for body mass, tail length, tibial length, organ masses). Afterward, catch-up growth occurred in all of those measures (defined as growth rate greater than controls, P < 0.001), although the catch-up was not complete at the time of the final measurement (P < 0.001).
Figure 3.
Growth plate structure and function during and after PTU treatment. Female rats were made hypothyroid by adding PTU to the drinking water from birth until 8 wk of age (open symbols). Untreated animals served as controls (closed symbols). All rats received depot leuprolide acetate every 6 wk, starting at 3 wk of age. The solid boxes represent time animals received PTU (0–8 wk of age, pertains to all graphs). The dotted line represents the transition period immediately after cessation of PTU. In control animals, all end points declined significantly with age (P < 0.001). In animals that had previously received PTU, this age-dependent decline was significantly delayed for all of these end points (P < 0.001). Quantitative histology (mean ± sem) was performed on Masson Trichrome-stained sections of the proximal tibial growth plate. An observer blinded to treatment and age measured growth plate height, resting zone height, number of resting zone chondrocytes per 0.2 mm growth plate width, number of proliferative and hypertrophic chondrocytes per column, terminal hypertrophic cell height, column density, and proliferative index in the proliferative zone. Proliferative index was measured by administering BrdU to the rats 12 and 2 h before the animals were killed and then identifying BrdU-labeled cells by immunohistochemistry. The proliferative index represents the number of labeled nuclei divided by total nuclei in intact chondrocyte columns.
Discontinuation of the PTU treatment was followed by catch-up growth
After the PTU treatment was discontinued, significant catch-up growth (defined as growth rate greater than controls) occurred in terms of body mass; tail length; tibia length; and heart, liver, and kidney masses (P < 0.001 for each; Fig. 2). At the last measurement, a statistically significant difference remained between the two groups in body mass, tail length, tibia length, and heart, liver, and kidney masses (P < 0.001 for each), indicating that the catch-up growth was incomplete at that time (Fig. 2). In control animals, mean uterine mass was 0.03 g at 3 wk of age and thereafter fluctuated between 0.10 and 0.27 g. In PTU-treated animals, uterine mass fluctuated between 0.08 and 0.20 g.
Catch-up growth after hypothyroidism is associated with a delay in the normal structural and functional senescent changes in the growth plate
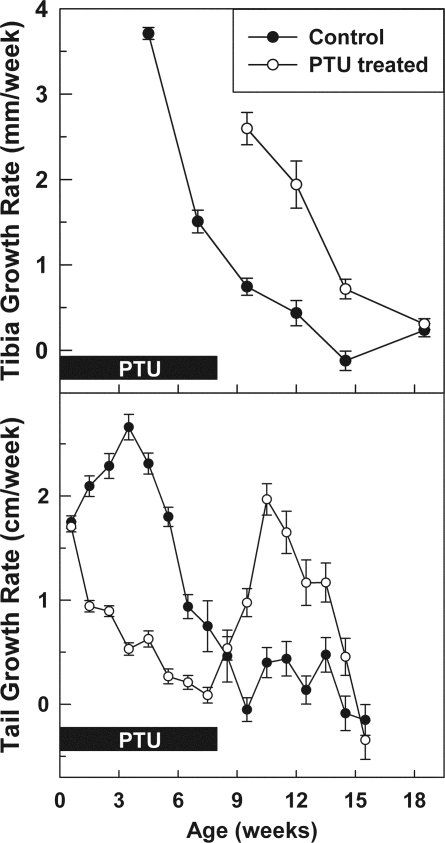
In the proximal tibial growth plates of untreated control animals, we observed significant age-dependent declines, from 3 to 21 wk of age, in growth plate height, resting zone height, number of resting zone chondrocytes, number of proliferative and hypertrophic chondrocytes per column, terminal hypertrophic cell height, column density, and chondrocyte proliferation rate (P < 0.001 for each, Figs. 3 and 4). In animals that had previously been hypothyroid, this age-dependent decline was significantly delayed for all of these measures of growth plate senescence (Figs. 3 and 4). Thus, beginning at 11 wk of age, in the animals that had previously received PTU, there was increased growth plate height, resting zone height, number of resting zone chondrocytes, number of proliferative and hypertrophic chondrocytes per column, terminal hypertrophic cell height, column density, and proliferation, compared with control animals (P < 0.001, Figs. 3 and 4), such that the curve for the previously hypothyroid animals appeared to be time shifted by approximately 5 wk, compared with the control animals. Similarly, there was a time shift of approximately 5 wk in the rate of longitudinal bone growth as assessed by tibial length and tail length (Fig. 5).
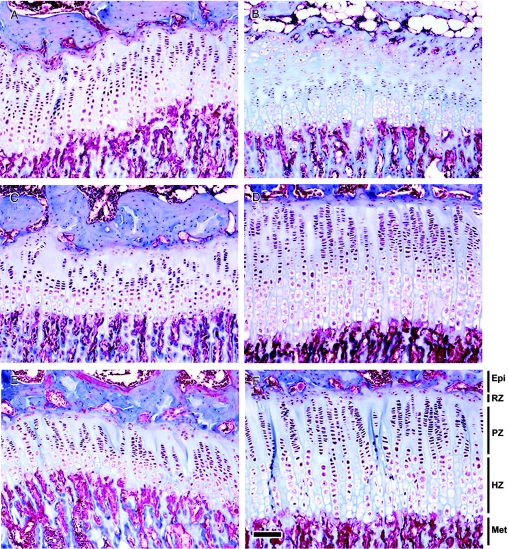
Figure 4.
Growth plate morphology during hypothyroidism and during catch-up growth. Female rats were made hypothyroid by adding PTU to the drinking water from birth until 8 wk of age. Untreated animals served as controls. All rats received depot leuprolide acetate every 6 wk, starting at 3 wk of age. Representative Masson Trichrome-stained sections of proximal tibial growth plates from 8- (A and B), 11- (C and D), and 13-wk-old (E and F) control (A, C, and E) and PTU-treated (B, D, and F) animals are shown. Hypothyroidism (8-wk time-point) affected the structure of the growth plate; the resting zone of PTU-treated animals was remarkably wide due to delayed epiphyseal ossification. PTU-treated animals exhibited a greater number of resting zone cells and a larger resting zone height, but a smaller overall growth plate height with fewer proliferative chondrocytes per column, compared with control animals. During catch-up growth (11 and 13 wk time point), the growth plate height, resting zone height, number of resting zone chondrocytes, number of proliferative and hypertrophic chondrocytes per column, terminal hypertrophic cell height, and column density were greater in animals that had previously received PTU than in controls. Epi, Epiphysis; RZ, resting zone; PZ, proliferative zone; HZ, hypertrophic zone; Met, metaphysic. Scale bar, 100 μm.
Figure 5.
Growth rate (mean ± sem) of the tibia and tail length during and after hypothyroidism. Female rats were made hypothyroid by adding propylthiouracil to the drinking water from birth until 8 wk of age (open symbols). Untreated animals served as controls (closed symbols). All rats received depot leuprolide acetate every 6 wk, starting at 3 wk of age. In control animals, the growth rate underwent the normal senescent decline beginning at 4 wk. In treated animals, during the treatment period (solid boxes), growth was inhibited. After recovery from the treatment, the growth rate was higher than in controls and then declined. In the animals that had previously been hypothyroid, the senescent decline in growth rate appears to be time shifted by approximately 5 wk, compared with control animals.
Catch-up growth after hypothyroidism is associated with a delay in age-dependent changes in gene expression
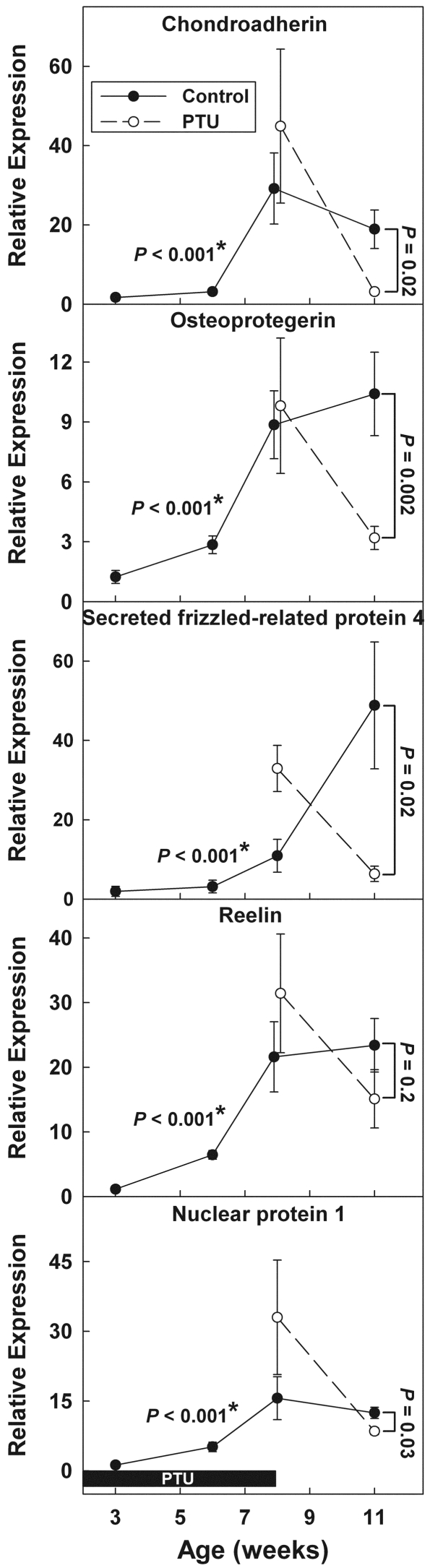
Based on previous screening analyses (microarray and/or real-time PCR; our unpublished data), we selected for study genes whose mRNA expression in growth plate chondrocytes changes markedly during growth plate senescence. If these changes in gene expression were part of the developmental program of growth plate senescence, then one would expect that hypothyroidism would delay these changes just as it delayed structural and functional markers of senescence. To test that prediction, we microdissected proximal tibial growth plates (including proliferative zone, prehypertrophic area, and proximal hypertrophic zone) from study animals, isolated RNA, and quantified specific mRNAs by real-time PCR. As expected, levels of chondroadherin, osteoprotegerin, secreted frizzled-related protein 4, reelin, and nuclear protein 1 increased with age in growth plates from control animals (P < 0.001, Fig. 6). In 11-wk-old animals that had previously received PTU, the expression levels were lower than in control animals (Fig. 6) for chondroadherin (P = 0.02), osteoprotegerin (P = 0.002), secreted frizzled-related protein 4 (P = 0.02), reelin (P = NS), and nuclear protein 1 (P = 0.03). These findings are consistent with the hypothesis that these changes in gene expression are part of the senescence program and therefore the increase in expression that occurred with age in control animals was delayed by previous growth inhibition in PTU-treated animals. At 8 wk of age, the mRNA levels might reflect not only this maturational delay but also the effects of concurrent hypothyroidism, and, indeed, the net effect on expression, compared with control animals, was variable (Fig. 6). At 8 wk of age, hypothyroidism was associated with increased secreted frizzled-related protein 4 mRNA levels, compared with controls (P = 0.01). Chondroadherin, osteoprotegerin, reelin, and nuclear protein 1 mRNA levels did not differ significantly from controls.
Figure 6.
Molecular markers of growth plate senescence. Female rats were made hypothyroid by adding PTU to the drinking water from birth until 8 wk of age. Untreated animals served as controls. All rats received depot leuprolide acetate every 6 wk, starting at 3 wk of age. The solid boxes represent time animals received PTU (0–8 wk of age, pertains to all graphs). The relative concentration (mean ± sem) of each mRNA is presented relative to the average concentration found in 3-wk-old control animals and adjusted for the amount of starting cDNA (using the calibrator gene 18S rRNA) as well as the efficiency of the real-time PCR. The levels of chondroadherin, osteoprotegerin, secreted frizzled-related protein 4, reelin, and nuclear protein 1 mRNA increased with age in microdissected growth plates collected from control animals (*, P < 0.001, by ANOVA). At 11 wk of age, the age-dependent rise in mRNA expression was delayed in the animals that had previously received PTU (by t test, P values shown at right side of each panel).
Discussion
In young rats, treatment with PTU caused hypothyroidism and inhibited growth. After release from hypothyroidism, catch-up growth was seen in body mass, tail length, tibial length, and heart, liver, and kidney masses. Control animals showed the normal senescent decline in tibial growth rate, chondrocyte proliferation rate, growth plate height, resting zone height, number of resting zone chondrocytes, number of proliferative zone chondrocytes per column, number of hypertrophic chondrocytes per column, terminal hypertrophic cell height, and column density. The senescent decline in every one of these variables was delayed in the animals that had previously been hypothyroid. In control animals, mRNA levels of chondroadherin, osteoprotegerin, secreted frizzled-related protein 4, reelin, and nuclear protein 1, increased markedly with age. During catch-up growth, animals that had previously been hypothyroid expressed chondroadherin, osteoprotegerin, secreted frizzled-related protein 4, and nuclear protein 1 mRNAs at levels similar to younger control animals. Thus, molecular markers of senescence, like the structural and functional markers of senescence, also appeared to be delayed by the previous hypothyroidism.
We attempted to minimize confounding factors that might affect growth. Hypothyroidism might have caused the rat pups to feed less robustly than normal and delayed weaning, and thus the growth inhibition may have reflected both the direct effects of thyroid hormone deficiency and the indirect effects of malnutrition. To minimize this indirect effect, we delayed weaning the relatively immature PTU-treated animals until 11 wk of age. We also attempted to minimize estrogen secretion by administering a long-acting GnRH agonist, leuprolide acetate, following a protocol described previously (22). However, uterine weight (approximately 0.16 g), although less than expected for normal mature rats (approximately 0.5 g) (26), exceeded that expected for ovariectomized rats (approximately 0.1 g) (26), suggesting incomplete suppression of the gonadal axis. If hypothyroidism altered the pace of sexual maturation, differing estrogen concentrations might have influenced the experimental endpoints.
The delay in senescence-related gene expression was assessed at 11 wk of age. Even though catch-up growth was occurring at this time-point, the thyroid hormone levels were not fully recovered. Differing levels of thyroid hormones could have influenced the expression of these senescence markers. However, some of the observed differences in gene expression (for example, chondroadherin and osteoprotegerin) at 11 wk had not been present at 8 wk when the hypothyroidism was more severe, suggesting that the observed differences at 11 wk are not simply due to incomplete recovery but rather to the prior history of hypothyroidism.
In the animals that had previously been hypothyroid, the observed delay in multiple functional, structural, and molecular markers of growth plate senescence strongly supports the hypothesis that hypothyroidism slows the developmental program of growth plate senescence. We previously showed evidence that glucocorticoid excess in the rabbit also slows growth plate senescence (8). The combined finding, that growth plate senescence is slowed by two different growth-inhibiting conditions in two different species, supports our model that growth plate senescence is not a function of time per se, but rather of growth, and therefore inhibition of growth also inhibits this developmental process. To explain this phenomenon at a cellular level, we proposed that growth plate chondrocytes have a finite proliferative capacity, which is gradually exhausted with age, causing growth plate senescence (3,5). The findings in the current study support this model.
Our findings also strongly support the hypothesis that catch-up growth is caused by delayed growth plate senescence. In control animals, the rate of longitudinal bone growth underwent the expected senescent decline with age. In animals that had previously been hypothyroid, that decline was delayed; the growth rate appeared to be shifted in time, compared with the control growth rate. The magnitude of this shift, approximately 5 wk, was similar in magnitude to the shift in other markers of growth plate senescence. Thus, in animals that had previously been hypothyroid, the growth plates resembled normal growth plates of animals 5 wk younger in their structural appearance, function, and molecular profile. Consequently, the growth rate during catch-up growth, although elevated for chronological age, appears to be normal for the degree of growth plate senescence. These findings therefore indicate that catch-up growth represents growth that is appropriate for a younger, less senescent growth plate. We had previously shown evidence that catch-up growth after glucocorticoid excess in the rabbit also appears to be due to a delayed senescent decline in the growth rate (8). The combined finding, that catch-up growth appears to be due to delayed growth plate senescence in two different species, after two different growth-inhibiting conditions, suggests that delayed senescence provides a general explanation for catch-growth. In children with celiac disease placed on a gluten-free diet, the pattern of catch-up growth is also consistent with the hypothesis of delayed growth plate senescence (27).
The current study substantiates and extends previous observations in several important ways. First, in the current study, we performed a far more complete assessment of the developmental state of the growth plate by looking at many more structural and functional markers of senescence than has been done previously. Second, in the current study, we combined, for the first time, molecular markers of growth plate senescence with structural and functional markers. The observation that these markers all behaved in concert supports the notion of growth plate senescence as an integrated developmental program. Third, in the current study, by choosing to study a different mammalian species and a different growth-inhibiting condition than in previous studies, we have shown evidence that the previous findings do not represent specific effects of glucocorticoid treatment in the rabbit but instead general, fundamental explanations for the causes of growth plate senescence and catch-up growth.
Our findings in the current study do not completely exclude the possibility that a systemic mechanism could be responsible for the observed catch-up growth (28). For example, an increase in growth hormone and/or IGF-I during the recovery period might account for the increase in growth rate, proliferation rate, and chondrocyte column height. However, at 11 wk of age, the serum IGF-I levels were actually lower in the rats undergoing catch-up growth than in controls. Furthermore, increased GH levels would not readily explain the observation that all variables were affected in a temporal pattern consistent with a time-shift. In addition, a systemic mechanism alone could not provide a general explanation for catch-up growth because it cannot readily explain the phenomenon of local catch-up growth that occurs in single growth plates after local growth inhibition by glucocorticoid (2). Although delayed growth plate senescence appears to cause catch-up growth in a variety of circumstances, other mechanisms, including hormonal mechanisms, might also contribute in some circumstances. Indeed, the observed catch-up growth in nonskeletal organs could be explained by a systemic mechanism. Alternatively, the observation might indicate that common, local cellular mechanisms limit growth in multiple tissues, including the growth plate, and thus, growth-inhibiting conditions may delay the exhaustion of the growth potential in multiple organs, resulting in subsequent catch-up growth.
Catch-up growth in tibia length and tail length, although striking, was incomplete. One possible explanation is that the rate of growth plate senescence may be determined by proliferation of stem-like cells in the resting zone (5,29) whereas the rate of growth is dependent on the proliferation rate of the nonstem cells in the proliferative zone. If hypothyroidism slows proliferation in the proliferative zone more than it slows proliferation in the resting zone, it might have a greater inhibitory effect on the rate of growth than on the rate of senescence. In this situation, each cell division of the stem-like cells would produce a smaller clone of proliferative zone chondrocytes, resulting in less efficient growth. In this situation, catch-up growth would be expected to be incomplete. Another possible explanation is that the number of stem-cell divisions tends to be similar in various hormonal and nutritional states but is not completely invariant.
mRNA levels for the genes studied were generally lower in PTU-treated animals than in controls at 11 wk of age during the recovery period, suggesting that the previous period of hypothyroidism had delayed the normal rise in gene expression; however, at 8 wk of age, when the animals were still receiving PTU, the mRNA levels tended to be greater in treated animals. However, the 8-wk time point is difficult to interpret because at this age the treated animals and controls differ in two ways. In treated animals, mRNA levels at this time might be affected not only by the preceding period of growth inhibition caused by hypothyroidism, which appeared to delay the normal rise in gene expression but also by the concurrent hypothyroidism. Taken together, the data at 8 and 11 wk of age suggest that a previous period of growth inhibition by hypothyroidism delays the rise in gene expression (as shown at 11 wk of age), but concurrent hypothyroidism increases expression of these genes. At the 8-wk time point, the stimulatory effect of concurrent hypothyroidism appears to outweigh the delaying effects of previous growth inhibition. Similar effects are seen for the structural and functional markers of senescence. For example, the number of proliferative chondrocytes per column is decreased during hypothyroidism, at 8 wk but is increased after hypothyroidism at 11 wk. The former presumably reflects primarily the growth-suppressive effects of concurrent hypothyroidism; the latter reflects the delaying effects of previous hypothyroidism.
In children with hypothyroidism, bone age is markedly delayed and using the bone age to predict adult height often overestimates the actual adult height (30). Our findings suggest an explanation for this overestimation. At the end of the PTU treatment, there was a striking delay in epiphyseal ossification that appeared to be out of proportion to the delay in growth plate senescence. Because bone age is based on the extent of cartilage ossification, the delay in bone age in hypothyroid children may exceed the delay in growth plate senescence. This divergence of bone age and growth plate senescence could explain the overoptimistic height prediction reported in hypothyroid children.
We conclude that growth inhibition due to hypothyroidism slows the entire developmental program of growth plate senescence, including multiple structural, functional, and molecular changes and that the subsequent catch-up growth represents the normal growth rate of younger, less senescent, growth plates. Combined with previous studies, the findings strongly support the concepts that growth plate senescence is a function of chondrocyte proliferation and that delayed growth plate senescence represents a general explanation for catch-up growth.
Footnotes
This work was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, National Institutes of Health. O.N. was supported by grants from the Swedish Research Council (K2007-52X-20316-01-4), the Swedish Society of Medical Research, the Magnus Bergvall Foundation, Sällskapet Barnavård, and Stiftelsen Frimurare Barnhuset i Stockholm.
First Published Online January 3, 2008
Abbreviations: BrdU, 5-Bromo-2′-deoxyuridine; PTU, propylthiouracil.
References
- Boersma B, Wit JM 1997 Catch-up growth. Endocr Rev 18:646–661 [DOI] [PubMed] [Google Scholar]
- Baron J, Klein KO, Colli MJ, Yanovski JA, Novosad JA, Bacher JD, Cutler Jr GB 1994 Catch-up growth after glucocorticoid excess: a mechanism intrinsic to the growth plate. Endocrinology 135:1367–1371 [DOI] [PubMed] [Google Scholar]
- Nilsson O, Baron J 2004 Fundamental limits on longitudinal bone growth: growth plate senescence and epiphyseal fusion. Trends Endocrinol Metab 15:370–374 [DOI] [PubMed] [Google Scholar]
- Walker KV, Kember NF 1972 Cell kinetics of growth cartilage in the rat tibia. II. Measurements during ageing. Cell Tissue Kinet 5:409–419 [DOI] [PubMed] [Google Scholar]
- Schrier L, Ferns SP, Barnes KM, Emons JA, Newman EI, Nilsson O, Baron J 2006 Depletion of resting zone chondrocytes during growth plate senescence. J Endocrinol 189:27–36 [DOI] [PubMed] [Google Scholar]
- Collado M, Blasco MA, Serrano M 2007 Cellular senescence in cancer and aging. Cell 130:223–233 [DOI] [PubMed] [Google Scholar]
- Nilsson O, Mitchum Jr RD, Schrier L, Ferns SP, Barnes KM, Troendle JF, Baron J 2005 Growth plate senescence is associated with loss of DNA methylation. J Endocrinol 186:241–249 [DOI] [PubMed] [Google Scholar]
- Gafni RI, Weise M, Robrecht DT, Meyers JL, Barnes KM, De Levi S, Baron J 2001 Catch-up growth is associated with delayed senescence of the growth plate in rabbits. Pediatr Res 50:618–623 [DOI] [PubMed] [Google Scholar]
- Mansson B, Wenglen C, Morgelin M, Saxne T, Heinegard D 2001 Association of chondroadherin with collagen type II. J Biol Chem 276:32883–32888 [DOI] [PubMed] [Google Scholar]
- Camper L, Heinegard D, Lundgren-Akerlund E 1997 Integrin α2β1 is a receptor for the cartilage matrix protein chondroadherin. J Cell Biol 138:1159–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K, Kitazawa R, Kurosaka M, Maeda S, Kitazawa S 2006 Expression profile of genes related to osteoclastogenesis in mouse growth plate and articular cartilage. Histochem Cell Biol 125:593–602 [DOI] [PubMed] [Google Scholar]
- Kawana F, Sasaki T 2003 Osteoclast differentiation and characteristic trabecular bone formation during growth plate destruction in osteoprotegerin-deficient mice. J Electron Microsc (Tokyo) 52:515–525 [DOI] [PubMed] [Google Scholar]
- Cundy T, Davidson J, Rutland MD, Stewart C, DePaoli AM 2005 Recombinant osteoprotegerin for juvenile Paget’s disease. N Engl J Med 353:918–923 [DOI] [PubMed] [Google Scholar]
- Horvath LG, Lelliott JE, Kench JG, Lee CS, Williams ED, Saunders DN, Grygiel JJ, Sutherland RL, Henshall SM 2007 Secreted frizzled-related protein 4 inhibits proliferation and metastatic potential in prostate cancer. Prostate 67:1081–1090 [DOI] [PubMed] [Google Scholar]
- Yang Y, Topol L, Lee H, Wu J 2003 Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development 130:1003–1015 [DOI] [PubMed] [Google Scholar]
- Andrade AC, Nilsson O, Barnes KM, Baron J 2007 Wnt gene expression in the post-natal growth plate: regulation with chondrocyte differentiation. Bone 40:1361–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Chen Y 2006 Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci 7:850–859 [DOI] [PubMed] [Google Scholar]
- Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, Martin ND, Walsh CA 2000 Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet 26:93–96 [DOI] [PubMed] [Google Scholar]
- Mallo GV, Fiedler F, Calvo EL, Ortiz EM, Vasseur S, Keim V, Morisset J, Iovanna JL 1997 Cloning and expression of the rat p8 cDNA, a new gene activated in pancreas during the acute phase of pancreatitis, pancreatic development, and regeneration, and which promotes cellular growth. J Biol Chem 272:32360–32369 [DOI] [PubMed] [Google Scholar]
- National Research Council 2003 Guide for the care and use of laboratory animals. Washington, DC: National Academy Press [Google Scholar]
- Meisami E 1984 Complete recovery of growth deficits after reversal of PTU-induced postnatal hypothyroidism in the female rat: a model for catch-up growth. Life Sci 34:1487–1496 [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Okada H, Heya T, Shimamoto T 1989 Controlled release of LHRH agonist, leuprolide acetate, from microcapsules: serum drug level profiles and pharmacological effects in animals. J Pharm Pharmacol 41:439–444 [DOI] [PubMed] [Google Scholar]
- Weise M, De-Levi S, Barnes KM, Gafni RI, Abad V, Baron J 2001 Effects of estrogen on growth plate senescence and epiphyseal fusion. Proc Natl Acad Sci USA 98:6871–6876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs C, Yanovski JA, Roth AH, Yu YM, Domene HM, Yano K, Cutler Jr GB, Baron J 1994 Dexamethasone increases growth hormone receptor messenger ribonucleic acid levels in liver and growth plate. Endocrinology 135:1113–1118 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW 2001 A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PM, Eriksen EF, Lind L, Orberg J, Sahlin L 2004 Estrogen supplementation modulates effects of the endocrine disrupting pollutant PCB126 in rat bone and uterus: diverging effects in ovariectomized and intact animals. Toxicology 199:129–136 [DOI] [PubMed] [Google Scholar]
- Emons JA, Boersma B, Baron J, Wit JM 2005 Catch-up growth: testing the hypothesis of delayed growth plate senescence in humans. J Pediatr 147:843–846 [DOI] [PubMed] [Google Scholar]
- Tanner JM 1963 Regulation of growth in size of mammals. Nature 199:845–850 [DOI] [PubMed] [Google Scholar]
- Abad V, Meyers JL, Weise M, Gafni RI, Barnes KM, Nilsson O, Bacher JD, Baron J 2002 The role of the resting zone in growth plate chondrogenesis. Endocrinology 143:1851–1857 [DOI] [PubMed] [Google Scholar]
- Boersma B, Otten BJ, Stoelinga GB, Wit JM 1996 Catch-up growth after prolonged hypothyroidism. Eur J Pediatr 155:362–367 [DOI] [PubMed] [Google Scholar]