Abstract
The human lutropin receptor (hLHR) and human TSH receptor (hTSHR) are G protein-coupled receptors that play key roles in reproductive and thyroid physiology, respectively. We show using a quantitative assessment of cAMP production as a function of cell surface receptor expression that the hTSHR possesses greater basal constitutive activity than the hLHR. Further studies were undertaken to test the hypothesis that different potential Gs-coupling motifs identified in IL2 of the hTSHR and hLHR contribute to their different basal constitutive activities. Although mutating the receptors to interchange their potential Gs-coupling motifs reversed their relative activities, we show this to be due to the swapping of one IL2 residue (Q476 in the hLHR; R531 in the hTSHR). Molecular dynamics simulations show that the effect of the hLHR(Q476R) mutation, switching the structural features of the hLHR toward those of the hTSHR, is greater than the switching effect of the hTSHR(R531Q) mutant toward the hLHR. The structural model of the hLHR(Q476R) mutant can be considered as a hybrid of wild-type (wt) hTSHR and constitutively active mutant hLHR forms. In this hLHR(Q476R) mutant, IL2 adopts a structure similar to IL2 of the wt hTSHR, but it shares with the hLHR constitutively active mutant the solvent exposure and the reciprocal arrangement of helices 3, 5, and 6, including the weakening of the wt native R3.50-D6.30 interaction. Our results suggest a H3-mediated structural connection between IL2 and the cytosolic extension of H6. Thus, IL2 contributes significantly to the inactive and active state ensembles of these G protein-coupled receptors.
THE HUMAN LUTROPIN receptor (hLHR) is a G protein-coupled receptor (GPCR) that plays a central role in reproductive endocrinology. Expressed primarily in the gonads, in females it stimulates androgen synthesis (which is used as substrate for the subsequent synthesis of estrogen) and progesterone production, and it mediates ovulation in response to pituitary LH. Its critical role in stimulating female sex steroid hormone synthesis is indispensable during pregnancy, when the hLHR of the corpus luteum responds to placental human chorionic gonadotropin (hCG), a hormone nearly identical to LH that is similarly recognized by the hLHR. In males the hLHR, in response to pituitary LH, stimulates androgen biosynthesis, which is essential for spermatogenesis. LH and hCG also have trophic effects on the gonads, and normal gonadal development depends on the appropriate expression and activity of the hLHR. The human TSH receptor (hTSHR) serves a very different physiological role. Expressed primarily in the thyroid, it mediates the actions of pituitary TSH by stimulating thyroid hormone synthesis. It too has trophic actions on the thyroid gland.
Despite their differing physiological functions, the hLHR and hTSHR [as well as the human follitropin receptor (hFSHR)] are structurally related glycoprotein hormone receptors, so named because they each bind the related glycoprotein hormones: LH or hCG to the hLHR, TSH to the hTSHR, and FSH to the hFSHR. The glycoprotein hormones are each composed of an identical α-subunit covalently associated with distinct, but related, β-subunits, in which the β-subunits of LH and hCG are nearly identical. Cloning of the cDNAs for each of the glycoprotein hormone receptors confirmed that, as one would have predicted, they too are closely related (1,2,3). Each receptor contains a serpentine region prototypical of GPCRs and a large extracellular domain composed of multiple leucine-rich repeats that confers high-affinity binding of hormone (reviewed in Refs. 4,5,6). The glycoprotein hormone receptors belong to the large family A of GPCRs. In recent years other receptors related to the glycoprotein hormone receptors have been cloned, and, together with the glycoprotein hormone receptors, these are now considered the leucine-rich repeat containing glycoprotein hormone receptor subfamily of GPCRs (7).
According to the probabilistic multistate model, the glycoprotein hormone receptors, as with other GPCRs, are thought to exist in the plasma membrane in equilibrium between ensembles of inactive and active states (8,9,10). The multistate model predicts that the relationship between conformational states and protein function are stochastic rather than deterministic as the allosteric models imply. According to the multistate model, the active state of the receptor cannot be attributed to individual conformational states but rather to an ensemble of states in the conformational space of the receptor. The multistate model further predicts that the topology and distribution of the ensemble change with activating mutations, ligand binding, receptor oligomerization, and receptor interactions with intracellular partners. Consistent with this model, when heterologous cells are transfected with cDNA encoding any of the wild-type (wt) glycoprotein hormone receptors, there is a detectable level of basal activity of the receptor, as measured by second messenger production, that is dependent on the density of cell surface receptor (11,12,13,14). The binding of agonist or the introduction of constitutively activating mutations (CAMs) causes a further increase in activation, likely due to induction of active state ensembles that differ from the active state ensembles of the unbound forms of the wt receptors (8,9,10).
Despite the structural similarities between the human glycoprotein hormone receptors, there are some notable functional differences. For example, the hFSHR is not as sensitive to mutations causing constitutive activity as the hLHR(11). It has also been shown that CAMs of the hFSHR, but not the hLHR, permit promiscuous activation of the receptor by other glycoprotein hormones (11,14,15,16,17,18). Furthermore, earlier studies have shown that cells transfected with increasing concentrations of plasmid encoding the hTSHR produced higher levels of basal cAMP than those transfected with the same concentrations of plasmid encoding the hLHR(19). Although it has been inferred from these data that the hTSHR possesses greater basal constitutive activity than the hLHR, further studies quantifying cell surface receptor levels are needed to determine the relative contributions of differing levels of basal constitutive activity vs. cell surface expression levels to the results observed.
In a bioinformatics approach, Möller et al. (20) had looked at the putative intracellular loops of 103 different GPCRs whose signaling pathways had been determined, and they identified a number of different potential amino acid motifs that were common among receptors signaling through Gs vs. Gq vs. Gi. In examining the predicted cytoplasmic ends of the transmembrane helices and intracellular loops of the hTSHR and hLHR, we identified in IL2 one such potential Gs-specific motif in the hLHR and a different potential Gs-specific motif in the hTSHR. The present studies were undertaken to determine if there is in fact a difference in the basal constitutive activities of the hTSHR and hLHR, and, if so, to determine if the motifs thus identified contribute to these different activities.
Materials and Methods
Plasmids and hormones
The cDNAs for the wt hLHR and wt hTSHR were kindly given to us by Ares Advanced Technology (Ares-Serono Group, Randolph, MA) and Dr. Gilbert Vassart (Institut de Recherche Interdisciplinaire en Biologie Humaine et Moléculaire and Service de Genetique Medicale, Campus Erasme, Brussels, Belgium), respectively, and were placed into pcDNA3.1(neo) (Invitrogen, Carlsbad, CA). The wt receptors, as well as all mutants thereof, were modified to contain a myc-epitope tag at the N terminus. Mutations were introduced using the PCR overlap method of site-directed mutagenesis (21,22). The plasmids were prepared using the QIAGEN maxiprep kit (QIAGEN, Valencia, CA), and the entire coding regions were sequenced by automated DNA sequencing (performed by the DNA Core Facility of the University of Iowa Carver College of Medicine, Iowa City, IA). The numbering of residues in transmembrane helices follows that proposed by Ballesteros and Weinstein (23), in which the first number indicates the H, and the numbers thereafter indicate the position of the helical residue relative to the most highly conserved residue within that H, which is denoted as 50.
Highly purified recombinant hCG was purchased from Dr. A. Parlow and the National Institute of Diabetes and Digestive and Kidney Diseases National Hormone and Pituitary Program or from Sigma-Aldrich (St. Louis, MO), and highly purified recombinant hTSH was purchased from Genzyme (Cambridge, MA). The specific activity for the hTSH was listed as 4–12 IU/mg. For the purposes of this study, a specific activity of 4 IU/mg was used for calculating the concentrations of hTSH.
Cells and transfections
Human embryonic kidney (HEK) 293 cells were obtained from the American Type Culture Collection (CRL 1573; Manassas, VA) and were maintained at 5% CO2 in growth media consisting of high-glucose DMEM containing 50 μg/ml gentamicin, 10 mm HEPES, and 10% newborn calf serum. Cells for experiments were plated onto 35-mm wells that had been precoated for 45 min with 0.1% gelatin in calcium and magnesium-free PBS (pH 7.4). Cells were transiently transfected at 50–70% confluency following the protocol of Chen and Okayama (24), except that the overnight precipitation was performed in a 5% CO2 atmosphere. Cells were then washed with Waymouth’s MB752/1 media modified to contain 50 μg/ml gentamicin and 1 mg/ml BSA, after which fresh growth media were added. The cells were used for experiments 24 h later. When transfecting cells with varying concentrations of plasmid-containing receptor cDNA, the total amount of plasmid was kept constant with empty vector.
Flow cytometry to evaluate cell surface receptor expression
HEK293 cells were plated and transfected as described previously. Forty-eight hours after transfection, cells were washed once with filtered PBS for immunohistochemistry (PBS-IH) [137 mm NaCl, 2.7 mm KCl, 1.4 mm KH2PO4, and 4.3 mm Na2HPO4 (pH 7.4)]. Cells were then detached by washing with PBS-IH and centrifuged at 500 × g to collect the cells. The supernatant was removed, and the cells were resuspended in PBS-IH and incubated 1 h at 4 C with or without anti-myc 9E10 monoclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted 1:20 with PBS-IH containing 0.5% BSA (PBS-IH/BSA). The cells were washed with PBS-IH/BSA and incubated an additional 1 h at 4 C with fluorescein isothiocyanate-conjugated goat antimouse antibody (Sigma-Aldrich) diluted 1:350 with PBS-IH containing 0.5% BSA. Cells were washed with PBS-IH/BSA, resuspended in 1 ml PBS-IH/BSA, and filtered through a 70-μm BD Falcon cell strainer (BD Biosciences, San Jose, CA) into a clean test tube on ice. A Becton Dickinson fluorescence-activated cell sorter DiVa (BD, Franklin Lakes, NJ) with a 488-nm wavelength laser was then used to quantify cell surface expression in 10,000 cells from each transfection. Control gating was set using cells transfected with empty vector and stained with antibody as described. The arbitrary fluorescence units describing total cell surface fluorescence was determined as the product of the percent cells gated and the geometric mean fluorescence of the sample minus the respective product in the control (empty vector) group.
125I-hCG binding assays to evaluate cell surface hLHR expression
HEK293 cells were plated onto gelatin-coated 35-mm wells and transiently transfected as described previously. On the day of the experiment, cells were washed two times with warm Waymouth’s MB752/1 containing 50 μg/ml gentamicin and 1 mg/ml BSA. To determine the maximal cell surface binding capacity, the cells were then incubated 1 h at room temperature in the same media containing a saturating concentration of 125I-hCG (500 ng/ml final concentration) with or without an excess of unlabeled crude hCG (50 IU/ml final concentration). The assay was terminated by washing the cells three times with cold Hanks’ Balanced Salt Solution modified to contain 50 μg/ml gentamicin and 1 mg/ml BSA. The cells were then solubilized in 0.5 n NaOH, transferred to plastic test tubes with cotton swabs, and counted in a γ-counter.
Determination of intracellular cAMP production
HEK293 cells were plated onto gelatin-coated 35-mm wells and transiently transfected as described previously. On the day of the experiment, cells were washed two times with warm Waymouth’s MB752/1 containing 50 μg/ml gentamicin and 1 mg/ml BSA, and placed into 1 ml of the same medium containing 0.5 mm isobutylmethylxanthine. After a 15-min preincubation at 37 C, buffer only (for the determination of basal cAMP) or hormone (hCG at a final concentration of 100 ng/ml or hTSH at a final concentration of 250 ng/ml, 1 mIU/ml) was added, and the cells were incubated a further 60 min at 37 C. The cells were then placed on ice, the media aspirated, and intracellular cAMP was extracted by the addition of 0.5 n perchloric acid containing 180 μg/ml theophylline and measured by RIA. All assays were performed with triplicate wells, and the cAMP in each well was determined in duplicate in the RIA.
Computational modeling of the hLHR and hTSHR
Computational modeling of the hLHR and hTSHR consisted of comparative modeling, followed by molecular dynamics (MD) simulations of selected models. Comparative was performed with the comparative modeling software Modeler (25), using the latest rhodopsin structure as a template [i.e. Protein Data Bank code: 1U19 (26)]. The modeled sequence includes the transmembrane helices, three intracellular loops (IL1, IL2, and IL3), three extracellular loops (EL1, EL2, and EL3), as well as the 323–358 ectodomain sequence, which can be reasonably modeled based upon the N terminus of rhodopsin. The details of comparative modeling of the updated model of the hLHR have been reported elsewhere (27). The hLHR model used in this study differs slightly from this last reported structure with respect to a slightly different alignment in the N terminus and IL2, as well as the MD setup. As for comparative modeling of the hLHR and hTSHR, briefly, a modified rhodopsin template was used in which the sequences 100–101 and 106–107 were deleted, which correspond to the H2/EL1 junction and the first two amino acids of H3, respectively. The sequence 236–242, corresponding to the C-terminal region of IL3, was deleted as well. During comparative modeling, α-helical restraints were imposed on the hLHR sequences 420–423 and 432–439, and the TSH receptor (TSHR) sequences 475–478 and 487–494.
For each receptor and from the selected sequence alignment (Fig. 1), 200 models were obtained by randomizing the cartesian coordinates of the model through a random number uniformly distributed in an interval from −4 Å to 4 Å (25). Two models were selected for the hLHR, and one model was selected for the hTSHR, based on low restraint violation and high stereochemical quality. These models were first completed by the addition of the polar hydrogens, and then subjected to automatic and manual rotation of the side-chain torsion angles when in non-allowed conformations, leading to five models for the hLHR and seven models for the TSHR, which were used as input structures for MD. MD simulations were performed with the CHARM program (28), using an implicit membrane-water model recently implemented in CHARM, i.e. the GBSW (29). Minimizations were performed using 1500 steps of steepest descent, followed by Adopted Basis Newton-Raphson minimization until the root mean square gradient was less than 0.001 kcal/mol Å. A disulfide bridge patch was applied to C439(3.25) and C514 (in EL2), for the hLHR, and between the corresponding C494(3.25) and C569 (in EL2), respectively, located in H3 and EL2. MD simulations were also performed with and without an additional disulfide patching between C336 and C353, for the hLHR, and the corresponding C390 and C408 for the TSHR. The bridged cysteine pairs lie in the ectodomain.
Figure 1.
Sequence alignment between rhodopsin and the two glycoprotein hormone receptors. Sequence alignment between bovine rhodopsin [Protein Data Bank code: 1U19 (26)] (i.e. template) on the one hand, and the hLHR (target) and hTSHR (target) that was used for comparative modeling. For space economy the template-target pairwise alignment used for comparative modeling has been combined in a unique alignment in this figure. The amino acid stretches 100–101, 106–107, and 236–242 have been deleted from the 1U19 template. The boxed sequences correspond to H8. Residues R3.50 and D6.30 of the hLHR and hTSHR are highlighted in gray.
The all-atom parameter set was used. The lengths of the bonds involving the hydrogen atoms were restrained by the SHAKE algorithm, allowing an integration time step of 0.001 psec. The systems were heated to 300 K with 7.5-K increases every 2,500 steps per 100,000 steps by randomly assigning velocities from the gaussian distribution. After heating, the system was allowed to equilibrate for 100 psec. The secondary structure of the H bundle was preserved by assigning distance restraints (i.e. minimum and maximum allowed distances of 2.7 and 3.0 Å, respectively) between the backbone oxygen atom of residue i and the backbone nitrogen atom of residue i+4, except for prolines. The scaling factor of such restraints was 10, and the force constant at 300 K was 10 kcal/mol Å. The receptor amino acids, which were found in non-canonical α-helical conformations in the input structure, a condition inherited from the rhodopsin template, were not subjected to any intrabackbone distance restraint. Short (100 psec) equilibrated MD runs were performed, and different input structures and different combinations of intrahelical distance restraints were probed. The latter tests consisted of applying distance restraints to different amino acid stretches in each helix. Different protonation states of H473 and H482(4.41), in the hLHR, and of H537, in the hTSHR, were probed as well. Finally, the computation conditions and the input receptor structure were chosen that, after MD simulation, produced average arrangements characterized by good stereochemical quality, as well as structural similarity to rhodopsin. For both receptors, the selected computational setup concerns with the protonated form of H4.41 and the presence of two disulfide bridges, i.e. the one inherited from rhodopsin structure and the one in the modeled portion of the ectodomain (see above in this paragraph for details of the disulfide patches). The selected input structure was used to produce the following mutants: D564(6.30)G and Q476R (in IL2), for the hLHR, and D619(6.30)G and R531Q (in IL2) for the hTSHR. wt and mutated structures were subjected to 1 nsec MD simulations, and the structures averaged over the entire 1000 psec trajectories were considered for the comparative analyses.
Results
In vitro experiments
Determinations of the absolute levels of cell surface receptor expression using ligand binding assays are not practical for the hTSHR, given the general difficulty in determining precise biological activities of the hTSH preparations used for iodination. Therefore, to measure quantitatively the relative levels of cell surface expression of the hLHR and hTSHR, each receptor was engineered with a myc epitope tag on its N terminus, a modification that has been shown to be well tolerated with respect to cell surface receptor expression, hormone binding, and hormone-stimulated cAMP production. Transfected cells were then assayed for cell surface receptor expression by flow cytometry using anti-myc monoclonal antibody. This antibody has specifically recognized the myc-tagged versions of hLHR and hTSHR (30). As would be expected (31), it was observed in these studies that some of the recombinantly expressed full-length cell surface myc-hTSHRs are cleaved in HEK293 cells, releasing the myc-labeled fragment A of the extracellular domain (30). However, flow cytometric analyses of nonpermeabilized cells with the anti-myc antibody would be expected to detect only intact full-length myc-hTSHRs on the cell surface. Indeed, flow cytometry has been used quite routinely in recent years for the quantification of relative cell surface hTSHR expression (32,33,34,35,36). Generally, investigators have used antibodies directed toward the hTSHR. However, the use of an anti-myc antibody to detect myc-labeled hTSHR and hLHR enables one to compare quantitatively the cell surface expression of the two receptors.
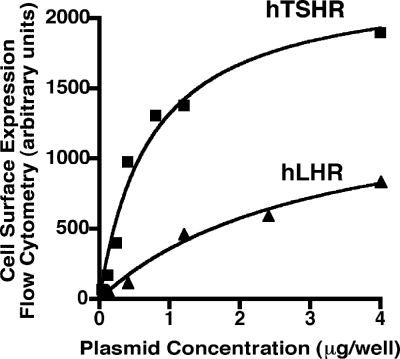
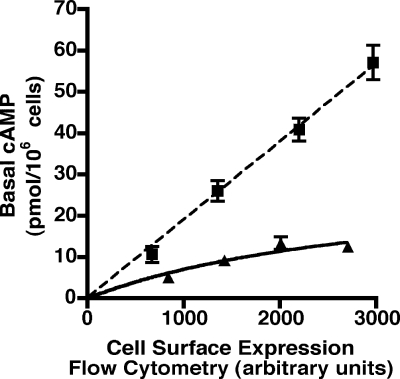
Cell surface expression of the hTSHR and hLHR plotted as a function of the plasmid concentrations used for transfection is shown in Fig. 2. These data show that, at any given plasmid concentration, the relative cell surface expression of the hTSHR is much greater than the hLHR. Similar results were obtained with several different plasmid preparations of each receptor cDNA. To determine whether the hLHR and hTSHR exhibit different basal constitutive activities, HEK293 cells were transiently transfected with increasing concentrations of plasmid encoding either myc-tagged hLHR or hTSHR. In the same experiment, basal cAMP concentrations and relative cell surface receptor expression levels were then assayed. Because the hTSHR is expressed at the plasma membrane more robustly than the hLHR, cells were transfected with lower plasmid concentrations of hTSHR cDNA than the hLHR cDNA so that the basal constitutive activities could be determined over a similar range of cell surface receptor expression. Data are presented as cAMP concentrations as a function of relative cell surface receptor expression. This approach, rather than a normalization of cAMP per amount of receptor expressed, was used because not all activities are linear relative to receptor expression. As shown in Fig. 3, when compared over a comparable range of cell surface receptor expression, the hTSHR exhibited a greater level of basal constitutive activity than the hLHR. At low receptor densities, the hTSHR appeared approximately 2-fold more active and at high receptor densities approximately 6-fold more active than the hLHR. Therefore, although at any given plasmid concentration the hTSHR is expressed at higher cell surface densities than the hLHR, it can nonetheless be concluded that, at the same cell surface receptor density, the hTSHR does indeed possess greater basal constitutive activity than the hLHR.
Figure 2.
Higher cell surface expression of the hTSHR compared with the hLHR. HEK293 cells were transiently transfected with increasing amounts of plasmid encoding myc-hLHR or myc-hTSHR, and relative cell surface receptor expression was determined by flow cytometry. The data shown are from one experiment that is representative of at least seven independent experiments.
Figure 3.
Greater intrinsic constitutive activity of the hTSHR compared with the hLHR. HEK293 cells were transiently transfected with increasing amounts of the indicated plasmids, which were myc-tagged. Within a given experiment, both cell surface receptor expression, as determined by flow cytometry, and basal cAMP production were assayed. The cAMP levels of cells transfected with empty vector have been subtracted from the cAMP values shown. The data shown are the mean ± sem of triplicate determinations within a given experiment that is representative of at least three independent experiments.
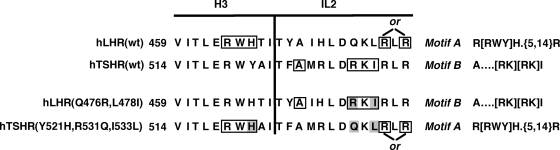
Examination of the three intracellular loops of the hLHR and hTSHR for potential Gs-coupling motifs compiled by Möller et al. (20) using a bioinformatics approach led us to identify one of these motifs in the hLHR and a different motif in the hTHSR. The motif described by Möller et al. (20) as R[RWY]H.{5,14}Rb is fulfilled by the boxed residues shown in the hLHR(wt) sequence in Fig. 4. To simplify discussion of the R[RWY]H.{5,14}R motif, we refer to it here simply as motif A. The motif described by Möller et al. (20) as A… . [RK][RK]Ic is fulfilled by the boxed residues shown in the hTSHR(wt) sequence in Fig. 4. For simplicity sake, we refer to this motif as motif B. Both motifs A and B are composed of residues from the cytoplasmic end of transmembrane H3 and IL2. We did not observe the other Gs-coupling motifs described by Möller et al. in the predicted intracellular regions of the hLHR or hTSHR. Therefore, the following experiments were performed to test the hypothesis that the higher basal constitutive activity of the hTSHR is conferred by motif B and the lower basal constitutive activity of the hLHR by motif A.
Figure 4.
Potential Gs-coupling motifs in the hLHR and hTSHR. Sequences of the predicted cytoplasmic end of TM3 and IL2 are shown for the hLHR and hTSHR. One potential Gs-coupling motif identified by Möller et al. (20), R[FWY]H.{5,14}R (referred to herein as motif A), is found in the hLHR as shown by the encircled residues. Another potential Gs-coupling motif identified by Möller et al. (20), A… . [RK][RK]I (referred to here as motif B), is found in the hTSHR as shown by encircled residues. Definitions of the nomenclature used by Möller et al. in the protein sequence pattern can be found at http://www.embl-heidelberg.de/∼chenna/elm_2.html. In the two mutants shown, motifs A and B have been switched. The mutated residues are shaded, and the newly created motifs are composed of the encircled residues.
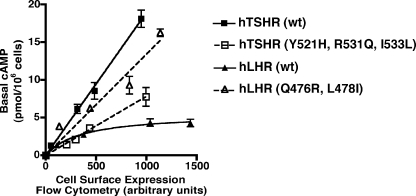
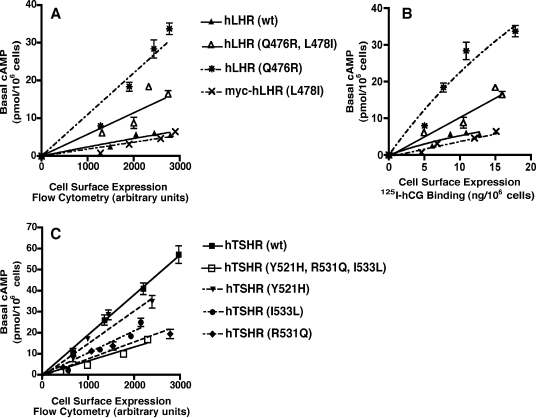
Toward this end, we constructed mutations of the hTSHR and hLHR that interchanged the potential Gs-coupling motifs (Fig. 4) and examined the basal constitutive activities of these mutants. When the hTSHR was mutated to hTSHR(Y521H,R531Q,I533L) to change motif B of the hTSHR to motif A of the hLHR, the basal activity of the hTSHR decreased markedly to a level similar to or just somewhat higher than the wt hLHR (Fig. 5). Although this decreased activity of the hTSHR may have resulted from the mutation of its motif B into motif A, it could also be reflective of a general deleterious effect of mutation of those three residues. Therefore, we also examined whether mutating the hLHR to change its motif A into the motif B found in the hTSHR would cause an increase in the basal activity of the hLHR. As shown in Fig. 5, hLHR(Q476R,L478I), which has been modified to now contain motif B of the hTSHR, exhibited increased basal constitutive activity compared with the wt hLHR.
Figure 5.
Interchanging motifs A and B between the IL2 regions of the hLHR and hTSHR reverses the basal constitutive activities. HEK293 cells were transiently transfected with increasing amounts of the indicated plasmids, all of which were myc-tagged. Within a given experiment, both cell surface receptor expression, as determined by flow cytometry, and basal cAMP production were assayed. The cAMP levels of cells transfected with empty vector have been subtracted from the cAMP values shown. The data shown are the mean ± sem of triplicate determinations within a given experiment that is representative of at least three independent experiments.
The data presented thus far suggest that the potential Gs-coupling motifs A and B in the hLHR and hTSHR, respectively, may be responsible for the different basal constitutive activities of these two glycoprotein hormone receptors, i.e. the decreased activity of hTSHR(Y521H,R531Q,I533L) may have resulted because motif B of the hTSHR was changed into motif A of the hLHR, and the increased activity of hLHR(Q476R,L478I) may have resulted because motif A of the hLHR was mutated to motif B of the hTSHR. However, it is also possible that the altered activities of these mutants were due to one or more of the individual amino acid substitutions independent of their context within a potential motif. Therefore, we examined the basal constitutive activities of the wt hLHR and the hLHR(Q476R,L478I) double mutant compared with the single mutants hLHR(Q476R) and hLHR(L478I). If hLHR(Q476R,L478I) exhibits greater constitutive activity than the wt hLHR because motif A has been switched to motif B, then we would expect that hLHR(Q476R) and hLHR(L478I) alone would have activities similar to the wt hLHR. As shown in Fig. 6A, mutation of L478I alone was without any effect on the basal constitutive activity of the hLHR. However, hLHR(Q476R) exhibited a greater level of constitutive activity than the double hLHR(Q476R,L478I) mutant. These data suggest that the increased activity of hLHR(Q476R,L478I) is due not to the interchanging of motif A to motif B but rather to the Q476R substitution in IL2. The same results were observed regardless of whether the cell surface expression of the wt and mutant hLHRs were quantified by flow cytometry or by 125I-hCG binding (compare Fig. 6, A and B), confirming the robustness of the flow cytometric analyses used for quantifying glycoprotein hormone receptor expression.
Figure 6.
Reversal of basal constitutive activity can be attributed to mutation of a single IL2 residue. HEK293 cells were transiently transfected with increasing amounts of the indicated plasmids, all of which were myc-tagged. Within a given experiment, both cell surface receptor expression, as determined by flow cytometry in A and C or 125I-hCG binding in B, and basal cAMP production were assayed. The cAMP levels of cells transfected with empty vector have been subtracted from the cAMP values shown. The data shown are the mean ± sem of triplicate determinations within a given experiment that is representative of at least three independent experiments. A, hLHR(Q476R,L478I) is compared with hLHR singly substituted mutants, and cell surface hLHR receptor expression is determined by flow cytometry. B, hLHR(Q476R,L478I) is compared with hLHR singly substituted mutants, and cell surface hLHR receptor expression is determined by 125I-hCG binding. C, hTSHR(Y521H,R531Q,I533L) is compared with hTSHR singly substituted mutants.
Similar analyses were performed on the hTSHR, comparing the basal constitutive activity of the triply substituted hTSHR(Y521H,R531Q,I533L) with hTSHR constructs containing only one of the three mutations. As shown in Fig. 6C, hTSHR(Y521H) had only slightly less basal constitutive activity than the wt hTSHR. However, hTSHR(I533L) and hTSHR(R531Q) both exhibited reduced basal constitutive activity, with R531Q being almost identical to that of the triply substituted hTSHR mutant. These data argue against the decreased activity of hTSHR(Y521H,R531Q,I533L) as being due to the switching of motif B to motif A. Rather, they suggest that individual substitutions, in particular R531Q, mediate the changes in basal activity for the hTSHR.
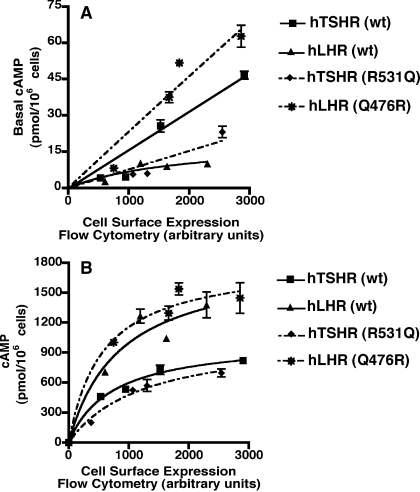
A quantitative comparison of hLHR(Q476R) with the wt hTSHR reveals that this IL2 mutant of the hLHR has even greater basal constitutive activity than the wt TSHR (Fig. 7A). A comparison of the hTSHR(R531Q) IL2 mutant with the wt hLHR shows that, although the effect of this mutation is to reduce significantly the basal constitutive activity of the hTSHR, it remains slightly more active than the wt hLHR. These data suggest that the same IL2 residue, Q476 in the hLHR and R531 in the hTSHR, confers different basal constitutive activities on each of the receptors. We further examined if the swapping of these residues also affects the hormone-stimulated responses (Fig. 7B). Interestingly, it was consistently observed that over a range of cell surface receptor expression, the wt hLHR generates a higher concentration of cAMP in response to a saturating concentration of hormone than the wt hTSHR. When one then looks at the effects of swapping this IL2 residue between the two receptors, the data shown in Fig. 7B indicate slight effects of the substitutions, resulting in an increase and decrease, respectively, in hormone-stimulated cAMP by the hLHR and hTSHR. However, we observed variability between these experiments, with results generally indicating little or no effect of the IL2 mutations on hormone-stimulated cAMP.
Figure 7.
Comparison of hLHR(Q476R) to the wt hTSHR and of hTSHR(R531Q) to the wt hLHR. HEK293 cells were transiently transfected with increasing amounts of the indicated plasmids, all of which were myc-tagged. Within a given experiment, both cell surface receptor expression, as determined by flow cytometry, and basal (A) or hormone-stimulated (B) cAMP production were assayed. The cAMP levels of cells transfected with empty vector have been subtracted from the cAMP values shown. The data shown are the mean ± sem of triplicate determinations within a given experiment that is representative of at least three independent experiments.
Taken altogether, the data presented thus far show that a nonconserved residue in IL2 of the hLHR and hTSHR (Q476 in the hLHR and R531 in the hTSHR) contributes to the relatively high basal activity of the hTSHR and lower basal activity of the hLHR, whereas having little or no impact on the hormone-stimulated activities of the two receptors. These results do not preclude other residues of the receptors as having roles in contributing to the basal constitutive activities of the receptors. However, given the structural similarities of these two glycoprotein hormone receptors, the identification of a residue that, when interchanged, causes reciprocal alterations in basal constitutive activity, provides a unique opportunity to determine by molecular modeling the structural basis underlying the differences in basal constitutive activities.
In silico experiments
Therefore, computational modeling of the hLHR and hTSHR was performed with the goal of comparing the structural hallmarks of the functionally different states in the two receptors. The study focused on the effects of the critical IL2 mutations identified in this study, Q476R for the hLHR and R531Q for the hTSHR, on the structure of IL2 and on the environment of the functionally important R3.50 of the E/D-R-Y/W motif. In addition, we compared these structures to those observed as a result of a D6.30G mutation, a substitution previously shown to cause constitutive activity of the hLHR and hTSHR (37,38). In line with previous work, changes in the environment of R3.50 were described in terms of perturbations in the native interaction pattern of R3.50, which is found interacting with D6.30 in the wt hLHR and hTSHR, and in terms of solvent accessibility of selected amino acids constituting the environment of the conserved arginine. In addition, mutational effects on the structure of IL2 were quantified in terms of Cα atom root mean-square deviation (Cα-RMSD) (Table 1). Specifically, Cα-RMSD values were computed on the IL2 from the average minimized structures of wt and mutated forms of the hLHR and hTSHR, leading to 12 pairwise comparisons. By definition, a smaller Cα-RMSD value indicates a greater similarity in structure between the IL2 being compared.
Table 1.
Structural similarity between IL2 from wt and mutant hLHR and hTSHR
| IL2 MATCHa | Cα-RMSD (Å)b |
|---|---|
| hLHR(wt)-hLHR(D6.30G) | 2.59 |
| hLHR(wt)-hLHR(Q476R) | 1.82 |
| hLHR(wt)-hTSHR(wt) | 2.39 |
| hLHR(wt)-hTSHR(R531Q) | 2.11 |
| hLHR(D6.30G)-hLHR(Q476R) | 2.00 |
| hLHR(D6.30G)-hTSHR(D6.30G) | 1.83 |
| hLHR(D6.30G)-hTSHR(R531Q) | 2.04 |
| hTSHR(wt)-hTSHR(D6.30G) | 0.90 |
| hTSHR(wt)-hTSHR(R531Q) | 0.78 |
| hTSHR(wt)-hLHR(Q476R) | 0.96 |
| hTSHR(D6.30G)-hTSHR(R531Q) | 0.51 |
| hTSHR(D6.30G)-hLHR(Q476R) | 0.98 |
Pairwise comparisons between the IL2 from the average minimized structures of the different hLHR and hTSHR forms. The amino acid stretches constituting the IL2 from the hLHR and hTSHR are, respectively, 469–481 and 524–536 (Fig. 1).
RMSD (Å) computed on the IL2 Cα atoms.
Consistent with previous studies on the hLHR (39), in the activating D6.30G mutation of both receptors, the breakage of the native R3.50-D6.30 interaction was found associated with the opening of a cytosolic crevice in between H3 and H6. This effect is properly described by the solvent accessible surface area (SAS) computed over selected amino acids. To facilitate the comparison between the hLHR and hTSHR, we selected the same amino acid positions for computation of the SAS index, i.e. R464(3.50), T467(3.53), and I468(3.54) for the hLHR, and R519(3.50), A522(3.53), and I523(3.54) for the hTSHR. These three positions hold the same amino acids in the two receptors, except for position 3.53, which holds a threonine in the hLHR and an alanine in the hTSHR. The SAShLHR is 58 Å2 in the wt receptor and 126 Å2 in the D6.30G CAM (Table 2 and Fig. 8). Following the same trend, the SAShTSHR is 71 Å2 in the wt receptor and 102 Å2 for the D6.30G CAM (Table 2 and Fig. 9). Intriguingly, the IL2 extracted from the average minimized structures of the D6.30G CAM of the hLHR and hTSHR is more similar (Cα-RMSD = 1.83 Å) than those extracted from the wt forms of the two receptors (Cα-RMSD = 2.39 Å; Table 1). Consistent with this trend, the IL2 from these two CAMs shares the loss of any charge-reinforced H bonds involving the unique and conserved lysine of the loop (i.e. the lysine at position i+1, where i is the mutation site; Figs. 4, 8, and 9).
Table 2.
SAS computed for wt and mutant hLHR and hTSHR
| Receptor formsa | SAS (Å2)b |
|---|---|
| hLHR(wt) | 58.0 |
| hLHR(D6.30G) | 126.0 |
| hLHR(Q476R) | 100.0 |
| hTSHR(wt) | 71.0 |
| hTSHR(D6.30G) | 102.0 |
| hTSHR(R531Q) | 76.0 |
Average minimized structures of wt and mutated hLHR and hTSHR.
SAS computed over R464(3.50), I468(3.34), and T467(3.35) for the hLHR, and R519(3.50), A522(3.53), and I523(3.54) for the hTSHR.
Figure 8.
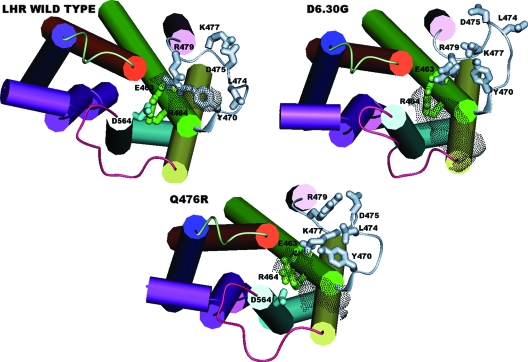
Computational models of the wt, D564(6.30)G, and Q476R forms of the hLHR. Shown are top views from the intracellular side of the hLHR models. The extracellular domains are not shown. Helices 1, 2, 3, 4, 5, 6, and 7 are colored in blue, orange, green, pink, yellow, cyan, and violet, respectively. H8 is colored in violet as well. The intracellular loops 1, 2, and 3 are colored in light green, gray, and purple, respectively. The side chains of hLHR E463(3.49), R464(3.50), and D564(6.30), as well as of selected amino acids in IL2 are shown in stick representation and colored according to their location. The side chain of the mutated IL2 Q476R residue is not shown because it does not perform any peculiar interaction and to rather highlight the effects of the mutation on other IL2 residues. The SAS computed over the amino acids at positions 3.50, 3.54, and 3.55 is also shown, represented by gray dots. SAS values are 58, 126, and 100 Å2 for the wt, D564(6.30), and Q476R forms of the hLHR, respectively.
Figure 9.
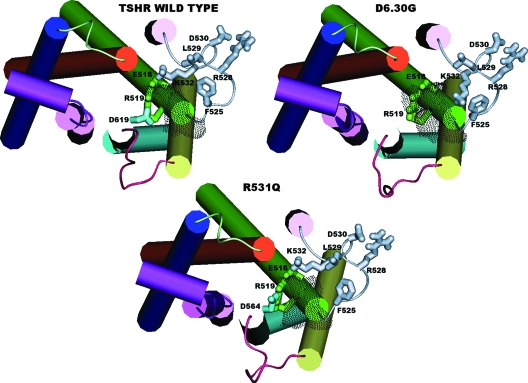
Computational models of the wt, D619(6.30)G, and R531Q forms of the hTSHR. Shown are top views from the intracellular side of the hTSHR models. The extracellular domains are not shown. Helices 1, 2, 3, 4, 5, 6, and 7 are colored in blue, orange, green, pink, yellow, cyan, and violet, respectively. H8 is colored in violet as well. The intracellular loops 1, 2, and 3 are colored in light green, gray, and purple, respectively. The side chains of hTSHR E518(3.49), R519(3.50), and D619(6.30), as well as of selected amino acids in IL2 are shown in stick representation and colored according to their location. The side chain of the mutated IL2 R531Q residue is not shown because it does not perform any peculiar interaction and to rather highlight the effects of the mutation on other IL2 residues. The SAS computed over the amino acids at positions 3.50, 3.54, and 3.55 is also shown, represented by gray dots. SAS values are 71, 102, and 76 Å2 for the wt, D619(6.30), and R531Q forms of the hTSHR, respectively.
With respect to the critical IL2 mutations, both Q476 in the hLHR and R531 in the hTSHR are directed toward the putative membrane phospholipids and make van der Waals interactions with the hydrophobic amino acid at position i-4 (Fig. 4). In the hLHR(Q476R) mutant, the substituting arginine makes long-range electrostatic interactions (without any H-bonding component) with the aspartate at position i-1. In contrast, in the hTSHR(R531Q) mutant, the substituting glutamine holds almost the same topology and interaction pattern as the original arginine. Intriguingly, the Q476R mutation confers to the hLHR IL2 striking structural similarities with IL2 of the wt hTSHR (Table 1 and Figs. 8 and 9). Indeed, the IL2 extracted from the average minimized structure of hLHR(Q476R) is more similar to IL2 from the wt and D6.30G CAM forms of hTSHR(Cα-RMSD = 0.96 and 0.98 Å, respectively) than to IL2 from the wt or D6.30G CAM forms of hLHR (Cα-RMSD = 1.82 and 2.0 Å, respectively; Table 1). Structural similarities between IL2 from the hLHR(Q476R) mutant and IL2 from the wt hTSHR include also the interaction pattern of almost all the amino acid side chains. In particular, the conserved lysine at position i+1 (i.e. K477 for the hLHR and K532 for the hTSHR) in the wt hLHR may be found interacting with the conserved aspartate at position i-1 (i.e. D475), whereas in the hLHR(Q476R) mutant, it interacts with E463(3.49), similar to the wt hTSHR (Figs. 8 and 9). In contrast to the hLHR(Q476R) mutant, the ability of the hTSHR(R531Q) mutation to switch the IL2 conformation toward that of the wt hLHR is less striking. In fact, the IL2 backbone conformation from this mutant is structurally similar to that from the wt hTSHR (Cα-RMSD = 0.78 Å; Table 1 and Fig. 8).
Consistent with in vitro experiments, the hLHR(Q476R) mutant shares with the D6.30G CAM forms of both the hLHR and hTSHR an increase in SAS compared with the wt receptors. In fact, whereas the SAS index of hLHR(Q476R) is 100 Å2, that of wt hLHR is only 58 Å2 (Table 2 and Fig. 8). This effect is associated with weakening of the native R3.50-D6.30 interaction. Interestingly and consistent with the SAS trend, in the hLHR(Q476R) mutant, the reciprocal arrangement of the cytosolic ends of H3, H5, and H6 resembles that of the D6.30G hLHR CAM rather than the wt hLHR(Fig. 8). In contrast to hLHR(Q476R), the hTSHR(R531Q) mutant retains the structural features of wt hTSHR as far as the interaction pattern of R3.50 and the SAS index are concerned (i.e. the SAS indices are 76 and 71 Å2 for the R531Q mutant and wt forms, respectively; Table 2 and Fig. 9).
Collectively, the local effects of hLHR(Q476R) and hTSHR(R531Q) mutations on the IL2 conformation, as well as the distal effects on the interaction pattern of R3.50 and on the SAS index, suggest that the hTSHR(R531Q) mutation is structurally more conservative than the hLHR(Q476R) mutation. The latter mutant appears to be a mixture of structural features from the wt hTSHR and the D6.30G hLHR CAM.
Discussion
Our understanding of GPCR activation has evolved in recent years to account for the observation that, even in the absence of agonist, cells expressing a GPCR will nonetheless exhibit a small degree of spontaneous receptor activity, a phenomenon referred to as basal constitutive activity of the GPCR. Basal constitutive activity has been reported for a large number of GPCRs (40). As has been reported previously (11,13,14) and shown here as well, both the hLHR and hTSHR exhibit basal constitutive activities that increase with increasing densities of cell surface receptor expression. For many GPCRs, the basal constitutive activity of the wt receptor can be further augmented by CAMs, many of which are associated with pathophysiological disorders (41). Numerous naturally occurring CAMs of the hLHR and hTSHR have been described (42,43). These mutations result in increases in the basal constitutive activities of the receptors and are associated with gonadotropin-independent precocious puberty in males (hLHR) (reviewed in Refs. 42 and 44) or with autonomously functioning thyroid adenomas and nonautoimmune autosomal dominant hyperthyroidism (hTSHR) (reviewed in Ref. 43). Inverse agonists, described for many GPCRs (40), cause a reduction in both basal and mutation-induced constitutive activity. One example of the physiological regulation of GPCR activity by an endogenous inverse agonist occurs at the melanocortin-4 receptor (MC4R). The hypothalamic MC4R, when activated by the agonist α-MSH, suppresses food intake. The effect of the inverse agonist agouti-related protein is to suppress the activity of the MC4R, thereby causing increased food intake. Interestingly, mutations have been identified in obese patients, but not in nonobese controls, that decrease the basal constitutive activity of the MC4R. Therefore, it has been proposed that the basal constitutive activity of the MC4R serves a physiological role in promoting the anorexigenic catabolic state necessary for normal body weight (45). Similarly, the ghrelin receptor also exhibits relatively high basal constitutive activity and a mutation that selectively decreases its constitutive activity segregated with short stature and obesity, also suggesting that its basal constitutive activity exerts a physiological role in maintaining normal growth and energy balance (46,47).
Although many naturally occurring loss-of-function inactivating mutations of the hLHR and hTSHR have been reported that result in decreased hormone responsiveness and possibly decreased basal activity as well (reviewed in Refs. 42 and 43), no naturally occurring mutations of these receptors have been described that selectively decrease only basal constitutive activities. Furthermore, although recently a monoclonal antibody raised to the extracellular domain of the hTSHR has been reported to have inverse agonist activity in that it reduces both the spontaneous constitutive basal activity of the wt hTSHR as well as CAMs of the hTSHR (36), there are no known endogenous inverse agonists of the hLHR or hTSHR. Therefore, it is not possible at this time to ascribe with certainty the physiological role of the basal constitutive activities of the hLHR and hTSHR. However, hormonal stimulation of the hLHR and hTSHR is associated with the growth and proliferation of target cells, as well as with hormone biosynthesis. Therefore, it is possible that hLHR and hTSHR basal constitutive activities may be involved in contributing to normal target cell growth and/or maintaining low levels of gonadal steroid hormones and thyroid hormone, respectively. Indeed, it has been suggested that the basal constitutive activities of GPCRs may generally contribute to the tone, or tonic regulation, of a physiological system in addition to agonists (48).
Previous studies have shown that heterologous cells expressing the hTSHR produce higher levels of cAMP than those expressing the hLHR (19). Because the cAMP data were analyzed relative to the concentrations of plasmid used to transfect the cells, the differences in cAMP production may have been due to different basal constitutive activities of the receptors and/or to different levels of cell surface receptor expression for a given amount of transfected plasmid. Our data suggest that both phenomena most likely contributed to the differences reported between the two receptors. As shown here using myc-tagged receptors quantified by flow cytometry, at a given concentration of plasmid transfected into the cells, the relative expression of the hTSHR is greater than that of the hLHR (Fig. 2). It should be noted that, despite any cleavage of the hTSHR that may release the myc-labeled fragment A (30,31), the resulting flow cytometric data would reflect only the full-length hTSHR on the cell surface. The data presented here show that, over a similar range of cell surface receptor levels, the hTSHR does indeed possess a greater intrinsic basal constitutive activity (2- to 6-fold higher) than the structurally related hLHR (Fig. 3). Whether this translates into the thyroid being subjected to higher basal activity of the hTSHR compared with the hLHR-expressing cells in the gonads cannot readily be extrapolated from these data in the absence of accurate quantification of the molar cell surface expression of the hTSHR and hLHR in their target tissues. Interestingly, the responsiveness of the hTSHR to a saturating concentration of agonist is approximately 2- to 3-fold less than that of the hormone-stimulated hLHR (Fig. 7B). Therefore, despite the greater basal activity of the hTSHR, the fold stimulation of cAMP production in response to agonist by the hTSHR is considerably less than that of the hLHR.
Although the hTSHR exhibits greater basal constitutive activity than the hLHR, the hFSHR possesses a lower degree of constitutive activity than the hLHR (11). Interestingly, the hFSHR has also been intrinsically less responsive to activating mutations (11), thereby potentially contributing to the relative paucity of naturally occurring activating mutations of the hFSHR that have been identified compared with the hLHR and hTSHR.
Using bioinformatics, Möller et al. (20) had compiled a list of many potential intracellular sequence motifs that were common to Gs-coupled receptors. Of those, we observed one motif (R[FWY]H.{5,14}R, which we termed motif A) in IL2 and the cytoplasmic end of TM3 of the hLHR and a different motif (A… . [RK][RK]I, which we termed motif B) in the comparable region of the hTSHR. Therefore, we hypothesized that these different Gs-stimulating motifs may be responsible at least in part for the different basal constitutive activities (as measured by increased Gs activation) of the hLHR and hTSHR. Our results show that the introduction of motif B into the hLHR increases its basal constitutive activity, and, conversely, the introduction of motif A into the hTSHR suppresses its basal constitutive activity. However, we further show that the different basal activities can be attributed to one particular residue in IL2 that contributes to each of the motifs. In the hLHR this residue corresponds to Q476, and in the hTSHR the residue at the comparable position is R531. Our data show that the interchange of the glutamine and arginine residues at this position renders the hLHR mutant even more active than the wt hTSHR and causes the basal activity of the hTSHR to be suppressed to levels not quite as low as those of the wt hLHR. Therefore, these data support a role for this nonconserved residue in IL2 in conferring the basal constitutive activity of these two related GPCRs. However, the interchanging of this IL2 residue on the hormone-stimulated activities of the hLHR and hTSHR was with little or no effect. Taken altogether, our data do not support a role for the R[FWY]H.{5,14}R or A… . [RK][RK]I motifs in mediating the basal constitutive activities of the hLHR and hTSHR, respectively.
Although our data show that the IL2 residue identified in this study (Q476 in the hLHR; R531 in the hTSHR) contributes to the different basal constitutive activities of the two receptors, these results are not meant to imply that this is the only residue in either receptor that modulates basal constitutive activity. For the hTSHR, it has been shown that a receptor construct lacking the extracellular domain possesses greater basal activity, suggesting a role for the extracellular domain in modulating the activity of the receptor (49,50). In addition, data from studies on the hTSHR in which alanine scanning mutagenesis was performed on residues in IL2 and IL3 suggest that substitutions of several residues cause a decrease in basal constitutive activity with little or no decrease in hTSH-stimulated cAMP accumulation (34,51). However, our results showing that reciprocal mutations of Q476 in the hLHR with R531 in the hTSHR lead to both a decrease in hTSHR basal constitutive activity as well as an increase in basal constitutive activity of the structurally related hLHR, provide a unique and compelling framework to characterize the structural features of these receptors that mark their different basal constitutive activities.
The results of the in silico experiments using MD simulations are consistent with the results of the in vitro experiments and suggest that the effect of the hLHR(Q476R) mutation, switching the conformational behavior of IL2 toward that of wt hTSHR, is greater than the switching effect of the hTSHR(R531Q) mutant toward the wt hLHR. The local effects of the hLHR(Q476R) and hTSHR(R531Q) mutations on the IL2 conformation, as well as the distal effects on the interaction pattern of R3.50 and on the SAS index, suggest that the hTSHR(R531Q) mutation is structurally more conservative than the hLHR(Q476R) mutation. Such a resistance of IL2 of the hTSHR to structural changes after the R531Q mutation may be due, at least in part, to the presence of an arginine at position i-3, which is engaged in persistent charge-reinforced H bonds with the aspartate at position i-1 in all the simulated forms of the hTSHR, i.e. the wt and the D6.30G and R531Q mutants (Fig. 9). In contrast, in the hLHR, position i-3 is occupied by a histidine that can eventually exist in different prototropic forms, but that has been considered in its neutral state in this study. A histidine at position i-3 is less forced than an arginine to be a partner of the aspartate at position i-1. This could result in more conformational degrees of freedom for the hLHR IL2 compared with the hTSHR IL2. The Cα-RMSD values reported in Table 1 further support this inference. Thus, consistent with functional data, the hLHR(Q476R) mutation shifts the IL2 conformation toward that of wt hTSHR(Table 1). In contrast, the cytosolic extensions of H3, H5, and H6 acquire a reciprocal arrangement and a degree of solvent accessibility similar to that of the D6.30G hLHR CAM (Fig. 8). The latter effect is properly marked by the SAS index that, in the hLHR(Q476R) mutant, is similar to the SAS of the D6.30G hLHR mutant and higher than the SAS of wt hLHR (Table 2 and Fig. 8).
Collectively, the hLHR(Q476R) mutant structurally resembles the wt hTSHR in some aspects and the D6.30G CAM of hLHR in others (Figs. 8 and 9). In this respect, the structural model of the hLHR(Q476R) mutant can be considered a hybrid of wt hTSHR and CAM hLHR forms (Figs. 8 and 9 and Table 1). In contrast, the hTSHR(R531Q) mutant does not resemble any of the hLHR forms, retaining almost all the structural features of wt hTSHR (Fig. 9 and Table 1).
The activating effect of the hLHR(Q476R) mutation is suggested to be due to an effect on the conformational preferences of IL2 (such that it resembles a mixture of the wt hTSHR and the D6.30G hLHR CAM) and, consequently, on the degree of solvent exposure of the cytosolic extension of H3, including the highly conserved R3.50. An intriguing result of this study is that, in both the hLHR and hTSHR, a structural connection exists between the cytosolic extension of H6, which holds D6.30, and IL2 and vice versa. The structural communication between these two domains appears to be mediated by the cytosolic end of H3 that holds the conserved arginine of the E/D-R-Y/W motif.
Acknowledgments
We thank Nathan Johnson for expert technical assistance.
Footnotes
This work was supported by National Institutes of Health Grant HD22196 (to D.L.S.) and Telethon Italy Grant S00068TELU (to F.F.).
Present address for T.M.: Deutsches Primatenzentrum, Goettingen, Lower Saxony D 37077, Germany.
Present address for D.M.: Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, Texas 75390.
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 27, 2007
R[FWY]H.{5,14}R refers to an arginine followed by an arginine, tryptophan, or tyrosine, followed by a histidine, followed by any five to 14 residues, and then an arginine (http://www.embl-heidelberg.de/∼chenna/elm_2.html).
A… . [RK][RK]I refers to an alanine followed by any four residues, followed by an arginine or lysine, followed by an arginine or lysine, followed by an isoleucine (http://www.embl-heidelberg.de/∼chenna/elm_2.html).
Abbreviations: CAM, Constitutively activating mutation; Cα-RMSD, Cα atom root mean-square deviation; GPCR, G protein-coupled receptor; H1, helix 1; hCG, human chorionic gonadotropin; HEK, human embryonic kidney; hFSHR, human follitropin receptor; hLHR, human lutropin receptor; hTSHR, human TSH receptor; IL1, 2, or 3, intracellular loop 1, 2, or 3; MC4R, melanocortin-4 receptor; MD, molecular dynamics; PBS-IH, PBS for immunohistochemistry; PBS-IH/BSA, PBS for immunohistochemistry containing 0.5% BSA; SAS, solvent accessible surface area; TSHR, TSH receptor; wt, wild type.
References
- McFarland KC, Sprengel R, Phillips HS, Kohler M, Rosemblit N, Nikolics K, Segaloff DL, Seeburg PH 1989 Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science 245:494–499 [DOI] [PubMed] [Google Scholar]
- Sprengel R, Braun T, Nikolics K, Segaloff DL, Seeburg PH 1990 The testicular receptor for follicle stimulating hormone: structure and functional expression of cloned cDNA. Mol Endocrinol 4:525–530 [DOI] [PubMed] [Google Scholar]
- Parmentier M, Libert F, Maenhaut C, Lefort A, Gerard C, Perret J, Van Sande J, Dumont JE, Vassart G 1989 Molecular cloning of the thyrotropin receptor. Science 246:1620–1622 [DOI] [PubMed] [Google Scholar]
- Ascoli M, Fanelli F, Segaloff DL 2002 The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev 23:141–174 [DOI] [PubMed] [Google Scholar]
- Simoni M, Gromoll J, Nieschlag E 1997 The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev 18:739–773 [DOI] [PubMed] [Google Scholar]
- Vassart G, Pardo L, Costagliola S 2004 A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci 29:119–126 [DOI] [PubMed] [Google Scholar]
- Hsu SY, Kudo M, Chen T, Nakabayashi K, Bhalla A, van der Spek PJ, van Duin M, Hsueh AJ 2000 The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol 14:1257–1271 [DOI] [PubMed] [Google Scholar]
- Onaran HO, Costa T 1997 Agonist efficacy and allosteric models of receptor action. Ann NY Acad Sci 812:98–115 [DOI] [PubMed] [Google Scholar]
- Kenakin T 2002 Efficacy at G-protein-coupled receptors. Nat Rev Drug Discov 1:103–110 [DOI] [PubMed] [Google Scholar]
- Onaran HO, Scheer A, Cotecchia S, Costa T 2000 A look into receptor efficacy: from the signalling network of the cell to the intramolecular motion of the receptor. In: Kenakin T, Angus JA, eds. The pharmacology of functional, biochemical, and recombinant receptor systems. Berlin: Springer; 217–259 [Google Scholar]
- Zhang M, Tao YX, Ryan GL, Feng X, Fanelli F, Segaloff DL 2007 Intrinsic differences in the response of the human lutropin receptor versus the human follitropin receptor to activating mutations. J Biol Chem 282:25527–25539 [DOI] [PubMed] [Google Scholar]
- Tao Y-X, Mizrachi D, Segaloff DL 2002 Chimeras of the rat and human FSH receptors (FSHRs) identify residues that permit or suppress transmembrane 6 mutation-induced constitutive activation of the FSHR via rearrangements of hydrophobic interactions between helices 6 and 7. Mol Endocrinol 16:1881–1892 [DOI] [PubMed] [Google Scholar]
- Zhang M, Mizrachi D, Fanelli F, Segaloff DL 2005 The formation of a salt bridge between helices 3 and 6 Is responsible for the constitutive activity and lack of hormone responsiveness of the naturally occurring L457R mutation of the human lutropin receptor. J Biol Chem 280:26169–26176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanelli L, Van Durme JJ, Smits G, Bonomi M, Rodien P, Devor EJ, Moffat-Wilson K, Pardo L, Vassart G, Costagliola S 2004 Modulation of ligand selectivity associated with activation of the transmembrane region of the human follitropin receptor. Mol Endocrinol 18:2061–2073 [DOI] [PubMed] [Google Scholar]
- Smits G, Olatunbosun O, Delbaere A, Pierson R, Vassart G, Costagliola S 2003 Ovarian hyperstimulation syndrome due to a mutation in the follicle-stimulating hormone receptor. N Engl J Med 349:760–766 [DOI] [PubMed] [Google Scholar]
- Vasseur C, Rodien P, Beau I, Desroches A, Gerard C, de Poncheville L, Chaplot S, Savagner F, Croue A, Mathieu E, Lahlou N, Descamps P, Misrahi M 2003 A chorionic gonadotropin-sensitive mutation in the follicle-stimulating hormone receptor as a cause of familial gestational spontaneous ovarian hyperstimulation syndrome. N Engl J Med 349:753–759 [DOI] [PubMed] [Google Scholar]
- Montanelli L, Delbaere A, Di Carlo C, Nappi C, Smits G, Vassart G, Costagliola S 2004 A mutation in the follicle-stimulating hormone receptor as a cause of familial spontaneous ovarian hyperstimulation syndrome. J Clin Endocrinol Metab 89:1255–1258 [PubMed] [Google Scholar]
- De Leener A, Montanelli L, Van Durme J, Chae H, Smits G, Vassart G, Costagliola S 2006 Presence and absence of follicle-stimulating hormone receptor mutations provide some insights into spontaneous ovarian hyperstimulation syndrome physiopathology. J Clin Endocrinol Metab 91:555–562 [DOI] [PubMed] [Google Scholar]
- Cetani F, Tonacchera M, Vassart G 1996 Differential effects of NaCl concentration on the constitutive activity of the thyrotropin and the luteinizing hormone/chorionic gonadotropin receptors. FEBS Lett 378:27–31 [DOI] [PubMed] [Google Scholar]
- Möller S, Vilo J, Croning MDR 2001 Prediction of the coupling specificity of G protein coupled receptors to their G proteins. Bioinformatics 17(Suppl 1):S174–S181 [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR 1989 Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59 [DOI] [PubMed] [Google Scholar]
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR 1989 Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61–68 [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Weinstein H 1995 Integrated methods for the construction of three-dimensional models of structure-function relations in G protein-coupled receptors. Meth Neurosci 25:366–428 [Google Scholar]
- Chen C, Okayama H 1987 High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 7:2745–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali A, Blundell TL 1993 Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234:779–815 [DOI] [PubMed] [Google Scholar]
- Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V 2004 The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J Mol Biol 342:571–583 [DOI] [PubMed] [Google Scholar]
- Fanelli F 2007 Dimerization of the lutropin receptor: insights from computational modeling. Mol Cell Endocrinol 260- 262:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKerell Jr AD, Bashford D, Bellott RL, Dunbrack Jr RL, Evanseck JD, Field MJ, Fisher S, Gao J, Guo H, Ha S, Josheph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick L, Ngo T, Nguyen DT, Prodhom B, Reiher III WE, Roux B, Schlendkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M 1998 All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B 102:3586–3616 [DOI] [PubMed] [Google Scholar]
- Im W, Feig M, Brooks III CL 2003 An implicit membrane generalized born theory for the study of structure, stability, and interactions of membrane proteins. Biophys J [Erratum (2004) 86:3330] 85:2900–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrachi D, Segaloff DL 2004 Intracellularly located misfolded glycoprotein hormone receptors associate with different chaperone proteins than their cognate wild-type receptors. Mol Endocrinol 18:1768–1777 [DOI] [PubMed] [Google Scholar]
- Rapoport B, McLachlan SM 2007 The thyrotropin receptor in Graves’ disease. Thyroid 17:911–922 [DOI] [PubMed] [Google Scholar]
- Smits G, Campillo M, Govaerts C, Janssens V, Richter C, Vassart G, Pardo L, Costagliola S 2003 Glycoprotein hormone receptors: determinants in leucine-rich repeats responsible for ligand specificity. EMBO J 22:2692–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urizar E, Claeysen S, Deupi X, Govaerts C, Costagliola S, Vassart G, Pardo L 2005 An activation switch in the rhodopsin family of G protein-coupled receptors: the thyrotropin receptor. J Biol Chem 280:17135–17141 [DOI] [PubMed] [Google Scholar]
- Claus M, Neumann S, Kleinau G, Krause G, Paschke R 2006 Structural determinants for G-protein activation and specificity in the third intracellular loop of the thyroid-stimulating hormone receptor. J Mol Med 84:943–954 [DOI] [PubMed] [Google Scholar]
- Tonacchera M, Agretti P, Pinchera A, Rosellini V, Perri A, Collecchi P, Vitti P, Chiovato L 2000 Congenital hypothyroidism with impaired thyroid response to thyrotropin (TSH) and absent circulating thyroglobulin: evidence for a new inactivating mutation of the TSH receptor gene. J Clin Endocrinol Metab 85:1001–1008 [DOI] [PubMed] [Google Scholar]
- Chen C-R, McLachlan SM, Rapoport B 2007 Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology 148:2375–2382 [DOI] [PubMed] [Google Scholar]
- Laue L, Chan W-C, Hsueh A, Kudo M, Hsu SY, Wu S, Blomberg L, Cutler GB 1995 Genetic heterogeneity of constitutively activating mutations of the human luteinizing hormone receptor in familial male-limited precocious puberty. Proc Natl Acad Sci USA 92:1906–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S, Mori T, Shenker A 1998 An anionic residue at position 564 is important for maintaining the inactive conformation of the human lutropin/choriogonadotropin receptor. Mol Pharmacol 53:894–901 [PubMed] [Google Scholar]
- Fanelli F, Verhoef-Post M, Timmerman M, Zeilemaker A, Martens JW, Themmen AP 2004 Insight into mutation-induced activation of the luteinizing hormone receptor: molecular simulations predict the functional behavior of engineered mutants at M398. Mol Endocrinol 18:1499–1508 [DOI] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K 2002 Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol 366:381–416 [DOI] [PubMed] [Google Scholar]
- Parnot C, Miserey-Lenkei S, Bardin S, Corvol P, Clauser E 2002 Lessons from constitutively active mutants of G protein-coupled receptors. Trends Endocrinol Metab 13:336–343 [DOI] [PubMed] [Google Scholar]
- Themmen AP 2005 An update of the pathophysiology of human gonadotrophin subunit and receptor gene mutations and polymorphisms. Reproduction 130:263–274 [DOI] [PubMed] [Google Scholar]
- Corvilain B, Van Sande J, Dumont JE, Vassart G 2001 Somatic and germline mutations of the TSH receptor and thyroid diseases. Clin Endocrinol (Oxf) 55:143–158 [PubMed] [Google Scholar]
- Latronico A, Segaloff D 1999 Naturally occurring mutations of the luteinizing-hormone receptor: lessons learned about reproductive physiology and G protein-coupled receptors. Am J Hum Genet 65:949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Lubrano-Berthelier L-B, Govaerts C, Picard F, Santiago P, Conklin B, Vaisse C 2004 Constitutive activity of the melanocortin-4 receptor is maintained by its N-terminal domain and plays a role in energy homeostasis in humans. J Clin Invest 114:1158–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantel J, Legendre M, Cabrol S, Hilal L, Hajaji Y, Morisset S, Nivot S, Vie-Luton M-P, Grouselle D, de Kerdanet M, Kadiri A, Epelbaum J, Le Bouc Y, Amselem S 2006 Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J Clin Invest 116:760–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst B, Schwartz TW 2006 Ghrelin receptor mutations–too little height and too much hunger. J Clin Invest 116:637–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ligt RAF, Kourounakis AP, Ijzerman AP 2000 Inverse agonism at G protein-coupled receptors: (patho)physiological relevance and implications for drug discover. Br J Pharmacol 130:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Tong KP, Fremont V, Chen J, Narayan P, Puett D, Weintraub BD, Szkudlinski MW 2000 The extracellular domain suppresses constitutive activity of the transmembrane domain of the human TSH receptor: implications for hormone-receptor interaction and antagonist design. Endocrinology 141:3514–3517 [DOI] [PubMed] [Google Scholar]
- Vlaeminck-Guillem V, Ho SC, Rodien P, Vassart G, Costagliola S 2002 Activation of the cAMP pathway by the TSH receptor involves switching of the ectodomain from a tethered inverse agonist to an agonist. Mol Endocrinol 16:736–746 [DOI] [PubMed] [Google Scholar]
- Neumann S, Krause G, Claus M, Paschke R 2005 Structural determinants for G protein activation and selectivity in the second intracellular loop of the thyrotropin receptor. Endocrinology 146:477–485 [DOI] [PubMed] [Google Scholar]