Abstract
Insulin transported across the blood-brain barrier (BBB) has many effects within the central nervous system. Insulin transport is not static but altered by obesity and inflammation. Lipopolysaccharide (LPS), derived from the cell walls of Gram-negative bacteria, enhances insulin transport across the BBB but also releases nitric oxide (NO), which opposes LPS-enhanced insulin transport. Here we determined the role of NO synthase (NOS) in mediating the effects of LPS on insulin BBB transport. The activity of all three NOS isoenzymes was stimulated in vivo by LPS. Endothelial NOS and inducible NOS together mediated the LPS-enhanced transport of insulin, whereas neuronal NOS (nNOS) opposed LPS-enhanced insulin transport. This dual pattern of NOS action was found in most brain regions with the exception of the striatum, which did not respond to LPS, and the parietal cortex, hippocampus, and pons medulla, which did not respond to nNOS inhibition. In vitro studies of a brain endothelial cell (BEC) monolayer BBB model showed that LPS did not directly affect insulin transport, whereas NO inhibited insulin transport. This suggests that the stimulatory effect of LPS and NOS on insulin transport is mediated through cells of the neurovascular unit other than BECs. Protein and mRNA levels of the isoenzymes indicated that the effects of LPS are mainly posttranslational. In conclusion, LPS affects insulin transport across the BBB by modulating NOS isoenzyme activity. NO released by endothelial NOS and inducible NOS acts indirectly to stimulate insulin transport, whereas NO released by nNOS acts directly on BECs to inhibit insulin transport.
INSULIN CIRCULATING in the blood is classically known for its ability to control levels of serum glucose. In contrast, insulin in the central nervous system (CNS) has effects on brain maturation (1,2), increases brain glucose use (3,4), promotes synthesis of acetylcholine (4), alters norepinephrine and dopamine levels and turnover in selective brain regions (5,6) in part by enhancing transport of tryptophan and tyrosine across the blood-brain barrier (BBB) (7), increases efferent sympathetic nerve activity (8), modulates neuronal responses in the olfactory bulb and amygdala to stimuli (9), affects pituitary sex hormone secretion (10), and alters auditory evoked potentials (11). CNS insulin is thought to have a significant role in cognition (12) and may be important in the pathophysiology and treatment of Alzheimer’s disease (13,14,15,16).
The effects of CNS insulin are independent of those of peripheral insulin and are not attributable to its effects on glucose levels. In fact, many of the effects of CNS insulin are opposite to those of peripheral insulin. For example, administration of insulin into the CNS induces hyperglycemia, hypoinsulinemia, and anorexia; decreases neuropeptide Y expression in the hypothalamus; and decreases body weight (17,18,19,20,21,22). Thus, insulin in the CNS to some degree may be acting as its own counterregulatory hormone and may induce a degree of insulin resistance. These effects are likely occurring at physiological levels of insulin because antibodies to insulin given directly into the brain enhance feeding (20,23) and increase body weight (20). Chronic deprivation of insulin action in the CNS in mice with selective knockouts of the insulin receptors in the CNS, but not in the peripheral tissues, also have increased food intake and mild diet-induced obesity with increased serum leptin and insulin levels as well as hypogonadal hypogonadism and dyslipidemia (10).
Despite this contrast between the actions of CNS and peripheral insulin, the source of CNS insulin is the pancreas. Insulin in blood is transported from the blood to the brain across the BBB by a saturable transport system (24). Thus, CNS and blood levels of insulin correlate (25,26). However, the rate of insulin transport across the BBB is not static. The BBB transport of insulin is impaired in obesity (27,28,29), enhanced in chemically induced diabetes (30), altered by starvation (31,32), decreased with dexamethasone treatment (33), and switched off during hibernation (34). It is likely therefore that the insulin transporter is regulated by unknown factors related to the CNS actions of insulin. Interestingly, all of these factors affecting insulin transport also have effects on the immune system or inflammatory status.
Lipopolysaccharide (LPS) enhances insulin transport across the BBB by 2- to 3-fold (35). LPS is the endotoxin derived from the cell wall of Gram-negative bacteria. It stimulates the innate immune system, releasing numerous cytokines and nitric oxide, inducing sickness behavior, and producing a sepsis-like picture (36,37,38,39). LPS administration, sickness behavior, and sepsis are each associated with insulin resistance (38,40,41,42,43). In fact, CNS insulin and peripheral LPS have many common effects. Thus, it may be that enhanced insulin transport across the BBB is one mechanism by which LPS induces anorexia, glycemic dysregulation, insulin resistance, dyslipidemia, learning and memory deficits, impaired sexual and reproductive functions, and impaired motivation.
Nitric oxide may provide a link between LPS and the BBB transport of insulin. Nitric oxide mediates or influences many of the effects of LPS, including those on insulin resistance (40). LPS and nitric oxide have many direct effects on BBB function and the brain endothelial cells (BECs), which comprise the BBB (44,45,46,47,48,49). LPS and nitric oxide also influence the BBB indirectly by affecting the microglia, astrocytes, and pericytes, which influence BBB activities and, along with the BBB, form the neurovascular unit (50). We have previously shown that the LPS-induced increase in insulin transport across the BBB is enhanced by N(1)-nitro-l-arginine methyl ester hydrochloride (L-NAME) (Sigma, St. Louis, MO), a general inhibitor of nitric oxide. Because L-NAME had no effect on insulin transport in the absence of LPS treatment, it seems likely that LPS is also releasing the nitric oxide that affects insulin transport.
Nitric oxide production is controlled by nitric oxide synthases (NOS), which generate it from l-arginine (51). There are three well-described NOS isoforms: neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). Which of these isoenzymes is modulated by LPS to affect insulin transport across the BBB is unclear. Here we examined the role that nitric oxide and each of the NOS isoforms play in mediating LPS-induced transport of insulin across the BBB.
Materials and Methods
Radioactive labeling of insulin and albumin
Human insulin (5 μg; Sigma) was radioactively labeled with 131I using the chloramine-T method. The radioactive insulin (I-Ins) was purified from unincorporated 131I by filtration on a Sephadex G-10 column. Over 90% of the radioactivity in the peak representing I-Ins could be precipitated with trichloroacetic acid, demonstrating a high degree of purification. Human serum albumin was labeled with 125I and purified in a similar manner and the radioactive albumin (I-Alb) precipitated over 90% with trichloroacetic acid.
Measuring of BBB permeability to iv I-Ins
All animal studies were conducted under approved protocols in an Association Assessment and Accreditation of Laboratory Animal Care-approved facility. Male CD-1 mice (Charles River, Wilmington, MA; 7–10 wk old; 25–40 g) were anesthetized with an ip injection of urethane (0.2 ml of 40% solution). The left jugular vein and the right carotid artery were exposed. Then the mice were given an injection of 0.2 ml of lactated Ringer’s solution (LR) containing 2 (106) cpm of I-Ins into the jugular vein. Five minutes after iv injection, blood was collected from the right carotid artery and the mice decapitated. Previous studies have shown that the radioactivity present in brain and blood represent intact insulin during this time period (24). In some studies, 2 (106) cpm of I-Alb was also included in the iv injection. The whole brain including the olfactory bulbs were removed immediately and weighed. The collected whole blood was centrifuged at 5000 × g at 4 C for 10 min and 50 μl of serum collected. The levels of radioactivity in the serum and whole brain were counted 3 min in a γ-counter that differentiates between 131I and 125I. The brain/serum ratio (microliters to grams) was calculated by the equation: brain to serum ratio (microliters to grams) = (counts per minute per gram of brain)/(counts per minute per microliter of serum).
In another group of mice, the brain was dissected after the method of Glowinski and Iversen (52) into the following 11 regions: olfactory bulb, frontal cortex, parietal cortex, occipital cortex, striatum, hippocampus, hypothalamus, thalamus, cerebellum, midbrain, and pons medulla. The regions were weighed, the levels of radioactivity measured, and the brain to serum ratio for each region determined with the equation above.
Pretreatment and injection protocols
We used a regimen previously established (35) of three ip injections of LPS (Salmonella typhimurium; Sigma) in determining the BBB permeability to insulin. Briefly, mice received three injections (at t = 0, 6, and 24 h) of LPS (3 mg/kg, dissolved in LR). BBB permeability to insulin as outlined above was studied 4 h after the third injection of LPS (28 h after first ip injection of LPS). Control mice received LR injections.
The effects of the specific NOS inhibitors 7-nitroindazole (7-NI; 10 mg/kg), aminoguanidine (AG; 30 mg/kg), and N (5)-(-iminoethyl)-l-ornithine (L-NIO; 10 mg/kg) were tested by giving each inhibitor in 0.2 ml LR solution 30 min before the LPS or LR injection. These inhibitors were purchased from Sigma. The effect of l-arginine (125 mg/kg; Sigma) was tested by giving it with the LPS injection.
Measurement of NOS isoenzyme mRNAs: quantitative RT-PCR
RNA was isolated from hemibrains using the RNeasy lipid tissue minikit protocol (QIAGEN, Valencia, CA). Total cDNA was produced by reverse transcription (RT) using the Taqman RT system (Applied Biosystems, Foster City, CA) of 0.2 μl of purified RNA, 3 μl 10 × RT buffer, 6.6 μl MgCl2, 6 μl 2.5 mm deoxynucleotide triphosphates, 1.5 μl random hexamers, 0.6 μl RNase inhibitor, and 0.75 μl Multiscribe RT. Samples were incubated for 10 min at 25 C, 30 min at 48 C, and 5 min at 95 C. Quantitative real-time PCR was performed in a Applied Biosystems 7300 real-time PCR system. Amplification was carried out in 25-μl reaction mixtures containing 1 μl of template cDNA, 0.5 μl of each 5 mm primer, 12.5 μl 2 × SYBR green master mix, and 10.5 μl PCR water. Cycling conditions were one cycle at 95 C for 10 min, followed by 50 cycles of 95 C for 15 sec and 60 C for 1 min followed by one cycle at 95 C for 15 sec, 60 C for 15 sec, and 95 C for 15 sec. Primers for quantitative real-time PCR were made using Primer 3 software (Whitehead Institute for Biomedical Research, Cambridge, MA), and primer efficiency was between 95 and 105%. Sequences were as follows: iNOS, forward, 5′-gacgagacggataggcagag, reverse, 5′-cttcaagcacctccaggaac; eNOS, forward, 5′-cacccagagcttttcttttctttgc, reverse, 5′-gcttacagaacccaggatgg; nNOS, forward, 5′-tatgtggcagaagctccaga, reverse, 5′-cggctggatttaggactttg; and β-actin, forward, 5′-ttcctccctggagaagag, reverse, 5′-tgccacaggattccatac. The relative amount of gene copies was extrapolated using the comparative cycle threshold method with β-actin as a normalizer and mouse standard RNA as a calibrator (Stratagene, La Jolla, CA).
Measurement of NOS isoenzyme proteins: Western blotting
After LPS treatment, brains were removed, weighed, and frozen at −80 C until they were processed. The brains were then homogenized on ice in 5 volumes (5 × the weight in grams) of lysis buffer containing (in mm) 20 Tris HCl, 150 NaCl, 2 EDTA, 1 EGTA, 0.5% Triton X-100, and protease inhibitor cocktail (Sigma). To remove cellular debris, the samples were centrifuged at 1000 × g for 10 min at 4 C and the supernatant was saved. The supernatant was shaken for 30 min at 4 C followed by centrifugation at 14,000 rpm for 20 min at 4 C. The supernatant was saved and protein concentration was determined using the Pierce BCA protein assay kit (Rockford, IL). For SDS-PAGE and Western blot analysis, proteins were separated on 3–8% Tris acetate gels (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose. NOS isoenzymes were detected by chemiluminescence (Pierce) using rabbit anti-nNOS (1:1,000), rabbit anti-iNOS (1:200), rabbit anti-eNOS (1:1,000), and horseradish peroxidase-conjugated antirabbit (1:10,000) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Semiquantifiable analysis of the protein was done using Image J software downloaded from the National Institutes of Health (Bethesda, MD). All protein densities were normalized to actin and expressed as percent of control.
Mouse brain endothelial cell (MBEC) monolayers
The protocol for isolating MBECs was modified from that for rat BECs (53,54,55). Brains from anesthetized CD-1 mice were cleaned of meninges and homogenized with a handheld scalpel. The homogenate was digested in a collagenase solution (1 mg/ml collagenase type 2 in 288 U/ml of DNase I; Sigma) at 37 C for 1 h. Neurons, astrocytes, and Schwann cells were removed by centrifuging in DMEM solution (Sigma) containing 20% BSA. The partially purified mixture was digested again (1 mg/ml collagenase/dispase with 288 U/ml DNase I at 37 C for 30 min). Finally, the endothelial cells were purified on a 33% Percoll gradient (Amersham Biosciences, Piscataway, NJ) centrifuged at 1000 × g for 10 min.
The MBECs were placed in culture dishes (Falcon, San Jose, CA) coated with 0.1 mg/ml collagen type 1 (Sigma) and 0.1 mg/ml fibronectin (Sigma) and incubated at 37 C with 5% CO2 (55,56) in endothelial cell culture medium [20% plasma derived serum (Quad Five, Ryegate, MT) containing 1 ng/ml basic fibroblast growth factor (Sigma) and DMEM] (53,57). Cell culture medium was changed every 2–3 d. MBECs were typically 70–80% confluent by d 7.
MBECs (4.0 × 104 cells/insert) cultured to 70–80% confluence were added to Transwell culture inserts (Costar, Cambridge, MA; 24-well format, 3470) and cultured for 3 more days. Transwells had a culture plate (abluminal side) volume of 0.6 ml, an insert volume of 0.1 ml, and polyester membrane pores of 0.4 mm. Transendothelial electrical resistance (TEER) was used to confirm confluence of monolayers on the day of study, and one of three treatments were added: 1) LPS was added to a final concentration of 1, 10, or 100 μg/ml (or an equal volume of LR as control); 2) serum obtained from mice treated with LPS or LR as above was added to a concentration of 10% serum; 3) 1% of mixed lymphocytes obtained from a cervical lymph node was added with LPS or LR (58). I-Ins was added to the luminal chambers 24 h after the treatments and the abluminal chamber sampled 10, 20, and 30 min after adding the I-Ins and I-Alb. Permeability was expressed in centimeters per minute by calculating the counts per minute cleared from the luminal to the abluminal chamber over time in units of milliliters per minute and multiplying by the surface area of the culture insert (0.33 cm2).
In other cultures, the nitric oxide donor S-nitroso-N-acetylpenicillamine (SNAP; Tocris Cookson, Inc., Ellisville, MO) was added to either the luminal or abluminal chamber to final concentrations of either 30 or 150 μm. The permeability of the MBECs to I-Ins and I-Alb was assessed simultaneously, and TEER was measured immediately before and after their assessment at 10, 20, 40, and 60 min.
Statistical analysis
Means are reported with their n and ses. Two-tailed t tests were used for comparison of two means. For more than two means, ANOVA followed by Newman-Keuls multiple comparisons test was used.
Results
In all studies, LPS caused a statistically significant increase in brain/serum ratios for I-Ins. Figure 1 shows statistical differences for I-Ins brain to serum ratios uncorrected for I-Alb brain to serum ratios [F(3,33) = 18.7, P < 0.001, n = 9/group]. LPS caused an increase of about 10 μl/g (P < 0.05) and, when combined with 7-NI, caused a similar further increase (P < 0.01). However, 7-NI in the absence of LPS was without effect. In comparison, neither AG nor L-NIO produced a statistically significant effect on I-Ins uptake either alone or in combination with LPS (data not shown). These results show that it is the nNOS isoenzyme responsible for the nitric oxide effect, which opposes LPS. The results also show that the nitric oxide effect is not constitutive.
Figure 1.
Effects of the nNOS inhibitor 7-NI (10 mg/kg) on LPS-induced increase in transport of I-Ins across the BBB. LR was used as the control injection. The first ip injection (LR or 7-NI) was followed 30 min later by the second ip injection (LR or LPS). The injection sequence was repeated twice (total of three sets of injections) and mice were studied 4 h after the last injection (28 h after the first injection). LPS caused a significant increase (*, P < 0.05) and 7-NI caused a further increase (**, P < 0.01) in I-Ins transport (n = 9/group).
Although neither AG nor L-NIO had a significant effect on LPS-enhanced insulin transport, they each arithmetically decreased the LPS effect by about 3 μl/g. This raised the possibility that simultaneous block by AG + L-NIO might together affect LPS-enhanced insulin transport. Figure 2 shows the effects of LPS on I-Ins uptake with or without combined AG + L-NIO treatment [(F3,20) = 14.3, P < 0.001, n = 4–7/group]. The combination of AG + L-NIO reversed the effect LPS (P < 0.001). In the absence of LPS, the combination did not differ from animals that received only LR. Taken together, these results show that LPS stimulates all three NOSs, but whereas nitric oxide produced from nNOS inhibits insulin transport, nitric oxide produced from both iNOS and eNOS stimulates insulin transport.
Figure 2.
Effects of AG (30 mg/kg) plus L-NIO (10 mg/kg), inhibitors of iNOS and eNOS, respectively, on the LPS-induced increase in I-Ins transport across the BBB. The two NOS inhibitors in combination prevented LPS from enhancing I-Ins transport (**, P < 0.01, n = 4–7/group).
To further assess the relative role of these three NOSs, we repeated the above studies in mice that were pretreated with the nitric oxide precursor arginine. Arginine is the substrate on which NOS acts to release citrulline and nitric oxide. ANOVA showed significant effects in the experiment with 7-NI [(F3,32) = 13.7, P < 0.001, n = 9–10/group] and with AG + L-NIO [(F3,33) = 12.2, P < 0.001, n = 7–9/group]. Pretreatment with arginine enhanced the LPS effect on I-Ins transport in both experiments (P < 0.05). The inhibitor 7-NI produced a nonsignificant increase in the LPS + arginine effect (Fig. 3), whereas the combination of inhibition with AG + L-NIO opposed the LPS + arginine effect (P < 0.01, Fig. 4). These results in general reinforce the findings of opposing roles for nNOS vs. iNOS/eNOS. They also suggest that eNOS or iNOS have excess capacity so that providing more substrate can enhance their production of NO, whereas nNOS is nearer maximum capacity. Alternatively, the cells expressing iNOS or eNOS may have more ready access to arginine than the cells expressing nNOS.
Figure 3.
Effects of l-arginine on LPS and 7-NI. l-arginine enhanced the LPS-induced increase in I-Ins transport, but addition of 7-NI did not result in a further statistically significant increase in transport. *, P < 0.05; **, P < 0.01 (n = 9–10/group).
Figure 4.
Effects of l-arginine (L-arg) on LPS and AG + L-NIO. These eNOS and iNOS inhibitors countered the increase in I-Ins transport induced by LPS + l-arginine. *, P < 0.05; **, P < 0.01 (n = 7–9/group).
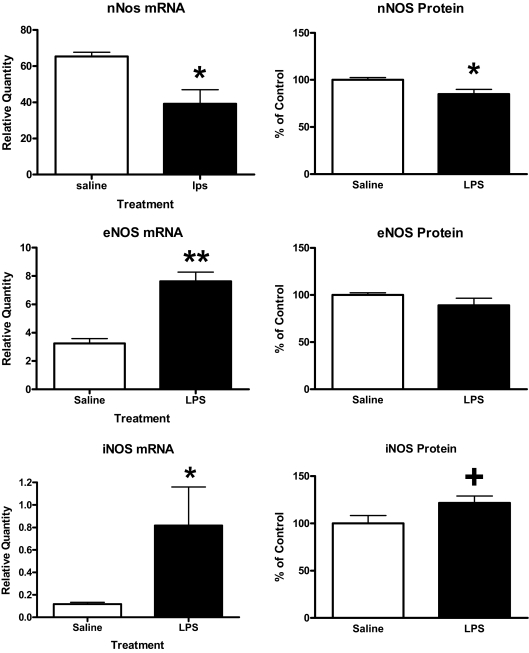
Figure 5 shows that LPS treatment suppressed both mRNA (t = 2.75, n = 3–4/group, df = 5, P < 0.05) and protein levels (t = 2.68, n = 8/group, df = 14, P < 0.05) for nNOS. However, LPS significantly increased iNOS mRNA (t = 2.83, n = 6–8/group, df = 12, P < 0.05) and eNOS mRNA (t = 6.35, n = 3–4/group, df = 5, P < 0.01) and produced a trend for an increase in iNOS protein (t = 1.94, n = 8/group, df = 14, <0.05p<0.1) with no effect on eNOS protein (n = 8/group).
Figure 5.
Effects of LPS on mRNA and protein levels of NOS isoenzymes. Left-hand panels show effects on mRNA (n = 3–4/group) as measured by RT-PCR, and right-hand panels show effects on protein levels (n = 6–8/group) as measured with Western blots. **, P < 0.01; *, P < 0.05; +, 0.05 < P < 0.10.
To determine whether the effects of LPS and 7-NI were uniform throughout the brain, we determined effects on 11 regions of the brain (n = 7–8/group). We first determined the effects of LPS and 7-NI on BBB disruption as determined by I-Alb. For whole brain and each of the 11 brain regions, LPS increased the I-Alb brain to serum ratio by about 50% (P < 0.01), consistent with BBB disruption. Treatment with 7-NI did not modify the LPS-induced disruption of the BBB except in the striatum in which 7-NI treatment blocked disruption (P < 0.05). The results for I-Ins reported for this study were corrected for the I-Alb space. For whole brain, the I-Ins brain to serum ratio more than tripled (P < 0.01), whereas 7-NI increased the LPS effect by another 22% (P < 0.05). Olfactory bulb, frontal cortex, thalamus, hypothalamus, occipital cortex, cerebellum, and midbrain showed the same pattern as whole brain: a statistically significant increase with LPS with a further statistically significant increase with 7-NI + LPS. All brain regions except striatum showed a statistically significant increase in I-Ins uptake with LPS. 7-NI + LPS produced a statistically significant increase in I-Ins uptake in comparison with LPS alone for all brain regions except striatum, parietal cortex, hippocampus, and pons medulla. 7-NI + LPS was statistically different from LR + LR for all regions except for hippocampus. Figure 6 shows results for selected brain regions illustrating each of these patterns.
Figure 6.
Brain region-specific effects of LPS and the nNOS inhibitor 7-NI. Most regions of the brain as represented by the olfactory bulb [F(3,21) = 20.1, P < 0.01] and hypothalamus [F(3,21) = 17.2, P < 0.01] had increases in I-Ins transport induced by LPS and 7-NI + LPS. Hippocampus [F(3,19) = 4.04, P < 0.05] and pons medulla [F(3,24) = 10.1, P < 0.01] had uptakes of I-Ins that were not further stimulated by 7-NI in the presence of LPS. *, P < 0.05; **, P < 0.01 (n = 7–8/group).
To determine whether LPS directly acted on the BBB, we cultured monolayers of brain endothelial cells (MBEC monolayer cultures) with LPS for 24 h. These monolayers consisted of over 90% BEC with small numbers of pericytes, a pluripotent cell found to modify the effects of glucose on insulin transport (59,60). LPS did not alter I-Ins transport in vitro. Serum from mice treated with LPS under the same regimen as produced an in vivo effect on I-Ins transport was added to the monolayer culture, but this also did not affect I-Ins transport. Finally, lymphocytes from cervical nodes was added to the culture with or without LPS and permeability to I-Ins determined 24 h later. We found that LPS in the presence of lymphocytes increased I-Ins transport across the BBB (Fig. 7, n = 5/group). We also showed that this transport was inhibited by unlabeled insulin. The two-way ANOVA was significant for lymphocyte treatment [F(1, 16) = 414, P < 0.001)], insulin treatment [F(1, 16) = 115, P < 0.001], and interaction [F(1, 16) = 7.8, P < 0.05]. Newman-Keuls multiple comparisons found each value in Fig. 7 different from the other three at the P < 0.001 level; Fig. 7 shows statistics for the two most relevant comparisons.
Figure 7.
LPS in the presence of lymphocytes enhanced the transport of I-In across MBECs in an in vitro model of the BBB. Results not shown showed that LPS in the absence of lymphoctyes had no effect on I-Ins transport. The increase was inhibited by unlabeled insulin, showing that it was the saturable transport of I-Ins that was enhanced. All groups were different from all the others at P < 0.001; only the two most relevant differences are indicated by ** (P < 0.01, n = 5/group).
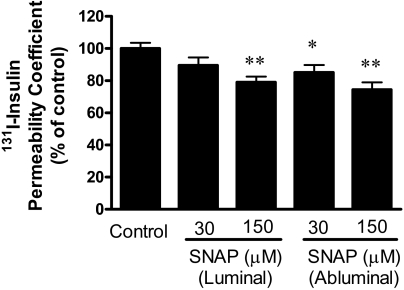
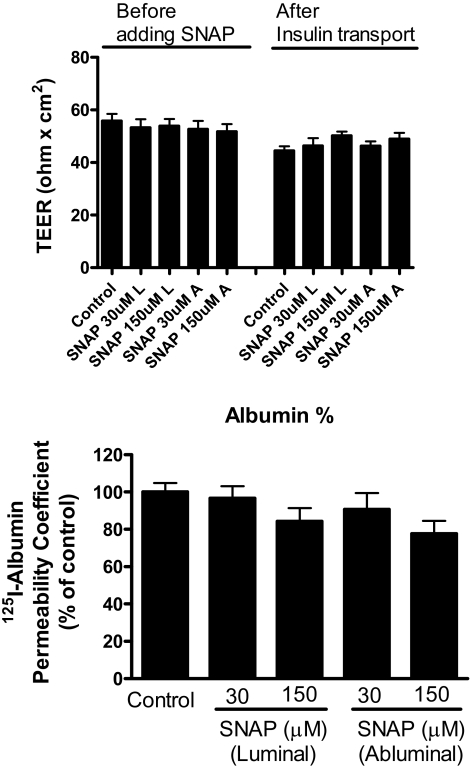
To determine whether nitric oxide directly acted on the BBB, we added the nitric oxide donor SNAP to MBEC monolayer cultures. Figure 8 shows that SNAP affected I-Ins transport [F(4,49) = 5.37, P < 0.005, n = 9–11/group]. SNAP added to the abluminal chamber at final concentrations of 30 μm (P < 0.05) and 150 (P < 0.01) significantly inhibited insulin transport, whereas 150 μm (P < 0.01), but not 30 μm, of SNAP added to the luminal chamber inhibited transport. SNAP had no effect on either TEER (Fig. 9, upper panel) or I-Alb (Fig. 9, lower panel) permeability in these cultures. Two-way ANOVA did show that TEER was slightly lower at the end of the experiment than at the beginning [F(1,110) = 14.2, P < 0.001, n = 9–11/group] but not for treatment or interaction. There were no statistically significant differences between groups when assessed by posttest. This suggests that there may have been a slight deterioration of BBB resistance during the study period.
Figure 8.
The nitric oxide donor SNAP inhibited insulin transport across the MBECs of the in vitro model of the BBB. SNAP was added to a concentration of either 30 or 150 μm to either the luminal or abluminal side of the MBECs and the luminal to abluminal transport of insulin assessed. *, P < 0.05; **, P < 0.01, n = 9–11/group.
Figure 9.
SNAP did not disrupt the BBB. Upper panel shows that SNAP did not affect TEER when added either to the luminal (L) or abluminal (A) chambers. Bottom panel shows SNAP did not affect permeability to albumin. Together, these measures indicate that neither paracellular nor transcytotic disruption of the BBB occurred (n = 9–11/group).
Discussion
Previous work has shown that treatment of mice with LPS increases the transport rate of insulin across the BBB by 2- to 3-fold. However, that work also showed that the increase in insulin transport is a net effect and that LPS is actually inducing both transport-enhancing and transport-inhibiting pathways. Evidence for an inhibitory pathway comes from the observation that mice treated with LPS plus the nitric oxide inhibitor L-NAME have an even greater increase in insulin transport than just those mice treated with LPS alone, whereas L-NAME in the absence of LPS was without effect. This indicated that LPS-stimulated nitric oxide inhibits insulin transport across the BBB. Here our primary objective was to determine which of the three NOS isoenzymes mediates the LPS effects on insulin transport.
We first used selective inhibitors against the three NOS isoenzymes. We found that treatment with the nNOS inhibitor 7-NI enhanced LPS-induced insulin transport. This showed that nNOS was responsible for the production of the nitric oxide, which inhibited insulin transport. However, the iNOS inhibitor AG and the eNOS inhibitor L-NIO were associated with arithmetic, nonstatistically significant decreases in the effect LPS. This suggested that these isoenzymes might be able to produce nitric oxide that enhanced the effect of LPS and so oppose the effect of nitric oxide produced by nNOS. To further investigate this, we gave AG and L-NIO together and found that the combination did decrease LPS-induced insulin transport. However, 7-NI, AG, L-NIO, or AG + L-NIO had no effect on insulin transport in the absence of LPS, showing that constitutive nitric oxide production is not affecting insulin transport. This first series of studies therefore showed that LPS stimulated all three NOS isoenzymes, that nitric oxide produced by iNOS and eNOS mediated an LPS-induced stimulation of insulin transport, and that the nitric oxide produced by nNOS opposed LPS-induced stimulation of insulin transport.
To further investigate these actions, we treated mice with the nitric oxide precursor l-arginine. Increasing the substrate that NOS uses to produce nitric oxide provided an opportunity to retest the dual nature of LPS-NOS effects and to measure reserve capacity of the enzymatic system. We found that LPS was more effective in stimulating insulin transport in mice pretreated with l-arginine. Additionally, blockade of eNOS and iNOS with AG + L-NIO inhibited LPS stimulation in l-arginine-treated mice. However, the inhibition of nNOS with 7-NI did not result in a statistically significant increase in the LPS + arginine-treated mice. It may be that nNOS is maximally stimulated in the absence of arginine, whereas eNOS and iNOS activity has reserve capacity that can be used by adding substrate. Alternatively, it could be that eNOS and iNOS have a more ready access to peripherally administered arginine than does nNOS. Either way, this suggests that the magnitude and perhaps even the direction of the LPS effect on insulin transport may depend on physiological factors that regulate NOS activity.
Opposing effects of NOS isoenzymes have been found in other circumstances. For example, eNOS activity seems protective in stroke, whereas nNOS activity seems harmful (51). In the kidney, eNOS promotes vasodilation, whereas iNOS inhibits eNOS and produces vasoconstriction (61). However, caution must be exercised in interpreting results based on the currently available pharmacologic inhibitors of isoenzymes. Although they are highly specific for inhibiting NOS in general, their selectivity for the isoenzymes is limited and depends partly on access and metabolism by the relevant tissues (51). Additionally, a given cell type can express more than one isoenzyme; for example, brain endothelial cells express both eNOS and iNOS (62,63). Despite these caveats, our results show opposing effects of nitric oxide on LPS-stimulated insulin transport, depending on which isoenzyme inhibitor is used. This, in turn, is evidence that nitric oxide is produced and acting on different components of the neurovascular axis that ultimately results in effects on the BBB transport of insulin into the brain.
We investigated the cellular level at which LPS induced its effects on NOS. We found that both mRNA and protein levels of nNOS were inhibited, not stimulated, by LPS. This suggests that the effects of LPS on nNOS are mediated through posttranslational mechanisms, possibly by agents released from other cell types to act in an allosterical fashion to regulate nNOS function. In contrast, levels of both eNOS and iNOS mRNA were significantly increased, although their protein levels were not. One shortcoming of these studies is that mRNA and protein levels were determined on whole brain samples and so included all cell types in the brain, whereas it is possible that the effects of these isoenzymes on insulin transport are mediated in a paracrine manner through specific cells close to the BBB.
The effects of LPS and nNOS inhibition were not completely uniform throughout brain. Seven of 11 brain regions behaved identically to whole brain; that is, insulin uptake increased with LPS treatment and increased still further with nNOS inhibition. Uptake by striatum did not increase with LPS and striatum, parietal cortex, hippocampus, and pons medulla did not increase to a statistically significant degree with nNOS inhibition. This shows that not all regions of the BBB respond identically to LPS or NOS inhibition. Previous work has shown that some regions of the BBB take up, but do not transport, insulin (64,65). Such nontransported uptake presumably represents binding to insulin receptors which are known to be present on brain endothelial cells and to affect various functions of the BBB (66,67,68,69,70,71). Typically, areas without transport are the midbrain, thalamus, and occipital cortex; these areas all had increased uptake with LPS treatment and nNOS inhibition. Therefore, it is probable that both the BBB receptor and transporter for insulin are sensitive to LPS and nitric oxide in most brain regions. Either LPS or nitric oxide could have been acting either directly on the BBB or indirectly though other cells of the neurovascular unit. The BECs that constitute the BBB are sensitive to both LPS and nitric oxide. For example, LPS stimulates cytokine release from MBECs (48) and nitric oxide has numerous effects on BBB function (49,72,73). Furthermore, LPS acts on BECs to induce release of nitric oxide, which can either increase or decrease the activity of another BBB transporter, p-glycoprotein (74,75). The ability of nitric oxide to both stimulate and inhibit insulin transport shows that its mechanisms of action are complex. However, nitric oxide is the same gaseous molecule whether synthesized from nNOS, eNOS, or iNOS and so should have the same direct effect on BBB function, regardless of the isoenzyme source. Therefore, either stimulation or inhibition must be mediated indirectly through other cell types (see Fig. 10).
Figure 10.
Working model of the relations among LPS, NOS isoenzymes, l-arginine, and insulin transport across the BBB. LPS stimulates all three NOS isoenzymes at cells other than the BEC. Nitric oxide (NO) generated from nNOS acts directly on the BECs comprising the BBB to inhibit insulin transport. Nitric oxide generated from eNOS and iNOS acts indirectly on the BBB to stimulate insulin transport. The stimulatory effect of eNOS/iNOS is enhanced by l-arginine, the substrate for NOS.
We tested the direct effects of LPS and nitric oxide on insulin transport across monolayer cultures of MBECs, a well established and widely used in vitro model of the BBB (54,70,76). In this model, the cells orient in a polarized fashion so that the surface against the collagen-coated insert has abluminal (brain side) properties, and the other surface has luminal (blood side) properties. These cells possess both eNOS and iNOS (63) and respond to LPS by releasing cytokines (48). We found here that LPS did not affect I-Ins transport. Therefore, LPS must be acting on some other cell type that, in turn, affects BEC insulin transport. Incubation of BMECs with serum from mice previously treated with LPS also showed that stable factors released into serum are not responsible for the enhanced transport of I-Ins. However, LPS-stimulated lymphocytes induced an increase in the saturable transport of I-Ins across the BBB. These results show that LPS does not act directly on BECs to enhance I-Ins transport but indirectly through some other cell type.
We then determined whether the BBB could directly respond to nitric oxide by adding SNAP, a source of nitric oxide, to the MBEC cultures. We found that SNAP added either to the luminal or abluminal chamber inhibited insulin transport across the BBB. SNAP did not disrupt the BBB either through the paracellular route as indicted by TEER or through the transcellular route as indicated by albumin. This shows that nitric oxide acts directly at the BBB to affect insulin transport. It also shows that it is the inhibition of insulin transport, the nNOS-mediated effect, that is the direct effect of nitric oxide on BBB transport of insulin. Thus, the stimulation of insulin transport as mediated by eNOS and iNOS must be indirectly mediated through non-BECs.
Figure 10 shows a diagram of the main findings. LPS acts at some cell other than the MBECs to stimulate nNOS-generated nitric oxide, which acts directly on the BBB to inhibit insulin transport. LPS also stimulates nitric oxide production from eNOS and iNOS, which acts indirectly to stimulate insulin transport.
Footnotes
Disclosure Statement: No author has any conflict of interest.
First Published Online January 10, 2008
Abbreviations: BBB, Blood-brain barrier; BEC, brain endothelial cell; CNS, central nervous system; eNOS, endothelial NOS; I-Alb, radioactive albumin; I-Ins, radioactive insulin; iNOS, inducible NOS; L-NAME, N(1)-nitro-l-arginine methyl ester; LPS, lipopolysaccharide; LR, lactated Ringer’s solution; MBEC, mouse brain endothelial cell; 7-NI, 7-nitroindazole; L-NIO, N (5)-(-iminoethyl)-l-ornithine; nNOS, neuronal NOS; NOS, nitric oxide synthase; RT, reverse transcription; SNAP, S-nitroso-N-acetylpenicillamine; TEER, transendothelial electrical resistance.
References
- Blasberg RG, Fenstermacher JD, Patlak CS 1983 Transport of α-aminoisobutyric acid across brain capillary and cellular membranes. J Cereb Blood Flow Metab 3:8–32 [DOI] [PubMed] [Google Scholar]
- Schubert M, Brazil DP, Burks DJ, Kushner JA, Ye J, Flint CL, Farhang-Fallah J, Dikkes P, Warot XM, Rio C, Corfas G, White MF 2003 Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci 23:7084–7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberg N, Hoyer S 1994 Short-term or long-term intracerebroventricular (i.c.v.) infusion of insulin exhibits a discrete anabolic effect on cerebral energy metabolism in the rat. Neurosci Lett 175:153–156 [DOI] [PubMed] [Google Scholar]
- Hoyer S 2003 Memory function and brain glucose metabolism. Pharmacopsychiatry 36:S62–S67 [DOI] [PubMed] [Google Scholar]
- Kwok RP, Juorio AV 1988 Effects of insulin on rat brain noradrenaline. Neurochem Res 13:887–892 [DOI] [PubMed] [Google Scholar]
- Montefusco O, Assini MC, Missale C 1983 Insulin-mediated effects of glucose on dopamine metabolism. Acta Diabetol Lat 20:71–77 [DOI] [PubMed] [Google Scholar]
- Tagliamonte A, DeMontis MG, Olianas M, Onali PL, Gessa GL 1976 Role of insulin in the transport of tyrosine and tryptophan from blood to brain. Adv Exp Med Biol 69:89–94 [DOI] [PubMed] [Google Scholar]
- Muntzel MS, Morgan DA, Mark AL, Johnson AK 1994 Intracerebroventricular insulin produces nonuniform regional increases in sympathetic nerve activity. Am J Physiol 267:R1350–R1355 [DOI] [PubMed] [Google Scholar]
- Cain DP 1975 Effects of insulin injection on responses of olfactory bulb and amygdala single units to odors. Brain Res 99:69–83 [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR 2000 Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122–2125 [DOI] [PubMed] [Google Scholar]
- Kern W, Born J, Schreiber H, Fehm HL 1999 Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes 48:557–563 [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Alkon DL 2001 Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol 177:125–134 [DOI] [PubMed] [Google Scholar]
- Hoyer S 1998 Is sporadic Alzheimer’s disease the brain type of non-insulin dependent diabetes mellitus? A challenging hypothesis. J Neural Transm 105:415–422 [DOI] [PubMed] [Google Scholar]
- Kurochkin IV, Goto S 1994 Alzheimer’s β-amyloid peptide specifically interacts with and is degraded by insulin degrading enzyme. FEBS Lett 345:33–37 [DOI] [PubMed] [Google Scholar]
- Solano DC, Sironi M, Bonfini C, Solerte SB, Govoni S, Racchi M 2000 Insulin regulates soluble amyloid precursor protein release via phosphatidyl inositol 3 kinase-dependent pathway. FASEB J 14:1015–1022 [DOI] [PubMed] [Google Scholar]
- Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A 1996 Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging 17:123–130 [DOI] [PubMed] [Google Scholar]
- Ajaya B, Haranath PS 1982 Effects of insulin administered into cerebrospinal fluid spaces on blood glucose in unanaesthetized and anaesthestized dogs. Indian J Med Res 75:607–615 [PubMed] [Google Scholar]
- Brief DJ, Davis JD 1984 Reduction of food intake and body weight by chronic intraventricular insulin infusion. Brain Res Bull 12:571–575 [DOI] [PubMed] [Google Scholar]
- Hatfield JS, Millard WJ, Smith CJV 1974 Short-term influence of intra-ventromedial hypothalamic administration of insulin on feeding in normal and diabetic rats. Pharmacol Biochem Behav 2:223–226 [DOI] [PubMed] [Google Scholar]
- McGowan MK, Andrews KM, Grossman SP 1992 Chronic intrahypothalamic infusions of insulin or insulin antibodies alter body weight and food intake in the rat. Physiol Behav 51:753–766 [DOI] [PubMed] [Google Scholar]
- Florant GL, Singer L, Scheurink AJW, Park CR, Richardson RD, Woods SC 1991 Intraventricular insulin reduces food intake and body weight of marmots during the summer feeding period. Physiol Behav 49:335–338 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Sipols AJ, Marks JL, Sanacora G, White JD, Scheurink A, Kahn SE, Baskin DG, Woods SC, Figlewicz DP, Porte Jr D 1992 Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology 130:3608–3615 [DOI] [PubMed] [Google Scholar]
- Strubbe JH, Mein CG 1977 Increased feeding in response to bilateral injection of insulin antibodies in the VMH. Physiol Behav 19:309–313 [DOI] [PubMed] [Google Scholar]
- Banks WA, Jaspan JB, Huang W, Kastin AJ 1997 Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides 18:1423–1429 [DOI] [PubMed] [Google Scholar]
- Woods SC, Porte Jr D 1977 Relationship between plasma and cerebrospinal fluid insulin levels of dogs. Am J Physiol 233:E331–E334 [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Baskin DG, Schwartz MW 2003 Insulin and the blood-brain barrier. Curr Pharm Des 9:795–800 [DOI] [PubMed] [Google Scholar]
- Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW 2000 Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes 49:1525–1533 [DOI] [PubMed] [Google Scholar]
- Stein LJ, Dorsa DM, Baskin DG, Figlewicz DP, Porte Jr D, Woods SC 1987 Reduced effect of experimental peripheral hyperinsulinemia to elevate cerebrospinal fluid insulin concentrations of Obese Zucker rats. Endocrinology 121:1611–1615 [DOI] [PubMed] [Google Scholar]
- Strubbe JH, Porte Jr D, Woods SC 1988 Insulin responses and glucose levels in plasma and cerebrospinal fluid during fasting and refeeding in the rat. Physiol Behav 44:205–208 [DOI] [PubMed] [Google Scholar]
- Banks WA, Jaspan JB, Kastin AJ 1997 Effect of diabetes mellitus on the permeability of the blood-brain barrier to insulin. Peptides 18:1577–1584 [DOI] [PubMed] [Google Scholar]
- Cashion MF, Banks WA, Kastin AJ 1996 Sequestration of centrally administered insulin by the brain: effects of starvation, aluminum, and TNF-α. Horm Behav 30:280–286 [DOI] [PubMed] [Google Scholar]
- Marks JL, Eastman CJ 1989 Effect of starvation on insulin receptors in rat brain. Neuroscience 30:551–556 [DOI] [PubMed] [Google Scholar]
- Baura GD, Foster DM, Kaiyala K, Porte Jr D, Kahn SE, Schwartz MW 1996 Insulin transport from plasma into the central nervous system is inhibited by dexamethasone in dogs. Diabetes 45:86–90 [DOI] [PubMed] [Google Scholar]
- Florant GL, Richardson RD, Mahan S, Singer L, Woods SC 1991 Seasonal changes in CSF insulin levels in marmots: insulin may not be a satiety signal for fasting in winter. Am J Physiol 260:R712–R716 [DOI] [PubMed] [Google Scholar]
- Xaio H, Banks WA, Niehoff ML, Morley JE 2001 Effect of LPS on the permeability of the blood-brain barrier to insulin. Brain Res 896:36–42 [DOI] [PubMed] [Google Scholar]
- Larson SJ, Dunn AJ 2001 Behavioral effects of cytokines. Brain Behav Immun 15:371–387 [DOI] [PubMed] [Google Scholar]
- Laye S, Parnet P, Goujon E, Dantzer R 1994 Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res 27:157–162 [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou J-H, Shen W-H, Johnson RW, Broussard SR 2003 Cytokine-induced sickness behavior. Brain Behav Immun 17:S112–S118 [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Karl IE 2003 Medical progress: the pathophysiology and treatment of sepsis. N Engl J Med 348:138–150 [DOI] [PubMed] [Google Scholar]
- Sugita H, Kaneki M, Tokunaga E, Sugita M, Koike C, Yasuhara S, Tompkins RG, Martyn JA 2002 Inducible nitric oxide synthase plays a role in LPS-induced hyperglycemia and insulin resistance. Am J Physiol 282:E386–E394 [DOI] [PubMed] [Google Scholar]
- Dahn MS, Lange MP, Mitchell RA, Lobdell K, Wilson RF 1987 Insulin production following injury and sepsis. J Trauma 27:1031–1038 [DOI] [PubMed] [Google Scholar]
- Agwunobi AO, Reid C, Mayeux R, Little RA, Carlson GL 2000 Insulin resistance and substrate utilizaton in human endotoxemia. J Clin Endocrinol Metab 85:3770–3778 [DOI] [PubMed] [Google Scholar]
- Roelfsema V, Thomas GB, Lin H, Breir BH, Maxwell L, Oliver MH, Heineman E, Clark RG, Gluckman PD 2001 The metabolic effects of endotoxin are differentially affected by the pattern of GH administration in the rat. J Endocrinol 171:173–181 [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Brennan JM, Vallance KL 1999 Adsorptive endocytosis of HIV-1gp120 by blood-brain barrier is enhanced by lipopolysaccharide. Exp Neurol 156:165–171 [DOI] [PubMed] [Google Scholar]
- Cao C, Watanabe Y, Yamagata K, Matsumura K 1997 Involvement of cyclooxygenase-2 in LPS-induced fever and regulation of its mRNA by LPS in the rat brain. Am J Physiol 272:R1712–R1725 [DOI] [PubMed] [Google Scholar]
- De Vries HE, Moor AC, Blom-Roosemalen MC, De Boer AG, Breimer DD, van Berkel TJ, Kuiper J 1994 Lymphocyte adhesion to brain capillary endothelial cells in vitro. J Neuroimmunol 52:1–8 [DOI] [PubMed] [Google Scholar]
- Nonaka N, Shioda S, Banks WA 2005 Effect of lipopolysaccharide on the transport of pituitary adenylate cyclase activating polypeptide across the blood-brain barrier. Exp Neurol 191:137–144 [DOI] [PubMed] [Google Scholar]
- Verma S, Nakaoke R, Dohgu S, Banks WA 2006 Release of cytokines by brain endothelial cells: a polarized response to lipopolysaccharide. Brain Behav Immun 20:449–455 [DOI] [PubMed] [Google Scholar]
- Utepbergenov DI, Mertsch K, Sporbert A, Tenz K, Paul M, Haseloff RF, Blasig IE 1998 Nitric oxide protects blood-brain barrier in vitro from hypoxia/reoxygenation-mediated injury. FEBS Lett 424:197–201 [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Ronnback L, Hansson E 2006 Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev 7:41–53 [DOI] [PubMed] [Google Scholar]
- Vallance P, Leiper J 2002 Blocking NO synthesis: how, where and why? Nat Rev Drug Discov 1:939–949 [DOI] [PubMed] [Google Scholar]
- Glowinski J, Iversen LL 1966 Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem 13:655–669 [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Hughes CCW, Revest PA, Greenwood J 1992 Development and characterization of a rat brain capillary endothelial culture: towards an in vitro blood-brain barrier. J Cell Sci 103:23–37 [DOI] [PubMed] [Google Scholar]
- Deli MA, Joo F 1996 Cultured vascular endothelial cells of the brain. Keio J Med 45:183–198 [DOI] [PubMed] [Google Scholar]
- Kis B, Kaiya H, Nishi R, Deli MA, Abraham CS, Yanagita T, Isse T, Gotoh S, Kobayashi H, Wada A, Niwa M, Kangawa K, Greenwood J, Yamashita H, Ueta Y 2002 Cerebral endothelial cells are a major source of adrenomedullin. J Neuroendocrinol 14:283–293 [DOI] [PubMed] [Google Scholar]
- Domotor E, Sipos I, Kittel A, Abbott NJ, Adam-Vizi V 1998 Improved growth of cultured brain microvascular endothelial cells on glass coated with a biological matrix. Neurochem Int 33:473–478 [DOI] [PubMed] [Google Scholar]
- Demeuse P, Kerkhofs A, Struys-Ponsar C, Knoops B, Remacle C, van den Bosch de Aguilar P 2002 Compartmentalized coculture of rat brain endothelial cells and astrocytes: a syngenic model to study the blood-brain barrier. J Neurosci Methods 121:21–31 [DOI] [PubMed] [Google Scholar]
- Maresh GA, Maness LM, Zadina JE, Kastin AJ 2001 In vitro demonstration of a saturable transport system for leptin across the blood-brain barrier. Life Sci 69:67–73 [DOI] [PubMed] [Google Scholar]
- Nakaoke R, Verma S, Niwa M, Dohgu S, Banks WA 2007 Glucose-regulated blood-brain barrier transport of insulin: pericyte-astrocyte-endothelial cell cross talk. Int J Neuroprot Neuroregener 3:195–200 [Google Scholar]
- Dore-Duffy P, Katychev A, Wang X, Van Buren E 2006 CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab 26:613–624 [DOI] [PubMed] [Google Scholar]
- Gabbai FB 2001 Effects of nitric oxide synthase blockers on renal function. Nephrol Dial Transplant 16(Suppl 1):13 [DOI] [PubMed] [Google Scholar]
- Shafer RA, Murphy S 1997 Activated astrocytes induce nitric oxide synthase-2 in cerebral endothelium via tumor necrosis factor α. GLIA 21:370–379 [DOI] [PubMed] [Google Scholar]
- Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, de Frutos T, Rodrigues-Feo JA, Guerra J, Caramelo C, Casado S, Lopez F 1998 Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res 83:279–286 [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ 1998 Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides 19:883–889 [DOI] [PubMed] [Google Scholar]
- Banks WA, Farr SA, Morley JE 2000 Permeability of the blood-brain barrier to albumin and insulin in the young and aged SAMP8 mouse. J Gerontol A Biol Sci Med Sci 55A:B601–B606 [DOI] [PubMed] [Google Scholar]
- Cangiano C, Cardelli-Cangiano P, Cascino A, Patrizi MA, Barberini F, Rossi F, Capocaccia L, Strom R 1983 On the stimulation by insulin of tryptophan transport across the blood-brain barrier. Biochem Int 7:617–627 [PubMed] [Google Scholar]
- Catalan RE, Martinez AM, Aragones MD, Miguel BG, Robles A 1988 Insulin action on brain microvessels; effect on alkaline phosphatase. Biochem Biophys Res Commun 150:583–590 [DOI] [PubMed] [Google Scholar]
- Frank HJL, Pardridge WM 1981 A direct in vitro demonstration of insulin binding to isolated brain microvessel. Diabetes 30:757–761 [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V 2001 Glucose and insulin increase the transport of leptin through the blood-brain barrier in normal mice but not in streptozotocin-diabetic mice. Neuroendocrinology 73:237–242 [DOI] [PubMed] [Google Scholar]
- Keller BT, Borchardt RT 1987 Cultured bovine brain capillary endothelial cells (BBCEC)—a blood-brain barrier model for studying the binding and internalization of insulin and insulin-like growth factor 1. Fed Proc 46:416 [Google Scholar]
- Miller DW, Borchardt RT 1991 Distribution of insulin binding sites on cultured bovine brain microvessel endothelial cells and their possible role in the transport of insulin across the blood-brain barrier. J Cell Biol 115:261a [Google Scholar]
- Minami T, Okazaki J, Kawabata A, Kuroda R, Okazaki Y 1998 Penetration of cisplatin into mouse brain by lipopolysaccharide. Toxicology 130:107–113 [DOI] [PubMed] [Google Scholar]
- Worrall NK, Chang K, LeJeune WS, Misko TP, Sullivan PM, Ferguson TB, Williamson JR 1997 TNF-α causes reversible in vivo systemic vascular barrier dysfunction via NO-dependent and -independent mechanisms. Am J Physiol 273:H2565–H2574 [DOI] [PubMed] [Google Scholar]
- Hartz AMS, Bauer B, Fricker G, Miller DS 2006 Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-α and lipopolysaccharide. Mol Pharmacol 69:462–470 [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AMS, Miller DS 2007 Tumor necrosis factor α and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol 71:667–675 [DOI] [PubMed] [Google Scholar]
- Audus KL, Borchardt RT 1987 Bovine brain microvessel endothelial cell monolayers as a model system for the blood-brain barrier. Biological approaches to the controlled delivery of drugs. Ann NY Acad Sci 507:9–18 [DOI] [PubMed] [Google Scholar]