Abstract
The present study was conducted to elucidate the possible molecular mechanisms involved in the antispermatogenic activity of l-CDB-4022, an indenopyridine. In this study 45-d-old male Sprague-Dawley rats were treated with a single oral dose of l-CDB-4022 (2.5 mg/kg) or vehicle, and blood and testes were collected at various time points. The rate of body weight gain was not affected, but a significant loss of testes weight was induced by l-CDB-4022. Serum hormones were assayed using specific RIAs or ELISAs, and testicular protein and RNA were analyzed by Western blotting and RT-PCR, respectively. There was a significant decrease in inhibin B and concomitant increase in FSH in serum from l-CDB-4022-treated rats, but serum levels of activin A, testosterone, and LH were unchanged. Western analysis of testicular lysates from l-CDB-4022-treated rats exhibited phosphorylation of ERK1/2 at 4 h and later time points. Loss of nectin/afadin complex occurred at 48 h, but there was an increase in levels of integrin-β1, N-cadherin, α-catenin, and β-catenin protein at 24 h and later time points. Increase in expression of Fas ligand and Fas receptor was detected 8 and 24 h after l-CDB-4022 treatment. The ratio of the membrane to soluble form of stem cell factor mRNA was decreased. Immunohistochemical analysis of testicular sections indicated a dramatic disruption of the Sertoli cell microtubule network in l-CDB-4022-treated rats. Collectively, these results suggest that l-CDB-4022 activates the MAPK pathway, reduces expression of prosurvival factors such as the membrane form of stem cell factor, alters expression of Sertoli-germ cell adherens junction proteins, disrupts Sertoli cell microtubule structure, and induces the proapoptotic factor, Fas, culminating in germ cell loss from the seminiferous epithelium.
THE INDENOPYRIDINE, CDB-4022 [4aRS,5SR,-9bRS]-2- ethyl-2,3,4,4a,5,9b-hexahydro-8-iodo-7-methyl-5-[4-carbo-methoxyphenyl]-1H-indeno[1,2-c]pyridine-hydro-
chloride, is in development as an oral male contraceptive. Previous studies have demonstrated that CDB-4022 exhibits antispermatogenic activity in various species, including mice, rats, dogs, and monkeys (1,2,3,4,5). Studies from our laboratory demonstrated that, in the rat, CDB-4022 induces irreversible infertility (6), whereas in the monkey, reversible severe oligospermia was observed (7). In the rat, CDB-4022 induced apoptosis of germ cells, resulting in the loss of fertility, unless endogenous testosterone was suppressed with a GnRH antagonist such as acyline before treatment with CDB-4022 (6,8). Prior treatment with Lupron Depot (TAP Pharmaceutical Products, Inc., Lake Forest, IL), a GnRH agonist, partially prevented the irreversible infertility induced by CDB-4022 in the rat (9). Serum inhibin B was undetectable in rats within a week after the treatment with CDB-4022, and there was a concomitant increase in FSH levels. There was no significant change in serum testosterone or LH levels after CDB-4022 treatment, suggesting that there was no effect on Leydig cell function (6). In the monkey, l-CDB-4022 induced reversible severe oligospermia without altering endogenous hormone levels except for a transient increase in inhibin B (7). Therefore, it appears that CDB-4022’s actions differ to some extent between these two species.
In our previous studies, infertility was induced by CDB-4022 in male rats 45 d of age and older (10). CDB-4022 is a racemic mixture of l- and d-isomers, and the l-isomer of CDB-4022 exhibits antispermatogenic activity (3). Ultrastructural evaluation of CDB-4022’s actions in the rat testes indicated that it has effects on Sertoli and germ cells, but not Leydig cells (11). Sertoli cell structure was disrupted within 3 h after treatment with 12.5 mg/kg CDB-4022 racemate. There was an increase in the amount of swollen smooth endoplasmic reticulum and a decrease in cytoplasmic ground substance in Sertoli cells at 6 and 12 h after treatment. Spermatocytes and spermatids were also affected within 3 h after treatment as judged by swelling of the nuclear envelope (11). However, it is still not clear how CDB-4022 affects the expression of junctional complex proteins involved in adhesion between Sertoli and germ cells, and which cell signaling pathways may be mediating these effects, resulting in loss of germ cells from the seminiferous epithelium.
The seminiferous epithelium of the testis is composed of Sertoli and germ cells. In the adult testis, the blood-testis barrier (BTB) divides the seminiferous epithelium into two compartments. One is a basal compartment in which spermatogonia, preleptotene, and leptotene spermatocytes reside. The other is an adluminal compartment in which meiotic spermatocytes and spermatids in various stages of spermatogenesis and spermiogenesis exist. Spermatogenesis, which takes place in the seminiferous epithelium, is associated with extensive junction restructuring to facilitate the movement of germ cells from the basal compartment to the luminal compartment of the epithelium (12,13). Sertoli cells provide crucial structural support to the germ cells and their translocation, by creating the BTB, secreting numerous growth factors and nutrients, and conducting other vital functions such as phagocytosis. Tight junctions are restricted to the BTB, but several anchoring junction types were also detected in the seminiferous epithelium. One type of anchoring junction is the adherens junction (AJ), which exists between Sertoli and germ cells. AJs include the protein complexes of cadherin/catenin, nectin/afadin, and integrin/laminin, which play a major role in spermatogenesis (14,15,16). These complexes are, in turn, connected to the actin cytoskeleton. The testis also has very dynamic prosurvival and proapoptotic systems that balance each other to regulate the extent of germ cell apoptosis. After exposure to an antispermatogenic agent, germ cell apoptosis is often increased, indicating that the seminiferous epithelium is dysfunctional and cannot provide support to germ cells, or that the germ cells were damaged. We hypothesized that CDB-4022 affects AJs, prosurvival and proapoptotic systems, and various cell signaling pathways in the rat testis, leading to loss of germ cells.
To test this hypothesis, we treated 45-d-old male Sprague Dawley rats with a single oral dose of the purified l-isomer of CDB-4022 (2.5 mg/kg) or vehicle and examined the effect of l-CDB-4022 on these endpoints. Blood and testes were collected at 4, 8, 24, 48, and 96 h, and 14 d after treatment. Serum was assayed for various hormones using specific RIAs or ELISAs, and testicular protein and RNA were analyzed by Western blotting and RT-PCR, respectively. Testicular sections were analyzed by immunohistochemistry for tyrosine tubulin expression. Here, we report that l-CDB-4022 affects Sertoli-germ cell interactions by altering the expression of AJ proteins, activating the MAPK pathway, reducing the expression of prosurvival factors such as the membrane form of stem cell factor (SCF), and inducing the apoptosis of germ cells by activation of the proapoptotic factor, Fas.
Materials and Methods
Animals
Male Sprague Dawley CD rats (Crl:CD(SD) were obtained from Charles River Laboratories (Kingston, NY). All rats were housed in polycarbonate, solid-floor cages with Bed-o-Cob or β-Chip bedding (Andersons Industrial Products Group, Maumee, OH), and received Purina laboratory rodent diet (product no. 5001; Purina, Richmond, IN) and tap water ad libitum. The photoperiod was a 14-h light, 10-h dark cycle. The environmental conditions of the animal rooms were maintained as recommended in the U.S. Institute of Laboratory Animal Resources’s Guide for the Care and Use of Laboratory Animals (17) to the maximum extent possible. BIOQUAL’s institutional animal care and use committee approved all study protocols.
Chemicals and reagents
l-CDB-4022 (also known as RTI-4587-073) was synthesized by Research Triangle Institute (Research Triangle Park, NC), and was considered more than 98% pure based on HPLC analysis. The CDB-4022 used in previous rat studies (6,8,9,10) was a racemic mixture of the l- and d-enantiomers. In this study the purified l-enantiomer was used because it is the active enantiomer. Needles, syringes, and surgical supplies were purchased from VWR International, Inc. (West Chester, PA). All other reagents and molecular biology grade chemicals were purchased from Sigma-Aldrich Inc. (St. Louis, MO). Superscript III RT-kit, PCR mix, and primers were purchased from Invitrogen Corp. (Carlsbad, CA).
Treatment
Male Sprague Dawley CD rats (45 d old) were treated with a single dose of l-CDB-4022 (2.5 mg/kg body weight) or vehicle (10% EtOH/sesame oil) by gavage. The 2.5-mg/kg dose was selected based on induction of irreversible infertility in adult male rats by the purified l-isomer (18). This was a maximally effective dose of purified l-CDB-4022. In previous studies the racemate was used that was maximally effective at a higher dose (6,11). Rats were euthanized by exsanguination at 4, 8, 24, 48, and 96 h, and 14 d after treatment. Blood and testes were collected from a group of eight rats (four rats per vehicle and four rats per l-CDB-4022) at each time point. Whole blood was allowed to clot for at least 2 h at 4 C, and serum was harvested by centrifugation (900 × g, 30 min, 2–6 C) and stored at −80 C until analyzed. The right testes were removed, weighed, and cut into two halves; one half was used for RNA, one for protein extraction. The left testes were fixed in Bouin’s solution for 24 h at 4 C for immunohistochemistry.
Immunoassays
All serum samples were analyzed in a single assay, ELISA or RIA. Inhibin B was determined in serum samples using an ultrasensitive Inhibin B ELISA assay kit (Serotec, Oxford, UK). The assay has been described in detail previously (6) and used to determine inhibin B levels in rat serum by our laboratory. Briefly, lyophilized human inhibin B extracted from follicular fluid (supplied with the kit) was reconstituted in castrate adult male rat serum and a standard curve generated by serial dilution of the human inhibin B in the same serum (6). The standard curve was calculated using a four-parameter curve fit (RiaSmart Data Reduction Program; PerkinElmer Life Sciences, Meriden, CT), and inhibin B levels in serum samples were determined. The limit of detection for the assay was 12.5 pg human inhibin B/ml, and the intraassay variation was 5.6%. Serum activin A was measured by specific ELISA (19) according to the manufacturer’s instructions (Oxford Bio-Innovations, Upper Heyford, Oxfordshire, UK). The standard used was human recombinant activin A provided in the kit. Lyophilized human recombinant activin A was diluted in castrate rat serum for the standard curve. A 6% sodium dodecyl sulfate solution was added (3% final concentration) to standard and serum samples, followed by boiling for 3 min. The samples were allowed to cool before the addition of H2O2 (2% final concentration) and subsequent 30 min incubation. Samples were added in duplicate to the anti-βA subunit monoclonal antibody coated plates and incubated overnight at room temperature. The plates were washed, and the second detection antibody (biotinylated-anti-βA subunit monoclonal antibody) was added for 2 h at room temperature. After washing, alkaline phosphatase linked to streptavidin was added to the wells and incubated at room temperature for 1 h. After further washes, the alkaline phosphatase activity was detected with an amplifier, after incubating with the substrate for 1 h at room temperature. The optical densities were read in the Molecular Devices microplate reader (Molecular Devices, Sunnyvale, CA) using a 490-nm filter, reference 620 nm. The standard curve was calculated using a four-parameter curve fit, and activin A levels in serum samples were determined. The limit of detection for the activin A assay was 39 pg human activin A/ml, and the intraassay variation was 1.8%.
Serum samples were assayed for testosterone using a commercially available coated-tube testosterone RIA (Coat-A-Count; Diagnostic Products Corp., Los Angeles, CA). The assay procedure followed the kit’s directions except that the incubation period was overnight at 2–6 C instead of 3 h at 37 C (8). This modification was made because it allowed a more accurate measurement of a known quantity of testosterone in castrate rat serum. No testosterone was detected in a serum pool from castrated rats. RIA data were analyzed using a four-parameter sigmoidal curve fit. The limit of detection in this assay was 0.06 ng testosterone/ml, and the intraassay variation was 6.6%. Levels of the rat gonadotropins, rat LH (rLH) and rat FSH (rFSH), were determined in serum samples by RIA using reagents supplied by the National Hormone and Peptide Program/National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) (supplied by Dr. A. F. Parlow) following the procedures received with the reagents. NIDDK-rFSH-RP-2 and NIDDK-rLH-RP3 were the standards for the FSH and LH assays, respectively. RIA data were analyzed using a four-parameter sigmoidal curve fit as mentioned previously. The limits of detection were 2.9 ng rFSH/ml and 0.39 ng rLH/ml based on 100 and 200 μl serum/tube, respectively. The intraassay coefficients of variation were 3.8 and 3.5% for the rFSH and rLH assays, respectively.
Western blotting
Testes were homogenized in lysis buffer [20 mm Tris-HCl (pH 7.5), 1 mm EDTA, 150 mm NaCl, 1% Triton X-100, 1 μg/ml leupeptin, 1 mm NaF, and 1 mm NaVO4] for protein extraction. The homogenate was then centrifuged at 14,000 rpm for 15 min at 4 C, and the supernatant was collected, aliquoted, and stored at −80 C until used. The protein concentration was determined using a Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA) and BSA as standard. Immunoblotting was performed using 100 μg protein per lane loaded onto NuPAGE 4–12% Bis-Tris gels (Invitrogen) under reducing conditions, and the separated proteins transferred onto polyvinylidene fluoride or nitrocellulose membranes. Membranes were then blocked with Tris-buffered saline-0.1% Tween 20 (TBS-T) (pH 7.5) containing 5% nonfat dry milk for 1 h at room temperature before incubating overnight at 4 C with various primary antibodies. The list of primary antibodies and dilutions used is presented in Table 1. After three 5-min washes in TBS-T, the membranes were incubated with matching secondary antibodies conjugated to horseradish peroxidase for 2 h at room temperature. The membranes were then washed three times for 5 min in TBS-T at room temperature. Supersignal West Dura Extended Duration Substrate (Pierce, Rockford, IL) was used for detection of protein bands by chemiluminescence using the ChemiGenius2 detector from Syngene (Frederick, MD) according to the manufacturer’s instructions.
Table 1.
Antibodies and dilutions
| Company | Antibody | Catalog no. | Working dilution | Use |
|---|---|---|---|---|
| Abcam, Inc. (Cambridge, MA) | Bax | Ab7977 | 1000 | WB |
| FasL | Ab15285 | 500 | WB | |
| BD Transduction Laboratories (BD Biosciences, San Jose, CA) | Integrin β1 | B610467 | 2500 | WB |
| Cell Signaling Technology, Inc. (Danvers, MA) | p44/42 MAPK | 9102 | 1000 | WB |
| Phospho-p44/42MAPK | 9101 | 1000 | WB | |
| Akt | 9272 | 1000 | WB | |
| Phospho-Akt | 9271 | 1000 | WB | |
| Phospho-p38MAPK | 4631 | 1000 | WB | |
| Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) | Nectin-3 | sc-14806 | 200 | WB |
| Fas | sc-1023 | 200 | WB | |
| β-catenin | sc-7199 | 200 | WB | |
| E-cadherin | sc-7870 | 200 | WB | |
| α-catenin | sc-7894 | 200 | WB | |
| N-cadherin | sc-7939 | 400 | WB | |
| Lamininγ-3 | sc-16601 | 200 | WB | |
| Sigma-Aldrich | Anti-l-Afadin | A0349 | 2000 | WB |
| Actin | A2547 | 1500 | WB | |
| Tyrosine tubulin | T9028 | 1500 | IHCWB |
IHC, Immunohistochemistry; WB, Western blotting.
Total RNA extraction and RT-PCR
Total RNA was extracted using an RNeasy Protect Midi Purification kit (QIAGEN, Inc., Germantown, MD) following the instructions provided with the kit. RNA (1 μg) was reverse transcribed using a Superscript III RT-kit from Invitrogen in a 20 μl reaction mix. cDNA was amplified by PCR using PCR mix from Invitrogen. The primer sequences for SCF (c-kit ligand), androgen receptor (AR), androgen-binding protein (ABP), Fas receptor (Fas), Fas ligand (FasL), claudin-11, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are as follows: SCF-FP 5′-cacttgcatttatcttcaactgc-3′, SCF-RP 5′-tccagtataaggctccaaaagc-3′, AR-FP 5′-attcctggatgggactgatg-3′, AR-RP 5′-tgccatcatttcaggaaagtc-3′, ABP-FP 5′-cagcaaaccctcttcctcc-3′, ABP-RP 5′-ttccatccacccatagcagcag-3′, Fas-FP 5′-ctgtggatcatggctgtcctgcct-3′, Fas-RP 5′-ctccagactttgtccttcattttc-3′, FasL-FP-5′-ggaatgggaagacacatatggaactgc-3′, FasL-RP 5′-catatctggccagtagtgcagtaattc-3′, Clau11-FP 5′-gattggcatcatcgtcacaacg-3′, Clau11-RP 5′-agccagcagaa-taaggagcaac-3′, and GAP-FP 5′-gggtggtgccaaaagggtc-3′, GAP-RP 5′-ggagttgctgttgaagtcaca-3′ (Invitrogen). PCR products were analyzed by electrophoresis in 1% agarose gels containing ethidium bromide.
Immunohistochemistry
The left testes preserved in Bouin’s solution were washed in 50 and 70% ethanol before embedding them in paraffin and subsequently sectioning them at approximately 5 μm. The sections were deparaffinized in xylene, rehydrated through a series of ethanol dilutions, and nonspecific sites were then blocked by incubating sections in 3% H2O2 in 50% methanol for 10 min. Sections were incubated with the blocking buffer (5% normal rabbit serum-PBS) for 30 min at room temperature. The blocking buffer was removed, and the sections were probed with the antityrosine-tubulin antibody at a 1:2000 dilution at room temperature for 16 h in a humidified chamber. After washing with PBS three times, the sections were incubated with biotinylated goat antirabbit IgG diluted 1:100 with PBS for 1 h at room temperature, followed by PBS wash and incubation with avidin-biotin-peroxidase complex (Vectastain ABC kit; Vector Laboratories, Burlingame, CA). Negative controls consisted of omission of primary antiserum that was replaced with normal rabbit serum diluted 1:2500 with PBS. Immunohistochemical reactions were visualized by incubating the sections with 3′3-diaminobenzidine (Sigma-Aldrich) for 5 min. The sections were then washed with 1× PBS to remove the 3′3-diaminobenzidine and counterstained with hematoxylin, mounted with Permount, examined and photographed using a SPOT Insight QE digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI) and software attached to a light microscope (Nikon Corp., Melville, NY). All images were analyzed using Adobe Photoshop (version 7.0; Adobe Systems, Inc., San Jose, CA).
Statistical analysis
Statistical analyses were performed using SigmaStat Software version 3.0 (SPSS, Inc., Chicago, IL). Results are presented as the mean ± se. Significant differences (P < 0.05) between vehicle- and l-CDB-4022-treated groups were determined with the Student’s t test for testes weights and serum hormone levels. Data from densitometric scanning of protein from immunoblots and SCF mRNA levels from RT-PCR gels of l-CDB-4022-treated rats at different times vs. controls were analyzed by one-way ANOVA.
Results
Effect of l-CDB-4022 on body and testis weight
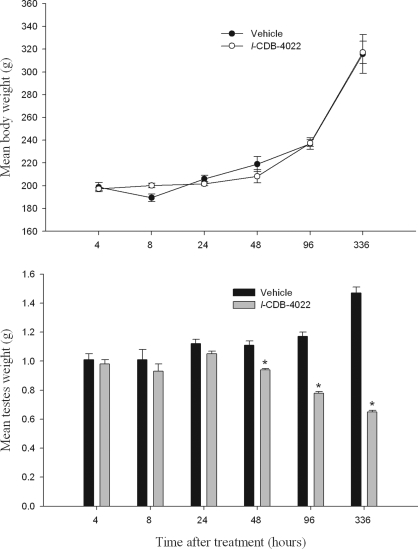
There were no clinical signs in the rats during the treatment period. The rate of body weight gain was not affected at any of the time points, but there was a progressive and significant (P < 0.001) loss of testes weight in l-CDB-4022-treated rats compared with vehicle-treated at 48 h and later time points (Fig. 1).
Figure 1.
Mean body (top panel) and testes (bottom panel) weights. Body and testes weights of male rats were measured at 4, 8, 24, 48, and 96 h, and 14 d after treatment with vehicle or l-CDB-4022 (2.5 mg/kg) at 45 d of age. There were four rats per time point in each treatment group. *, Significantly different from the vehicle-treated rats at the same time point (P < 0.001).
Effect of l-CDB-4022 on serum hormone levels
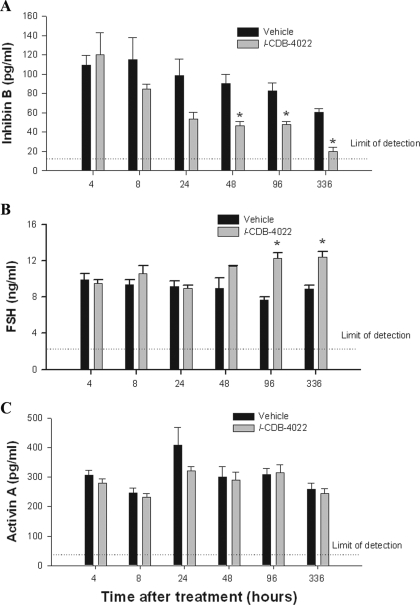
Circulating hormone levels were evaluated to assess the effect of l-CDB-4022 on testicular and pituitary gland function. Serum inhibin B levels were decreased progressively, and showed a statistically significant (P < 0.05) decrease at 48 and 96 h in rats treated with l-CDB-4022 compared with vehicle-treated rats, and decreased close to nondetectable levels by 14 d (Fig. 2A). There was a significant increase (P < 0.05) in serum FSH levels at 96 h and 14 d (Fig. 2B). The inverse relationship between FSH and inhibin B is presumably due to the negative feedback regulation between inhibin B and FSH. There was no effect of l-CDB-4022 treatment on serum levels of activin A (Fig. 2C), testosterone, or LH (data not shown).
Figure 2.
Serum hormone analysis. Serum levels of inhibin B (A) FSH (B), and activin A (C) in male rats after treatment with a single oral dose of l-CDB-4022 (2.5 mg/kg) or vehicle (10% ethanol/sesame oil) at 45 d of age. Data represent the mean ± se (n = 4). *, FSH and inhibin B levels in l-CDB-4022-treated rats were significantly different from those in vehicle-treated rats (P < 0.005).
Activation of the MAPK pathway
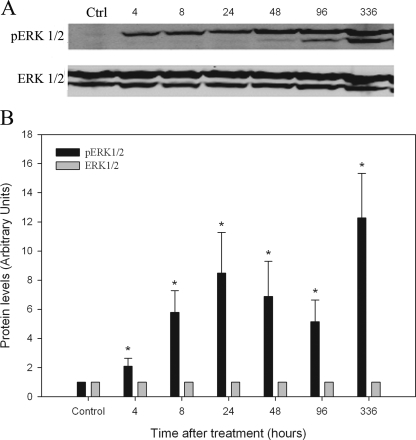
To understand the effect of l-CDB-4022 on various cell signaling pathways in the rat testis, we assessed the activation (phosphorylation) of phosphatidylinositol 3-kinase (PI3-K)/AKT, p38 MAPK, and ERK1/2 by immunoblotting. There was no activation of PI3-K, AKT, or p38 MAPK proteins in testicular lysates from l-CDB-4022-treated rats (data not shown), but ERK1/2 proteins were activated (Fig. 3). ERK1/2 are members of the MAPK family, which constitutes a cascade of serine/threonine-specific protein kinases that respond to extracellular stimuli (mitogens) and regulate various cellular activities, such as gene expression, mitosis, differentiation, and cell survival/apoptosis (20,21,22,23,24). Phosphorylation of ERK1/2 was not observed in vehicle-treated rat testicular lysates, whereas the testicular lysates from l-CDB-4022D-treated rats exhibited activation of ERK1/2 when probed with antibodies specific to phospho-ERK1/2 (Fig. 3). The activation of ERK1/2 proteins occurred at the earliest time point in the study, 4 h, and increased at later time points. Reprobing of the membranes using an antibody that detects total ERK1/2 protein confirmed that l-CDB-4022 treatment altered the activation of ERK1/2, but not protein expression (Fig. 3).
Figure 3.
Western analysis for activation of ERK1/2. A, Testicular proteins in lysates (100 μg) from male rats treated with a single oral dose of l-CDB-4022 (2.5 mg/kg) or vehicle (10% ethanol/sesame oil) at 45 d of age were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunostained with a phosphoERK1/2 primary antibody. Membranes were stripped and reprobed with ERK1/2 antibody to assess the protein expression. B, All immunoblots were densitometrically scanned for the levels of pERK1/2 and ERK1/2. The levels of pERK1/2 and ERK1/2 in control (Ctrl) rats were arbitrarily set at 1.0. Each bar is the mean ± se of data from four blots (four rats per time point). *, P < 0.05.
Effect of l-CDB-4022 on expression of prosurvival factor, SCF
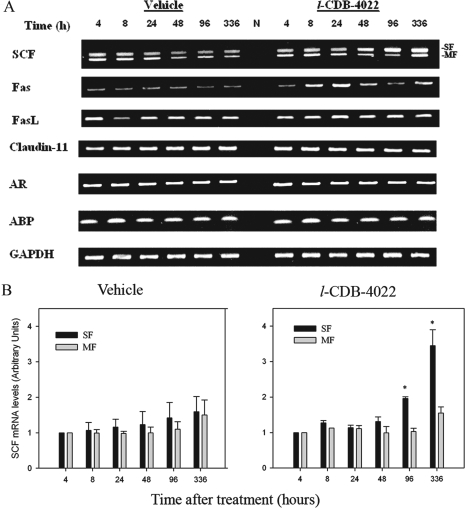
SCF is a Sertoli cell-specific protein ligand that binds to the c-kit receptor on differentiating spermatogonia (25,26). The c-kit receptor, a tyrosine kinase, binds to SCF, and undergoes dimerization and autophosphorylation activating subsequent downstream signaling pathways such as the JAK/STAT pathway, the PI3-kinase pathway, and the Ras-Raf-MAP kinase cascade (26,27). We evaluated the effect of l-CDB-4022 on SCF expression by RT-PCR analysis. Primers for SCF were designed spanning different exons to amplify two main forms of SCF transcripts (membrane and soluble forms) expressed in testis due to alternate mRNA splicing. There was a decrease in the ratio of the membrane to the soluble form of SCF mRNA in l-CDB-4022- treated rats compared with the vehicle-treated rats (Fig. 4A). Quantification of SCF mRNA isoforms from three RT-PCR experiments by densitometry indicated that the soluble form of SCF increased significantly (2- to 4-fold) with l-CDB-4022 treatment at 96 h and 14 d compared with vehicle-treated animals (Fig. 4B). There was no significant increase in the membrane form at any time point. Published reports indicate that SCF plays a major role in spermatogenesis in rodent testis, preferentially as a membrane form rather than a soluble form (28).
Figure 4.
RT-PCR analysis of various testicular genes. A, Total RNA (1 μg) was reverse transcribed to cDNA, and used in PCR for amplification of SCF, AR, Fas, FasL, claudin-11, and ABP mRNA transcripts as described in Materials and Methods. GAPDH was amplified as an internal control. The gels are representative of three RT-PCR experiments. N, Negative RT control. B, The RT-PCR gels were densitometrically scanned for the mRNA levels of SCF. The levels of soluble and membrane forms of SCF at 4 h were arbitrarily set at 1.0. Each bar is mean ± se of three RT-PCR experiments (three rats per time point). *, P < 0.05. SF, Soluble form; MF, membrane form.
Effect of l-CDB-4022 on expression of AR, ABP, and claudin-11 genes
Testicular genes such as AR, ABP, and claudin-11 play an important role in spermatogenesis. AR and ABP play a critical role in mediating androgen-dependent germ cell development in the testis, and claudin-11 is a hormonally regulated (FSH, testosterone) (29) key factor in the establishment of the hematotesticular barrier. We evaluated the levels of mRNA for AR, ABP, and claudin-11 by RT-PCR to assess the effect of l-CDB-4022 on gene expression. There was no change in the expression of these genes in l-CDB-4022-treated rats compared with vehicle-treated rats (Fig. 4A).
l-CDB-4022 induced germ cell apoptosis by the Fas-mediated pathway
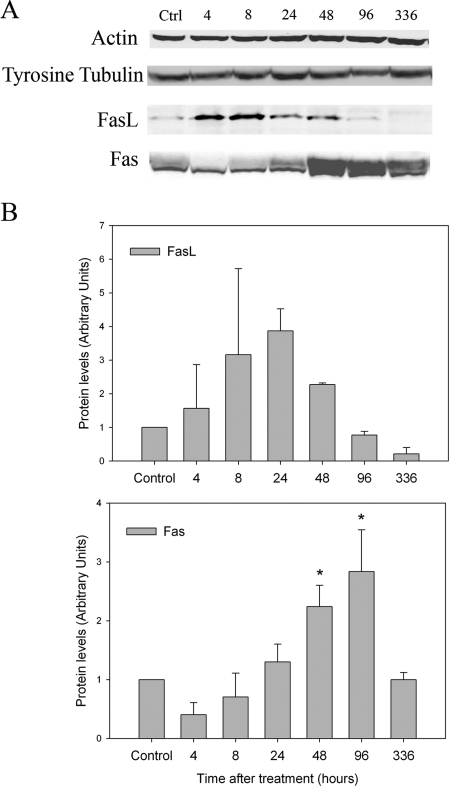
To assess how l-CDB-4022 induced apoptosis of germ cells in the rat testis, testicular lysates were analyzed by immunoblotting for proapoptotic proteins such as Bax, FasL, and Fas. Bax is a proapoptotic protein member of the Bcl-2 family that regulates apoptosis (30). FasL is a member of the TNF family, and is expressed in various cells and tissues (31,32). FasL binds to its receptor Fas, forms a death-inducing signaling complex, and causes apoptosis of Fas-bearing cells (33). In testis, FasL is specifically expressed in Sertoli cells, and Fas, in germ cells (34). There was no change in Bax protein expression in testicular lysates from l-CDB-4022-treated rats (data not shown). On the contrary, there was a significant increase in expression of FasL protein at time points, 4, 8, and 24 h, decreasing to nondetectable levels at later time points (Fig. 5). Fas expression followed a similar pattern except that the increase in its expression occurred at later time points, 24, 48, and 96 h, after l-CDB-4022 treatment and decreased by 14 d. RT-PCR analysis exhibits an increase in expression of Fas mRNA at 8, 24, and 48 h, indicating a correlation with the results of Western blotting, but there was no visible increase in FasL mRNA (Fig. 4).
Figure 5.
Western analysis for actin, tyrosinated tubulin, FasL, and Fas. A, Testicular proteins (100 μg) in lysates from male rats treated with a single oral dose of l-CDB-4022 (2.5 mg/kg) or vehicle (10% ethanol/sesame oil) at 45 d of age were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunostained with actin, tyrosinated tubulin, FasL, and Fas antibodies. B, The immunoblots were densitometrically scanned for the protein levels of FasL (top panel) and Fas (bottom panel). The levels of FasL and Fas in control (Ctrl) rats were arbitrarily set at 1.0. Each bar is mean ± se of data from four blots (four rats per time point). *, P < 0.05.
Effect of l-CDB-4022 on AJ proteins
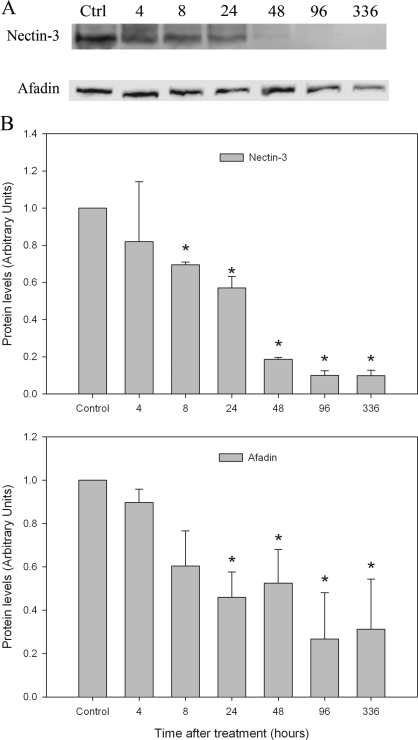
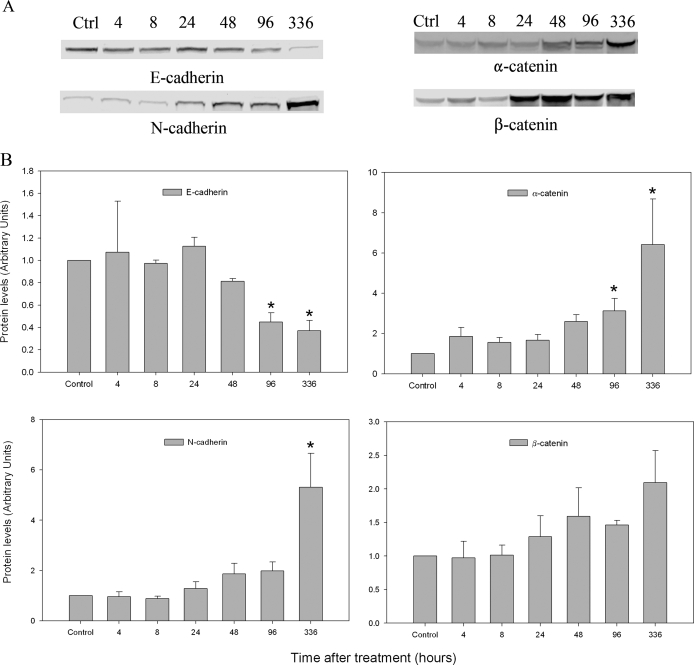
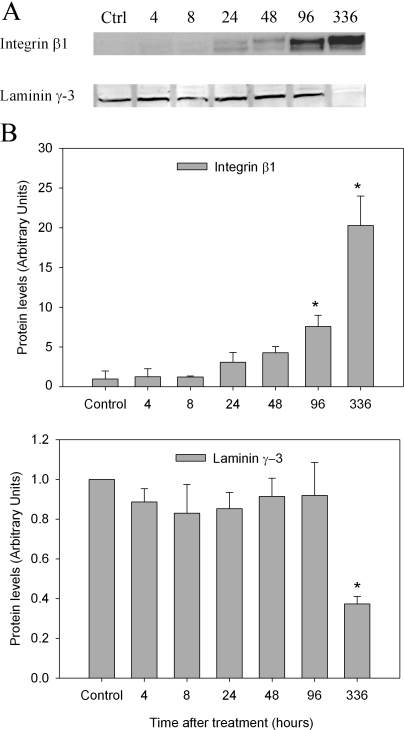
Three classes of AJ proteins have been identified in the seminiferous epithelium of rat testis (14,15,16). They are the nectin/afadin complex, cadherin/catenin complex, and integrin/laminin complex. To determine whether l-CDB-4022 affected these AJs, testicular lysates were analyzed by immunoblotting. Significant loss of nectin-3 and afadin proteins occurred at 8 and 24 h after treatment, respectively, in l-CDB-4022-treated rats (Fig. 6). Cadherins/catenins are calcium-dependent cell adhesion molecules, which play an important role in cell adhesion. N-cadherin expression was significantly increased in testicular lysates from l-CDB-4022-treated rats at 14 d, whereas there was a decrease in E-cadherin expression at 96 h and 14 d (Fig. 7). There was a significant increase in expression of α-catenin at 96 h and 14 d. An increase in expression of β-catenin was also observed at 48 h and later (Fig. 7). Integrin-β1 showed a pattern of increase similar to that of α-catenin, whereas γ-laminin expression remained unchanged at all time points except at 14 d. There was a significant decrease (∼50%) in γ-laminin at 14 d after treatment, indicating the loss of elongated spermatids from the testis by this time (Fig. 8).
Figure 6.
Western analysis for nectin-3 and afadin. A, Testicular proteins (100 μg) in lysates were analyzed by Western blotting using nectin-3 and l-afadin antibodies. B, The immunoblots were densitometrically scanned for the protein levels of nectin-3 (top panel) and afadin (bottom panel). The levels of nectin-3 and afadin in control (Ctrl) rats were arbitrarily set at 1.0. Each bar is the mean ± se of data from four blots (four rats per time point). *, P < 0.05.
Figure 7.
Western analysis for cadherins and catenins. A, Testicular proteins (100 μg) in lysates were analyzed for cadherins and catenins by Western blotting. B, The immunoblots were densitometrically scanned for the protein levels of N (bottom-left panel) and E-cadherin (top-left panel), and α (top-right panel) and β-catenin (bottom-right panel). The levels of cadherins and catenins in control (Ctrl) rats were arbitrarily set at 1.0. Each bar is the mean ± se of data from four blots (four rats per time point). *, P < 0.05.
Figure 8.
Western analysis for integrin-β1 and γ-laminin. A, Testicular proteins (100 μg) in lysates were analyzed for integrin-β1 and γ-laminin by Western blotting. B, The immunoblots were densitometrically scanned for the protein levels of integrin and laminin. The levels of integrin-β1 (top panel) and γ-laminin (bottom panel) in control (Ctrl) rats were arbitrarily set at 1.0. Each bar is the mean ± se of data from four blots (four rats per time point). *, P < 0.05.
Effect of l-CDB-4022 on Sertoli cell microtubules
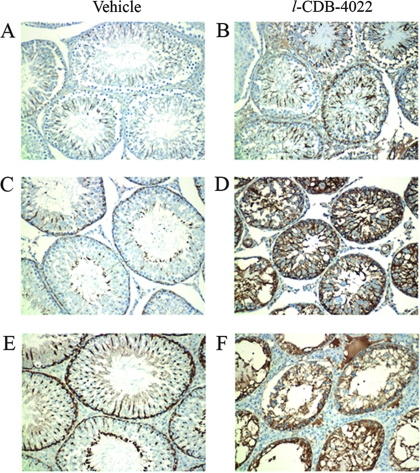
To determine whether the Sertoli cell microtubules were sensitive to l-CDB-4022, testicular sections from both vehicle- and l-CDB-4022-treated rats were used for immunohistochemical staining of tyrosinated tubulin (α-tubulin). Tyrosine tubulin was used as a Sertoli cell-specific control protein in Western blotting and immunohistochemistry (35). Testicular sections from vehicle-treated rats exhibited spokes of dark staining for tyrosinated tubulin with a classical radial pattern representing the Sertoli cell microtubules oriented along the longitudinal axis of the cell (Fig. 9). In contrast, dramatic disruption of the Sertoli cell microtubule network, as judged by compact and darker staining, was observed in testicular sections from l-CDB-4022-treated rats. The collapsed microtubules were clearly visible at 96 h with sloughed material in the lumen. By 14 d the lumen appeared to be clear, indicating that germ cell loss from the seminiferous epithelium was complete. However, there was no alteration in protein levels of tyrosinated tubulin in testicular lysates from l-CDB-4022-treated rats analyzed by Western blotting (Fig. 5).
Figure 9.
Immunohistochemical localization of tyrosine tubulin in representative sections of testes from vehicle (A, C, and E) and l-CDB-4022 (B, D, and F)-treated rats at 8 h (A and B), 96 h (C and D), and 14 d (E and F).
Discussion
The indenopyridine, l-CDB-4022, induced testicular atrophy and germ cell loss from the seminiferous epithelium in the rat testis after a single oral dose. Although l-CDB-4022 induced a significant loss in weight of the testes, there was no effect on the rate of body weight gain or clinical signs in the male rats. The data in the present study agree with those from previous studies (6,10) showing that a single oral dose of CDB-4022 decreased serum inhibin B levels paralleled by an increase in serum FSH. In our study there was no change in serum activin A levels in l-CDB-4022-treated rats. There are some reports demonstrating a reciprocal relationship between activin A and inhibin B levels in primary rat Sertoli cells treated with an inflammatory agent (36,37). However, in our study serum inhibin B levels exhibited a reciprocal relationship with FSH, but not activin A in l-CDB-4022-treated rats. Unaltered levels of serum LH and testosterone in l-CDB-4022-treated rats indicate that this compound did not have any effect on Leydig cells.
MAPKs are signal transducers of various pathways that regulate different cellular functions. ERKs, c-Jun N-terminal kinases (JNKs), and p38 MAPKs are the subfamilies of the MAPK pathway (38). They are found in almost all cell types, activated by a variety of growth factors and hormones producing cell-specific signals. In general, activation of ERK1/2 proteins in a cell results in cell growth and differentiation, whereas activation of JNKs and p38 MAPKs plays a role in mediating stress-induced signaling (39,40). However, recent evidence suggests that activation of the ERKs can also be stimulated by a variety of stress stimuli such as heat shock (41). Heat shock can activate ERK1/2 in monkey testis, which in turn leads to apoptosis of germ cells, but does not activate JNKs or p38 MAPKs (41,42). A constitutively activated ERK1 mutant induced dedifferentiation and growth inhibition in madin-darby canine kidney-C7 cells (43), indicating that activated ERK1 plays an important role in cellular function.
In our study l-CDB-4022 induced activation of ERK1/2 as early as 4 h with an increase in activation with time, indicating that these proteins play an important role in l-CDB-4022’s action. Both ERK1 and ERK2 are expressed in Sertoli and germ cells in the rat testis (44), and play an important role in spermatogenesis by modulating germ cell adhesion and motility (45). It has been shown that FSH and testosterone also stimulate ERK1/2 in primary rat Sertoli cells (46). ERK was down-regulated in the rat testis by phthalate, an environmental toxicant, which causes germ cell apoptosis (47). On the contrary, activation of ERK1/2 also seems to be an early step in the action of most antispermatogenic agents, including l-CDB-4022, and may be related to the disruption of the apical ectoplasmic specialization adhesion function, leading to germ cell loss and dedifferentiation of Sertoli cells (41,48). However, it is indeed intriguing that there was no effect of l-CDB-4022 on other related signaling pathways such as p38 MAPK and PI3K/AKT in the rat testis.
l-CDB-4022 induced an apparent alteration in the ratio of the two alternately spliced products of the SCF gene. SCF is specifically produced by Sertoli cells, and the membrane form is the predominant form during spermatogenesis. SCF interacts with the c-kit receptor, a transmembrane tyrosine kinase expressed by spermatogonia and Leydig cells (27). In transgenic mouse models, a mutation that results in expression of soluble SCF only causes infertility, anemia, and loss of pigmentation (49). Rats exposed to other antispermatogenic agents such as 2,5-hexanedione also exhibit germ cell loss and a decrease in the ratio of transmembrane to soluble SCF expression (50). Reversal of 2,5-hexanedione-induced germ cell loss with a GnRH agonist increases the ratio of transmembrane to soluble SCF expression (51). In our study, unlike humans (52), no correlation was found between soluble SCF and serum testosterone levels in l-CDB-treated rats, as there was no change in serum testosterone levels. The apparent alteration in SCF isoform levels in l-CDB-4022-treated rats indicates that the loss of specific adhesive interactions between Sertoli and germ cells occurred as the soluble form became predominant with l-CDB-4022 treatment. However, further research is needed to determine the importance of SCF in the action of l-CDB-4022.
l-CDB-4022 induces activation of ERK1/2 and alters the SCF isoform ratio, leading to the possible loss of germ cell adhesion with Sertoli cells, which in turn results in apoptosis and sloughing of germ cells from the seminiferous epithelium. When rat testicular lysates were analyzed for various apoptotic proteins, activation of the Fas-mediated pathway was observed. Fas-mediated apoptosis is a well-recognized signal transduction pathway in which a ligand interaction with its receptor triggers cell death (32,33). The Fas system is involved in maintaining homeostasis in various systems, cell-mediated toxicity, and control of immune-privileged sites (32,53). After l-CDB-4022 treatment, FasL was up-regulated in the testis at early time points, followed by Fas activation at later time points. The highest FasL expression was detected at 8 h after l-CDB-4022 exposure, whereas germ cell apoptosis was not observed until 96 h. Up-regulation of Fas in germ cells also appears to be an essential step in l-CDB-4022’s action, and the mechanisms leading to an increase in both FasL and Fas levels are under investigation.
Expression of most of the AJ proteins examined in this study was affected by l-CDB-4022 treatment. Loss of nectin-3 protein was observed in l-CDB-4022-treated rats at 8 h, followed by loss of its partner protein, afadin, at a later time point, indicating that this calcium-independent protein complex plays a major role in germ cell adhesion. Nectin-3 and afadin colocalize with the actin filament (F-actin) that underlies Sertoli cell-spermatid junctions (54). There is evidence suggesting that nectin and E-cadherin are associated through afadin and the α- and β-catenin complex, and that the nectin-afadin system is involved in the formation of AJs cooperatively with the E-cadherin-catenin system (55,56). The loss of E-cadherin in l-CDB-4022-treated rats supports the association between nectin-afadin and E-cadherin. On the contrary, N-cadherin, integrin-β1, and both α- and β-catenin were induced in l-CDB-4022-treated rats, suggesting that the increase in expression of these proteins is to compensate for the loss of adhesion between Sertoli and germ cells. Previous studies with compounds such as AF-2364 and di-(2-ethylhexyl) phthalate also showed an up-regulation of N-cadherin, and α- and β-catenin expression in the testis (57,58). The loss of germ cells was observed at 96 h and later in l-CDB-4022-treated rats, leading to testicular atrophy and reduction in testis weight to about one third to one half that of control. There was a possible enrichment of proteins from Sertoli cells, spermatogonia, or spermatocytes in testicular lysates that were being analyzed from l-CDB-4022-treated rats due to the loss of mature germ cells, instead of being induced by treatment, especially at 96 h and 14 d. To exclude that possibility, we analyzed the testicular lysates by immunoblotting using specific antibodies to Sertoli cell proteins such as tyrosine tubulin and actin. There was no difference in expression of these proteins in l-CDB-4022-treated rats compared with vehicle-treated. In addition, there was no change in expression of claudin-11, a Sertoli cell-specific gene, by RT-PCR.
Tubulin is a major component of microtubules in the Sertoli cell cytoskeleton. Our results demonstrated that these microtubules were sensitive to l-CDB-4022 treatment, and the disruption was evident with the disappearance of the classical radial pattern of spokes. Surprisingly, there was no change in tyrosinated tubulin levels by Western blotting, indicating that l-CDB-4022 disrupted the microtubule structure without altering the protein levels. Our results are in contrast with previous reports on microtubule disruptors, carbendazim and colchine, in the rat (59). Those compounds induced disruption of the Sertoli cell cytoskeleton and reduced immunostaining of tyrosinated tubulin.
In conclusion, l-CDB-4022 affects multiple proteins and pathways in the rat testis, leading to germ cell loss. l-CDB-4022 induces rapid activation of the ERK-MAPK signaling pathway, followed by down-regulation of prosurvival factors such as the membrane form of SCF, and up-regulation of the Fas-mediated apoptotic pathway. l-CDB-4022 also affects various AJ proteins that are involved in Sertoli-germ cell adhesion and disrupts Sertoli cell microtubule structure. Additional experiments will be required to determine whether the BTB has been disrupted in male rats by l-CDB-4022, leading to irreversible infertility.
Note Added in Proof
Since this manuscript has been communicated, Chen et al. (60) reported that serum inhibin B levels were suppressed and activin A levels were unchanged in adult male rats treated with CDB-4022 (racemate) similar to our results. However, they also reported a 2-fold decrease in serum testosterone levels, which was not observed in this study or our previous studies.
Acknowledgments
We thank the following technicians for their technical expertise: Bruce Till, David Gropp, Margaret Krol, and Trung Pham of BIOQUAL, Inc. We also thank Dr. Richard Blye of the Contraception and Reproductive Health Branch, National Institute of Child Health and Human Development, for review of this manuscript.
Footnotes
This work was supported by National Institute of Child Health and Human Development contract NO1-HD-2-3338 awarded to BIOQUAL, Inc.
This work was presented in part at the 89th Annual Meeting of the Endocrine Society, Toronto, Canada, 2007.
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 3, 2008
Abbreviations: ABP, Androgen-binding protein; AJ, adherens junction; AR, androgen receptor; BTB, blood-testis barrier; Fas, Fas receptor; FasL, Fas ligand; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; JNK, c-Jun N-terminal kinase; PI3, phosphatidylinositol 3-kinase; SCF, stem cell factor; TBS-T, Tris-buffered saline-0.1% Tween 20.
References
- Suter KE, Hovel C, Gradient F, Fluckiger E 1977 Antispermatogenic activity of an indenopyridine derivative. Experientia 33:810 (Abstract) [Google Scholar]
- Hodel C, Suter K 1978 Reversible inhibition of spermatogenesis with an indenopyridine (20–438). Arch Toxicol Suppl 1:323–326 [PubMed] [Google Scholar]
- Cook CE, Wani MC, Jump JM, Lee Y-W, Fail PA, Anderson SA, Gu Y-Q, Petrow V 1995 Structure activity studies of 2,3,4,4α,5,9β-hexahydroindeno[1,2-c]pyridines as antispermatogenic agents for male contraception. J Med Chem 38:753–763 [DOI] [PubMed] [Google Scholar]
- Cook CE, Jump JM, Zhang P, Stephens JR, Lee Y-W, Fail PA, Anderson SA 1997 Exceptionally potent antispermatogenic compounds from 8-halogenation of (4αRS,5SR,9βRS)-hexahydroindeno-[1,2-c] pyridines. J Med Chem 40:2111–2112 [DOI] [PubMed] [Google Scholar]
- Bjordahl JA, Jost LK, Fail PA, Cook CE, Evenson DP 1997 Flow cytometric analysis of the antispermatogenic effects of an indenopyridine derivative. Biol Reprod 54(Suppl 1):212 (Abstract 518) [Google Scholar]
- Hild SA, Reel JR, Larner JM, Blye RP 2001 Disruption of spermatogenesis and Sertoli cell structure and function by the indenopyridine CDB-4022 in rats. Biol Reprod 65:1771–1779 [DOI] [PubMed] [Google Scholar]
- Hild SA, Marshall GR, Attardi BJ, Hess RA, Schlatt S, Simorangkir DR, Ramaswamy S, Koduri S, Reel JR, Plant TM 2007 Development of l-CDB-4022 as a nonsteroidal male oral contraceptive: induction and recovery from severe oligospermia in the adult male cynomolgus monkey (Macaca fascicularis). Endocrinology 148:1784–1796 [DOI] [PubMed] [Google Scholar]
- Hild SA, Attardi BJ, Reel JR 2004 The ability of a gonadotropin-releasing hormone antagonist, acyline, to prevent irreversible infertility induced by the indenopyridine, CDB-4022, in adult male rats: the role of testosterone. Biol Reprod 71:348–358 [DOI] [PubMed] [Google Scholar]
- Hild SA, Meistrich ML, Blye RP, Reel JR 2001 Lupron Depot prevention of antispermatogenic/antifertility activity of the indenopyridine, CDB-4022, in the rat. Biol Reprod 65:165–172 [DOI] [PubMed] [Google Scholar]
- Hild SA, Reel JR, Age-dependent effects of CDB-4022 on testicular function and fertility in male rats. Proc 33rd Annual Meeting of the Society for the Study of Reproduction, University of Wisconsin, Madison, WI, July 15–18, 2000, p 337 (Abstract 588) [Google Scholar]
- Hild SA, Reel JR, Dykstra MJ, Mann PC, Marshall GR 2007 Acute adverse effects of the indenopyridine CDB-4022 on the ultrastructure of Sertoli cells, spermatocytes, and spermatids in rat testes: comparison to the known Sertoli cell toxicant Di-n-pentylphthalate (DPP). J Androl 28:621–629 [DOI] [PubMed] [Google Scholar]
- Russell LD, Peterson RN 1985 Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol 94:177–211 [DOI] [PubMed] [Google Scholar]
- Russell, LD 1977 Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat 148:313–328 [DOI] [PubMed] [Google Scholar]
- Vogl AW, Pfeiffer DC, Redenbach DM 1991 Ectoplasmic (“junctional”) specializations in mammalian Sertoli cells: influence on spermatogenic cells. Ann NY Acad Sci 637:175–202 [DOI] [PubMed] [Google Scholar]
- Toyama Y, Maekawa M, Yuasa S 2003 Ectoplasmic specializations in the Sertoli cell: new vistas based on genetic defects and testicular toxicology. Anat Sci Int 78:1–16 [DOI] [PubMed] [Google Scholar]
- Siu MK, Mruk DD, Lee WM, Cheng CY 2003 Adhering junction dynamics in the testis are regulated by an interplay of β 1-integrin and focal adhesion complex-associated proteins. Endocrinology 144:2141–2163 [DOI] [PubMed] [Google Scholar]
- U.S. Institute of Laboratory Animal Resources 1996 Guide for the care and use of laboratory animals. 7th ed. Washington, DC: National Academy Press [Google Scholar]
- Hild SA, Attardi BJ, Burgenson J, Reel JR, Antispermatogenic activity of the purified enantiomers of the indenopyridine CDB-4022 function and fertility in male rats. Proc 36th Annual Meeting of the Society for the Study of Reproduction, Cincinnati, OH, July 19–22, 2003, p 134 (Abstract 55) [Google Scholar]
- Knight PG, Muttukrishna S, Groome NP 1996 Development and application of a two-site enzyme immunoassay for the determination of ’total’ activin-A concentrations in serum and follicular fluid. J Endocrinol 148:267–279 [DOI] [PubMed] [Google Scholar]
- Davis RJ 1993 The mitogen-activated protein kinase signal transduction pathway. J Biol Chem 268:14553–14556 [PubMed] [Google Scholar]
- Cobb MH, Goldsmith EJ 1995 How MAP kinases are regulated. J Biol Chem 270:14843–14846 [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG 1995 The MAPK signaling cascade. FASEB J 9:726–735 [PubMed] [Google Scholar]
- Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S 2003 MAPK pathways in radiation responses. Oncogene 22:5885–5896 [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J 2001 Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81:807–869 [DOI] [PubMed] [Google Scholar]
- Hakovirta H, Yan W, Kaleva M, Zhang F, Vanttinen K, Morris PL, Soder M, Parvinen M, Toppari J 1999 Function of stem cell factor as a survival factor of spermatogonia and localization of messenger ribonucleic acid in the rat seminiferous epithelium. Endocrinology 140:1492–1498 [DOI] [PubMed] [Google Scholar]
- Yan W, Linderborg J, Suominen J, Toppari J 1999 Stage-specific regulation of stem cell factor gene expression in the rat seminiferous epithelium. Endocrinology 140:1499–1504 [DOI] [PubMed] [Google Scholar]
- Mauduit C, Hamamah S, Benahmed M 1999 Stem cell factor/c-kit system in spermatogenesis. Hum Reprod Update 5:535–545 [DOI] [PubMed] [Google Scholar]
- Tajima Y, Onoue H, Kitamura Y, Nishimune Y1991 Biologically active kit ligand growth factor is produced by mouse Sertoli cells and is defective in Sld mutant mice. Development 113:1031–1035. [DOI] [PubMed] [Google Scholar]
- Hellani A, Ji J, Mauduit C, Deschildre C, Tabone E, Benahmed M 2000 Developmental and hormonal regulation of the expression of oligodendrocyte-specific protein/claudin 11 in mouse testis. Endocrinology 141:3012–3019 [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ 1997 Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619 [DOI] [PubMed] [Google Scholar]
- Nagata S 1997 Apoptosis by death factor. Cell 88:355–365 [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM 1998 Death receptors: signaling and modulation. Science 281:1305–1308 [DOI] [PubMed] [Google Scholar]
- Pinkoski MJ, Green DR 1999 Fas ligand, death gene. Cell Death Differ 6:1174–1181 [DOI] [PubMed] [Google Scholar]
- Lee J, Richburg JH, Younkin SC, Boekelheide K 1997 The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology 138:2081–2088 [DOI] [PubMed] [Google Scholar]
- Oke BO, Suarez-Quian CA 1993 Localization of secretory, membrane-associated and cytoskeletal proteins in rat testis using an improved immunocytochemical protocol that employs polyester wax. Biol Reprod 48:621–631 [DOI] [PubMed] [Google Scholar]
- Okuma Y, Saito K, O’Connor AE, Phillips DJ, de Kretser DM, Hedger MP 2005 Reciprocal regulation of activin A and inhibin B by interleukin-1 (IL-1) and follicle-stimulating hormone (FSH) in rat Sertoli cells in vitro. J Endocrinol 185:99–110 [DOI] [PubMed] [Google Scholar]
- Okuma Y, O’Connor AE, Muir JA, Stanton PG, de Kretser DM, Hedger MP 2005 Regulation of activin A and inhibin B secretion by inflammatory mediators in adult rat Sertoli cell cultures. J Endocrinol 187:125–134 [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R 2002 Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911–1912 [DOI] [PubMed] [Google Scholar]
- Cobb MH 1999 MAP kinase pathways. Prog Biophys Mol Biol 71:479–500 [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M 2001 Mammalian MAP kinase signalling cascades. Nature 410:37–40 [DOI] [PubMed] [Google Scholar]
- Zhang XS, Zhang ZH, Jin X, Wei P, Hu XQ, Chen M, Lu CL, Lue YH, Hu ZY, Sinha Hikim AP, Swerdloff RS, Wang C, Liu YX 2006 Dedifferentiation of adult monkey Sertoli cells through activation of extracellularly regulated kinase 1/2 induced by heat treatment. Endocrinology 147:1237–1245 [DOI] [PubMed] [Google Scholar]
- Zhang XS, Yan W, Linderborg J, Suominen J, Toppari J Zhang ZH, Guo SH, Yang W, Zhang ZQ, Yuan JX, Jin X, Hu ZY, Liu YX 2006 Activation of extracellular signal-related kinases 1 and 2 in Sertoli cells in experimentally cryptorchid rhesus monkeys. Asian J Androl 8:265–272 [DOI] [PubMed] [Google Scholar]
- Schramek H, Feifel E, Healy E, Pollack V 1997 Constitutively active mutant of the mitogen-activated protein kinase kinase MEK1 induces epithelial dedifferentiation and growth inhibition in madin-darby canine kidney-C7 cells. J Biol Chem 272:11426–11433 [DOI] [PubMed] [Google Scholar]
- Chapin RE, Wine RN, Harria, MW, Borchers CH, Haseman, JK 2001 Structure and control of a cell-cell adhesion complex associated with spermiation in rat seminiferous epithelium. J Androl 22:1020–1052 [DOI] [PubMed] [Google Scholar]
- Sun QY, Breitbart H, Schatten H 1999 Role of the MAPK cascade in mammalian germ cells. Reprod Fertil Dev 11:443–450 [DOI] [PubMed] [Google Scholar]
- Fix C, Jordan C, Cano P, Walker W 2004 Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc Natl Acad Sci USA 101:10919–10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya N, Dufour JM, Vo MN, Okita J, Okita R, Kim KH 2005 Differential effects of phthalates on the testis and the liver. Biol Reprod 72:745–754 [DOI] [PubMed] [Google Scholar]
- Lee NP, Cheng CY 2004 Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: is this a potential target for male contraceptive development? Hum Reprod Update 10:349–369 [DOI] [PubMed] [Google Scholar]
- Brannan CI, Bedell MA, Resnick JL, Eppig JJ, Handel MA, Williams DE, Lyman SD, Donovan PJ, Jenkins NA, Copeland NG 1992 Developmental abnormalities in Steel17H mice result from a splicing defect in the steel factor cytoplasmic tail. Genes Dev 6:1832–1842 [DOI] [PubMed] [Google Scholar]
- Allard EK, Blanchard KT, Boekelheide K 1996 Exogenous stem cell factor (SCF) compensates for altered endogenous SCF expression in 2,5-hexanedione-induced testicular atrophy in rats. Biol Reprod 55:185–193 [DOI] [PubMed] [Google Scholar]
- Blanchard KT, Lee J, Boekelheide K 1998 Leuprolide, a gonadotropin-releasing hormone agonist, reestablishes spermatogenesis after 2,5-hexanedione-induced irreversible testicular injury in the rat, resulting in normalized stem cell factor expression. Endocrinology 139:236–244 [DOI] [PubMed] [Google Scholar]
- Fox RA, Sigman M, and Boekelheide K 2000 Transmembrane versus soluble stem cell factor expression in human testis. J Androl 21:579–585 [PubMed] [Google Scholar]
- Giammona CJ, Sawhney P, Chandrasekaran Y, Richburg JH 2002 Death receptor response in rodent testis after mono-(2-ethylhexyl) phthalate exposure. Toxicol Appl Pharmacol 185:119–127 [DOI] [PubMed] [Google Scholar]
- Ozaki-Kuroda K, Nakanishi H, Ohta H, Tanaka H, Kurihara H, Mueller S, Irie K, Iked, W, Sasaki T, Wimmer E, Nishimune Y, Takai Y 2002 Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol 12:1145–1150 [DOI] [PubMed] [Google Scholar]
- Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, Nagafuchi A, Tsukita S, Takai Y 2000 Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol 150:1161–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, Nishioka H, Aoki J, Nomoto A, Mizoguchi A, Takai Y 1999 Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a P.D.Z. domain-containing protein. J Cell Biol 145:539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobarzo CM, Lustig L, Ponzio R, Denduchis B 2006 Effect of di-(2-ethylhexyl) phthalate on N-cadherin and catenin protein expression in rat testis. Reprod Toxicol 22:77–86 [DOI] [PubMed] [Google Scholar]
- Zhang J, Wong CH, Xia W, Mruk DD, Lee NP, Lee WM, Cheng CY 2005 Regulation of Sertoli-germ cell adherens junction dynamics via changes in protein-protein interactions of the N-cadherin-β-catenin protein complex, which are possibly mediated by c-Src and myotubularin-related protein 2: an in vivo study using an androgen suppression model. Endocrinology 146:1268–1284 [DOI] [PubMed] [Google Scholar]
- Correa LM, Miller MG 2001 Microtubule depolymerization in rat seminiferous epithelium is associated with diminished tyrosination of α-tubulin. Biol Reprod 64:1644–1652 [DOI] [PubMed] [Google Scholar]
- Chen YC, Cochrum RK, Tseng MT, Ghooray DT, Moore JP, Winters SJ, Clark BJ 2007 Effects of CDB-4022 on Leydig cell function in adult male rats. Biol Reprod 77:1017–1026 [DOI] [PubMed] [Google Scholar]