Abstract
Our objective was to evaluate whether excessive brain glycogen deposition might follow episodes of acute hypoglycemia (AH) and thus play a role in the hypoglycemia-associated autonomic failure seen in diabetic patients receiving intensive insulin treatment. We determined brain glucose and glycogen recovery kinetics after AH and recurrent hypoglycemia (RH), an established animal model of counterregulatory failure. A single bout of insulin-induced AH or RH for 3 consecutive days was used to deplete brain glucose and glycogen stores in rats. After microwave fixation and glycogen extraction, regional recovery kinetics in the brain was determined using a biochemical assay. Both AH and RH treatments reduced glycogen levels in the cerebellum, cortex, and hypothalamus from control levels of 7.78 ± 0.55, 5.4 ± 0.38, and 4.45 ± 0.37 μmol/g, respectively, to approximately 50% corresponding to a net glycogen utilization rate between 0.6 and 1.2 μmol/g·h. After hypoglycemia, glycogen levels returned to baseline within 6 h in both the AH and the RH group. However, recovery of brain glycogen tended to be faster in rats exposed to RH. This effect followed more rapid recovery of brain glucose levels in the RH group, despite similar blood glucose levels in both groups. There was no statistically significant increase above baseline glycogen levels in either group. In particular, brain glycogen was not increased 24 h after the last of recurrent episodes of hypoglycemia, when a significant counterregulatory defect could be documented during a hyperinsulinemic hypoglycemic clamp study. We conclude that glycogen supercompensation is not a major contributory factor to the pathogenesis of hypoglycemia-associated autonomic failure.
MODERN INTENSIVE INSULIN therapy of diabetes has led to a significant reduction of long-term complications attributed to hyperglycemia (1,2); however, the major limitation to intensive therapy in type 1 diabetic patients is the risk of hypoglycemia, which results from defective glucose counterregulation and reduced awareness of hypoglycemia (3). Even though these clinical syndromes have been well described over the past years, the underlying mechanisms causing them remain controversial and poorly understood. It is becoming increasingly clear that the brain plays a dominant role in the activation of glucose counterregulatory defense systems and past studies have identified regions within the hypothalamus, the ventromedial hypothalamus in particular, as centers that relay and generate the central response to low blood glucose (4,5,6,7). It has been proposed that the failure to mount an adequate counterregulatory response to hypoglycemia may be due to a discrepancy between systemic and central energy substrate availability. This hypothesis is supported by data showing that direct microinjection of glucose or lactate into the ventromedial hypothalamus of hypoglycemic rats was able to significantly reduce counterregulatory hormone responses (6,8).
One physiologically available energy source that could provide the brain with additional fuel to sustain normal neuronal activity when systemic glucose levels are low and brain glucose has declined is astrocytic glycogen. The potential importance of central nervous system glycogen stores has recently received increased attention. Technical advances that have improved the ability to preserve glycogen during fixation have suggested the existence of much higher glycogen levels than previously anticipated (9,10,11,12). High-powered focused microwave devices used for fast and complete fixation of brain tissue in situ have allowed for subsequent in vitro measurement of the total glycogen pool in a particular region of the brain (13,14,15). Studies using in vivo nuclear magnetic resonance (NMR) spectroscopy have provided dynamic measurements of glycogen levels in humans and rodents under different glycemic conditions and have determined the kinetics of how acute hypoglycemia (AH) depletes whole-brain glycogen stores in an attempt to maintain normal function (16,17,18). These studies suggest that brain glycogen stores begin to decline when brain glucose drops to near undetectable levels (19,20) and that after hypoglycemia, whole-brain glycogen stores markedly increase above baseline levels, so-called supercompensation. A similar increment in brain glycogen is seen after transient brain hypoxia and sleep deprivation (21,22). It was speculated that this increase of brain energy substrate might alter glycemic thresholds for hypoglycemic counterregulation as well as the changes of other brain functions associated with the phenomenon of hypoglycemia-associated autonomic failure, or HAAF (3,19,23).
Some regions of the brain have been shown to be more susceptible to hypoglycemia-induced injury than others. They vary in their contribution to the physiological response to hypoglycemia (24,25). Whether these differences can be correlated to changes in local glucose availability or different glycogen levels in those regions is not clear. Most biochemical analyses of brain glycogen levels were done on whole-brain extracts and did not offer the spatial resolution to answer this question.
In vivo NMR experiments to measure glycogen pool dynamics depend on prior isotopomer labeling that may lead to an underestimation of the absolute glycogen levels (26) and have, furthermore, not provided information about deeper, fuel-sensing regions located within the hypothalamus. The study presented here was undertaken to identify differences in glucose as well as glycogen kinetics among the cerebral cortex, the cerebellum, a part of the brain recently implied to be affected by hypoglycemia (27), and particularly the hypothalamus in the context of acute and recurrent hypoglycemia (RH). Other regions shown to be of importance to glucose counterregulation during hypoglycemia located within the brainstem were not included in this study because of technical limitations during microwaving. Because previous studies have demonstrated a substantial influence of changes in neuronal activity on glucose (28) as well as glycogen levels (29), we studied awake, freely moving and ad libitum-fed rats that were not subject to an infusion of glucose before or after hypoglycemia to prevent inadvertent changes in glucose and glycogen levels, a possible complication of magnetic resonance spectroscopy studies during which animals are anesthetized. We measured brain glycogen levels at the end of a hypoglycemic episode to characterize the decline from baseline and then at 6 h, when supercompensation was shown to occur in rats in vivo analyzed by NMR (19) and in vitro in cultured astrocytes (30). In addition, brain glycogen was measured 24 h after the end of hypoglycemia, a time when the counterregulatory defect to hypoglycemia is usually documented. To confirm that the 3-d RH regimen used in our study would result in a loss of counterregulation we have seen in previous studies (31,32), we determined the epinephrine and norepinephrine responses to hypoglycemia in a parallel group of animals that underwent a hyperinsulinemic hypoglycemic clamp at the same time point when other animals were killed for brain glycogen analysis.
Materials and Methods
Animals
Adult male Sprague Dawley rats (Charles River, Wilmington, MA) of 270–300 g weight were housed and cared for in the Yale Animal Resource Center, fed a standard pellet diet (Prolab 3000; Agway, Syracuse, NY), and maintained on a 12-h light, 12-h dark cycle. Experimental protocols are in accordance with Yale Animal Care and Use Committee guidelines.
AH
After a 1-wk acclimatization period, nonfasted rats were injected on the morning of the day of study, 2 h after the beginning of the light cycle with an ip dose of 10 U/kg human regular insulin (Eli Lilly, Indianapolis, IN). During the ensuing 3-h period of hypoglycemia, food was withheld, and tail vein glucose levels were measured hourly and fell in the targeted range of 30–40 mg/dl, or 1.9 ± 0.27 mmol/liter. The animals had free access to water and were allowed to move around in their cages during that time. Five animals were killed at 0, 6, and 24 h after the end of a single bout of hypoglycemia. Although the first group was killed immediately after the end of hypoglycemia, the other two groups studied 6 and 24 h after hypoglycemia had free access to food during their recovery period.
RH
On the morning of three consecutive days, nonfasted rats were injected ip with regular insulin at a dose of 10 U/kg to produce 3-h periods of sustained hypoglycemia in the same fashion as for the previous group. At the end of each period, the rats were given free access to food. The time points for killing were 0, 6, and 24 h after the hypoglycemic episode on d 3, with n = 7, n = 6, and n = 12 animals per group, respectively. The two animals that did not recover from hypoglycemia and went on to develop a seizure were not included in this study.
Controls
Two groups of control animals received ip 0.9% saline injections on one or three consecutive days, had no access to food for each 3-h episode, and were subsequently killed at the same time points and under the same conditions as described above.
Tissue preparation
After quick anesthesia in a bell jar using a mixture of 30% vol/vol isoflurane in propylene glycol (Sigma Chemical Co., St. Louis, MO), brain tissue was fixed in situ using focused high-energy microwave irradiation (9.5 kW, 1.7 sec). The 30- to 40-mg blocks of tissue from cerebellum, hypothalamus (4-mm-thick coronal slice was cut 2 mm in front of and 2 mm caudal to the optic chiasm) and cortex (bilateral parietal area) were carefully dissected using razor blades and an acrylic rat brain matrix and stored at −80 C until assayed for glycogen content.
Glucose/glycogen
Glucose and glycogen content of brain tissue were determined using a method similar to that described by Cruz and Dienel (29). After weighing and homogenization of the tissue in 0.03 n HCl, background glucose levels were measured. Homogenates were then boiled for 45 min, pH was brought to 4.9 using 3 m sodium acetate buffer, and samples were incubated for 2 h at 37 C with 20 U/ml amyloglycosidase (Roche Diagnostics, Alameda, CA) to release all glucosyl units from glycogen. After neutralization, glucose levels were quantified using an AmplexRed glucose oxidase-based assay (Molecular Probes, Eugene, OR) that was quantified on a Victor3 fluorescence microplate reader (Perkin-Elmer, Wellesley, MA). After subtraction of background glucose levels, glycogen concentrations were calculated and expressed as micromoles per gram wet weight frozen tissue.
Lactate
Aliquots of the glucose extract samples were run on a CMA600 microdialysis analyzer (CMA, North Chelmsford, MA) to rule out postmortem glycogenolysis. Our samples showed lactate concentrations lower than 2.5 μmol/g consistent with complete tissue fixation during the microwaving process (26).
Insulin clamp and catecholamine assay
A group of animals pretreated according to the RH protocol (n = 14) was used to determine the degree of altered ability to counterregulate to hypoglycemia, when compared with control animals (n = 8). During a 90-min hyperinsulinemic-hypoglycemic clamp, a constant infusion of regular human insulin (Eli Lilly, Indianapolis, IN; 50 mU/kg·min) and a variable 20% glucose infusion were used to maintain plasma glucose levels at 45 mg/dl. Blood samples were collected every 30 min for subsequent measurement of catecholamine responses and comparison with controls (n = 8). Plasma catecholamine concentrations were analyzed by HPLC using electrochemical detection.
Statistics
Data were analyzed with SPSS version 12.0. Group comparisons were made using ANOVA with the Dunnett correction for multiple comparisons. Statistical significance was assumed at the P < 0.05 threshold.
Additional post hoc analyses were carried out using the Tukey-Kramer correction. Glycogen recovery rates were calculated as a linear function of the difference in glycogen between the values at the end of the last hypoglycemic episode and at 6 h of recovery.
Results
Brain glucose concentrations after AH and RH
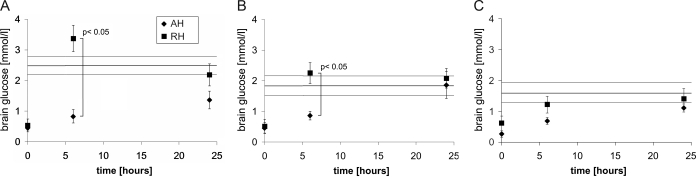
The average brain glucose levels across all regions measured dropped from control levels of 2.01 ± 0.19 μmol/g to statistically significant lower levels by the end of the last hypoglycemic episode in both the AH (0.37 ± 0.06 μmol/g; P < 0.0001) as well as the RH (0.55 ± 0.12 μmol/g; P < 0.0001) group. Brain glucose levels at 6 h after the end of hypoglycemia were significantly different between the two treatment groups with AH vs. RH (0.79 ± 0.09 vs. 2.28 ± 0.29 μmol/g; P = 0.0013). These glucose levels among the groups did reveal a time dependence of the return to baseline values after the end of a hypoglycemic episode that varied depending on the pretreatment with either a single bout or recurrent bouts of hypoglycemia; effective recovery rates during the first 6 h after the last hypoglycemic episode were 0.07 μmol/g in the AH and 0.29 μmol/g in the RH group, which were statistically different from each other (P < 0.0001). Figure 1 shows how these changes can further be attributed to glucose changes in the individual regions that were analyzed: cerebellum, cortex and hypothalamus. The most significant contribution of the faster glucose recovery at 6 h in RH animals vs. the AH group comes from cerebellum (3.37 ± 0.43 μmol/g RH vs. 0.83 ± 0.22 μmol/g AH) and cortex (2.25 ± 0.34 RH vs. 0.86 ± 0.14 AH) and to a smaller extent the hypothalamus (1.22 ± 0.27 RH vs. 0.69 ± 0.11 AH). In contrast, there was no significant difference in brain glucose levels between both groups and controls by 24 h. To further assess the mechanism for the more rapid recovery of brain glucose levels at 6 h, we measured the recovery of plasma glucose levels in catheterized animals from a similar group of rats before the time when brain glucose levels were measured. Plasma glucose levels rebounded quickly and averaged 156 mg/dl in the AH group and 159 mg/dl in the RH group (n = 8 and n = 6). Plasma glucose levels over the 3 h immediately preceding brain glucose measurements at 6 h were 143 and 139 mg/dl for AH and RH, respectively (P = 0.66).
Figure 1.
Recovery of glucose levels in cerebellum (A), cortex (B), and hypothalamus (C) after the end of a single (AH) or three (RH) hypoglycemic episodes. Measurements were made immediately at the end of hypoglycemia and at 6 and 24 h. Error bars indicate ±sem. Control brain glucose levels ± sem are indicated by a continuous solid line.
Brain glycogen depletion by hypoglycemia
Because of the nature of our experiments as endpoint measurements, we were not able to determine baseline glycogen levels intra-individually but instead compared those of a saline-injected control group with all of our other measurements. Comparison of glycogen levels between controls and immediately at the end of a single 3-h episode of AH revealed a statistically significant decrease in glycogen levels in all three regions to approximately 50% of what they were at baseline as shown in Table 1. Resulting effective glycogenolysis rates for 3 h of hypoglycemia are 1.2, 1.1, and 0.6 μmol/g·h in cerebellum, cortex, and hypothalamus, respectively. However, no significant difference in the reduction of glycogen levels could be observed when animals were pretreated with three episodes of RH vs. only one (AH).
Table 1.
Regional brain glycogen concentrations in controls and their depletion after 3 h of AH and RH expressed as micromoles per gram wet weight tissue
| Control levels (μmol/g) | AH (μmol/g) | Significance level (Dunnett) | RH (μmol/g) | Significance level (Dunnett) | |
|---|---|---|---|---|---|
| Cerebellum | 7.78 ± 0.55 (n = 17) | 4.2 ± 0.92 (n = 6) | P = 0.005 | 3.9 ± 0.75 (n = 9) | P = 0.0005 |
| Cortex | 5.4 ± 0.38 (n = 16) | 2.0 ± 0.61 (n = 5) | P = 0.0001 | 2.71 ± 0.5 (n = 9) | P = 0.0003 |
| Hypothalamus | 4.45 ± 0.37 (n = 14) | 2.69 ± 0.57 (n = 6) | P = 0.03 | 2.41 ± 0.53 (n = 7) | P = 0.008 |
Brain glycogen recovery after AH and RH
When animal groups were given free access to food after an AH episode, glycogen levels began to rebound and reached levels not significantly different from baseline within 6 h. A comparison of brain glycogen recovery between animals exposed to AH and RH is shown in Fig. 2.
Figure 2.
Recovery time course of brain glycogen levels after depletion by one acute episode of hypoglycemia (n = 5 for each time point, indicated by diamonds) as well as after RH (n = 10, indicated by squares) in cerebellum (A), cortex (B), and hypothalamus (C). Values are expressed in micromoles per gram wet weight. Error bars indicate ±sem. Control glycogen levels ± sem in cerebellum, 7.78 ± 0.55 μmol/g (n = 17); in cortex, 5.4 ± 0.38 μmol/g (n = 16), and in the hypothalamus, 4.45 ± 0.37 μmol/g (n = 14), are indicated by continuous solid lines.
Unrestrained and with free access to food, both treatment groups showed recovery of glycogen stores back to baseline within 6 h, and levels were not different from sham-injected controls. The faster recovery rate among RH animals showed a strong trend that approached statistical significance (P = 0.056). Lower measured glycogen levels at 6 h in the cortex after AH remain statistically indistinguishable from control levels. Similarly, the accumulation of glycogen in the cortex 6 and 24 h after exposure to RH remain statistically insignificant from the control levels [6.8 ± 0.7 μmol/g (6 h) and 6.8 ± 0.5 μmol/g (24 h) vs. 5.4 ± 0.38 μmol/g (control); P = 0.51 Dunnett and P = 0.26 Dunnett, respectively].
The direct comparison of cortical glycogen levels between the AH and RH group at 24 h after the last episode of hypoglycemia in the cortex (4.04 ± 0.77 vs. 6.8 ± 0.5 μmol/g) showed a trend toward a statistically significant difference with P = 0.0682. None of the other comparisons between areas among AH- and RH-pretreated animals resulted in a statistical difference.
Impaired sympathoadrenal response after RH
To establish that three episodes of insulin-induced hypoglycemia on subsequent days negatively impact the ability to mount a sympathoadrenal response to hypoglycemia, we measured norepinephrine and epinephrine release under hypoglycemic clamp conditions. As expected, the profile of the epinephrine response to hypoglycemia shown in Fig. 3 reveals a delayed peak as well as a reduction by 40% in animals exposed to RH (2026 ± 193 vs. 3369 ± 321 pg/ml; P = 0.01). No statistically significant effect on norepinephrine levels, with 439 ± 95 pg/ml in control and 542 ± 36 pg/ml in RH animals, was observed.
Figure 3.
Epinephrine response during a hyperinsulinemic-hypoglycemic clamp 24 h after the last episode of hypoglycemia. Comparison is between animals exposed to antecedent RH and saline-injected controls (control). Error bars indicate sem.
Discussion
The impairment of glucose counterregulation after recurring episodes of hypoglycemia remains a poorly understood phenomenon that limits effective control of blood glucose with intensified insulin regimens. Because it is recognized that the brain plays a central role in initiation of the counterregulatory response, one potential mechanism could be excessive accumulation of brain glycogen (12). Brain glycogen represents a store of glucosyl units that is able to buffer the high metabolic requirement during sudden bursts of neuronal activity (33,34). This mostly astrocytic pool of energy substrate has further been shown to be available to brain metabolism under conditions of sleep deprivation (22,35,36). The increased availability of glycogen as a source of central energy substrate may be able to sustain normal neuronal function during times when peripheral as well as central glucose becomes otherwise limited (19). This was first suggested when in vivo NMR experiments in rodents exposed to a single episode of hypoglycemia were able to reproduce (3,19) the overshoot in glycogen levels after its depletion during hypoglycemia, a phenomenon that was initially observed in astrocyte cultures (30).
Because different stressors are able to change the observed glycogen concentration in the brain to a significant degree (29), we studied the glycogen store dynamics in animals exposed to 3-h hypoglycemic episodes on three consecutive days, an animal model that exhibits an impaired sympathoadrenal response to hypoglycemia (Fig. 3), much like that seen in humans with counterregulatory failure (32,37). Animals were awake and moving about in their cages without restraint for the entire time of preconditioning, and they had free access to food after each episode of hypoglycemia. The goal was to avoid restraint-related stress, which would increase epinephrine levels, a known stimulus for glycogenolysis, as well as the problems encountered in other studies where anesthetized animals received large amounts of iv glucose before and after hypoglycemia that could interfere with normal glucose homeostasis as well as brain glycogen measurements (18).
Recent biochemical studies of brain glycogen pools in rodents exposed to sleep deprivation have underlined the importance of differentiation between brain regions when reporting regional differences in glycogen depletion across the brain. In one case, cortical glycogen levels were resistant to the sleep deprivation-induced depletion observed in cerebellum and the brainstem (36), and those changes were further found to be mouse strain dependent (35). Furthermore, a recent comparison of the impact of different anesthetic agents on brain glycogen content suggested that local glycogen depletion in superficial structures such as the cortex (mainly observed by in vivo NMR) may not be detected in whole-brain extracts, especially at lower glycogen concentrations (38). To avoid these shortcomings, we increased our spatial resolution by reducing the size of the respective volume of brain tissue to measure the glycogen pool kinetics within three regions that have previously been implicated in altered responses to hypoglycemia, using a highly sensitive biochemical assay. These include the cortex (27), cerebellum (24,27), and the hypothalamus (6,27,39).
Average brain glucose in euglycemic control animals was 2.01 ± 0.19 μmol/g. Baseline levels in the hypothalamus of 1.61 ± 0.33 μmol/g correspond well with previous findings from in vivo NMR studies that measured a larger brain volume (20) and from microdialysis studies of extracellular fluid in smaller areas like the ventromedial nucleus of the hypothalamus (40). We found no significant difference in brain glucose levels between the AH-and the RH-pretreated group at the end of hypoglycemia. However, thereafter, we observed a significantly faster recovery of glucose levels in the cerebellum and cortex of animals exposed to RH. The hypothalamus also showed a similar trend, but this difference did not reach statistical significance. These differences were not accounted for by higher postprandial blood glucose levels in the RH animals before measurement, suggesting increased glucose transporter expression levels or higher blood flow (20). These observations are consistent with previous data from our lab that revealed higher extracellular fluid glucose levels in RH animals during a cognitive challenge (41) as well as data demonstrating increased brain glucose uptake after RH in humans (42).
We then went on to measure brain glycogen content. In the cerebellum, the observed levels were at 7.78 ± 0.51 μmol/g, nearly 2-fold higher than in cortex and hypothalamus. This might be explained by previous reports of a higher level of glucose uptake yet a lower metabolic rate for glucose in the cerebellum compared with other regions of the brain (43,44). In each region, we observed a 50% reduction in those levels after AH. The resulting rates of glycogen utilization of 1.2 μmol/g·h to 0.6 μmol/g·h correspond well with previous observations of 1.5 μmol/g·h for whole brain (26) but point to regional differences that may have different physiological consequences. Exposure to repeated episodes of hypoglycemia much like those encountered during intensive insulin therapy of type 1 diabetic patients (45,46) produced similar depletion kinetics of brain glycogen as was seen after only a single hypoglycemic episode. This suggests that no differential induction of phosphorylase activity occurs and that previous exposure to hypoglycemia neither facilitates nor impairs access to glucose from glycogen during subsequent episodes.
Previous studies in mice have demonstrated that tissue glycogen stores are able to extend the period of normal neuronal activity, when no glucose is available (47). Thus, increasing brain glycogen to higher levels than normal might confer a relative resistance to hypoglycemia-induced neuronal injury and contribute to the altered sympathoadrenal response to AH in animals exposed to RH. To address this possibility, we studied awake animals with free access to food to avoid the potential confounding effect of severe systemic hyperglycemia that may occur in magnetic resonance spectroscopy studies of animals receiving isotopically labeled glucose infusions before and after hypoglycemia. Such high brain glucose levels do not normally occur in awake animals that stop feeding once a sufficient level of satiety has been reached. In the present study, we found that within 6 h after the end of hypoglycemia, brain glycogen concentrations returned to control levels, and we did not observe supercompensation, the severalfold increase of brain glycogen levels beyond baseline. A significant increase in glycogen beyond control levels was not observed in any brain region analyzed at 24 h after the last of three RH episodes, the time when we were able to show a diminished sympathetic response during a hypoglycemic clamp (Fig. 2). There was a small nonsignificant increase in glycogen levels in the cortex, the region predominantly contributing to the typically superficial brain structures measured in NMR experiments that have shown supercompensation in previous studies (19). Deeper brain structures, especially the hypothalamus that contains the centers predominantly associated with the modulation of counterregulatory responses (31,48,49), showed the same glycogen levels in AH and RH groups, suggesting that glycogen content alone cannot be the dominant factor causing the different sympathoadrenal responses.
Although the slope of glycogen recovery between AH and RH did not reach statistical significance (0.12 μmol/g·h AH vs. 0.43 μmol/g·h RH; P = 0.056), there appeared to be a pronounced time dependence of glucose recovery (P < 0.0001) in the cerebellum and cortex that is likely providing the substrate for new glycogen synthesis in the respective area (Fig. 1). This finding supports previous reports of a direct relationship between brain glucose and glycogen levels (18,26). Brain glycogen levels could be artificially increased above baseline levels if only sufficient amounts of glucose were provided. This effect may be the basis for previous reports of brain glycogen supercompensation that was shown in the context of infusion of substantial amounts of isotopically labeled glucose. It could further provide the explanation for the differences between our reported cortical and hypothalamic glycogen levels and a recent study that did observe supercompensation in those two regions. That study used icv injected 2-deoxyglucose to induce local recurrent neuroglycopenia (50), which is known to produce systemic hyperglycemia that in turn could have provided extra substrate for glycogen synthesis.
We conclude that increased deposition of brain glycogen (supercompensation) is not likely to be the dominant factor leading to impaired hormonal counterregulation in response to RH. On the other hand, RH appears to promote glycogen recovery as a result of more rapid restoration of brain glucose levels after hypoglycemia. This may be due to changes in blood-brain barrier transport capacity or regional blood flow.
Acknowledgments
We are grateful to Ralph Jacob, Aida Grozsmann, Andrea Belous, Maya Davis, Wanling Zhu, Ajin Wang, Yuyan Ding, and Xiaoning Fan for their technical support and assistance.
Footnotes
This work was supported by research grants from the National Institutes of Health (DK20495 and P30DK45735) as well as the Juvenile Diabetes Research Foundation Center for the Study of Hypoglycemia at Yale. R.I.H. is the recipient of a Ruth L. Kirschstein National Research Service Award (F32DK077461-01).
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 10, 2008
Abbreviations: AH, Acute hypoglycemia; NMR, nuclear magnetic resonance; RH, recurrent hypoglycemia.
References
- 1997 Hypoglycemia in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. Diabetes 46:271–286 [PubMed] [Google Scholar]
- 1998 Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet [Erratum (1999) 354:602] 352:837–853 [PubMed] [Google Scholar]
- Cryer PE 2004 Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 350:2272–2279 [DOI] [PubMed] [Google Scholar]
- de Vries MG, Lawson MA, Beverly JL 2005 Hypoglycemia-induced noradrenergic activation in the VMH is a result of decreased ambient glucose. Am J Physiol Regul Integr Comp Physiol 289:R977–R981 [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Fan X, Ding Y, Zhu W, Jacob RJ, Sherwin RS 2004 Potential role for AMP-activated protein kinase in hypoglycemia sensing in the ventromedial hypothalamus. Diabetes 53:1953–1958 [DOI] [PubMed] [Google Scholar]
- Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI 1997 Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 99:361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gao J, Yan J, Owyang C, Li Y 2004 Hypothalamus-brain stem circuitry responsible for vagal efferent signaling to the pancreas evoked by hypoglycemia in rat. J Neurophysiol 91:1734–1747 [DOI] [PubMed] [Google Scholar]
- Borg MA, Tamborlane WV, Shulman GI, Sherwin RS 2003 Local lactate perfusion of the ventromedial hypothalamus suppresses hypoglycemic counterregulation. Diabetes 52:663–666 [DOI] [PubMed] [Google Scholar]
- Brown AM, Ransom BR 2007 Astrocyte glycogen and brain energy metabolism. Glia 55:1263–1271 [DOI] [PubMed] [Google Scholar]
- Brown AM 2004 Brain glycogen re-awakened. J Neurochem 89:537–552 [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ 2007 Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55:1251–1262 [DOI] [PubMed] [Google Scholar]
- Gruetter R 2003 Glycogen: the forgotten cerebral energy store. J Neurosci Res 74:179–183 [DOI] [PubMed] [Google Scholar]
- Passonneau JV, Lauderdale VR 1974 A comparison of three methods of glycogen measurement in tissues. Anal Biochem 60:405–412 [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Swanson RA 1987 The regional distribution of glycogen in rat brain fixed by microwave irradiation. Brain Res 417:172–174 [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MM, Sagar SM, Sharp FR 1992 Sensory stimulation induces local cerebral glycogenolysis: demonstration by autoradiography. Neuroscience 51:451–461 [DOI] [PubMed] [Google Scholar]
- Gruetter R 2002 In vivo 13C NMR studies of compartmentalized cerebral carbohydrate metabolism. Neurochem Int 41:143–154 [DOI] [PubMed] [Google Scholar]
- Oz G, Henry PG, Seaquist ER, Gruetter R 2003 Direct, noninvasive measurement of brain glycogen metabolism in humans. Neurochem Int 43:323–329 [DOI] [PubMed] [Google Scholar]
- Choi IY, Tkac I, Ugurbil K, Gruetter R 1999 Noninvasive measurements of [1-13C]glycogen concentrations and metabolism in rat brain in vivo. J Neurochem 73:1300–1308 [DOI] [PubMed] [Google Scholar]
- Choi IY, Seaquist ER, Gruetter R 2003 Effect of hypoglycemia on brain glycogen metabolism in vivo. J Neurosci Res 72:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Lee SP, Kim SG, Gruetter R 2001 In vivo measurements of brain glucose transport using the reversible Michaelis-Menten model and simultaneous measurements of cerebral blood flow changes during hypoglycemia. J Cereb Blood Flow Metab 21:653–663 [DOI] [PubMed] [Google Scholar]
- Brucklacher RM, Vannucci RC, Vannucci SJ 2002 Hypoxic preconditioning increases brain glycogen and delays energy depletion from hypoxia-ischemia in the immature rat. Dev Neurosci 24:411–417 [DOI] [PubMed] [Google Scholar]
- Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD 2002 Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci 22:5581–5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer PE 2006 Hypoglycemia in diabetes: pathophysiological mechanisms and diurnal variation. Prog Brain Res 153:361–365 [DOI] [PubMed] [Google Scholar]
- Agardh CD, Kalimo H, Olsson Y, Siesjo BK 1981 Hypoglycemic brain injury: metabolic and structural findings in rat cerebellar cortex during profound insulin-induced hypoglycemia and in the recovery period following glucose administration. J Cereb Blood Flow Metab 1:71–84 [DOI] [PubMed] [Google Scholar]
- Auer RN 2004 Hypoglycemic brain damage. Metab Brain Dis 19:169–175 [DOI] [PubMed] [Google Scholar]
- Morgenthaler FD, Koski DM, Kraftsik R, Henry PG, Gruetter R 2006 Biochemical quantification of total brain glycogen concentration in rats under different glycemic states. Neurochem Int 48:616–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham EM, Dunn JT, Smith D, Sutcliffe-Goulden J, Reed LJ, Marsden PK, Amiel SA 2005 Differential changes in brain glucose metabolism during hypoglycaemia accompany loss of hypoglycaemia awareness in men with type 1 diabetes mellitus. An [11C]-3-O-methyl-d-glucose PET study. Diabetologia 48:2080–2089 [DOI] [PubMed] [Google Scholar]
- McNay EC, McCarty RC, Gold PE 2001 Fluctuations in brain glucose concentration during behavioral testing: dissociations between brain areas and between brain and blood. Neurobiol Learn Mem 75:325–337 [DOI] [PubMed] [Google Scholar]
- Cruz NF, Dienel GA 2002 High glycogen levels in brains of rats with minimal environmental stimuli: implications for metabolic contributions of working astrocytes. J Cereb Blood Flow Metab 22:1476–1489 [DOI] [PubMed] [Google Scholar]
- Sorg O, Magistretti PJ 1992 Vasoactive intestinal peptide and noradrenaline exert long-term control on glycogen levels in astrocytes: blockade by protein synthesis inhibition. J Neurosci 12:4923–4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon RJ, Fan X, Cheng H, McNay E, Chan O, Shaw M, Ding Y, Zhu W, Sherwin RS 2006 Activation of AMP-activated protein kinase within the ventromedial hypothalamus amplifies counterregulatory hormone responses in rats with defective counterregulation. Diabetes 55:1755–1760 [DOI] [PubMed] [Google Scholar]
- Flanagan DE, Keshavarz T, Evans ML, Flanagan S, Fan X, Jacob RJ, Sherwin RS 2003 Role of corticotrophin-releasing hormone in the impairment of counterregulatory responses to hypoglycemia. Diabetes 52:605–613 [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA 2006 Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab 27:219–249 [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF 2006 Astrocyte activation in working brain: energy supplied by minor substrates. Neurochem Int 48:586–595 [DOI] [PubMed] [Google Scholar]
- Franken P, Gip P, Hagiwara G, Ruby NF, Heller HC 2003 Changes in brain glycogen after sleep deprivation vary with genotype. Am J Physiol Regul Integr Comp Physiol 285:R413–R419 [DOI] [PubMed] [Google Scholar]
- Franken P, Gip P, Hagiwara G, Ruby NF, Heller HC 2006 Glycogen content in the cerebral cortex increases with sleep loss in C57BL/6J mice. Neurosci Lett 402:176–179 [DOI] [PubMed] [Google Scholar]
- Amiel SA, Tamborlane WV, Simonson DC, Sherwin RS 1987 Defective glucose counterregulation after strict glycemic control of insulin-dependent diabetes mellitus. N Engl J Med 316:1376–1383 [DOI] [PubMed] [Google Scholar]
- Lei H, Morgenthaler F, Yue T, Gruetter R 2007 Direct validation of in vivo localized 13C MRS measurements of brain glycogen. Magn Reson Med 57:243–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranston I, Reed LJ, Marsden PK, Amiel SA 2001 Changes in regional brain 18F-fluorodeoxyglucose uptake at hypoglycemia in type 1 diabetic men associated with hypoglycemia unawareness and counter-regulatory failure. Diabetes 50:2329–2336 [DOI] [PubMed] [Google Scholar]
- de Vries MG, Arseneau LM, Lawson ME, Beverly JL 2003 Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes 52:2767–2773 [DOI] [PubMed] [Google Scholar]
- McNay EC, Sherwin RS 2004 Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes 53:418–425 [DOI] [PubMed] [Google Scholar]
- Boyle PJ, Nagy RJ, O’Connor AM, Kempers SF, Yeo RA, Qualls C 1994 Adaptation in brain glucose uptake following recurrent hypoglycemia. Proc Natl Acad Sci USA 91:9352–9356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranston I, Marsden P, Matyka K, Evans M, Lomas J, Sonksen P, Maisey M, Amiel SA 1998 Regional differences in cerebral blood flow and glucose utilization in diabetic man: the effect of insulin. J Cereb Blood Flow Metab 18:130–140 [DOI] [PubMed] [Google Scholar]
- Ratcheson RA, Blank AC, Ferrendelli JA 1981 Regionally selective metabolic effects of hypoglycemia in brain. J Neurochem 36:1952–1958 [DOI] [PubMed] [Google Scholar]
- Cryer PE 1992 Iatrogenic hypoglycemia as a cause of hypoglycemia-associated autonomic failure in IDDM. A vicious cycle. Diabetes 41:255–260 [DOI] [PubMed] [Google Scholar]
- Davis MR, Shamoon H 1991 Counterregulatory adaptation to recurrent hypoglycemia in normal humans. J Clin Endocrinol Metab 73:995–1001 [DOI] [PubMed] [Google Scholar]
- Brown AM, Sickmann HM, Fosgerau K, Lund TM, Schousboe A, Waagepetersen HS, Ransom BR 2005 Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. J Neurosci Res 79:74–80 [DOI] [PubMed] [Google Scholar]
- Kang L, Dunn-Meynell AA, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eiki J, Zhang BB, Levin BE 2006 Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes 55:412–420 [DOI] [PubMed] [Google Scholar]
- Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L 2005 Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 11:320–327 [DOI] [PubMed] [Google Scholar]
- Alquier T, Kawashima J, Tsuji Y, Kahn BB 2007 Role of hypothalamic adenosine 5′-monophosphate-activated protein kinase in the impaired counterregulatory response induced by repetitive neuroglucopenia. Endocrinology 148:1367–1375 [DOI] [PubMed] [Google Scholar]