Abstract
Bone loss resulting from chronic ethanol (EtOH) abuse is frequently accompanied by altered vitamin D3 homeostasis. In the current study, we examined EtOH effects in a female rat model in which control or EtOH-containing diets were infused intragastrically. EtOH treatment reduced plasma 1,25-dihydroxycholecalciferol (1,25 (OH)2 D3) coincident with a decrease in renal CYP27B1 (25(OH)D3 1α-hydroxylase) mRNA and an increase in expression of renal CYP24A1 (1,25 (OH)2 D3- 24-hydroxylase). EtOH induction of CYP24A1 occurred as a result of increased transcription and was also observed in vitro in primary cultures of rat renal proximal tubule cells (RPTCs) and in NRK-52E cells. Synergistic induction of CYP24A1 by EtOH in combination with 1,25 (OH)2 D3 was observed. The major EtOH metabolizing enzymes, alcohol dehydrogenase-1 and CYP2E1, were induced by EtOH in RPTCs. Inhibition of EtOH metabolism by 4-methylpyrazole inhibited the induction of CYP24A1 mRNA. CYP24A1 mRNA induction in RPTCs was also inhibited by the protein synthesis inhibitor cycloheximide. CYP24A1 was also induced after hydrogen peroxide treatment, and EtOH treatment of RPTCs resulted in production of reactive oxygen species as measured by flow cytometry using the fluorescent probe dichlorofluorescin acetate. In addition, inhibition of MAPK signaling pathways with the MAPK kinase inhibitor U0126 or the p38 inhibitor SB203580 inhibited EtOH induction of CYP24A1. Our data suggest that EtOH reduces circulating 1,25 (OH)2 D3 concentrations as the result of CYP24A1 induction that is mediated via MAPK activation resulting from renal oxidative stress produced by local metabolism of EtOH via CYP2E1 and antidiuretic hormone-1.
CHRONIC CONSUMPTION of ethanol (EtOH) is an important risk factor in the development of osteoporosis in both men and women, and results in reduced bone mineral density and increased fracture risk (1). Although moderate EtOH consumption has increased bone density (2), it is clear that EtOH has direct effects on bone cells resulting in both inhibition of osteoblastogenesis (3,4,5,6) and stimulation of osteoclast differentiation and activation (7,8,9); indirect effects on the skeleton also occur as the result of EtOH-induced endocrine disruption. Previously, we have shown that EtOH suppresses plasma estradiol in cycling animals, contributing to increased bone resorption (10) and that chronic EtOH treatment interferes with the GH/IGF-I axis, which is known to stimulate bone formation (11,12,13,14). However, an additional consequence of both acute and chronic alcohol consumption is hypocalcemia (15,16,17,18). This appears to be the result of EtOH effects on the homeostasis of mineral regulating hormones and in particular vitamin D3, which functions to stimulate intestinal absorption of calcium in addition to EtOH-mediated inhibition of intestinal calcium transport independent of vitamin D (15,19). The active form of vitamin D3, 1,25-dihydroxycholecalciferol (1,25 (OH)2 D3), is synthesized primarily in the kidney by the mitochondrial cytochrome P450-dependent monooxygenase 25-hydroxycholecalciferol (25 (OH) D3)-1α-hydroxylase (CYP27B1) from its precursor 25 (OH) D3 (20). A second mitochondrial P450-dependent monooxygenase, CYP24A1, also found mainly in the kidney is responsible for inactivation of 1,25 (OH)2 D3 by a series of successive oxidation and carbon-carbon bond cleavage reactions beginning with C24 oxidation (21).
The plasma concentrations of 1,25 (OH)2 D3 are tightly regulated as the result of feedback loops controlling renal CYP27B1 and CYP24A1 expression (22). A major feedback loop involves down-regulation of CYP27B1 and up-regulation of CYP24A1 by 1,25 (OH)2 D3 itself (22,23,24). Chronic EtOH abuse has resulted in reduced plasma 1,25 (OH)2 D3 in some studies of alcoholics (25,26,27), and similar reductions of plasma 1,25 (OH)2 D3 have been reported in some animal models of chronic EtOH exposure (28,29,30,31). In contrast, other studies have observed no changes in vitamin D3 metabolites after short-term feeding of EtOH for 3 wk (32). One early report in chickens demonstrated that EtOH decreased activity of renal CYP27B1 and increased the activity of CYP24A1 (33), and at least one report suggested decreased CYP27B1 activity in rats fed EtOH for 4 wk (34). However, the molecular mechanisms underlying the effect of EtOH on vitamin D3 metabolism remain unclear.
In the current study, we investigated the effects of EtOH on renal CYP27B1 and CYP24A1 expression in a model in which EtOH is administered chronically to female rats as part of liquid diets via intragastric infusion during the dark phase of the lighting cycle when the animals are normally awake and eating. This total enteral nutrition (TEN) model overcomes the reduced food intake caused by rodent aversion to EtOH, and provides complete control of caloric intake, EtOH dose, and diet composition. In addition, overnight infusion allows us to mimic human consumption patterns without compromising normal sleep cycles. We have previously shown that chronic EtOH treatment in this model significantly reduces serum 1,25 (OH)2 D3 concentrations under a number of physiological conditions (30,31). We examined renal CYP27B1 and CYP24A1 expression in these animals, and regulation of CYP24A1 expression by EtOH, 1,25 (OH)2 D3, and hydrogen peroxide (H2O2) in vitro in both rat renal proximal tubule cells (RPTCs) and in the renal NRK-52E cell line. The current data suggest that EtOH reduces plasma 1,25 (OH)2 D3 as a result of reduced synthesis by CYP27B1 and increased degradation via CYP24A1. Induction of CYP24A1 appears to involve increased sensitization to feedback by 1,25 (OH)2 D3 as a consequence of renal oxidative stress produced by EtOH metabolism and subsequent activation of MAPK pathways.
Materials and Methods
Animals and experimental treatments
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. Time-impregnated female Sprague Dawley rats (250–300 g) and age-matched nonpregnant controls were obtained from Charles River Laboratories (Wilmington, MA). Animals were housed in an Association Assessment and Accreditation of Laboratory Animal Care approved animal facility. Pregnant and cycling rats were intragastrically cannulated and infused for 15 d with either control or EtOH-containing diets at a dose of 13 g/kg/d as described previously (30,35). In addition, in a second study designed to examine vitamin D3 homeostasis during the anabolic phase of bone rebuilding in dams after weaning, time-impregnated rats were surgically cannulated on gestational d 12, and had ad libitum access to commercial rodent chow (Harlan Teklad 8640; Harlan Sprague Dawley Inc., Indianapolis, IN) and water until their offspring were weaned. At birth, litters were culled to five male and five female pups per dam, and litter weights were equalized. Lactation continued until postnatal d (PND) 17, at which time the pups were weaned and the dams fed either control or EtOH diets at 13 g/kg/d via TEN. Body weight gains, alterations in bone density, and biochemical parameters in female cycling, pregnant, and post-weaning rats exposed to EtOH in these studies have been published previously (30,31,36). Diets were made isocaloric by substituting EtOH for carbohydrate calories and met caloric and nutritional guidelines recommended for rodents by the National Research Council. Twenty-four-hour urine EtOH concentrations (UECs) were measured daily using an Analox Instruments GL5 Analyzer fitted with an amperometric electrode sensor (Analox Instruments Ltd., London, UK). After 1- or 4-wk diet infusion after weaning, seven to nine rats per group were euthanized under anesthesia, and trunk blood was collected. Detailed analyses of bone density (peripheral quantitative computerized tomography (pQCT) and micro-CT, μCT), static and dynamic histomorphometry, and biochemical analyses have been recently published (31,36). Serum was prepared and stored at −20 C until analysis. Kidneys were excised, snap frozen in liquid nitrogen, and stored at −70 C for RNA extraction and apoprotein analysis (12).
Renal cell cultures
RPTCs were prepared from control ad libitum chow-fed female rats and cultured as described previously (37). Briefly, kidneys were aseptically dissected, and renal cortices were minced and treated with collagenase (1 mg/ml) in Hanks’ buffered solution for 30 min. Large undigested pieces were allowed to settle, and the resulting supernatant of cells was mixed with 10% horse serum in Hanks’ buffered solution. Cell pellets obtained after centrifugation (500 g for 7 min at room temperature) were washed once with DMEM and then suspended in growth media for plating. Cells were grown in DMEM containing 4 mm glutamine, 5 μg/ml transferrin, 5 μg/ml insulin, 50 nm hydrocortisone, 100 U/ml penicillin, 10 mg/ml streptomycin, 1 mg/ml glucose, and 10% fetal bovine serum. Cells were allowed to grow for 5 d before respective treatments. In addition, in some experiments the rat kidney epithelial cell line, NRK-52E, was used. NRK-52E cells were obtained from Dr. Alexi G. Basnakian (University of Arkansas for Medical Sciences) and cultured under previously described conditions with DMEM containing 10% fetal bovine serum, 100 U/ml penicillin, and 10 mg/ml streptomycin. Cells were treated with 1–100 mm EtOH, 0.1–10 nm 1,25 (OH)2 D3 alone or in combination with EtOH for 12–24 h. In some experiments, RPTCs were preincubated for 60 min with 5 μg/ml of the protein synthesis inhibitor cycloheximide, 100 μm of the alcohol dehydrogenase (ADH)-1/CYP2E1 inhibitor 4-methylpyrazole (4-MP), 10 μm of the MAPK kinase (MEK1)/MEK2/MEK5 inhibitor U0126, or 50 μm of the p38-MAPK inhibitor SB203580. In addition, NRK-52E cells were exposed to H2O2 at concentrations of 1–500 μm for 24 h. All cell culture experiments were conducted in triplicate.
Serum vitamin D metabolites
Serum 25 (OH) D3 and 1,25 (OH)2 D3 concentrations were measured radioimmunometrically using commercially available RIA kits from DiaSorin Inc. (Stillwater, MN).
Analysis of gene expression
Total RNA was isolated from kidney using TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH) according to the manufacturer’s recommendation. Additional RNA cleanup and DNAse digestion was performed using RNeasy mini columns (QIAGEN, Inc., Valencia, CA). RNA quality was ascertained spectrophotometrically (ratio A260/A280) and also by checking the ratio of 18S to 28S RNA using the RNA NanoChip on a 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Total RNA (1 μg) was reverse transcribed using IScript cDNA synthesis (Bio-Rad Laboratories, Hercules, CA) according to standard protocols (12). Subsequent real-time PCR analysis used the 2× SYBR green master mix and was monitored on an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA). Gene-specific primers were: CYP27B1 forward primer, CTGTGAACCCTGGTCCTATG, and reverse primer, CTTCTTTGATCACCAGCCTTTAGCA; CYP24A1 forward primer, CCTTTAGGTCT-TGGCTGTTTCT, and reverse primer, AAGGACCACTTGTTCAGCTCACT; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward primer, TGAGGTGACCGCATCTTCTTG, and reverse primer, TGGTAACCA-GGCGTCCGATA; ADH-1 forward primer, CACCAAACCCATCCAGGAAGT, and reverse primer, GCATGCTGAATGGCAGCTTAA; and CYP2E1 forward primer, GGAACATTTTTCAGCTGGATTTG, and reverse primer, TTGTGGTTCAGTAGCACCTCCTT. They were designed using Primer Express Software (Applied Biosystems). The relative amounts of gene expression were quantitated using a standard curve according to the manufacturer’s instructions. Gene expression was normalized using the expression of the GAPDH mRNA. Intron-specific primers for CYP24A1 (forward primer, TTGGGATGAGGGAGAATCTCA, and reverse primer, TGGGCAAGATTTAACCCTAACG) were designed using Primer Express Software (Applied Biosystems) and were used in RT-PCR analysis of CYP24A1 heterogeneous nuclear RNA (hnRNA) expression. CYP24A1 hnRNA expression was normalized using the expression of the GAPDH mRNA.
Analysis of CYP24A1 apoprotein expression
Polyclonal antirat CYP24A1 antibodies were raised in rabbits using the CYP24A1-specific amino acid sequence: AQRRLLQEVQSVLP (amino acids 348 to 361, NP_963966) linked to keyhole limpet hemocyanin by Biosynthesis (Lewisville, TX). Western blots were performed on whole kidney homogenates. Ten micrograms of total protein were separated by SDS-PAGE and transferred to nitrocellulose membranes. After blocking with 5% milk, blots were incubated with primary antibody, followed by horseradish peroxidase-linked goat-antirabbit secondary antibodies (1:4000; Sigma Chemical Co., St. Louis, MO) for 2 h, respectively, at room temperature and cross-reactive protein visualized using chemiluminescence (West Pico Supersignal; Pierce, Rockford, IL). Equivalent protein loading was verified in parallel SDS-PAGE gels stained for total protein with Coomassie blue. Western blots were quantified by densitometric scanning using Quantity One software (Bio-Rad Laboratories). Specificity of the CYP24A1 antibody was verified by molecular mass of the recognized protein band approximately 59 kDa and induction by 0.1 nm 1,25 (OH)2 D3 in RPTCs. Immunostaining for CYP24A1 in RPTCs was performed using fixed cells after treatment with or without 100 mm EtOH for 24 h. Cells were grown on coverslips in six-well plates and fixed using 10% buffered formalin for 15 min after treatment with 100 mm EtOH, blocked with normal goat serum in 2% BSA-PBS for 30 min, and then reacted with the rabbit anti-CYP24A1 antibody for 60 min. After three washes in PBS, cells were incubated with biotinylated goat-antirabbit IgG, which was then labeled with streptavidin-conjugated Alexa 488 (1:200 for 20 min; Molecular Probes, Carlsbad, CA) and counterstained with 4′,6-diamidino-2-phenylindole (Molecular Probes). Sections were visualized using a Zeiss Axiovert 200M (Carl Zeiss Inc., Thornwood, NY) inverted fluorescence microscope.
Flow cytometric measurement of reactive oxygen species (ROS)
Generation of ROS in NRK-52E cells after EtOH treatment was detected by flow cytometry using the cell-permeable dye 2,7-dichlorodihydrofluorescein diacetate (DCF-DA) (Sigma-Aldrich, St. Louis, MO), which becomes fluorescent upon reaction with ROS. DCF-DA was dissolved in dimethyl sulfoxide at a stock concentration of 50 mm. NRK-52E cells were pretreated with 10 μm DCF-DA for 30 min before the treatment with 100 mm EtOH. The cells were continuously treated for 24 h and then washed three times with PBS before they were harvested. Washed cells were suspended in 500 μl PBS and kept on ice until flow cytometric analysis. ROS measurement was immediately performed using FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) with 488-nm excitation. The signals were obtained using a 530-nm band-pass filter for DCF-DA. Each determination was based on the mean fluorescence intensity of at least 5000 cells.
Data and statistical analysis
Data are presented as means ± sem. Comparison between multiple groups was accomplished by either the Student’s t test or one-way ANOVA, followed by Student-Newman-Keuls post hoc analyses. The effect of EtOH and 1,25 (OH)2 D3, and the interaction thereof were determined at each time point or dose of 1,25 (OH)2 D3 using two-way ANOVA, followed by Student-Newman-Keuls post hoc analyses. Statistical significance was set at P < 0.05. SigmaStat software package version 3.0 (Systat Software, Inc., San Jose, CA) was used to perform all statistical tests.
Results
EtOH intake reduces serum 1,25 (OH)2 D3 and expression of renal CYP27A1 and increased expression of renal CYP24A1
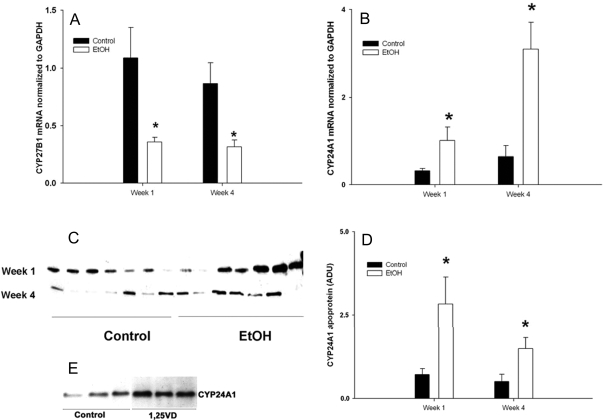
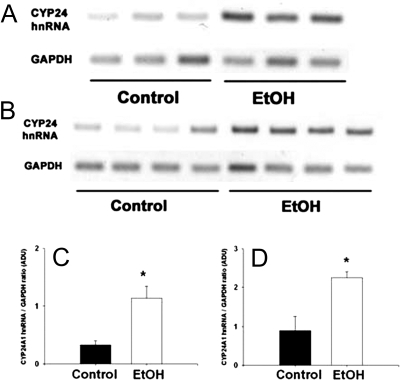
EtOH consumption by cycling female rats, pregnant females, and dams during the post-weaning period using the TEN model resulted in no differences in growth rate compared with controls and average UECs of 150–300 mg/dl (30,31,36). We have previously demonstrated that UECs mirror blood EtOH concentrations (BECs) as a result of EtOH equilibration with body water (11,35,38). These concentrations are readily attainable in human alcoholics (39). Under multiple physiological conditions examined such as cycling, pregnant, or after lactation, EtOH-treated female rats had reduced bone mineral density (30,31,36) and reduced plasma concentrations of 1,25 (OH)2 D3 (P < 0.05). In contrast, serum 25 (OH) D3 was either increased (P < 0.05) or unchanged (Table 1). Analysis of renal CYP27A1 mRNA expression from control and EtOH-treated dams at 1 and 4 wk after weaning by real-time RT-PCR demonstrated a 50% decrease in EtOH-treated rats (P < 0.05) (Fig. 1A), whereas CYP24A1 mRNA expression in the same samples demonstrated a substantial 4- to 6-fold increase (Fig. 1B). Induction of CYP24A1 in the EtOH-treated group (P < 0.05) was verified at the level of apoprotein expression in kidney homogenates by Western blotting with a rabbit polyclonal antibody raised against a peptide sequence specific for rat CYP24A1 (Fig. 1, C and D). The specificity of this antibody for CYP24A1 was verified by recognition of a protein band of the correct molecular mass (∼59 kDa) present in whole kidney lysates, but not found in liver lysates. Furthermore, we found increased expression of this band in Western blots of RPTC cell lysates treated with 0.1 nm 1,25 (OH)2 D3 for 24 h (P < 0.05; Fig. 1E). In addition, expression of renal CYP24A1 hnRNA in young rats fed EtOH-containing diets for 1 or 4 wk revealed an approximate 3.3- and 2.5-fold increase, respectively (P < 0.05). The data shown in Fig. 2 indicate that induction of CYP24A1 mRNA by EtOH treatment occurred at the level of increased gene transcription.
Table 1.
Serum concentrations of vitamin D3 metabolites in EtOH-fed female rats in varying physiological states
| 25 (OH) vitamin D3 (ng/ml)
|
1, 25 (OH)2 vitamin D3 (pg/ml)
|
|||
|---|---|---|---|---|
| Control | EtOH | Control | EtOH | |
| Cycling female | 28.2 ± 3.7 | 28.5 ± 2.4 | 86.3 ± 8.7 | 42.7 ± 10.4a |
| Pregnant female | 11.2 ± 0.9 | 17.8 ± 1.6a | 143.7 ± 8.0 | 82.4 ± 5.9a |
| After weaning | 11.8 ± 0.4 | 18.1 ± 1.1a | 84.4 ± 7.8 | 16.8 ± 3.5a |
Data were obtained from cycling, pregnant, or post-weaning rats fed either 0 (nonpregnant, n = 5; pregnant, n = 12; post-weaning, n = 8) or 13 g/kg·d EtOH (nonpregnant, n = 4; pregnant, n = 20; post-weaning, n = 8) as part of liquid diets via total enteral nutrition. Pregnant rats or weight-matched cycling females were fed diets from gestation d 4–20 (or an equivalent duration in cycling females). Post-weaning female rats were fed control or EtOH diets from PND 17 for a period of 2 wk. Serum levels of 25 (OH) D3 and 1,25 (OH)2 D3 were determined using RIA. For comparisons between control and EtOH treatments, statistical differences were determined using the Student’s t test.
P < 0.05 compared with control animals.
Figure 1.
Renal CYP27B1 mRNA (A), CYP24A1 mRNA (B), and CYP24A1 apoprotein (C), and densitometric quantitation of CYP24A1 protein levels (D) from post-weaning rats fed control or EtOH-containing liquid diets via TEN. Control or EtOH-containing diets were intragastrically infused to groups of female rats infused with either control or EtOH (13 g/kg·d) containing diets from PND 17 as described in Materials and Methods. Gene expression of CYP27B1 and CYP24A1 was monitored using real-time RT-PCR and normalized GAPDH mRNA. Western blots of CYP24A1 (seven per group per time point) were performed in whole tissue homogenates using procedures described in Materials and Methods. E, Immunoblots of CYP24A1 apoprotein expression from whole cell lysates of RPTCs treated with vehicle or 1,25 (OH)2 D3 (0.1 nm) for 24 h. Each lane represents an individual sample. Statistical differences were determined using the Student’s t test. *, P < 0.05 compared with control (non-EtOH) rats at the same time point. The number of rats was nine, seven, six, and seven for control and EtOH-fed rats at wk 1 and 4, respectively.
Figure 2.
hnRNA expression of renal CYP24A1 at 1 (A and C) and 4 (B and D) wk after consumption of either control or EtOH-containing diets. Control or EtOH-containing diets were intragastrically infused to groups of female rats infused with either control or EtOH (13 g/kg·d) containing diets from PND 17 as described in Materials and Methods. hnRNA expression was monitored using RT-PCR and normalized GAPDH mRNA. Each lane represents an individual animal. Data are represented as means ± sem. Statistical differences were determined using the Student’s t test. *, P < 0.05 compared with control (non-EtOH) rats at the same time point.
CYP24A1 expression is up-regulated by in vitro EtOH treatment of rat RPTCs
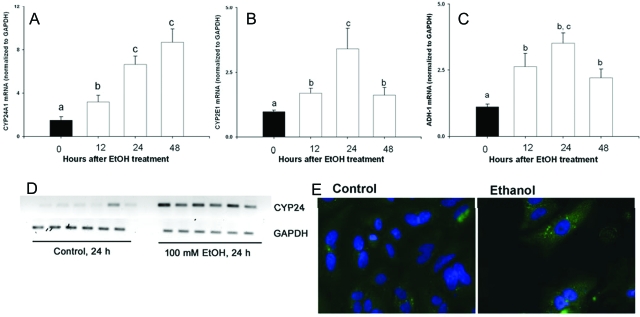
To determine if EtOH-mediated induction of CYP24A1 occurs as a consequence of direct EtOH action on renal tubule cells, we used RPTCs as an in vitro assay system. Treatment of RPTCs with 1–100 mm EtOH for 12–48 h resulted in significant increases in expression of CYP24A1 mRNA as determined by real-time RT-PCR. Expression of CYP24 mRNA/GAPDH mRNA of control was 1.0 ± 0.11, 1 mm EtOH was 2.7 ± 0.44 (P < 0.05), and 10 mm EtOH was 4.2 ± 1.2 (P < 0.05), compared with a 7- to 8-fold increase observed after exposure to 100 mm EtOH for 24 h (P < 0.05) (Fig. 3A). This was accompanied by increased gene expression of the major EtOH metabolizing enzymes, CYP2E1 and ADH-1, in RPTCs after EtOH exposure (Fig. 3, B and C; P < 0.05). Consistent with in vivo findings, the in vitro expression of CYP24A1 hnRNA (P < 0.05; Fig. 3D) and CYP24A1 apoprotein expression as determined by fluorescence immunohistochemistry were also increased after EtOH treatment (P < 0.05; Fig. 3E).
Figure 3.
Gene expression of CYP24A1 (A), CYP2E1 (B), and ADH-1 (C) mRNA in RPTC cultures treated with either 100 mm EtOH for 0, 12, 24, or 48 h. Expression of mRNA was assessed using real-time RT-PCR, and data were normalized to expression of GAPDH mRNA (n = 6 per group per time point). D, CYP24A1 hnRNA and GAPDH mRNA assessed in cells treated with either vehicle or 100 mm EtOH for 24 h as described in Materials and Methods using intron-specific primers resulting in an amplicon of 150 bp. E, Immunostaining for CYP24A1 apoprotein in RPTCs treated with either vehicle or 100 mm EtOH for 24 h as described in Materials and Methods. Data are represented as means ± sem. Differing superscripts indicate significant differences by one-way ANOVA, followed by all pair-wise comparison using the Student-Newman-Keuls method (P < 0.05). For comparisons between control and EtOH treatments, statistical differences were determined using the Student’s t test. *, P < 0.05 compared with vehicle-treated cells at the same time point.
CYP24A1 mRNA expression is induced synergistically by EtOH and 1,25 (OH)2 D3 in RPTCs and NRK-52E cells
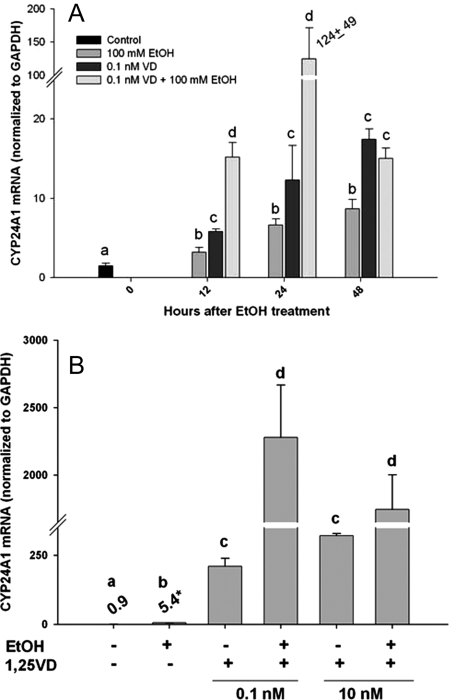
1,25 (OH)2 D3 is one of the most potent inducers of CYP24A1 gene transcription. To investigate whether the constitutive sensitivity to induction of CYP24A1 by 1,25 (OH)2 D3 is altered by EtOH, we performed experiments of cotreatment of RPTCs with or without 100 mm EtOH and 0.1 nm 1,25 (OH)2 D3, and monitored CYP24A1 gene expression. As expected, both EtOH and 1,25 (OH)2 D3 alone resulted in an approximate 8- to 10-fold induction of gene expression at 24 h (P < 0.05; Fig. 4A). However, the combination of EtOH and 1,25 (OH)2 D3 led to a remarkable synergistic increase (∼125-fold at 24 h) in CYP24A1 mRNA expression, which was much greater than either agent alone or in addition (Fig. 4A). To examine if a similar synergism in 1,25 (OH)2 D3-mediated induction of CYP24A1 was observed in other renal cells, we conducted studies using the renal epithelial NRK-52E cell line. As reported previously, NRK-52E cells are highly responsive to 1,25 (OH)2 D3-mediated CYP24A1 induction (40). Similar to the findings from the RPTCs, EtOH alone led to an approximate 6-fold increase in CYP24A1 mRNA, 24 h after treatment. At both doses of 1,25 (OH)2 D3 tested in the experiments, CYP24A1 mRNA was highly increased. Furthermore, cotreatment of EtOH and 1,25 (OH)2 D3 (0.1 nm) led to an approximate 2200-fold induction of CYP24A1 mRNA at 24 h, suggesting a remarkable transcriptional synergism (P < 0.001; Fig. 4B).
Figure 4.
Gene expression of CYP24A1 mRNA in RPTCs (A) or NRK-52E cells (B) after treatment with vehicle or EtOH (100 mm) with or without 1,25 (OH)2 D3 (0.1 or 10 nm). RPTCs were treated for 0, 12, 24, or 48 h, whereas NRK-52E cells were treated for 24 h. Treatments were performed at least in triplicate, whereas six for EtOH treatments at each time point. Expression of CYP24A1 mRNA was normalized to expression of GAPDH mRNA. Data are represented as means ± sem. The effect of EtOH and 1,25 (OH)2 D3, and the interaction thereof were determined at each time point or dose of 1,25 (OH)2 D3 using two-way ANOVA, followed by Student-Newman-Keuls post hoc analyses. Differing superscripts indicate significant differences (P < 0.05).
Induction of CYP24A1 mRNA by EtOH in RTPCs requires de novo protein synthesis and is dependent on EtOH metabolism
To investigate further the underlying mechanisms of EtOH-mediated induction of CYP24A1, we used the protein synthesis inhibitor, cycloheximide, in RPTCs. Increasing doses of EtOH from 50–100 mm for 24 h revealed almost maximal induction of CYP24A1 mRNA expression at the lowest dose (P < 0.05; Fig. 5). Pretreatment with cycloheximide almost completely abolished EtOH induction of CYP24A1 mRNA, suggesting the requirement of new protein synthesis (P < 0.05). In addition, pretreatment with the ADH-1/CYP2E1 inhibitor 4-MP also blunted EtOH induction of CYP24A1 mRNA expression (P < 0.05; Fig. 5), suggesting that EtOH metabolism might be required for maximal induction of CYP24A1.
Figure 5.
Gene expression of CYP24A1 mRNA in RPTCs after treatment with vehicle or EtOH (50, 75, or 100 mm), with or without cycloheximide (CHX) (5 μg/ml) or 4-MP (100 μm). RPTCs were treated for 24 h with EtOH. Treatments were performed with at least five for treatments. Expression of CYP24A1 mRNA was normalized to expression of GAPDH mRNA. Data are represented as means ± sem. The effect of EtOH and cycloheximide or 4-MP, and the interaction thereof were determined using two-way ANOVA, followed by Student-Newman-Keuls post hoc analyses. Differing superscripts indicate significant differences (P < 0.05).
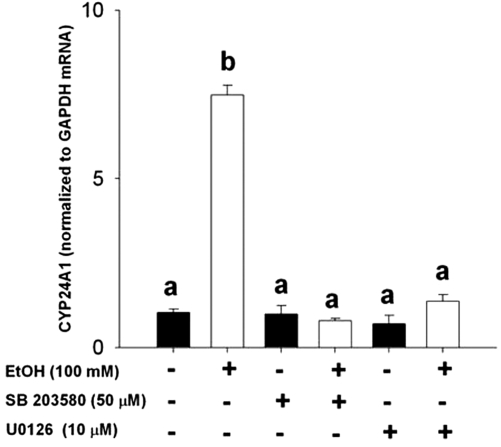
EtOH induction of CYP24A1 mRNA requires MAPK activation
Several recent studies have implicated alterations in MAPK signaling in mediating EtOH effects in a variety of tissues (41). In addition, CYP24A1 gene expression has been suggested to be affected by MAPK activation. To determine if EtOH-mediated induction of CYP24A1 mRNA involves MAPKs, we used chemical inhibitors of MEK1/2/5 and p38-MAPK. Cotreatment of RPTCs with either the MEK1/MEK2/MEK5 inhibitor U0126 or with the p38 inhibitor SB203580 resulted in the complete loss of induction of CYP24A1 mRNA expression after 24-h treatment with EtOH (P < 0.05; Fig. 6), implicating a potentially important mechanistic role of MAPK in mediating this effect.
Figure 6.
Gene expression of CYP24A1 mRNA in RPTCs after treatment with vehicle or EtOH, with or without MEK1/2 inhibitor U0126 (10 μm) or p38-MAPK inhibitor SB203580 (50 μm). RPTCs were treated for 2 h with respective inhibitors, before 24 h treatment with EtOH (100 mm). Treatments were performed with at least five. Expression of CYP24A1 mRNA was normalized to expression of GAPDH mRNA. Data are represented as means ± sem. The effect of EtOH and U0126 or SB203580, and the interaction thereof were determined using two-way ANOVA, followed by Student-Newman-Keuls post hoc analyses. Differing superscripts indicate significant differences (P < 0.05).
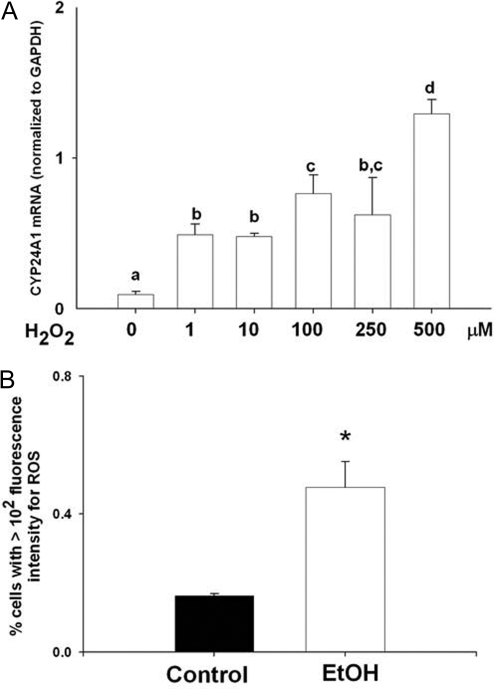
Cellular oxidative status affects CYP24A1 gene expression
Chronic EtOH intake results in production of ROS in several cell types. ROS modulate the activity of many signal transduction pathways, including MAPK. Treatment of NRK-52E cells with increasing concentrations of H2O2 (1–500 μm) to produce oxidative stress resulted in dose-responsive induction of CYP24A1 mRNA expression (Fig. 7A; P < 0.05). In addition, we examined whether treatment with EtOH led to intracellular ROS formation. Treatment of NRK-52E cells with 100 mm EtOH for 24 h resulted in increased generation of ROS (Fig. 7B; P < 0.05).
Figure 7.
A, Gene expression of CYP24A1 mRNA in RPTCs after treatment with vehicle or H2O2 (1–500 μm) for 24 h. All treatments were performed in triplicates. Expression of CYP24A1 mRNA was normalized to expression of GAPDH mRNA. B, Flow cytometric analyses of ROS generation in RPTCs treated with EtOH (100 mm) for 24 h using DCF-DA fluorescence. ROS generation was estimated in triplicate by measuring florescence intensity from at least 5000 cells, using FACSCalibur flow cytometer with 488 and 530-nm excitation and emission wavelengths, respectively. Data are represented as means ± sem. Differing superscripts indicate significant differences by one-way ANOVA, followed by all pair-wise comparison using the Student-Newman-Keuls method (P < 0.05). For comparisons between control and EtOH treatments, statistical differences were determined using the Student’s t test. *, P < 0.05 compared with vehicle-treated cells.
Discussion
The vitamin D hormone system plays an important role in a diverse range of cellular actions, including maintaining mineral and skeletal homeostasis, cell proliferation and differentiation, and modulation of immune responses and development (42,43). As a result, disruption of vitamin D homeostasis has been observed in and may be causally linked to a variety of pathological conditions, including osteoporosis, certain cancers, and other immune disorders. Chronic consumption of alcohol continues to result in substantial morbidity, and significantly increases the risk of liver disease, osteopenia, and cancer among other diseases. Therefore, it is reasonable to consider that direct effects of EtOH leading to disruption of vitamin D homeostasis may play a role in these pathologies. In the current study, we demonstrate that chronic EtOH consumption by female rats results in significantly reduced serum 1,25 (OH)2 D3 levels, independent of physiological status. EtOH levels attained in the present studies are in the physiologically relevant range. For example, EtOH levels in the post-weaning rats (170 mg/dl, 37 mm) are moderately high, being approximately two times greater than the level considered legally intoxicated in most states but are easily attainable in human alcoholics (39). In the intragastric infusion model, BECs and UECs mirror each other as EtOH equilibrates with body water, and monitoring UECs is an accurate, convenient, and noninvasive method of tracking BECs (11,38). Thus, the results reported here are considered applicable to chronic drinkers. However, considering that the rat requires relatively greater EtOH concentrations for most reported biological effects and effects of EtOH on CYP24 mRNA expression in vitro were observed at EtOH concentrations as low as 1 mm, it is easily conceivable that the effects reported here are relevant to women who consume alcohol at levels much lower than those required to achieve these high BECs.
Alterations in circulating 1,25 (OH)2 D3 levels were coincident with down-regulation of CYP27B1 and up-regulation of CYP24A1 in the kidney. These data are consistent with previous reports of the inhibitory effects of EtOH treatment on renal 25 (OH) D3 1α-hydroxylase activity in chickens and rats (33,34), and inductive effects of EtOH treatment on 1,25 (OH)2 D3 24-hydroxylase activity in chickens (33). We have focused on the regulation of renal CYP24A1 by EtOH and demonstrated for the first time that EtOH induction of CYP24A1 is mediated at the level of gene transcription because steady-state renal hnRNA expression was increased in addition to mRNA and apoprotein. We have also shown that the effects of EtOH on renal CYP24A1 expression are indirect at the cellular level using RPTCs and NRK-52E cells. It appears that CYP24A1 mRNA induction by EtOH requires the synthesis of a protein intermediate because induction was abolished by the protein synthesis inhibitor cycloheximide. Furthermore, these effects are, at least in part, dependent on cellular metabolism of EtOH by ADH-1 or CYP2E1, as suggested by the inhibition CYP24A1 mRNA expression by the ADH/CYPE1 inhibitor 4-MP (44,45). In addition, our data show significant synergism in renal CYP24 induction between EtOH and 1,25 (OH)2 D3.
Previous studies of CYP24 promoter regulation have shown that CYP24A1 transcriptional up-regulation by 1,25 (OH)2 D3 involves activation of a number of MAPK pathways, including ERK1/2 and ERK5 (46). It has been proposed that interactions of 1,25 (OH)2 D3 with its receptor at the plasma membrane results in activation of Ras and downstream activation of ERK1/2 and ERK5 via MEK1/2 and MEKK3/MEK5, respectively. ERK1/2 has been proposed to phosphorylate RXR and ERK5 to phosphorylate the VD/RXR coactivator Ets1, which in turn results in full transcriptional activation of the CYP24A1 gene via two vitamin D responsive element sites located within the −300-bp region of the CYP24A1 promoter (46). 1,25 (OH)2 D3 induction of CYP24A1 was inhibited by the MEK1/2, MEK5 inhibitor U0126. Interestingly, the renal ERK5 pathway has responded to oxidative stress produced by H2O2 treatment, whereas the ERK1/2 pathway was unaffected (46). We have demonstrated that H2O2 treatment of NRK-52E cells results in induction of CYP24A1 mRNA and that EtOH treatment of renal cells can also result in the production of ROS. Moreover, we have shown that EtOH induction of CYP24A1 mRNA is markedly reduced by treatment with U0126. It is likely that ROS production occurs as the result of EtOH metabolism and effects on coupling of mitochondrial electron transport (47,48). Similar oxidative stress in response to EtOH treatment has occurred in many other tissues, including liver, testis, and brain (49,50,51). Our data are consistent with the hypothesis that EtOH stimulates the production of ROS in renal cells, which in turn activates the ERK5 signaling pathway to affect CYP24A1 transcription. This proposed pathway would not only explain the suppression of EtOH induction of CYP24A1 mRNA by U0126, it also provides a possible explanation for the synergistic interaction of EtOH with 1,25 (OH)2 D3 in CYP24A1 mRNA induction. However, more than one MAPK pathway also seems to be involved in EtOH induction of CYP24A1. We also provide evidence that p38-MAPK mediated pathways are involved in EtOH induction of renal CYP24A1. Treatment of renal cells with the p38α, β, and δ inhibitor SB203580 also reversed EtOH induction of CYP24A1 mRNA, whereas this inhibitor has been ineffective in inhibiting induction of CYP24A1 by 1,25 (OH)2 D3 (46).
The increase in CYP24A1 expression produced by EtOH itself and the increased sensitivity of renal CYP24A1 expression to 1,25 (OH)2 D3 feedback in the presence of EtOH described previously would result in increased 1,25 (OH)2 D3 catabolism. It would be interesting to determine if a similar increased sensitivity to 1,25 (OH)2 D3 feedback is also responsible for our observed suppression of renal CYP27B1 mRNA expression and, thus, reduced 1,25 (OH)2 D3 synthesis. The consequence of these effects of EtOH on feedback loops regulating 1,25 (OH)2 D3 synthesis and degradation would be a lower homeostatic set point for plasma 1,25 (OH)2 D3 and the reduced plasma concentrations of the bioactive VD that we observed in EtOH-treated rats. An additional consequence of increased expression of renal CYP24A1 would be expected to be increased production of 24-hydroxylated VD metabolites, such as 24,25 (OH) D3 (22). It has been suggested that 24,25 (OH) D3 and other CYP24A1 products may have endocrine actions separate from 1,25 (OH)2 D3, including important effects on chondrocyte differentiation and cartilage formation (52,53). We have previously reported effects on chondrocyte proliferation and altered morphology in the tibial growth plate of rats chronically exposed to EtOH in the TEN model (30). The role of altered VD homeostasis in this aspect of EtOH action on the skeleton also requires further investigation. Furthermore, 1,25 (OH)2 D3 also has pleiotropic actions on many cellular processes, such as regulation of proliferation and differentiation, and modulation of immune responsiveness and of central nervous system function. Thus, disrupted VD homeostasis may also play an important role in other health effects of EtOH, such as increased cancer risk, immune dysfunction, and teratogenic effects of fetal exposure.
In conclusion, we report that chronic EtOH intake results in reduced serum 1,25 (OH)2 D3 concentrations as a result of impaired renal synthesis and/or increased degradation of 1,25 (OH)2 D3, an effect that appears to be in part due to sensitization to 1,25 (OH)2 D3 feedback on these pathways. This may be mediated via increased renal oxidative stress and activation of MAPKs. Detailed analyses of EtOH effects and ROS actions on renal MAPK signaling converging on the CYP24 promoter are in progress.
Acknowledgments
We thank Matt Ferguson, Tammy Dallari, and Mark Robinette for their technical assistance.
Footnotes
This study was supported in part by National Institutes of Health Grant R01AA012928 (to M.J.J.R.).
Disclosure Statement: The authors have nothing to declare.
First Published Online December 27, 2007
Abbreviations: ADH, Alcohol dehydrogenase-1; BEC, blood ethanol concentrations; DCF-DA, 2,7-dichlorodihydrofluorescein diacetate; 1,25 (OH)2 D3, 1,25-dihydroxycholecalciferol; EtOH, ethanol; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hnRNA, heterogeneous nuclear RNA; H2O2, hydrogen peroxide; MEK, MAPK kinase; 4-MP, 4-methylpyrazole; PND, postnatal d; ROS, reactive oxygen species; RPTC, renal proximal tubule cell; TEN, total enteral nutrition; UEC, urine ethanol concentration.
References
- Chakkalakal DA 2005 Alcohol-induced bone loss and deficient bone repair. Alcohol Clin Exp Res 29:2077–2090 [DOI] [PubMed] [Google Scholar]
- Holbrook TL, Barrett-Connor E 1993 A prospective study of alcohol consumption and bone mineral density. BMJ 306:1506–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friday KE, Howard GA 1991 Ethanol inhibits human bone cell proliferation and function in vitro. Metabolism 40:562–565 [DOI] [PubMed] [Google Scholar]
- Gong Z, Wezeman FH 2004 Inhibitory effect of alcohol on osteogenic differentiation in human bone marrow-derived mesenchymal stem cells. Alcohol Clin Exp Res 28:468–479 [DOI] [PubMed] [Google Scholar]
- Chavassieux P, Serre CM, Vergnaud P, Delmas PD, Meunier PJ 1993 In vitro evaluation of dose-effects of ethanol on human osteoblastic cells. Bone Miner 22:95–103 [DOI] [PubMed] [Google Scholar]
- Perrien DS, Brown EC, Fletcher TW, Irby DJ, Aronson J, Gao GG, Skinner RA, Hogue WR, Feige U, Suva LJ, Ronis MJ, Badger TM, Lumpkin Jr CK 2002 Interleukin-1 and tumor necrosis factor antagonists attenuate ethanol-induced inhibition of bone formation in a rat model of distraction osteogenesis. J Pharmacol Exp Ther 303:904–908 [DOI] [PubMed] [Google Scholar]
- Farley JR, Fitzsimmons R, Taylor AK, Jorch UM, Lau KH 1985 Direct effects of ethanol on bone resorption and formation in vitro. Arch Biochem Biophys 238:305–314 [DOI] [PubMed] [Google Scholar]
- Cheung RC, Gray C, Boyde A, Jones SJ 1995 Effects of ethanol on bone cells in vitro resulting in increased resorption. Bone 16:143–147 [PubMed] [Google Scholar]
- Dai J, Lin D, Zhang J, Habib P, Smith P, Murtha J, Fu Z, Yao Z, Qi Y, Keller ET 2000 Chronic alcohol ingestion induces osteoclastogenesis and bone loss through IL-6 in mice. J Clin Invest 106:887–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Haley RL, Hidestrand M, Shankar K, Liu X, Lumpkin CK, Simpson PM, Badger TM, Ronis MJ 2006 Estradiol protects against ethanol-induced bone loss by inhibiting up-regulation of receptor activator of nuclear factor-κB ligand in osteoblasts. J Pharmacol Exp Ther 319:1182–1190 [DOI] [PubMed] [Google Scholar]
- Badger TM, Ronis MJ, Lumpkin CK, Valentine CR, Shahare M, Irby D, Huang J, Mercado C, Thomas P, Ingelman-Sundberg M 1993 Effects of chronic ethanol on growth hormone secretion and hepatic cytochrome P450 isozymes of the rat. J Pharmacol Exp Ther 264:438–447 [PubMed] [Google Scholar]
- Shankar K, Hidestrand M, Liu X, Xiao R, Skinner CM, Simmen FA, Badger TM, Ronis MJ 2006 Physiologic and genomic analyses of nutrition-ethanol interactions during gestation: implications for fetal ethanol toxicity. Exp Biol Med (Maywood) 231:1379–1397 [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Aronson J, Gao GG, Hogue W, Skinner RA, Badger TM, Lumpkin Jr CK 2001 Skeletal effects of developmental lead exposure in rats. Toxicol Sci 62:321–329 [DOI] [PubMed] [Google Scholar]
- Salih MA, Orhii PB, Chen C, Kalu DN 1999 Growth hormone and the expression of mRNAs for matrix proteins and oncogenes in bone. Mol Cell Endocrinol 147:149–159 [DOI] [PubMed] [Google Scholar]
- Sampson HW 1997 Alcohol, osteoporosis, and bone regulating hormones. Alcohol Clin Exp Res 21:400–403 [DOI] [PubMed] [Google Scholar]
- Shan JH, Bowser EN, Hargis GK, Wongsurawat N, Banerjee P, Henderson WJ, Williams GA 1978 Effect of ethanol on parathyroid hormone secretion in the rat. Metabolism 27:257–260 [DOI] [PubMed] [Google Scholar]
- Keiver K, Weinberg J 2003 Effect of duration of alcohol consumption on calcium and bone metabolism during pregnancy in the rat. Alcohol Clin Exp Res 27:1507–1519 [DOI] [PubMed] [Google Scholar]
- Keiver K, Duggal S, Simpson ME 2005 Ethanol administration results in a prolonged decrease in blood ionized calcium levels in the rat. Alcohol 37:173–178 [DOI] [PubMed] [Google Scholar]
- Krawitt EL, Sampson HW, Katagiri CA 1975 Effect of 1,25-dihydroxycholecalciferol on ethanol mediated suppression of calcium absorption. Calcif Tissue Res 18:119–124 [DOI] [PubMed] [Google Scholar]
- Miller WL, Portale AA 2000 Vitamin D 1α-hydroxylase. Trends Endocrinol Metab 11:315–319 [DOI] [PubMed] [Google Scholar]
- Sakaki T, Sawada N, Nonaka Y, Ohyama Y, Inouye K 1999 Metabolic studies using recombinant Escherichia coli cells producing rat mitochondrial CYP24 CYP24 can convert 1α,25-dihydroxyvitamin D3 to calcitroic acid. Eur J Biochem 262:43–48 [DOI] [PubMed] [Google Scholar]
- Omdahl JL, Morris HA, May BK 2002 Hydroxylase enzymes of the vitamin D pathway: expression, function, and regulation. Annu Rev Nutr 22:139–166 [DOI] [PubMed] [Google Scholar]
- Zierold C, Mings JA, DeLuca HF 2003 Regulation of 25-hydroxyvitamin D3-24-hydroxylase mRNA by 1,25-dihydroxyvitamin D3 and parathyroid hormone. J Cell Biochem 88:234–237 [DOI] [PubMed] [Google Scholar]
- Henry HL 2001 The 25(OH)D(3)/1α,25(OH)(2)D(3)-24R-hydroxylase: a catabolic or biosynthetic enzyme? Steroids 66:391–398 [DOI] [PubMed] [Google Scholar]
- Laitinen K, Valimaki M 1991 Alcohol and bone. Calcif Tissue Int 49(Suppl):S70–S73 [DOI] [PubMed] [Google Scholar]
- Laitinen K, Valimaki M, Lamberg-Allardt C, Kivisaari L, Lalla M, Karkkainen M, Ylikahri R 1990 Deranged vitamin D metabolism but normal bone mineral density in Finnish noncirrhotic male alcoholics. Alcohol Clin Exp Res 14:551–556 [DOI] [PubMed] [Google Scholar]
- Lund B, Sorensen OH, Hilden M, Lund B 1977 The hepatic conversion of vitamin D in alcoholics with varying degrees of liver affection. Acta Med Scand 202:221–224 [DOI] [PubMed] [Google Scholar]
- Turner RT, Aloia RC, Segel LD, Hannon KS, Bell NH 1988 Chronic alcohol treatment results in disturbed vitamin D metabolism and skeletal abnormalities in rats. Alcohol Clin Exp Res 12:159–162 [DOI] [PubMed] [Google Scholar]
- Keiver K, Herbert L, Weinberg J 1996 Effect of maternal ethanol consumption on maternal and fetal calcium metabolism. Alcohol Clin Exp Res 20:1305–1312 [DOI] [PubMed] [Google Scholar]
- Shankar K, Hidestrand M, Haley R, Skinner RA, Hogue W, Jo CH, Simpson P, Lumpkin Jr CK, Aronson J, Badger TM, Ronis MJ 2006 Different molecular mechanisms underlie ethanol-induced bone loss in cycling and pregnant rats. Endocrinology 147:166–178 [DOI] [PubMed] [Google Scholar]
- Shankar K, Hidestrand M, Badger TM, Ronis MJJ, Ethanol-induced bone loss and disruption of vitamin D homeostasis is accompanied by induction of renal 1,25 dihydroxycolecalciferol-24-hydroxylase. Program of the 86th Annual Meeting of The Endocrine Society, New Orleans, LA, 2004, p 371 (Abstract) [Google Scholar]
- Turner RT, Greene VS, Bell NH 1987 Demonstration that ethanol inhibits bone matrix synthesis and mineralization in the rat. J Bone Miner Res 2:61–66 [DOI] [PubMed] [Google Scholar]
- Kent JC, Devlin RD, Gutteridge DH, Retallack RW 1979 Effect of alcohol on renal vitamin D metabolism in chickens. Biochem Biophys Res Commun 89:155–161 [DOI] [PubMed] [Google Scholar]
- Peng T, Lobaugh B, Lester GE, Hirsch PF 1990 Ethanol inhibits renal 25-hydroxyvitamin D-1-a-hydroxylase in rats. J Bone Miner Res 5:245 [Google Scholar]
- Badger TM, Hidestrand M, Shankar K, McGuinn WD, Ronis MJ 2005 The effects of pregnancy on ethanol clearance. Life Sci 77:2111–2126 [DOI] [PubMed] [Google Scholar]
- Shankar K, Hidestrand M, Liu X, Chen JR, Haley R, Perrien DS, Skinner RA, Lumpkin CK, Badger TM, Ronis MJ 29 October 2007 Chronic ethanol consumption inhibits post-lactational anabolic bone rebuilding in female rats. J Bone Miner Res [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Bergin E, Levine JS, Koh JS, Lieberthal W 2000 Mouse proximal tubular cell-cell adhesion inhibits apoptosis by a cadherin-dependent mechanism. Am J Physiol Renal Physiol 278:F758–F768 [DOI] [PubMed] [Google Scholar]
- Badger TM, Crouch J, Irby D, Hakkak R, Shahare M 1993 Episodic excretion of ethanol during chronic intragastric ethanol infusion in the male rat: continuous vs. cyclic ethanol and nutrient infusions. J Pharmacol Exp Ther 264:938–943 [PubMed] [Google Scholar]
- Wadstein J, Skude G 1979 Serum ethanol, hepatic enzymes and length of debauch in chronic alcoholics. Acta Med Scand 205:317–318 [DOI] [PubMed] [Google Scholar]
- Armbrecht HJ, Chen ML, Hodam TL, Boltz MA 1997 Induction of 24-hydroxylase cytochrome P450 mRNA by 1,25-dihydroxyvitamin D and phorbol esters in normal rat kidney (NRK-52E) cells. J Endocrinol 153:199–205 [DOI] [PubMed] [Google Scholar]
- Aroor AR, Shukla SD 2004 MAP kinase signaling in diverse effects of ethanol. Life Sci 74:2339–2364 [DOI] [PubMed] [Google Scholar]
- Lin R, White JH 2004 The pleiotropic actions of vitamin D. Bioessays 26:21–28 [DOI] [PubMed] [Google Scholar]
- Sutton AL, MacDonald PN 2003 Vitamin D: more than a “bone-a-fide” hormone. Mol Endocrinol 17:777–791 [DOI] [PubMed] [Google Scholar]
- Sarkola T, Iles MR, Kohlenberg-Mueller K, Eriksson CJ 2002 Ethanol, acetaldehyde, acetate, and lactate levels after alcohol intake in white men and women: effect of 4-methylpyrazole. Alcohol Clin Exp Res 26:239–245 [PubMed] [Google Scholar]
- Choi D, Leininger-Muller B, Kim YC, Leroy P, Siest G, Wellman M 2002 Differential role of CYP2E1 binders and isoniazid on CYP2E1 protein modification in NADPH-dependent microsomal oxidative reactions: free radical scavenging ability of isoniazid. Free Radic Res 36:893–903 [DOI] [PubMed] [Google Scholar]
- Dwivedi PP, Hii CS, Ferrante A, Tan J, Der CJ, Omdahl JL, Morris HA, May BK 2002 Role of MAP kinases in the 1,25-dihydroxyvitamin D3-induced transactivation of the rat cytochrome P450C24 (CYP24) promoter. Specific functions for ERK1/ERK2 and ERK5. J Biol Chem 277:29643–29653 [DOI] [PubMed] [Google Scholar]
- Arteel GE 2003 Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology 124:778–790 [DOI] [PubMed] [Google Scholar]
- Sastre J, Serviddio G, Pereda J, Minana JB, Arduini A, Vendemiale G, Poli G, Pallardo FV, Vina J 2007 Mitochondrial function in liver disease. Front Biosci 12:1200–1209 [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Butura A, Sampey BP, Shankar K, Prior RL, Korourian S, Albano E, Ingelman-Sundberg M, Petersen DR, Badger TM 2005 Effects of N-acetylcysteine on ethanol-induced hepatotoxicity in rats fed via total enteral nutrition. Free Radic Biol Med 39:619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanvermez R, Demir S, Tuncel OK, Alvur M, Agar E 2005 Alcohol-induced oxidative stress and reduction in oxidation by ascorbate/L-cys/L-met in the testis, ovary, kidney, and lung of rat. Adv Ther 22:548–558 [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM 2003 Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci USA 100:7919–7924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen JP, van den Bemd GJ, van Driel M, Buurman CJ, Pols HA 2001 24,25-Dihydroxyvitamin D(3) and bone metabolism. Steroids 66:375–380 [DOI] [PubMed] [Google Scholar]
- Dean DD, Boyan BD, Schwart Z, Muniz OE, Carreno MR, Maeda S, Howell DS 2001 Effect of 1α,25-dihydroxyvitamin D3 and 24R,25-dihydroxyvitamin D3 on metalloproteinase activity and cell maturation in growth plate cartilage in vivo. Endocrine 14:311–323 [DOI] [PubMed] [Google Scholar]