Abstract
GnRH neurons play a pivotal role in the central regulation of fertility. Kisspeptin greatly increases GnRH/LH release and GnRH neuron firing activity and may be involved in estradiol feedback, but the neurobiological mechanisms for these actions are unknown. G protein-coupled receptor 54, the receptor for kisspeptin, is expressed by GnRH neurons as well as other hypothalamic neurons, suggesting both direct and indirect effects are possible. To investigate this and determine whether kisspeptin activation of GnRH neurons is estradiol sensitive, we recorded the firing rate of GnRH neurons in brain slices from adult female mice that were ovariectomized (OVX) and either treated with estradiol (E) capsules (OVX+E) or left without further treatment. Kisspeptin increased GnRH neuronal activity in a dose-dependent manner in cells from both OVX and OVX+E mice, and estradiol significantly potentiated the response. To begin to distinguish direct from indirect actions of kisspeptin, fast synaptic transmission mediated by ionotropic γ-aminobutyric acid and glutamate receptors was pharmacologically blocked (blockade). Blockade reduced GnRH response to kisspeptin in OVX+E but not in OVX mice. Actions of kisspeptin were also assessed using whole-cell voltage- and current-clamp recording in slices from OVX animals. Kisspeptin application depolarized GnRH neurons in current-clamp and generated inward current in voltage-clamp recordings, even after blocking action potential-dependent neural communication, consistent with a direct effect. Blockers of potassium channels abolished the inward current. Together our data indicate that kisspeptin activates GnRH neurons via both direct and transsynaptic mechanisms and that transsynaptic mechanisms are either enabled and/or potentiated by estradiol.
GNRH NEURONS FORM the final common pathway for the control of fertility. GnRH released from axon terminals stimulates secretion of LH and FSH from the anterior pituitary gland. These gonadotropins promote steroidogenesis and gametogenesis. Steroids, in turn, feed back at the pituitary and hypothalamus. Estradiol feedback is particularly interesting because estradiol has both negative and positive feedback effects, with the latter being critical for generating the neural signal for ovulation (1,2,3,4,5,6,7,8). Whether estradiol effects are direct, transsynaptic, or both is under debate. GT1 GnRH neuronal cell lines express both estrogen receptor (ER)-α and -β (9,10). In contrast, only ERβ has been detected in native GnRH neurons (11,12,13). Whether GnRH neurons themselves express ER, there is good evidence that estradiol regulation of GnRH neurons can be transsynaptic because estradiol regulates synaptic transmission to these cells (14) and neurons reported to be afferent to GnRH neurons express both ERα and ERβ (15,16,17,18).
Of particular interest with regard to estradiol feedback regulation of GnRH neurons is recent work on the neuromodulator kisspeptin. Kisspeptin, also known as metastin, is a natural ligand for G protein-coupled receptor (GPR) 54 (19,20). The KiSS-1 gene encodes a 145-amino acid peptide that is cleaved into amidated C-terminal 54, 14, 13, and 10 amino acid products, all of which activate GPR54 (20). Thirty percent of patients with idiopathic hypogonadotropic hypogonadism have loss-of-function mutations in the GPR54 gene (21). These GPR54-inactivating mutations in humans as well as GPR54 knockouts in mice cause failure to initiate puberty and thus lead to infertility (21,22). Likewise, KiSS-1 knockout mice showed abnormal sexual maturation consistent with disruptions in this system being a cause of hypogonadotropic hypogonadism (23). Notably, some patients with idiopathic hypogonadotropic hypogonadism are responsive to exogenous GnRH, suggesting the defect can be in the abnormality of GnRH synthesis, secretion, or activity (24). Both kisspeptin and GPR54 are highly expressed in the hypothalamus (25,26,27), within areas known to regulate GnRH neuronal activity (18,28), and GnRH neurons express GPR54 (29,30,31,32). Kisspeptin increases GnRH and LH release and GnRH neuron firing activity (31,32,33,34,35,36). Estradiol regulates expression of GPR54 as well as KiSS-1 mRNA (18,28,37), making kisspeptin a likely transsynaptic modulator for conveying estradiol feedback to GnRH neurons.
Despite these advances, little is known about the neurobiological mechanisms engaged by kisspeptin to regulate GnRH neurons. Because GnRH neurons express GPR54, a broad assumption has been made that kisspeptin action is direct on GnRH neurons. Although this is a likely mechanism, it is also important to bear in mind that GPR54 is expressed in other parts of the hypothalamus; thus, kisspeptin may have transsynaptic effects on GnRH neurons. Furthermore, although estradiol is known to alter KiSS-1 mRNA, whether it alters the response of GnRH neurons to kisspeptin is not known. Here we used electrophysiological approaches to test the hypothesis that kisspeptin acts both directly and transsynaptically to regulate GnRH neurons and that estradiol modulates the response of GnRH neurons to kisspeptin.
Materials and Methods
Animals
Transgenic female mice, in which green fluorescent protein (GFP) was genetically targeted to GnRH neurons were used for these studies (38). Mice were housed on a 14-h light, 10-h dark cycle, with lights off at 1630 h, and were maintained on Harlan 2916 rodent chow (Harlan, Bartonsville, IL) and water ad libitum. All procedures were approved by the Animal Care and Use Committee of the University of Virginia and were conducted within the guidelines of the National Research Council’s Guide for the Care and Use of Laboratory Animals. Adult female GnRH-GFP mice were ovariectomized (OVX) under isoflurane (Abbott Laboratories, North Chicago, IL) anesthesia. Postoperative analgesia was provided by a long-acting local anesthetic (0.25% bupivacaine; 7.5 μl/site; Abbott Laboratories). At the time of surgery, some mice received sc SILASTIC (Dow Corning, Midland, MI) capsules containing 0.625 μg estradiol (E) in sesame oil (OVX+E). All recordings were done 2–4 d after surgery in the morning during the time of E-negative feedback (39).
Brain slice preparation and recordings
All chemicals were from Sigma Chemical Co. (St. Louis, MO) unless noted. Brain slices were prepared using modifications (40) of a previously described method (41). Briefly, all solutions were bubbled with a 95% O2-5% CO2 mixture throughout the experiments and for at least 15 min before exposure to the tissue. The brain was rapidly removed and placed in ice-cold, high-sucrose saline solution containing 250 mm sucrose, 3.5 mm KCl, 26 mm NaHCO3, 10 mm glucose, 1.25 mm Na2HPO4, 1.2 mm MgSO4, and 2.5 mm MgCl2. Coronal 300-μm brain slices were cut with a Vibratome 3000 (Technical Products International, Inc., St. Louis, MO). Slices were incubated for 30 min at 30–32 C in a solution of 50% high-sucrose saline and 50% normal saline containing (in mm) 135 mm NaCl, 26 mm NaHCO3, 3.5 mm KCl, 10 mm glucose, 1.3 mm Na2HPO4, 1.2 mm MgSO4, and 2.5 mm CaCl2 (pH 7.4) and were then transferred to a solution of 100% NS at room temperature and kept at least 30 min and no more than 6 h before recording.
For recording, individual brain slices were placed in a recording chamber continuously superfused with oxygenated normal saline solution and kept at 29–31 C. Cells were visualized with an Olympus BX50WI upright fluorescent microscope with infrared differential interference contrast (Opelco, Dulles, VA). GnRH neurons were identified by brief illumination at 470 nm to visualize the GFP signal. Recording pipettes were pulled from borosilicate glass capillaries (1.65 mm outer diameter; 1.12 mm inner diameter; World Precision Instruments, Inc., Sarasota, FL) using a Flaming/Brown P-97 (Sutter Instrument, Novato, CA) and had resistances from 1.5 to 4 mΩ when filled with the appropriate solution (see below). Pipettes were placed in contact with a GnRH neuron using an MP-285 or MP-225 micromanipulator (Sutter Instruments). Current and voltage traces were obtained using an EPC-8 amplifier (HEKA, Mahone Bay, Nova Scotia, Canada) with the PulseControl XOP (Instrutech, Port Washington, NY) running in Igor Pro (Wavemetrics, Lake Oswego, OR), or using one head stage of an EPC-10 dual amplifier (HEKA) controlled by PatchMaster (HEKA) for some whole-cell recordings.
To test the effects of kisspeptin on firing activity of GnRH neurons and unidentified neurons within the medial preoptic area, we used a minimally invasive electrophysiological method, targeted extracellular recordings. This type of recording does not alter the intracellular milieu; thus, the cell response to synaptic inputs that remain within the brain slice is undisturbed. Recording pipettes were filled with normal HEPES-buffered solution containing 150 mm NaCl, 10 mm HEPES, 10 mm glucose, 2.5 mm CaCl2, 1.3 mm MgCl2, and 3.5 mm KCl. Initial resistances ranged from 6 to 30 mΩ and either remained stable or increased during recording up to as high as 50 mΩ. Recordings were made in voltage-clamp mode with a pipette holding potential of 0 mV; at low seal resistance, the amplifier potential does not influence the cell. In this type of recording, we detect action currents, which are not action potentials per se, although they accurately reflect changes in the action potential firing rate. For simplicity, we used the phrase firing rate and/or firing activity to refer to these events.
We used the human decapeptide form of kisspeptin, which differs from the murine sequence by only one conservative amino acid substitution (kisspeptin-10; Phoenix Pharmaceuticals, Burlingame, CA). The effects of kisspeptin have been reported to be long lasting (31,32). We thus used several drug application strategies to enable effective washout to establish reversibility; the response to kisspeptin was prolonged with all application methods attempted. The initial response to kisspeptin was tested using a 5-min bath application. For dose-response studies, cells were recorded for a 10-min control period to establish spontaneous firing rate, and then kisspeptin (0.1, 1, 10, 20, 100 nm) was bath applied for 1 min and washed out for 15 min. Attempts to perform dose-response curves by treating each cell with multiple doses suggested down-regulation of the response. As a result, only one dose per cell and one cell per brain slice was examined. No more than three cells per animal were recorded.
Targeted extracellular recordings were also used to investigate the role of fast synaptic transmission in kisspeptin action on GnRH neurons. Slices were exposed via the bath to a cocktail of 100 μm picrotoxin to block γ-aminobutyric acid (GABA)A receptors, and 20 μm 6-cyano-7-nitroquinoxaline-2,3-dione and 20 μm D(−)2-amino-5-phosphonovaleric acid to block α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid- and N-methyl-d-aspartic acid-type glutamate receptors, respectively. Spontaneous firing activity was recorded for a 10-min control period, 5-min exposure to 1 nm kisspeptin, and then 15 more minutes of control solution as a wash.
To investigate effects of kisspeptin on membrane potential and current of GnRH neurons, we performed whole-cell recordings in voltage- and current-clamp modes to monitor the changes in membrane current and potential, respectively, induced by kisspeptin. Reported values are not corrected for an estimated −13 mV liquid junction potential (42). In current-clamp recordings, the membrane capacitance was compensated and cells had an initial membrane potential negative to −55 mV without current injection and action potential amplitude of greater than 90 mV. In voltage-clamp recordings, membrane potential was held at −60 mV. During whole-cell recordings, input resistance, series resistance, and membrane capacitance were continually measured. Only recordings with stable input resistance greater than 500 mΩ, series resistance less than 20 mΩ, and stable membrane capacitance were used for analysis. Recording pipettes (3–4 mΩ) were filled with a solution containing the following (in mm): 125 K-gluconate, 20 KCl, 10 HEPES, 5 EGTA, 4 MgATP, 0.4 NaGTP, and 0.1 CaCl2 (pH 7.2) (300 mOsm). In these studies, the effects of kisspeptin were tested by adding 20 μl of 1 μm of kisspeptin solution to the recording chamber.
Additional whole-cell voltage-clamp recordings were done in the presence of 0.5 μm tetrodotoxin and the fast synaptic transmission blockade cocktail to minimize presynaptic influence on GnRH neurons. In these recordings, membrane potential was clamped at −60 mV and holding current changes were monitored; estradiol modulation was tested by examining neurons from OVX and OVX+E mice. After a stable pretreatment period, kisspeptin (10 nm) was added via the bath for 5 min. To begin to identify the underlying conductance altered by kisspeptin, additional recordings were done in the presence of 5 mm 4-aminopyridine and 20 mm tetraethylammonium to block voltage-dependent potassium currents (43). Additional cells were recorded at −96 mV, the reversal potential for potassium current under our experimental conditions. Because there was no difference in the magnitude of inward current between OVX and OVX+E mice, recordings to examine the underlying conductance were performed on GnRH neurons from OVX+E mice.
Data analysis
Using programs written for Igor Pro (41), extracellularly recorded events were counted and binned at 1-min intervals to identify changes in firing rate of GnRH neurons. Binned event data were analyzed for the mean firing rate before treatment (control) during kisspeptin application and during washout. Mean firing rate was determined by dividing the total number of events detected before, during, and after kisspeptin treatment by the duration of recording in each condition; 2 min were skipped after drug changes to eliminate transition periods. Fold change after treatment was calculated for all targeted extracellular recordings because of the inherent difference in firing rate of GnRH neurons from OVX and OVX+E mice (39). Groups were compared using two-way ANOVA followed by Bonferroni post hoc test. For whole-cell recordings, change in amplitude of membrane potential (current clamp) or inward current (voltage clamp) was measured from the pretreatment baseline and compared using paired t test with each cell serving as its own control. For whole-cell recording in which the effects of estradiol were also evaluated, two-way ANOVA was used as above. Significance was set at P < 0.05 and all data are reported as mean ± sem.
Results
Estradiol enhances GnRH neuron response to kisspeptin
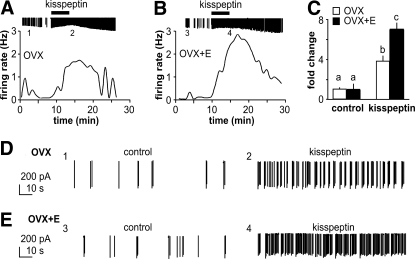
To examine estradiol sensitivity of the GnRH neuron response to kisspeptin, we performed extracellular recordings. Activity in these recordings reflects both intrinsic changes in the recorded cell and changes in activity of neural afferents remaining in the brain slice, providing a measure of the integrated response of the GnRH neuron to kisspeptin. Figure 1 shows representative recordings of GnRH neurons from OVX (Fig. 1A) and OVX+E (Fig. 1B) female mice; expanded time scales of firing activity are shown in Fig. 1, D and E. Kisspeptin (1 nm) increased firing activity of GnRH neurons from both OVX and OVX+E animals (OVX, n = 10, P < 0.01; OVX+E, n = 10, P < 0.001). There was a significant interaction with estradiol, with cells from OVX mice responding less than those from OVX+E mice (Fig. 1C, P < 0.001).
Figure 1.
Kisspeptin action on GnRH neurons from OVX and OVX+E animals. A and B, Representative traces of GnRH firing activity changes over time. Graph shows firing rate over time; downward lines on the top are individual action currents recorded, and kisspeptin (1 nm) application is marked by the black bar. Numbers 1–4 below action current records in A and B indicate areas examined in detail in D and E, respectively. C, Mean ± sem response; different letters indicate P < 0.001 with two-way ANOVA followed by Bonferroni post hoc test. D and E, One-minute excerpts of action currents to examine firing pattern during control period (1 and 3) and during kisspeptin treatment (2 and 4) from OVX (D) and OVX+E (E) mice.
Kisspeptin increases GnRH neuronal activity in dose-dependent and estradiol-sensitive manner
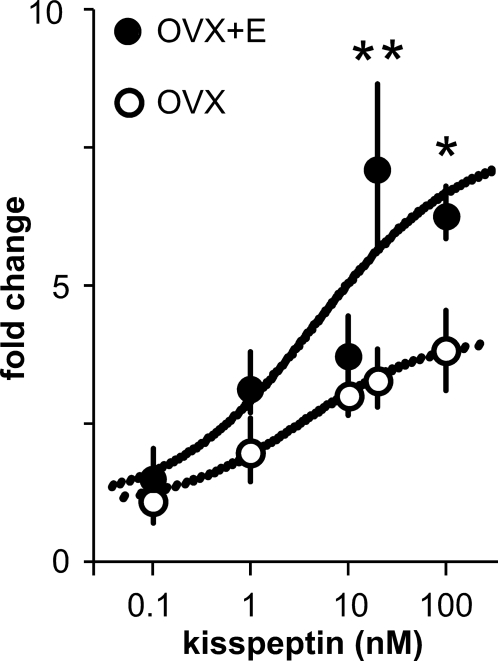
To determine the dose-response of GnRH neuron activity to kisspeptin and whether estradiol modulates this response, we performed additional extracellular recordings of GnRH neurons from OVX and OVX+E mice. Because of the prolonged response to a 5-min application of kisspeptin in the first experiment, we reduced the duration to 1 min in an attempt to enhance the ability to wash out the drug. As shown in Fig. 2, kisspeptin increases activity of GnRH neurons from both OVX and OVX+E mice in a dose-dependent manner. The EC50 was not different between neurons from OVX (3.6 nm) and OVX+E (4.5 nm) mice (P > 0.2). Estradiol increased maximum response to kisspeptin (20 nm OVX response, 3.3 ± 0.5-fold, n = 6, OVX+E response, 7.1 ± 1.5-fold, n = 7, P < 0.001; 100 nm OVX response, 3.8 ± 0.2-fold, n = 6; OVX+E response, 6.3 ± 0.5-fold, n = 6, P < 0.05). These results confirm the data in Fig. 1, demonstrating that estradiol potentiates the GnRH neuron response to kisspeptin.
Figure 2.
Estradiol enhances maximum firing rate response of GnRH neurons to kisspeptin. Mean ± sem (**, P < 0.001; *, P < 0.05) response of GnRH neurons from OVX+E and OVX mice to kisspeptin showing curve fit.
Kisspeptin excites non-GnRH neurons in the medial preoptic area
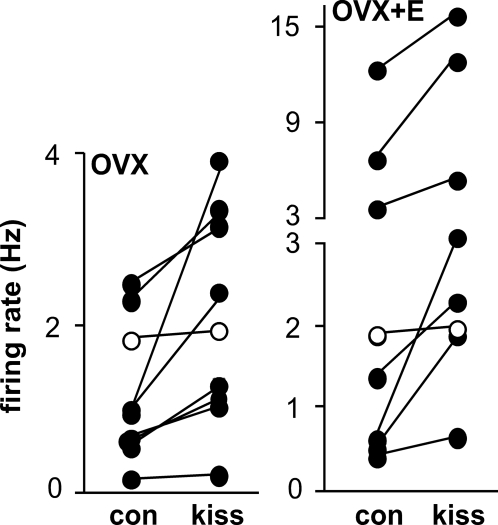
The widespread expression of GPR54 suggests kisspeptin may affect other cell types. To test whether kisspeptin alters firing rate of non-GnRH neurons, we recorded unidentified cells in the medial preoptic area from OVX and OVX+E mice. The dose-response data achieved with 1-min application used in the previous experiment appropriately reached a plateau at high doses, but the short duration kisspeptin application did not reduce the duration of the biological response. We thus returned to a 5-min duration. As Fig. 3 shows, kisspeptin (10 nm) increased firing activity of most unidentified neuronal cells from both OVX and OVX+E animals (OVX, n = 9, control, 1.1 ± 0.2 Hz, kisspeptin, 1.9 ± 0.4 Hz, P < 0.002; OVX+E, n = 8, control, 3.3 ± 1.4 Hz, kisspeptin, 5.4 ± 2.0 Hz, P < 0.002). These results suggest that kisspeptin could regulate GnRH neurons through altering synaptic transmission.
Figure 3.
Kisspeptin increases firing rate of non-GnRH neurons. Cells from OVX mice (n = 9, P < 0.002) are on the left, and from OVX+E mice (n = 8, P < 0.002) on the right. Mean firing rate before and after kisspeptin are shown for each cell, connected by lines. The white circles indicate cells that did not change firing rate by at least 30% in response to kisspeptin. Kiss, Kisspeptin; con, control.
Blocking fast synaptic transmission reduces response to kisspeptin in GnRH neurons from OVX+E but not OVX mice
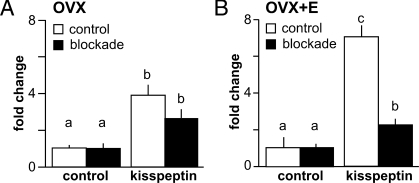
To determine whether fast synaptic transmission is important for the stimulatory action of kisspeptin on GnRH neurons and whether this is dependent on estradiol milieu, we blocked ionotropic GABA and glutamate receptors (blockade). Both classes of receptors were blocked simultaneously to avoid imbalances between excitatory and inhibitory neurotransmission within the brain slice network (44,45). After blockade, GnRH neurons from both OVX and OVX+E mice still responded to kisspeptin with increased firing, suggesting either direct effects on GnRH neurons or indirect activation via neuromodulators as opposed to mediators of fast synaptic transmission such as GABA and glutamate (Fig. 4). In GnRH neurons from OVX+E mice, however, blockade of fast synaptic transmission significantly reduced the response to kisspeptin (n = 10, P < 0.001, Fig. 4B). In contrast, response of GnRH neurons from OVX mice was similar with and without blockade (n = 10, P > 0.05) (Fig. 4A). These data suggest excitatory action of kisspeptin on GnRH neurons from OVX+E, but not OVX, mice is partially mediated by GPR54-expressing afferents.
Figure 4.
Blockade of fast synaptic transmission decreases response to kisspeptin in an estradiol-dependent manner. Mean ± sem firing rate response of GnRH neurons from OVX (A) and OVX+E (B) animals are recorded in the absence (white bars, control) and presence of fast GABA and glutamate receptor blockers (black bars, blockade). Different letters indicate P < 0.05 with two-way ANOVA followed by Bonferroni post hoc test.
Kisspeptin depolarizes GnRH neurons and generates an inward current
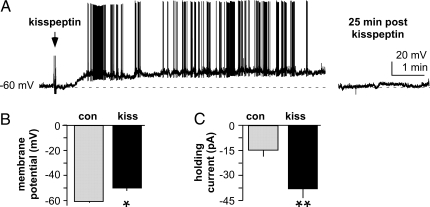
To further examine actions of kisspeptin on GnRH neurons, we performed whole-cell recordings of GnRH-GFP neurons from female mice. During recording, 20 μl of 1 μm kisspeptin was added to the recording chamber as a single drop. Based on the volume of the solution in the chamber (∼1.5 ml), the maximum concentration reached is approximately 10 nm, although the 5- to 6-ml/min flow rate makes it unlikely this level was achieved. In current-clamp mode, kisspeptin application caused a marked persistent depolarization (four of five cells; Fig. 5, A and B, P < 0.006), and upon this depolarization, action potentials were generated (Fig. 5A). In voltage-clamp, kisspeptin induced a significant inward current in GnRH neurons (Fig. 5C, n = 4, P < 0.001). These data indicate that kisspeptin action on GnRH neurons can be studied in the whole-cell configuration. Even with this brief drug application, however, the response was prolonged. We thus returned to standard bath application for the next experiments to test whether this effect is direct because dosing is more consistent.
Figure 5.
Kisspeptin increases activity of GnRH neurons recorded in the whole-cell configuration. A, Current-clamp recording in which drop of kisspeptin added to the bath (arrow) depolarizes the GnRH neuron by about −15 mV (upward slope), upon which action potentials are produced (upward spikes). B and C, Mean ± sem response. *, P < 0.006; **, P < 0.001.
Kisspeptin generates an inward current in GnRH neurons, regardless of estradiol milieu
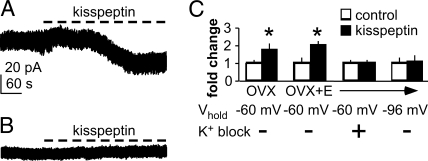
The above studies suggest a direct action of kisspeptin on GnRH neurons, but changes in neurotransmission and neuromodulation cannot be excluded. To isolate kisspeptin action on GnRH neurons, voltage-clamp recordings were done in the presence of both the blockade cocktail used above and tetrodotoxin, which blocks action potential firing and thus minimizes presynaptic release. Tetrodotoxin in combination with blockade of the receptors conveying fast synaptic transmission to remove the influence of spontaneous transmitter release results in near pharmacological isolation of cells. Kisspeptin significantly increased inward current in GnRH neurons from both OVX (n = 7, P < 0.001) and OVX+E mice (n = 8, P < 0.001) (Fig. 6, A and C). There was no difference in the magnitude of the change in holding current change between GnRH neurons from OVX and OVX+E mice (P > 0.6), suggesting that the inward current change evoked by kisspeptin is not estradiol sensitive.
Figure 6.
Kisspeptin generates an inward current in GnRH neurons. All recordings were done in the presence of tetrodotoxin and blockers of fast synaptic transmission. A, Kisspeptin generates a slow inward current in GnRH neurons held at −60 mV under control conditions. B, Pretreatment with the potassium channel blockers abolishes this effect. C, Mean ± sem fold change in holding current showing inward current generated by kisspeptin in GnRH neurons from OVX and OVX+E mice and abolition of the current by K+ block or holding the cells at the reversal potential for potassium, −96 mV. *, P < 0.001.
To begin to characterize the conductances that might be responsible for the increase in inward current caused by kisspeptin, we examined candidate channel blockers. First, we noted that the change in current caused by kisspeptin was similar in the absence (Fig. 5C) and presence (Fig. 6, A and C) of tetrodotoxin, suggesting it was unlikely to be a voltage-gated sodium channel. Because potassium channels play a major role in setting the membrane potentials of cells (46), we examined these next. When blockers of voltage-dependent potassium currents were added to the bath before kisspeptin, there was no change in holding current (n = 5, P > 0.2, Fig. 6, B and C). Furthermore, when kisspeptin was applied under control conditions to cells held at the reversal potential for potassium (−96 mV), there was no change in holding current (n = 4, P > 0.2, Fig. 6C), although input resistance still increased. Together these data suggest that kisspeptin closes potassium channels in GnRH neurons to generate the inward current that leads to depolarization of these cells and that this effect is not dependent on estradiol.
Discussion
Kisspeptin is a neuropeptide recently discovered to have a strong stimulatory effect on GnRH neurons and to possibly playing a major role in conveying steroid feedback regulation to GnRH neurons. The action of kisspeptin can be direct because GnRH neurons express GPR54 (29,30,31,32); however, it may also be transsynaptic via GPR54-expressing afferents because this receptor is widely expressed within the hypothalamus (25,26,27). Here we demonstrate kisspeptin action on GnRH neuronal activity is dose dependent and enhanced by estradiol. In the presence of estradiol, kisspeptin activation of GnRH neurons is likely via both transsynaptic and direct mechanisms, and in the absence of estradiol indirect kisspeptin action is reduced.
Our data support previous work showing kisspeptin increased GnRH neuron activity in proestrous female mice (31). The present work extends those data in several ways. First, the dose-response curve revealed that lower doses of kisspeptin were effective. Second, the comparison of OVX and OVX+E mice shows a role for estradiol in increasing sensitivity to kisspeptin. Third, cells recorded in the whole-cell configuration responded to kisspeptin, indicating that dialysis of the cell inherent in this method does not preclude kisspeptin action. This is important because whole-cell recordings are valuable for future studies of specific mechanisms.
Our work further began to probe the neurobiological mechanisms for kisspeptin action. GPR54 is expressed not only on GnRH neurons (29,30,31,32) but also in other parts of the hypothalamus (25,26,27), suggesting kisspeptin can have widespread effects on neurotransmission and neuromodulation, affecting GnRH neurons both directly and indirectly. Consistent with this postulate, blockade of fast synaptic transmission reduced the response to kisspeptin in GnRH neurons from OVX+E mice. In contrast, GnRH neurons from OVX mice responded similarly in the presence and absence of these receptor blockers. Under conditions of pharmacological isolation of GnRH neurons in brain slices to minimize presynaptic influence, kisspeptin caused a similar increase in inward current in GnRH neurons from OVX and OVX+E mice. Induction of the inward current in GnRH neurons by kisspeptin was abolished by either pharmacological blockade of voltage-gated potassium channels or holding the cell at reversal potential for potassium. Together these data suggest direct effects of kisspeptin on GnRH neurons are similar with and without estradiol and that kisspeptin action involves changes in potassium channel conductances.
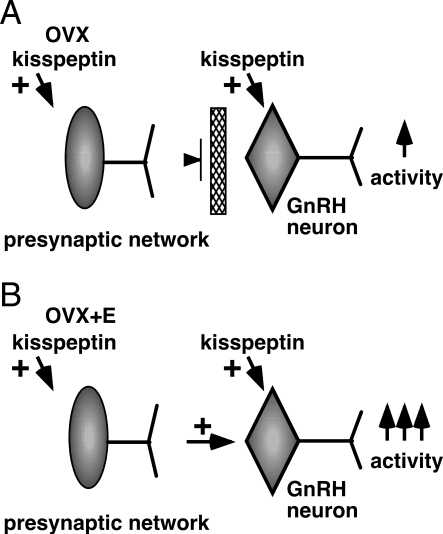
Unlike the direct effects of kisspeptin, experiments with fast synaptic transmission blockade indicate that indirect effects of this peptide were enhanced by estradiol treatment. In this regard, kisspeptin may act directly on primary afferents of GnRH neurons, or through multisynaptic pathways altering neuromodulator release onto GnRH neurons. Kisspeptin increased firing activity of unidentified hypothalamic neurons from both OVX and OVX+E mice, indicating estradiol is not needed for the response in non-GnRH neurons. Yet blocking fast synaptic transmission had no effect on the ability of kisspeptin to increase GnRH neuron activity in OVX mice. Together these observations suggest estradiol enables the connection between the presynaptic network affected by kisspeptin and GnRH neurons. Removal of estradiol by ovariectomy causes a disconnection between the activation of presynaptic network elements by kisspeptin and the conveying of these signals to GnRH neurons (Fig. 7). The action of estradiol to increase response to kisspeptin by engaging indirect mechanisms may be an important component of the indirect regulation of GnRH neurons by this steroid.
Figure 7.
Postulated role of estradiol in kisspeptin action on GnRH neurons. A, In the absence of estradiol, kisspeptin directly increases GnRH firing activity as well as activity of nearby unidentified neurons, but the lack of estradiol disconnects the afferent network so that only direct action of kisspeptin on GnRH neurons occurs. B, In the presence of estradiol, interactions of the afferent network and GnRH neurons are enabled. Thus, kisspeptin not only acts directly but also through afferent inputs to increase GnRH neuronal activity, and its action is more profound.
In this regard, estradiol modulation of the functional network afferent to GnRH neurons has been observed. Specifically, both GABA and glutamate transmission are altered in a diurnal manner in the presence of estradiol (14,47). The diurnal changes in GABA transmission at least are dependent on the presence of estradiol as the rate of transmission remains constant in OVX mice. These changes might be due to estradiol modulation of synapse density because studies in rats showed that estradiol is capable of changing synapse density during the estrous cycle (48). In general, during the proestrous phase of the cycle, when estradiol levels are high, synapse density was higher than in the estrous phase of the cycle. Additionally, when female rats were ovariectomized synapse density in the hippocampus was lower, compared with OVX and E-treated rats (48). These studies suggest an estradiol-induced plasticity of the indirect actions of kisspeptin in altering GnRH neuron activity.
Other work supports a link between estradiol and kisspeptin action. In mice kisspeptin expression is differentially regulated by estradiol in two distinct nuclei. Specifically, KiSS-1 expression increased after OVX and decreased after estradiol replacement in the arcuate nucleus, whereas the opposite effect was observed in the anteroventral periventricular nucleus (AVPV) (18). Furthermore, in female rats during induced LH surges, there is increased coexpression of KiSS-1 and the transcription factor Fos, a marker of neuronal activity, in the AVPV. This was correlated with increased Fos expression in GnRH neurons (49). A preliminary report suggests similar findings in mice; Fos expression in KiSS-1 neurons in the AVPV was observed during positive but not negative feedback (50). The above data suggested that kisspeptin neurons in the AVPV are targets for transcriptional activation by estradiol and may be one means to convey estradiol stimulatory action to induce the GnRH/LH surge. The AVPV expresses GPR54 (51), and neurons in this region use both GABA and glutamate as transmitters and project to GnRH neurons (52), suggesting a possible source of indirect action of kisspeptin via changes in fast synaptic transmission.
A recent report in GPR54 knockout mice demonstrated these mice can still exhibit positive feedback to estradiol (53). This suggests signaling through GPR54 is not crucial for estradiol-positive feedback, at least in mice. One possible explanation is that the estradiol-kisspeptin feedback loop in one of several redundant mechanisms for generating positive feedback. In this regard, as mentioned above, increased transmission via GABA and glutamate are also hallmarks of positive feedback as is an increase in vasoactive intestinal polypeptide action (54,55,56). Other factors, such as catecholamines, neuropeptide Y, and vasopressin, have also been implicated in surge generation (57,58,59,60,61,62). Given the importance of the surge for passing on genetic material, it is not surprising that multiple redundant systems exist and that loss of one component can be compensated by others. Of note, the increases in GABA and glutamate transmission as well as vasoactive intestinal polypeptide action also occur only in subpopulations of GnRH neurons (14,47,56).
In summary, our data suggest that kisspeptin can activate GnRH neurons directly and in the presence of estradiol also through the presynaptic network. The enhanced response to kisspeptin in the presence of estradiol could contribute along with increased kisspeptin neuron activity to the positive feedback response of a subpopulation of GnRH neurons. Future studies will identify the transsynaptic mechanisms enabled by kisspeptin as well as the direct mechanisms through which kisspeptin acts on GnRH neurons.
Acknowledgments
We thank Debra Fisher for expert technical assistance and Catherine Christian, Alison Roland, and Pei-San Tsai for editorial comments.
Footnotes
This work was supported by National Institute of Child Health and Human Development/National Institutes of Health Grant R01 HD41469.
Disclosure Statement: J.P.-F., Z.C., and S.M.M. have nothing to disclose.
First Published Online December 27, 2007
Abbreviations: AVPV, Anteroventral periventricular nucleus; E, estradiol; ER, estrogen receptor; GABA, γ-aminobutyric acid; GFP, green fluorescent protein; GPR, G-protein coupled receptor; KiSS, kisspeptin; OVX, ovariectomized.
References
- Levine JE, Ramirez VD 1980 In vivo release of luteinizing hormone-releasing hormone estimated with push-pull cannulae from the mediobasal hypothalami of ovariectomized, steroid-primed rats. Endocrinology 107:1782–1790 [DOI] [PubMed] [Google Scholar]
- Caraty A, Locatelli A, Martin GB 1989 Biphasic response in the secretion of gonadotrophin-releasing hormone in ovariectomized ewes injected with oestradiol. J Endocrinol 123:375–382 [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Cummins JT, Thomas GB, Clarke IJ 1987 Steroid feedback inhibition of pulsatile secretion of gonadotropin-releasing hormone in the ewe. Biol Reprod 36:1207–1218 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Karsch FJ 1990 The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology 127:1375–1384 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Locatelli A, Karsch FJ 1991 Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 129:1175–1182 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Brand RC, Karsch FJ 1992 Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology 130:2978–2984 [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Padmanabhan V, Thrun LA, Karsch FJ 1997 Estradiol requirements for induction and maintenance of the gonadotropin-releasing hormone surge: implications for neuroendocrine processing of the estradiol signal. Endocrinology 138:5408–5414 [DOI] [PubMed] [Google Scholar]
- Wintermantel TM CR, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE 2006 Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy D, Angelini NL, Belsham DD 1999 Estrogen directly represses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-α (ERα)- and ERβ-expressing GT1–7 GnRH neurons. Endocrinology 140:5045–5053 [DOI] [PubMed] [Google Scholar]
- Radovick S, Ticknor CM, Nakayama Y, Notides AC, Rahman A, Weintraub BD, Cutler Jr GB, Wondisford FE 1991 Evidence for direct estrogen regulation of the human gonadotropin-releasing hormone gene. J Clin Invest 88:1649–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL 2000 Detection of estrogen receptor-β messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 141:3506–3509 [DOI] [PubMed] [Google Scholar]
- Skynner MJ, Sim JA, Herbison AE 1999 Detection of estrogen receptor α and β messenger ribonucleic acids in adult gonadotropin-releasing hormone neurons. Endocrinology 140:5195–5201 [DOI] [PubMed] [Google Scholar]
- Skinner DC, Dufourny L 2005 Oestrogen receptor β-immunoreactive neurones in the ovine hypothalamus: distribution and colocalisation with gonadotropin-releasing hormone. J Neuroedocrinol 17:29–39 [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM 2007 Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci 27:1913–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SL, Ottem EN, Carpenter CD 2003 Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod 69:1771–1778 [DOI] [PubMed] [Google Scholar]
- Eyigor O, Lin W, Jennes L 2004 Identification of neurons in the female rat hypothalamus that express oestrogen receptor-α and vesicular glutamate transporter-2. J Neuroendocrinol 16:26–31 [DOI] [PubMed] [Google Scholar]
- Flugge G, Oertel WH, Wuttke W 1986 Evidence for estrogen-receptive GABAergic neurons in the preoptic/anterior hypothalamic area of the rat brain. Neuroendocrinology 43:1–5 [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA 2005 Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- Hori A, Honda S, Asada M, Ohtaki T, Oda K, Watanabe T, Shintani Y, Yamada T, Suenaga M, Kitada C, Onda H, Kurokawa T, Nishimura O, Fujino M 2001 Metastin suppresses the motility and growth of CHO cells transfected with its receptor. Biochem Biophys Res Commun 286:958–963 [DOI] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M 2001 Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 31:613–617 [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley Jr WF, Aparicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel J-C, Matsuda F, Chaussain J-L, Milgrom E 2003 Hypogonadotropic hypogonadism due to loss of function of the Kiss1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapatto R PJ, Zhang D, Chan Y-M, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara BS 2007 Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148:4927–4936 [DOI] [PubMed] [Google Scholar]
- Hoffman AR, Crowley Jr WF 1982 Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med 307:1237–1241 [DOI] [PubMed] [Google Scholar]
- Brailoiu GC, Dun SL, Ohsawa M, Yin D, Yang J, Chang JK, Brailoiu E, Dun NJ 2005 KiSS-1 expression and metastin-like immunoreactivity in the rat brain. J Comp Neurol 17:314–329 [DOI] [PubMed] [Google Scholar]
- Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC 2001 AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem 276:28969–28975 [DOI] [PubMed] [Google Scholar]
- Lee DK, Nguyen T, O’Neill GP, Cheng R, Liu Y, Howard AD, Coulombe N, Tan CP, Tang-Nguyen AT, George SR, O’Dowd BF 1999 Discovery of a receptor related to the galanin receptors. FEBS Lett 446:103–107 [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA 2005 Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- Parhar IS, Ogawa S, Sakuma Y 2004 Laser-captured single digoxigenin-labeled neurons of gonadotropin-releasing hormone types reveal a novel G protein-coupled receptor (Gpr54) during maturation in cichlid fish. Endocrinology 145:3613–3618 [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA 2004 Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80:264–272 [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE 2005 Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA 2005 Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA 2004 A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR 2004 Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinology 16:850–858 [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M 2005 Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 146:3917–3925 [DOI] [PubMed] [Google Scholar]
- Tovar S, Vazquez MJ, Navarro VM, Fernandez-Fernandez R, Castellano JM, Vigo E, Roa J, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M 2006 Effects of single or repeated intravenous administration of kisspeptin upon dynamic LH secretion in conscious male rats. Endocrinology 147:2696–2704 [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M 2004 Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145:4565–4574 [DOI] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM 2000 Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419 [DOI] [PubMed] [Google Scholar]
- Christian CA, Mobley JL, Moenter SM 2005 Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA 102:15682–15687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Moenter SM 2005 Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: a possible local feedback circuit. J Neurosci 25:5740–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM 2002 Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology 143:2284–2292 [DOI] [PubMed] [Google Scholar]
- Barry PH 1994 JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods 51:107–116 [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Moenter SM 2002 Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol 16:2255–2265 [DOI] [PubMed] [Google Scholar]
- Salazar P, Tapia R, Rogawski MA 2003 Effects of neurosteroids on epileptiform activity induced by picrotoxin and 4-aminopyridine in the rat hippocampal slice. Epilepsy Res 55:71–82 [DOI] [PubMed] [Google Scholar]
- Feng Z, Durand DM 2005 Decrease in synaptic transmission can reverse the propagation direction of epileptiform activity in hippocampus in vivo. J Neurophysiology 93:1158–1164 [DOI] [PubMed] [Google Scholar]
- Hille B 2001 Ionic channels of excitable membranes. 3rd ed. Sunderland, UK: Sinauer Associates, Inc. [Google Scholar]
- Christian CA, Moenter SM, Diurnal changes in NMDA and AMPA/KA receptor-mediated glutamate transmission to gonadotropin-releasing hormone (GnRH) neurons are associated with the GnRH/LH surge. Abstract viewer/itinerary planner, Proc 37th Annual Meeting of the Society for Neuroscience, San Diego, CA, 2007 (Abstract 518.4) [Google Scholar]
- Woolley CS, McEwen BS 1992 Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci 12:2549–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA 2006 Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Le W, Smith JT, Popa S, Clifton D, Steiner RA, Fos activation of KISS1 neurons is linked to the LH surge. Abstract viewer/itinerary planner, Proc 36th Annual Meeting of the Society for Neuroscience, Atlanta, GA, 2006 (Abstract 776.6) [Google Scholar]
- Adachi S, Yamada S, Takatsu S, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda KI 2007 Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53:367–378 [DOI] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Petersen SL 2002 Glutamatergic signaling through the N-methyl-d-aspartate receptor directly activates medial subpopulations of luteinizing hormone-releasing hormone (LHRH) neurons, but does not appear to mediate the effects of estradiol on LHRH gene expression. Endocrinology 143:4837–4845 [DOI] [PubMed] [Google Scholar]
- Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA 2007 The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci 27:12088–12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Beek EM, Swarts HJM, Wiegant VM 1999 Central administration of antiserum to vasoactive intestinal peptide delays and reduces luteinizing hormone and prolactin surges in ovariectomized, estrogen-treated rats. Neuroendocrinology 69:227–237 [DOI] [PubMed] [Google Scholar]
- van der Beek EM, van Oudheusden HJ, Buijs RM, van der Donk HA, van den Hurk R, Wiegant VM 1994 Preferential induction of c-fos immunoreactivity in vasoactive intestinal polypeptide-innervated gonadotropin-releasing hormone neurons during a steroid-induced luteinizing hormone surge in the female rat. Endocrinology 134:2636–2644 [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM, Vasoactive intestinal polypeptide (VIP) can excite GnRH neurons in an estradiol-dependent manner. Proc 38th Annual Meeting of the Society for the Study of Reproduction, Quebec City, Quebec, Canada, 2005, p 115 (Abstract) [Google Scholar]
- Pau KY, Spies HG 1986 Estrogen-dependent effects of norepinephrine on hypothalamic gonadotropin-releasing hormone release in the rabbit. Brain Res 399:15–23 [DOI] [PubMed] [Google Scholar]
- Temel S, Lin W, Lakhlani S, Jennes L 2002 Expression of estrogen receptor-α and cFos in norepinephrine and epinephrine neurons of young and middle-aged rats during the steroid-induced luteinizing hormone surge. Endocrinology 143:3974–3983 [DOI] [PubMed] [Google Scholar]
- Woller MJ, Terasawa E 1992 Estradiol enhances the action of neuropeptide Y on in vivo luteinizing hormone-releasing hormone release in the ovariectomized rhesus monkey. Neuroendocrinology 56:921–925 [DOI] [PubMed] [Google Scholar]
- Mizuno M, Gearing M, Terasawa E 2000 The role of neuropeptide Y in the progesterone-induced luteinizing hormone-releasing hormone surge in vivo in ovariectomized female rhesus monkeys. Endocrinology 141:1772–1779 [DOI] [PubMed] [Google Scholar]
- Palm IF, van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A 1999 Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience 93:659–666 [DOI] [PubMed] [Google Scholar]
- Palm IF, van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A 2001 The stimulatory effect of vasopressin on the luteinizing hormone surge in ovariectomized, estradiol-treated rats is time-dependent. Brain Res 901:109–116 [DOI] [PubMed] [Google Scholar]