Abstract
Stimulation of prostate growth is a major concern with testosterone therapy in older hypogonadal men. As a result, nonsteroidal selective androgen receptor modulators with anabolic activity but less prostate stimulation are being developed. Anabolic steroids might exhibit similar tissue selectivity. We hypothesized the anabolic steroid 19-nor-4-androstenediol-3β,17β-diol (3β,19-NA) would increase muscle, lean body mass (LBM), and bone mineral density (BMD) with little stimulation of prostate growth. Male Sprague Dawley rats were implanted with SILASTIC brand (Dow Corning, Midland, MI) capsules containing 3β,19-NA (4, 8, or 16 cm), dihydrotestosterone (DHT) (8 cm), 19-nortestosterone (16 cm), or four empty capsules after undergoing either a sham operation (intact) or orchidectomy (ORX). Serum gonadotropins, measured after 4, 8, or 24 wk of treatment, were significantly lower in 3β,19-NA-treated vs. untreated, intact, and ORX rats (P < 0.05), and testosterone was lowered by 3β,19-NA-treatment of intact animals. LBM and BMD were assessed after 20 wk, and 4 wk later, rats were killed for levator ani muscle and prostate weights. Compared with ORX rats, 3β,19-NA-treated rats had dose-dependent higher levator ani muscle weights, LBM, and BMD, which were similar to intact and DHT-treated rats at the highest 3β,19-NA dose. In contrast, prostate weights in all 3β,19-NA-treated groups were similar to ORX rats and lower than intact and DHT- and 19-nortestosterone-treated rats even at the highest 3β,19-NA dose. In summary, 3β,19-NA increases muscle and bone mass without significant stimulation of prostate growth, suggesting it may have some properties of a steroidal selective androgen receptor modulator. Anabolic steroids such as 3β,19-NA should be studied further to determine their mechanisms of tissue selectivity and effects in men.
A SIGNIFICANT CONCERN regarding androgen replacement therapy in older men is stimulation of prostate growth because the prostate is considered a highly androgen-sensitive tissue. Ideally, androgen replacement therapy would provide the anabolic benefits of testosterone including increasing muscle mass, decreasing fat mass, improving physical performance (1), and increasing bone mineral density (BMD) (2) without stimulating prostate growth. Small molecules with such tissue selectivity, termed selective androgen receptor modulators (SARMs), are currently under development (3).
Anabolic steroid analogs of naturally occurring androgens (termed anabolic steroids) were developed to promote anabolic actions, primarily muscle growth, with relatively less androgenic activity, i.e. SARM-like activity. Recently, anabolic steroids, such as 19-nortestosterone (19-NT, also called nandrolone) and oxandrolone, have been demonstrated to stimulate muscle mass in wasting conditions (e.g. HIV and chronic kidney disease) (4,5,6). Commonly, anabolic steroids are abused by athletes and nonathletes to enhance muscle mass and strength, physical performance, and appearance (7).
19-Nor-4-androstene-3β,17β-diol (3β,19-NA, also known as bolandiol or 3β,17β-dihydroxyestr-4-ene, abbreviated as estren-β) is an anabolic steroid that until recently was available as a dietary supplement (referred to as 19-norandrostenediol or 17β-diol) and used by athletes to enhance performance. It has been suggested that the anabolic activity of 3β,19-NA is attributable to its metabolism to 19-NT (8), a potent anabolic steroid, and that such conversion occurs in vivo in humans (9,10,11,12). 19-NT has been suggested to be a pure androgen, in that it is minimally aromatized and its 5α-reduced metabolite has very little androgenic activity (9,13,14,15), although this latter point is controversial (16). Furthermore, the end-organ effects of 3β,19-NA administration in men are poorly characterized.
The in vivo effects of the diastereomer of 3β,19-NA, estr-4-ene-3α,17β-diol (estren-α) in rodents is controversial (17,18,19). Some studies suggest estren-α has tissue-specific properties that make it more active in bone, for example, compared with reproductive organs (20,21,22), supporting estren-α as a SARM or selective estrogen receptor modulator. However, a more recent paper did not corroborate these findings (17). Furthermore, the latter authors reported that 3β,19-NA (estren-β), similar to estren-α, lacked tissue selectivity in their mouse model (17). This is in contrast to previous data from Saunders and Drill (23) demonstrating greater myotrophic vs. androgenic effects from im injection of 3β,19-NA in rats.
In this study, we sought to determine the effects of 3β19-NA administration on endogenous reproductive hormone production, body composition, including BMD and muscle mass, the androgen-sensitive levator ani muscle, and reproductive tissues in a Sprague Dawley rat model. Based on its similarity to 19-NT, we hypothesized that 3β,19-NA could act as a SARM with greater anabolic activity in muscle and bone compared with growth-stimulatory effects on reproductive tissues. Furthermore, we compared the activity of 3β,19-NA to high-dose 19-NT and dihydrotestosterone (DHT) in vivo to explore whether these androgens demonstrate different tissue selectivity, suggesting unique mechanisms of action.
Materials and Methods
Animals
Male Sprague Dawley rats were purchased (Harlan Sprague Dawley, Inc., Indianapolis, IN) and housed in an American Association for Accreditation of Laboratory Animal Care-accredited facility at the Veteran’s Administration Puget Sound Health Care System (Seattle, WA). Animals were individually housed in polycarbonate rat cages containing corncob bedding in a light- and temperature-controlled room on a 12-h light, 12-h dark cycle (lights off from 1800–0600 h). Animals had ad libitum access to Purina rodent chow (5001; Ralston Purina Co., St. Louis, MO) and tap water. All animal experiments were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Veteran’s Administration Puget Sound Health Care System Institutional Animal Care and Use Committee.
Hormone delivery
SILASTIC brand capsules were made using medical grade SILASTIC brand tubing (no. 602-285; id, 0.062 in.; od, 0.125 in.), filled with 3β,19-NA, DHT, or 19-NT (all from Steraloids, Newport, RI), both ends plugged with silicone adhesive (Dow Corning; no. 891), and incubated overnight in sterile saline at 4 C before implantation. Capsules were filled to 4 cm in length for all hormones. A variable number of 3β,19-NA-filled capsules (one, two, or four capsules) were implanted in each experiment as outlined below to generate a dose response. Rats implanted with an empty, two DHT-filled, or four 19-NT-filled capsules were used for comparison groups. Each steroid-filled capsule contained approximately 48 mg steroid hormone.
Procedures
Bilateral orchidectomy (ORX) with sparing of the epididymal fat pad or sham operation (a 1- to 2-cm abdominal incision) was performed using aseptic procedures under isoflurane anesthesia. At the time of surgery, steroid-filled or empty SILASTIC brand capsules were implanted sc in the intrascapular region.
Experimental design: short-term studies in intact and ORX rats
To determine the effect of high-dose 3β,19-NA on the reproductive hormones (LH, FSH, testosterone) and androgen-sensitive tissues (testes, levator ani, prostate, and seminal vesicles), two groups of intact adult male Sprague Dawley rats weighing 280–300 g each (10 rats per group) underwent sham operation and were implanted with either four 4-cm empty SILASTIC brand capsules (intact) or four 4-cm 3β, 19-NA-filled SILASTIC brand capsules (intact plus 16 cm 3β,19-NA). Eight weeks after surgery, the rats were killed by decapitation, and trunk blood was collected for measurement of serum testosterone, LH, and FSH levels. The testes, levator ani (an androgen-responsive skeletal muscle that is used as a classical endpoint for anabolic activity of anabolic steroids), and prostate and seminal vesicles (androgen-dependent reproductive glands that are used as classical endpoints of androgenic activity of anabolic steroids) (24) were dissected and weighed. In all cases, data for organ weights are presented after correction for total body weight because ORX and high-dose androgen treatment in rats is associated with significant reductions in total body weight gain over time (25,26,27).
A parallel experiment was conducted to determine the dose-response effects of 3β,19-NA on pituitary gonadotropins (LH and FSH) and androgen-sensitive tissues (levator ani muscle, prostate, and seminal vesicles) in ORX animals. Three groups of adult male Sprague Dawley rats weighing 285–310 g each (10 rats per group) were ORX and implanted with one (ORX plus 4 cm 3β,19-NA), two (ORX plus 8 cm 3β,19-NA), or four (ORX plus 16 cm 3β,19-NA) 4-cm 3β,19-NA-filled SILASTIC brand capsules. A group of male ORX Sprague Dawley rats (285–310 g, n = 5) implanted with four 4-cm empty SILASTIC brand capsules was included as a control (ORX). Four weeks after surgery, the rats were killed, and trunk blood was collected for measurement of serum hormones, and the levator ani muscle, prostate, and seminal vesicles were dissected and weighed.
Experimental design: long-term studies in intact and ORX rats
To determine the effects of long-term administration of various doses of 3β,19-NA compared with high-dose DHT and 19-NT on body composition and androgen-sensitive tissues, 3-wk-old prepubertal male Sprague Dawley rats were divided into seven groups (n = 28–29 per group). One group of animals underwent a sham operation, and the remainder were ORX. The sham-operated animals were implanted with a 4-cm empty SILASTIC brand capsules (intact). The ORX animals were implanted with either four 4-cm empty SILASTIC brand capsules (ORX), one (ORX plus 4 cm 3β,19-NA), two (ORX plus 8 cm 3β,19-NA), or four (ORX plus 16 cm 3β,19-NA) 3β,19-NA-filled SILASTIC brand capsules; four 4-cm 19-NT-filled SILASTIC brand capsules (ORX plus 16 cm 19-NT); or two 4-cm DHT-filled SILASTIC brand capsules (ORX plus 8 cm DHT). Twenty weeks after surgery, body composition was measured by dual-energy x-ray absorptiometry (DEXA) during pentobarbital anesthesia. After 4 wk of recovery from the anesthesia used for the DEXA, the rats were killed, and the levator ani, prostate, and seminal vesicles were dissected and weighed. As in the short-term studies, correction was made for total body weight for comparisons of organ weights between treatment groups.
Hormone assays
Serum was stored at −30 C until hormone assays were performed in duplicate. All samples from each experiment were analyzed together for each assay. Testosterone was measured by fluoroimmunoassay (Delfia; PerkinElmer, Waltham, MA) (28). Assay sensitivity was 0.35 ng/ml, inter- and intraassay coefficients of variation were 5.3 and 6.7%, respectively. Rat FSH was measured using an immunoradiometric assay kit (American Laboratory Products Co., Windham, NH). The limit of detection for FSH was 0.2 ng/ml. The mean intra- and interassay coefficients of variation for FSH were 2.3 and 7.8%, respectively, as reported by the manufacturer. Rat LH was measured using a modified, sensitive immunofluorometric assay described by Haavisto (29). The rat LH standard HIDDK rLH RP-3 was obtained from the National Institutes of Health (Bethesda, MD), capture antibody 518 B7 kindly provided by Dr. J. F. Roser (Department of Animal Science, University of California, Davis, California), and tracer antibody anti-hLH 5303 obtained from Medix Biochemica (Kauniainen, Finland) and labeled with europium using a Delfia labeling kit (PerkinElmer). The assay buffer and streptavidin-labeled plates were obtained from PerkinElmer. The lower limit of detection and quantitation for the LH assay was 0.04 ng/ml. The mean intra- and interassay coefficients of variation were 4.6 and 3.8%, respectively.
Measurements of body composition and BMD
Body composition (lean mass, fat mass, and BMD) were measured by DEXA (QDR 4500; Hologic, Inc., Waltham, MA) as described previously (30). Nonfasted rats were weighed, anesthetized with an ip injection of 60 mg/kg pentobarbital, and received body temperature support with the use of warm pads. Animals were placed on the scanning platform in dorsal recumbent, positioned, and scanned using the Rat Whole Body software package (version 5.67; Choplogic, Inc., Waltham, MA). Intraassay coefficients of variation were 0.6, 5.6, and 1.7% for lean mass, fat mass, and BMD respectively. Interassay coefficients of variation were 1.0, 6.0, and 1.5% for lean mass, fat mass, and BMD, respectively. Because body weights of animals in different experimental groups varied, both lean mass and fat mass were normalized to total body weight. BMD is bone mineral content normalized for body surface area.
Statistical analyses
Hormone levels, tissue weights, and body composition (lean and fat mass and BMD) were compared among treatment groups in each study by two-way ANOVA. Post hoc testing by Fisher’s protected least significant difference test was performed for between-treatment differences separately for each experimental group. Values for the lower limit of quantitation were used in calculating mean values when results were below this level. Results are reported as the mean ± sem, and P < 0.05 was considered significant. The statistical software package used was StatView version V5.0.1 for the Macintosh (Abacus Concepts, Inc., Berkeley, CA; and SAS Institute, Inc., Cary, NC).
Results
3β,19-NA suppresses the hypothalamic-pituitary-testicular axis
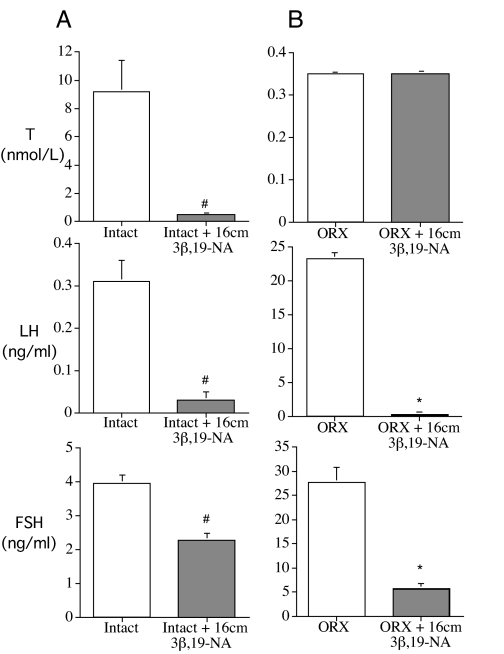
After 8 wk of treatment, 3β,19-NA produced a chemical castration in intact animals. Testosterone was significantly suppressed in 3β,19-NA-treated intact rats compared with intact animals implanted with empty capsules (0.5 ± 0.1 nmol/liter for intact plus 16 cm 3β,19-NA vs. 9.2 ± 2.2 nmol/liter for intact). Likewise, 3β,19-NA-treated rats had significantly lower serum LH (0.04 ± 0.01 ng/ml for intact plus 16 cm 3β,19-NA vs. 0.28 ± 0.04 ng/ml for intact) and FSH (2.27 ± 0.20 ng/ml for intact plus 16 cm 3β,19-NA vs. 3.95 ± 0.2 ng/ml for intact) levels compared with untreated intact animals (Fig. 1A).
Figure 1.
Serum levels of hypothalamic-pituitary-testicular axis hormones testosterone (T), rat LH, and rat FSH in rats. Note different y-axis scales in each panel. A, Sham-operated animals implanted with 16-cm empty SILASTIC brand capsules (intact) or 16-cm 3β,19-NA-filled SILASTIC brand capsules for 8 wk. B, ORX animals implanted with 16-cm empty SILASTIC brand capsules or 16-cm 3β,19-NA-filled SILASTIC brand capsules for 4 wk. #, P < 0.05 vs. intact; *, P < 0.05 vs. ORX plus placebo. Error bars show sem.
Similarly, in ORX animals, administration of 3β,19-NA for 4 wk prevented the increase in LH and FSH associated with surgical castration without increasing testosterone levels (Fig. 1B). Gonadotropin suppression by 3β,19-NA in ORX rats was dose responsive (LH, 23.3 ± 0.8 ng/ml for ORX vs. 19.8 ± 1.4 ng/ml for ORX plus 4 cm 3β,19-NA, 6.6 ± 2.0 ng/ml for ORX plus 8 cm 3β,19-NA, and 0.3 ± 0.1 ng/ml for ORX plus 16 cm 3β,19-NA, data not shown). Likewise, 24 wk after ORX, there was a dose-responsive suppression of LH production by 3β,19-NA compared with untreated animals (LH, 7.1 ± 0.8 ng/ml for ORX plus 4 cm 3β,19-NA, 1.3 ± 0.2 ng/ml for ORX plus 8 cm 3β,19-NA, and 0.04 ± 0.01 ng/ml for ORX plus 16 cm 3β,19-NA vs. 18.3 ± 1.3 ng/ml for ORX).
Selective effects of 3β,19-NA on androgen-sensitive tissues
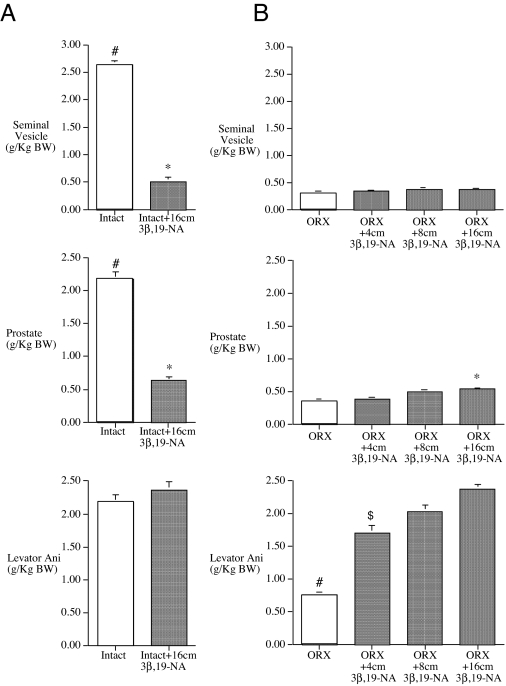
After 8 wk of treatment, 3β,19-NA-treated intact rats demonstrated markedly lower prostate, seminal vesicle (Fig. 2A), and testis weights (6.83 ± 0.2 g/kg for intact plus 16 cm 3β,19-NA vs. 8.23 ± 0.23 g/kg) compared with untreated, intact animals. In contrast, levator ani weight, an androgen-sensitive skeletal muscle biomarker of anabolic activity, in 3β,19-NA-treated intact rats was similar to that in untreated, intact animals (Fig. 2A) despite markedly suppressed serum testosterone levels (Fig. 1) and reproductive gland weights (Fig. 2A).
Figure 2.
Weight of androgen-responsive tissues divided by body weight for intact and ORX animals either treated with either empty or various doses of 3β,19-NA-filled SILASTIC brand capsules for 8 wk (intact) (A) or 4 wk (ORX) (B). #, P < 0.05 vs. all other groups; *, P < 0.05 vs. ORX plus placebo; $, P < 0.05 vs. intact. Error bars show sem.
Similar results were observed in ORX rats treated with increasing doses of 3β,19-NA for 4 wk. At all doses, 3β,19-NA failed to prevent the loss of seminal vesicle weight associated with ORX (Fig. 2B). 3β,19-NA produced a limited stimulation of the prostate with only rats treated with the highest dose (ORX plus 16 cm 3β,19-NA) having slightly higher prostate weight compared with that in untreated, ORX animals (0.54 ± 0.4 g/kg for ORX plus 16 cm 3β,19-NA vs. 0.34 ± 0.3 g/kg for ORX). Prostate weights for all doses of 3β,19-NA-treated ORX animals remained significantly less than that in intact animals (Fig. 2B). In contrast, all 3β,19-NA-treated ORX rats had significantly higher levator ani muscle weights compared with untreated ORX rats, which were similar to that of untreated intact animals in the initial 8-wk study (Fig. 2A).
Long-term effects of 3β,19-NA on body composition and BMD
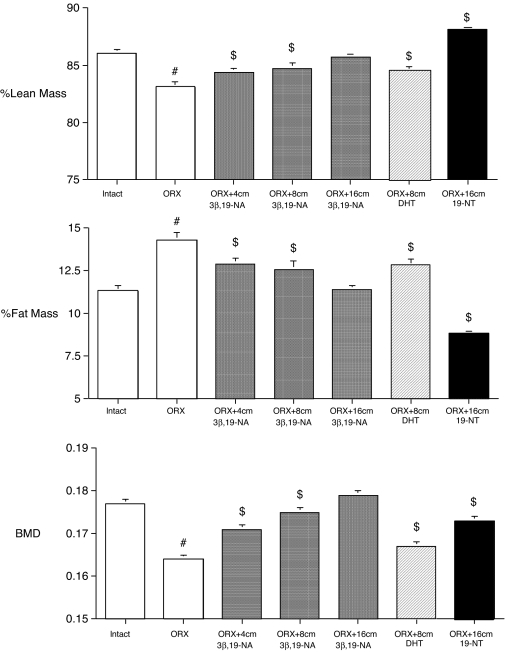
Twenty weeks after surgery, untreated ORX rats had a lower percentage lean mass and BMD but a higher percentage fat mass, compared with intact animals (Fig. 3). However, ORX rats who received 3β,19-NA capsules at the time of ORX maintained their body composition and BMD in a progressive, dose-dependent fashion, compared with untreated ORX animals. Moreover, in rats that received the highest dose of 3β,19-NA (ORX plus 16 cm 3β,19-NA), percentage lean and fat mass and BMD were no different from those of untreated, intact animals (Fig. 3).
Figure 3.
Percent lean mass, percent fat mass, and BMD measured by DEXA at 20 wk in sham-operated (intact plus placebo), ORX, and ORX rats treated with 4-, 8-, or 16-cm 3β,19-NA; 8-cm DHT; and 16-cm 19-NT SILASTIC brand capsules. $, P < 0.05 vs. intact; #, P < 0.05 vs. all other groups. Error bars show sem.
High-dose DHT-treated ORX rats (ORX plus 8 cm DHT), had a slight but significantly higher percentage of lean mass and BMD and a lower percentage of fat mass compared with the untreated ORX animals (Fig. 3). However, this dose of DHT treatment in ORX rats did not restore body composition and BMD to the levels of untreated (intact) animals. In contrast, high-dose 19-NT-treated ORX rats (ORX plus 16 cm 19-NT) had a significantly higher percentage of lean mass and lower percentage of fat mass compared with both the untreated ORX and sham-operated (intact) animals. Although 19-NT-treated rats had a significantly higher BMD compared with the untreated ORX animals and similar to lower-dose 3β,19-NA-treated rats, BMD was not restored to the level of untreated intact animals or animals treated with the highest dose of 3β,19-NA (ORX plus 16 cm 3β,19-NA) (Fig. 3).
Long-term tissue-selective effects of 3β,19-NA on androgen-responsive tissues
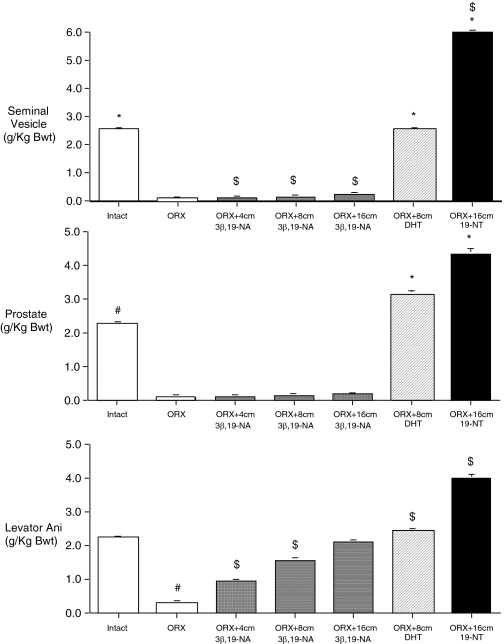
In the same experiment, 24 wk after surgery, seminal vesicle and prostate weights and levator ani weight were markedly lower in untreated ORX compared with untreated intact rats (Fig. 4). 3β,19-NA-treated ORX animals demonstrated dose-dependent higher levator ani weights but seminal vesicle and prostate weights that were not significantly different from those in untreated ORX rats (Fig. 4). The weight of the levator ani muscle in the highest-dose 3β,19-NA group (ORX plus 16 cm 3β,19-NA = 1.035 ± 0.017 g) was not significantly different from the untreated intact group (intact = 1.151 ± 0.027 g), whereas seminal vesicle and prostate weights for all doses of 3β,19-NA remained significantly lower than that of untreated intact and similar to ORX rats.
Figure 4.
Weight of androgen-responsive tissues divided by body weight after 24 wk of treatment. Sham-operated animals had four empty capsules placed (intact). ORX animals had four empty capsules placed (ORX) or were treated with 4-, 8-, or 16-cm 3β,19-NA-; 8-cm DHT-; or 16-cm 19-NT-filled SILASTIC brand capsules. *, P < 0.05 vs. ORX; $, P < 0.05 vs. intact; #, P < 0.05 vs. all other groups. Error bars show sem.
In contrast to 3β,19-NA, 24 wk of high-dose DHT (ORX plus 8 cm DHT) and 19-NT (ORX plus 16 cm 19-NT) treatment resulted in significantly higher weights of the seminal vesicles, prostate, and levator ani compared with the untreated ORX animals. In fact, the final weights of these androgen-sensitive tissues were equal to or significantly greater than those of the untreated intact group after treatment with DHT or 19-NT (Fig. 4). A dose of DHT that maintained levator ani weight similar to that in intact rats resulted in seminal vesicle and prostate weights that were higher than those in intact rats. In contrast, high-dose 3β,19-NA maintained levator ani weight similar to that in DHT and intact rats but resulted in seminal vesicle and prostate weights that were similar to untreated ORX animals. Although maintenance of levator ani weight suggests significant androgenic activity of DHT on muscle, anabolic activity was not corroborated by percentage of lean mass, which remained significantly lower in DHT-treated rats compared with that in intact animals.
Discussion
In this study, we found that long-term administration of 3β,19-NA to ORX rats prevented the loss in lean body mass, BMD, and levator ani muscle weight and the gain in fat mass associated with castration in a dose-dependent fashion. These effects were similar to those observed after administration of DHT and 19-NT to ORX animals. However, 3β,19-NA administration resulted in little or no stimulation of the prostate and seminal vesicle weights at doses effective at preventing reductions in lean mass, BMD, and levator ani muscle weight and increases in fat mass that occurred after ORX. Furthermore, the in vivo action of 3β,19-NA on body composition and bone could not be attributed to endogenous testosterone action, because 3β,19-NA administration to ORX rats resulted in maintenance of body composition and bone mass and to adult intact animals resulted in maintenance of muscle mass despite castrate circulating testosterone levels, i.e. chemical castration. The suppressive effect of 3β,19-NA at the level of the pituitary was confirmed in ORX rats, where 3β,19-NA administration suppressed high levels of FSH and LH production resulting from loss of negative feedback in the absence of endogenous testosterone production from the testis.
The goal of SARM development is to produce molecules that provide the anabolic benefits of androgens to men while avoiding or minimizing growth stimulation to the prostate (3,31,32). In the ORX rat model presented here, it appears that 3β,19-NA may fulfill some of these criteria at the doses given. After 8 wk of treatment in ORX rats (Fig. 2B), an 8-cm implant of 3β,19-NA maintained levator ani weight, whereas the loss of prostate was similar to ORX animals. Similar selectivity was achieved with longer treatment using higher doses of 3β,19-NA (16 cm 3β,19-NA for 24 wk, Fig. 4). Whether the observed pharmacodynamic response to 3β,19-NA implants, which likely provide constant stimulation of receptors, might be altered with diurnal or pulsatile administration is not known and will be the subject of additional studies.
It is possible that the activity of 3β,19-NA is mediated via its conversion by 3-β hydroxysteroid dehydrogenase to the anabolic steroid 19-NT, as has been suggested by others (33,34), because increased urinary metabolites of 19-NT can be measured after oral 3β,19-NA dosing in men (35,36,37). 19-NT has some properties of a SARM, with greater anabolic activity for muscle compared with prostate (14), perhaps due to the low androgenicity of its 5α-reduced metabolite, 5α-dihydronortestosterone (38). However, the effects of 19-NT on bone metabolism are unclear. Estrogens are thought to play an important role in maintaining bone in men, and 19-NT is purported to be a nonaromatizable androgen (10,15), although this is controversial (39). Studies in aging male rats suggested 19-NT is as potent as testosterone at maintaining BMD (40), but in men receiving glucocorticoids, 19-NT was much less potent than more efficiently aromatized androgens on bone (10). Although we have limited dosing information, in our study, 3β,19-NA was more effective than 19-NT at maintaining BMD compared with prostate weight (Figs. 3 and 4) in this in vivo rat model. It is possible that 19-NT used at lower doses than those used here might maintain muscle and bone mass in ORX rats at doses that are relatively prostate sparing (14); thus, our data cannot rule out the possibility that 3β,19-NA action is mediated after in vivo conversion to 19-NT.
An alternative or additional explanation for our findings is that some or all of the 3β,19-NA administered is aromatized or 5α-reduced before conversion to 19-NT and that these metabolites mediate the bioactivity of 3β,19-NA compared with 19-NT. In addition, tissue-specific metabolism or cofactor recruitment might play a role in specific end-organ effects. Additional studies comparing the serum concentrations of these 19-norsteroids, their metabolites, and their dosing effects will be necessary to determine this aspect of 3β,19-NA action and to determine whether there are differences in the metabolism of 3β,19-NA in rodents and men.
The specific mechanism of action of 3β,19-NA in cells is not well characterized. 3β,19-NA appears to act through the androgen receptor, with a much higher binding affinity for the androgen receptor than the estrogen receptor in vitro (17) and similar bioactivity in estrogen receptor-deficient animals compared with wild-type controls (17). It has recently been proposed that the α-isomer of 3β,19-NA, estren-α, exerts tissue-specific effects by recruiting nongenomic signaling pathways after binding to the androgen/estrogen receptor (41). However, both the mechanism of action and tissue specificity of estren-α has been called into question by Windahl et al. (17). In contrast to results from Kousteni et al. (20), Windahl et al. (17) found that in 12-wk-old C57BL/6 ORX mice, estren-α was as effective as DHT in preventing bone loss and turnover after ORX and in stimulating seminal vesicle weights. Furthermore, the activity of estren-α was blocked by antiandrogens and similar in ERα knockout mice, implying that its actions were mediated primarily through the androgen receptor. Results were reported as similar for experiments using 3β,19-NA (estren-β) implants, although the authors show data suggesting that 3β,19-NA has a greater affinity for the androgen receptor than estren-α (17).
Although only the present study has characterized the effects of 3β,19-NA on the prostate, the reason for the inconsistent results between the two previous studies with regard to the effects of 3β,19-NA and estren-α on seminal vesicle weights is not clear. It is possible that these discrepancies are attributable to differences in dosing or delivery of 3β,19-NA, as has been postulated (19). However, in contrast to Windahl et al. (17), direct comparison of similar doses 3β,19-NA and DHT in our study confirmed the relative lack of stimulation of seminal vesicles (and prostate) weight despite greater maintenance of muscle and bone mass in ORX rats treated with 3β,19-NA compared with DHT (Fig. 4). Future studies including examination of tissue hormone levels will help determine the role of dosing in these results or whether differential signaling by unique androgens is a critical factor in apparent tissue selectivity.
Until recently, 3β,19-NA was available as a dietary supplement that was marketed to increase muscle mass and performance and shorten recovery time. However, there are very few human trials examining the effects of 3β,19-NA in men. van Gammeren et al. (33,34) reported two small, randomized, placebo-controlled trials in resistance-trained athletes supplemented orally with a combination of 19-norandrostenedione and 3β,19-NA (also known as 19-norandrostenediol) for 8 wk. In neither of these studies did oral administration of 19-norsteroids result in changes in body composition or muscle performance. However, serum levels of 3β,19-NA and its metabolites were not measured, and the bioavailability of oral 3β,19-NA is not known. In addition, these negative results may have been confounded by studying resistance-trained athletes who may have nearly maximized their percent lean body mass at the start of the study and may have been taking other supplements before the study or the relatively short time (8 wk) of drug exposure. In neither of these studies was endocrine or reproductive function examined; thus, the effects of 3β,19-NA in men on these parameters are not known.
In summary, 3β,19-NA is a potent steroid acting to maintain muscle and bone with markedly reduced prostatic and reproductive activity in both ORX and intact rats. 3β,19-NA had a direct effect on the hypothalamus and/or pituitary, reducing serum LH and FSH concentrations in both intact and ORX animals; thus, its anabolic effects were not due to increased testosterone production. These tissue-specific characteristics suggest that 3β,19-NA may share some properties with SARMs, similar to that of 17α-methyl-19-nortestosterone, in this rodent model. However, the mechanism by which the apparent tissue selectivity of 3β,19-NA is not known. The action of 3β,19-NA may have a weaker direct effect on selective tissues or require conversion to 19-NT such that a higher dose is required to significantly increase the mass of androgenic tissues (seminal vesicles and prostate) compared with muscle and bone. More studies examining the tissue-specific effects of 3β,19-NA and its metabolism in men are warranted.
Acknowledgments
We thank Dr. John K. Amory for helpful discussions and careful reading of this manuscript and Dr. J. F. Roser for providing antibody for the rat LH assay.
Footnotes
This work was supported by Drug Enforcement Administration Grant 03-P-103 and the Department of Veterans Affairs. S.T.P. is supported by the National Institute of Aging, a Division of the National Institutes of Health, through Grant K23 AG027238 and by the Endocrine Society Solvay Clinical Research Award.
Disclosure Summary: S.T.P. has received grant support from GlaxoSmithKline and Ascend Therapeutics. A.M.M. has consulted for GlaxoSmithKline, QuatRx, Solvay, Amgen, Threshold, and GTx Pharmaceuticals and received grant support from GlaxoSmithKline, Ascend Therapeutics, Ardana, and Solvay Pharmaceuticals.
First Published Online December 20, 2007
Abbreviations: BMD, Bone mineral density; DHT, dihydrotestosterone; DEXA, dual-energy x-ray absorptiometry; estren-α, estr-4-ene-3α,17β-diol; estren-β, 3β,17β-dihydroxyestr-4-ene; ORX, orchidectomy; 3β,19-NA, 19-nor-4-androstene-3β,17β-diol; 19-NT, 19-nortestosterone; SARM, selective androgen receptor modulator.
References
- Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL 2005 Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab 90:1502–1510 [DOI] [PubMed] [Google Scholar]
- Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL 2004 Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab 89:503–510 [DOI] [PubMed] [Google Scholar]
- Gao W, Dalton JT 2007 Expanding the therapeutic use of androgens via selective androgen receptor modulators (SARMs). Drug Discov Today 12:241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J, Batterham MJ, Rekers H, Harms MK, Geurts TB, Helmyr PM, Silva de Mendonca J, Falleiros Carvalho LH, Panos G, Pinchera A, Aiuti F, Lee C, Horban A, Gatell J, Phanuphak P, Prasithsirikul W, Gazzard B, Bloch M, Danner SA 2006 Effects of nandrolone decanoate compared with placebo or testosterone on HIV-associated wasting. HIV Med 7:146–155 [DOI] [PubMed] [Google Scholar]
- Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T 2006 Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J Am Soc Nephrol 17:2307–2314 [DOI] [PubMed] [Google Scholar]
- Orr R, Fiatarone Singh M 2004 The anabolic androgenic steroid oxandrolone in the treatment of wasting and catabolic disorders: review of efficacy and safety. Drugs 64:725–750 [DOI] [PubMed] [Google Scholar]
- Yesalis CE 2001 Use of steroids for self-enhancement: an epidemiologic/societal perspective. AIDS Read 11:157–160 [PubMed] [Google Scholar]
- Schrader Y, Thevis M, Schanzer W 2006 Quantitative determination of metabolic products of 19-norandrostenediol in human plasma using gas chromatography/mass spectrometry. Drug Metab Dispos 34:1328–1335 [DOI] [PubMed] [Google Scholar]
- Chung T, Kelleher S, Liu PY, Conway AJ, Kritharides L, Handelsman DJ 2007 Effects of testosterone and nandrolone on cardiac function: a randomized, placebo-controlled study. Clin Endocrinol (Oxf) 66:235–245 [DOI] [PubMed] [Google Scholar]
- Crawford BA, Liu PY, Kean MT, Bleasel JF, Handelsman DJ 2003 Randomized placebo-controlled trial of androgen effects on muscle and bone in men requiring long-term systemic glucocorticoid treatment. J Clin Endocrinol Metab 88:3167–3176 [DOI] [PubMed] [Google Scholar]
- Frisoli Jr A, Chaves PH, Pinheiro MM, Szejnfeld VL 2005 The effect of nandrolone decanoate on bone mineral density, muscle mass, and hemoglobin levels in elderly women with osteoporosis: a double-blind, randomized, placebo-controlled clinical trial. J Gerontol A Biol Sci Med Sci 60:648–653 [DOI] [PubMed] [Google Scholar]
- Kuipers H, Wijnen JA, Hartgens F, Willems SM 1991 Influence of anabolic steroids on body composition, blood pressure, lipid profile and liver functions in body builders. Int J Sports Med 12:413–418 [DOI] [PubMed] [Google Scholar]
- Toth M, Zakar T 1982 Relative binding affinities of testosterone, 19-nortestosterone and their 5α-reduced derivatives to the androgen receptor and to other androgen-binding proteins: a suggested role of 5α-reductive steroid metabolism in the dissociation of “myotropic” and “androgenic” activities of 19-nortestosterone. J Steroid Biochem 17:653–660 [DOI] [PubMed] [Google Scholar]
- Sundaram K, Kumar N, Monder C, Bardin CW 1995 Different patterns of metabolism determine the relative anabolic activity of 19-norandrogens. J Steroid Biochem Mol Biol 53:253–257 [DOI] [PubMed] [Google Scholar]
- Behre HM, Kliesch S, Lemcke B, von Eckardstein S, Nieschlag E 2001 Suppression of spermatogenesis to azoospermia by combined administration of GnRH antagonist and 19-nortestosterone cannot be maintained by this non-aromatizable androgen alone. Hum Reprod 16:2570–2577 [DOI] [PubMed] [Google Scholar]
- Cunningham GR, Tindall DJ, Means AR 1979 Differences in steroid specificity for rat androgen binding protein and the cytoplasmic receptor. Steroids 33:261–276 [DOI] [PubMed] [Google Scholar]
- Windahl SH, Galien R, Chiusaroli R, Clement-Lacroix P, Morvan F, Lepescheux L, Nique F, Horne WC, Resche-Rigon M, Baron R 2006 Bone protection by estrens occurs through non-tissue-selective activation of the androgen receptor. J Clin Invest 116:2500–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill US 2006 You say estren, I say estrogen. Let’s call the whole replacement off! J Clin Invest 116:2327–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC, Jilka RL, Kousteni S, Bellido T, Weinstein RS, O’Brien CA, Plotkin L, Han L 2006 Response to Windahl et al. J Clin Invest 116:2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O’Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC 2002 Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science 298:843–846 [DOI] [PubMed] [Google Scholar]
- Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, Manolagas SC 2003 Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest 111:1651–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC, Kousteni S 2001 Perspective: nonreproductive sites of action of reproductive hormones. Endocrinology 142:2200–2204 [DOI] [PubMed] [Google Scholar]
- Saunders FJ, Drill VA 1956 The myotrophic and androgenic effects of 17-ethyl-19-nortestosterone and related compounds. Endocrinology 58:567–572 [DOI] [PubMed] [Google Scholar]
- Hershberger LG, Shipley EG, Meyer RK 1953 Myotrophic activity of 19-nortestosterone and other steroids determined by modified levator ani muscle method. Proc Soc Exp Biol Med 83:175–180 [DOI] [PubMed] [Google Scholar]
- Gao W, Kim J, Dalton JT 2006 Pharmacokinetics and pharmacodynamics of nonsteroidal androgen receptor ligands. Pharm Res 23:1641–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward CJ 1993 A re-evaluation of the anabolic effect of testosterone in rats: interactions with gonadectomy, adrenalectomy and hypophysectomy. Acta Endocrinol (Copenh) 128:473–477 [DOI] [PubMed] [Google Scholar]
- Gentry RT, Wade GN 1976 Androgenic control of food intake and body weight in male rats. J Comp Physiol Psychol 90:18–25 [DOI] [PubMed] [Google Scholar]
- Sohn EH, Wolden-Hanson T, Matsumoto AM 2002 Testosterone (T)-induced changes in arcuate nucleus cocaine-amphetamine-regulated transcript and NPY mRNA are attenuated in old compared to young male brown Norway rats: contribution of T to age-related changes in cocaine-amphetamine-regulated transcript and NPY gene expression. Endocrinology 143:954–963 [DOI] [PubMed] [Google Scholar]
- Haavisto AM, Pettersson K, Bergendahl M, Perheentupa A, Roser JF, Huhtaniemi I 1993 A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology 132:1687–1691 [DOI] [PubMed] [Google Scholar]
- Wolden-Hanson T, Marck BT, Smith L, Matsumoto AM 1999 Cross-sectional and longitudinal analysis of age-associated changes in body composition of male Brown Norway rats: association of serum leptin levels with peripheral adiposity. J Gerontol A Biol Sci Med Sci 54:B99–B107 [DOI] [PubMed] [Google Scholar]
- Miner JN, Chang W, Chapman MS, Finn PD, Hong MH, Lopez FJ, Marschke KB, Rosen J, Schrader W, Turner R, van Oeveren A, Viveros H, Zhi L, Negro-Vilar A 2007 An orally active selective androgen receptor modulator is efficacious on bone, muscle, and sex function with reduced impact on prostate. Endocrinology 148:363–373 [DOI] [PubMed] [Google Scholar]
- Ostrowski J, Kuhns JE, Lupisella JA, Manfredi MC, Beehler BC, Krystek Jr SR, Bi Y, Sun C, Seethala R, Golla R, Sleph PG, Fura A, An Y, Kish KF, Sack JS, Mookhtiar KA, Grover GJ, Hamann LG 2007 Pharmacological and x-ray structural characterization of a novel selective androgen receptor modulator: potent hyperanabolic stimulation of skeletal muscle with hypostimulation of prostate in rats. Endocrinology 148:4–12 [DOI] [PubMed] [Google Scholar]
- van Gammeren D, Falk D, Antonio J 2002 Effects of norandrostenedione and norandrostenediol in resistance-trained men. Nutrition 18:734–737 [DOI] [PubMed] [Google Scholar]
- van Gammeren D, Falk D, Antonio J 2001 The effects of supplementation with 19-nor-4-androstene-3,17-dione and 19-nor-4-androstene-3,17-diol on body composition and athletic performance in previously weight-trained male athletes. Eur J Appl Physiol 84:426–431 [DOI] [PubMed] [Google Scholar]
- Colker CM, Antonio J, Kalman D 2001 The metabolism of orally ingested 19-nor-4-androstene-3,17-dione and 19-nor-4-androstene-3,17-diol in healthy, resistance-trained men. J Strength Cond Res 15:144–147 [PubMed] [Google Scholar]
- Tseng YL, Kuo FH, Sun KH 2005 Quantification and profiling of 19-norandrosterone and 19-noretiocholanolone in human urine after consumption of a nutritional supplement and norsteroids. J Anal Toxicol 29:124–134 [DOI] [PubMed] [Google Scholar]
- Tseng YL, Sun CY, Kuo FH 2006 Detection and quantification of glucuro- and sulfoconjugated metabolites in human urine following oral administration of xenobiotic 19-norsteroids. Steroids 71:817–827 [DOI] [PubMed] [Google Scholar]
- Kumar N, Sundaram K, Bardin CW 1995 Feedback regulation of gonadotropins by androgens in rats: is 5α-reduction involved? J Steroid Biochem Mol Biol 52:105–112 [DOI] [PubMed] [Google Scholar]
- de Gooyer ME, Oppers-Tiemissen HM, Leysen D, Verheul HA, Kloosterboer HJ 2003 Tibolone is not converted by human aromatase to 7α-methyl-17α-ethynylestradiol (7α-MEE): analyses with sensitive bioassays for estrogens and androgens and with LC-MSMS. Steroids 68:235–243 [DOI] [PubMed] [Google Scholar]
- Vanderschueren D, Van Herck E, Suiker AM, Visser WJ, Schot LP, Bouillon R 1992 Bone and mineral metabolism in aged male rats: short and long term effects of androgen deficiency. Endocrinology 130:2906–2916 [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Kousteni S, Chen JR, Schuller M, Plotkin L, Bellido T 2004 Kinase-mediated transcription, activators of nongenotropic estrogen-like signaling (ANGELS), and osteoporosis: a different perspective on the HRT dilemma. Kidney Int Suppl 91:S41–S49 [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Kousteni S, Jilka RL 2002 Sex steroids and bone. Recent Prog Horm Res 57:385–409 [DOI] [PubMed] [Google Scholar]