Abstract
Ectopic or tertiary lymphoid tissues develop at sites of inflammation or infection in peripheral, non-lymphoid organs. These tissues are architecturally similar to conventional secondary lymphoid organs, with separated B and T cell areas, specialized populations of dendritic cells, well-differentiated stromal cells and high endothelial venules. Ectopic lymphoid tissues are often associated with the local pathology that results from chronic infection or chronic inflammation. However, there are also examples in which ectopic lymphoid tissues appear to contribute to local protective immune responses. Here we review how ectopic lymphoid structures develop and function in the context of local immunity and pathology.
Keywords: ectopic lymphoid tissue, germinal center, BALT, inflammation, autoimmunity
1. Introduction
Secondary lymphoid organs are an integral part of the immune system and are important sites of immune activation. Encapsulated lymph nodes (LNs) are found along lymphatic vessels that drain regional tissues, while mucosal lymphoid organs, including Peyer’s patches and Nasal Associated Lymphoid Tissue (NALT) are not encapsulated and are found directly beneath the mucosal epithelium. Despite their structural differences, lymphoid organs are organized in a similar fashion, with B cell follicles, separated T cell areas, multiple populations of dendritic cells and specialized stromal cells. Each secondary lymphoid organ has the ability to recruit naïve lymphocytes from the blood as well as activated antigen presenting cells from the surrounding tissues or mucosal surfaces. Moreover, the specific architecture of each secondary lymphoid organ has evolved to maximize encounters between antigen, antigen presenting cells and lymphocytes in order to efficiently generate rapid and robust adaptive immune responses (reviewed in [1–5].
The development of LNs as well as many of the mucosal lymphoid organs is pre-programmed and does not require pathogen-induced inflammation or any type of adaptive immune response (reviewed in [6,7]). In contrast, tertiary lymphoid tissues, also known as ectopic lymphoid tissues, develop in adults in response to chronic inflammation, chronic infection or autoimmunity (previously reviewed in [8]). These tissues can develop in nearly every organ in the body and do not appear in predictable sites. Moreover, these tissues are not encapsulated like LNs, nor are they typically sub-mucosal tissues like Peyer’s patches or NALT. Instead they are often embedded in non-lymphoid organs at sites of inflammation or infection.
Many of the cellular and molecular signals that control the development of secondary lymphoid organs have been identified, and not surprisingly, many of these signals are also involved in the development of ectopic lymphoid tissues (reviewed in [6,8]). However, the development of ectopic follicles is not identical that of secondary lymphoid organs and it is not well understood what triggers their formation. Here we propose that secondary and tertiary lymphoid tissues are part of a spectrum of lymphoid tissues that ranges from highly organized encapsulated tissues, which develop at specific sites according to pre-programmed developmental pathways, to granulomas and ectopic follicles, which develop only after local inflammation or infection at a variety of sites in multiple tissues. All of these lymphoid tissues participate in immune responses to some degree and while ectopic follicles have been primarily associated with pathology and detrimental autoimmune responses, they are also likely to participate in protective immune responses to local infections.
2. Current model of secondary lymphoid organ development
The development of lymphoid organs has received considerable attention since the discovery that mice lacking molecules in the lymphotoxin (LT) signaling pathway do not develop LNs and Peyer’s patches [9–13]. It is now generally accepted that Lymphoid Tissue inducer (LTi) cells are instrumental for the initiation of lymphoid organ development [14,15]. LTi cells are derived from common lymphoid progenitors in fetal liver [16] and can be distinguished from other lymphocytes based on their surface phenotype (CD3−CD4+CD45+IL-7R+c-Kit+)[17]. Not all LTi cells are functionally equivalent, as the IL-7R is important for the expansion and differentiation of LTi cells at mucosal sites, such as Peyer’s patches [18,19], whereas signaling through RANK is important for the differentiation and survival of LTi cells that home to LNs [20]. LTi cells also express the chemokine receptors, CCR7 and CXCR5 [21], which promote their homing to sites of future lymphoid organ development and lead to their interactions with local mesenchymal VCAM+ICAM+ LN organizer cells [19,22,23]. LTi cells express LTαβ and signal through the LTβR on LN organizer cells. In turn, the responding mesenchymal cells produce homeostatic chemokines, including CCL19, CCL21 and CXCL13, which accelerate the recruitment of LTi cells as well as other lymphocytes and promote the development of adult lymphoid architecture [23–27]. The expression of homeostatic chemokines by stromal cells and the expression of LTαβ on recirculating lymphocytes, particularly B cells [28,29], are maintained by positive feedback, in which LT controls chemokine expression by stromal cells and chemokine signaling on lymphocytes maintains surface expression of LTαβ on lymphocytes [30]. Thus, the disruption of any part of this loop leads to impaired lymphoid organ development and architecture [21,30,31].
LT also controls the differentiation of stromal cells and high endothelial venules (HEVs)[28,32–34] and is important for the expression of various adhesion molecules on vascular endothelial cells and stromal cells. For example, the expression of MAdCAM, PNAd, VCAM-1 and ICAM-1 is controlled, in part, by the activities of LTα, LTαβ and TNF signaling through LTR and TNFR1 [35–37]. Similar pathways control the expression of the sulfotransferases that generate the PNAd epitope as well as the expression of GlyCAM and MAdCAM, which are common protein substrates for post-translational sulfation [37–39]. Thus, LT signaling is essential for numerous aspects of secondary lymphoid organ development, lymphoid architecture and the proper recirculation of lymphocytes.
3. Transgenic models of tertiary lymphoid organogenesis
Much of what we know about the formation of tertiary lymphoid tissues comes from transgenic mice in which various chemokines and cytokines are expressed at ectopic sites. For example, LTαexpressed in the pancreas under the control of the rat insulin promoter leads to inflammatory lesions in the kidney and pancreas [40]. These areas are organized like secondary lymphoid tissues with separated T and B cell zones, specialized stromal cells and HEVs. These ectopic tissues also appear to be functional, as they respond to antigen, form germinal centers and promote isotype switching in B cells [40]. However, on their own, they do not lead to diabetes. LTα expression in the pancreas also promotes the expression of CXCL13 and CCL21, which appear to organize B and T cells zones. In this model, the expression of CXCL13 and CCL21 is dependent on TNFR1, and not LTβ, suggesting that signals through the TNFR1 rather than the LTβR are sufficient for at least the minimal development of this tertiary lymphoid tissue [41].
Although LTα alone can clearly initiate tertiary lymphoid organogenesis, the simultaneous expression of LTα and LTβ much more efficiently promotes the formation of ectopic lymphoid tissues [39]. Mice that express both LTα and LTβ in the pancreas have much larger infiltrates in the pancreatic islets than those in LTα single transgenic mice [39]. Separation into T and B cell areas is more distinct and large FDC networks are visible in all B cell areas. In addition, CCL21, CCL19 and CXCL13 are expressed at higher levels in the islets of LTαβ double transgenic mice than in the islets of LTα single transgenic mice. Moreover, PNAd is strongly expressed on the luminal surface of HEV in LTαβ double transgenic mice, whereas it is primarily expressed on the abluminal surface of HEVs in LTα single transgenic mice [39]. These data highlight distinct roles of LTα and LTαβ in lymphoid organogenesis [39].
In addition to LTαβ, LIGHT also signals through the LTβR and plays a role in the expression of homeostatic chemokines and the formation of tertiary lymphoid structures in some situations [42–44]. LIGHT expression is induced in the islets of older NOD mice concomitantly with the appearance of ectopic lymphoid tissues and the development of diabetes [45]. Blockade of LTβR signaling disrupts the organization of tertiary structures and prevents diabetes. Conversely the transgenic expression of LIGHT in the pancreas of NOD mice promotes the rapid formation of ectopic follicles and results in accelerated onset of diabetes [45]. Thus, the expression of LTα, LTβ and LIGHT can all contribute to the formation of tertiary lymphoid structures via signaling through TNFR1 and LTβR [45].
As mentioned above, the expression of LTα, LTβ and the expression of homeostatic chemokines are linked via a positive feedback loop that is critical for the development of ectopic lymphoid tissues [30]. Mice expressing CXCL13 in the pancreas develop tertiary lymphoid tissues that contain B and T cell zones, HEVs, stromal cells and CCL21 [46]. Development of these features is dependent on B lymphocytes and LTαβ[46]. The expression of CCL21 in the pancreas also promotes the development of tertiary lymphoid tissues in an LTβR-dependent manner [26,47,48]. In contrast, the ectopic expression of CCL19 in pancreatic islets only leads to small infiltrates composed of lymphocytes and dendritic cells with HEVs and stromal cells [26]. Similarly, the ectopic expression of CXCL12 in the pancreas promotes the accumulation of small infiltrates that contain relatively few T cells, but enriched in dendritic cells, B cells, and plasma cells [26]. Thus, the homeostatic chemokines, CXCL12, CXCL13, CCL19 and CCL21, are not equal in their ability to promote tertiary lymphoid organogenesis. In part, this may be explained by their differential ability to promote LTαβ expression on lymphocytes. CXCL13 triggers expression of LTαβ on B cells, while CCL19 and CCL21, but not CXCL12 promote LTαβ expression on CD4 T cells [26]. Interestingly, LTαβ can also be induced on naive T cells by IL-4 and IL-7, suggesting that there may be multiple ways to engage the chemokine/lymphotoxin loop and to trigger the formation of tertiary lymphoid tissues [26].
The pancreas is not the only organ in which the ectopic expression of cytokines and chemokines leads to the development of tertiary lymphoid organogenesis. Mice expressing LTα via the rat insulin promoter also develop tertiary lymphoid tissues in the kidney [40]. Moreover, mice expressing CCL21 under control of the thyroglobulin promoter develop organized lymphoid tissues in the thyroid [49–51]. These tissues have separated B and T cell areas as well as HEVs and lymphatic vessels surrounding the follicles [50]. Although HEVs expressing PNAd are frequently observed in the thyroid tissue, lymphocyte recruitment is independent of L-selectin and LTα, but does require CCR7 [49]. On the other hand, the ability to form segregated B and T cell areas in the thyroid, express PNAd and develop lymphatic vessels is dependent on LTβR signaling and LTα expression as well as on the activities of lymphocytes [50]. Interestingly, LT-expressing CD4 T cells rather than B cells are important for the initiation of lymphangiogenesis in this model [50]. In contrast, the transgenic over-expression of CCL21 in the skin does not lead to the development of tertiary lymphoid tissues [47], even though CCL21 is highly expressed at this site. Although the numbers of B cells, T cells and Langerhans cells in the skin are unchanged by the CCL21 transgene, the Langerhans cells are retained in the skin when activated by topically applied FITC [47]. Thus, the CCL21 transgene is functional, even though it does not promote the formation of ectopic lymphoid tissue. This implies that there are differences in the mechanisms that lead to the development of ectopic lymphoid tissues in different organs, which probably reflects cell types and vascular beds that are present in each organ.
Although many studies focus on the role of LTα, LTαβ and homeostatic chemokines in the development of tertiary lymphoid tissues, the transgenic over-expression of cytokines that are not obviously part of the lymphotoxin/homeostatic chemokine axis can also promote the development of tertiary lymphoid organs. For example, the transgenic co-expression of IL-6 and IL-6R under the Class I and β-actin promoters leads to the development of organized inducible Bronchus-Associated Lymphoid Tissue (iBALT) in the peri-bronchial and peri-vascular regions of the lung [52]. Similar to its expression in ectopic lymphoid tissues that develop in other transgenic models, CXCL13 is expressed in the B cell follicles of iBALT. However, the signaling pathways that link IL-6 and CXCL13 are not known [52]. As in pancreatic islets and thyroid tissue, the IL-6-induced formation of ectopic lymphoid tissue in the lung does not seem to lead to local pathology or autoimmunity. However, this is not always the case. For example, the over-expression of IL-5 in the respiratory epithelium also promotes the development of organized iBALT [53]. Unlike the chemokine or LT-dependent development of tertiary lymphoid tissues in the pancreas and thyroid, which do not lead to diabetes or thyroiditis, the IL-5-dependent development of BALT in the lung leads to goblet cell hyperplasia, epithelial hypertrophy, focal collagen deposition and accumulation of eosinophils in peribronchial areas and in the airway lumen - all signs of lung pathology [53]. Moreover, IL-5 transgenic mice exhibit airway hyperresponsiveness to methacholine in the absence of antigen challenge [53]. Thus, in some cases, the transgene-induced formation of tertiary lymphoid tissues is accompanied by pathology. This is probably related to the nature of the transgene and whether it is a homeostatic cytokine/chemokine, like LTα, LTβ, CXCL13 and CCL21 or whether it is an effector cytokine, like IL-5.
4. The role of LTi cells in the formation of ectopic lymphoid tissues
Although the organogenesis of LNs and Peyer’s patches clearly requires LTi cells [54–57], it is less clear whether LTi cells are required for the formation of ectopic follicles. In transgenic mice that express CXCL13 in the pancreas, CD4+CD3− cells are the first hematopoietic cell types recruited to the islets very early after birth [46], suggesting that LTi cells may be involved in the development of the tertiary lymphoid tissues that will eventually develop in this location. However, it is unclear whether this is due to a coincidence in timing or due to a causal relationship between LTi cells and the development of tertiary lymphoid tissues. More direct evidence for the involvement of LTi cells in the formation of ectopic lymphoid tissues comes from the generation of transgenic mice that over-express IL-7 [58]. These mice have abnormally high numbers of LTi cells and develop a much higher number of LNs and Peyer’s patches than normal mice [58]. They also develop ectopic lymphoid tissues in pancreas and salivary gland. Interestingly, IL-7 by itself is not sufficient to drive the development of these ectopic lymphoid tissues, since IL-7 transgenic mice crossed to RORγ−/− mice, which lack LTi cells [54], do not develop any secondary lymphoid organs or ectopic lymphoid tissues [58]. Thus, in this model, the development of ectopic follicles is dependent on LTi cells.
Importantly, IL-7 is also implicated in the formation of ectopic follicles in patients with rheumatoid arthritis, who have high concentrations of IL-7 in serum and the inflamed synovium of affected joints [59,60]. While IL-7 in these conditions may be contributing to ectopic follicle formation by promoting the survival, expansion or differentiation of LTi cells, IL-7 is also likely to de-regulate T and B cell development, longevity and function [61–63]. As a result, the precise role of IL-7 in the development of ectopic lymphoid tissues in these patients remains difficult to determine.
If LTi cells are required for the development of ectopic lymphoid follicles, then there may be a developmental window during which tertiary lymphoid tissue development is dependent on the availability of LTi cells. This is clearly the case for the development of conventional secondary lymphoid organs, which occurs during a developmental window that varies depending on the particular lymphoid organ [64]. For example, mesenteric LNs develop first (around day E9–E10), followed by brachial (day E13), axillary (day E15) inguinal (day E16) and popliteal LNs (day E17)[64]. Mucosal lymphoid organs, such as Peyer’s patches and NALT appear to develop last, as disruption of lymphotoxin signaling just before birth blocks Peyer’s patch formation [64] while the development of NALT can be recovered in neonatal Id2−/− mice by the administration of LTi cells [56]. There is also evidence that the formation of ectopic lymphoid tissues also occurs during a defined developmental window shortly after birth. For example, the transfer of cells from disrupted neonatal LNs to ectopic sites, such as the skin, triggers the de novo development of lymphoid tissues [65,66]. However, while the ectopic administration of LTi cells to neonatal mice results in lymphoid tissues with segregated B and T cell areas, follicular dendritic cells (FDCs) and HEVs, the administration of LTi cells to ectopic sites in adult mice results in generation of disorganized accumulations of lymphocytes without FDCs or HEVs [65,66]. Thus, it appears that interactions between LTi cells and immature mesenchymal cells during a neonatal period is important for the proper formation of ectopic lymphoid tissues under some circumstances.
On the other hand, recent studies using mice that express CCL21 in the thyroid suggest that LTi cells are not required for the development of ectopic lymphoid tissues [67]. Instead, the first cells that appear in the thyroid are CD3+CD4+ T cells, which interact with local dendritic cells. Subsequently, PNAd+ HEVs appear, homeostatic and inflammatory chemokines are produced and lymphocytes and dendritic cells are recruited and organized into tertiary lymphoid tissue [67]. Importantly, when CCL21 transgenic mice are crossed to Id2−/− mice, which lack LTi cells [57], tertiary lymphoid tissues still form in the thyroid, despite the absence of conventional secondary lymphoid organs [67]. These data suggest that the formation of tertiary lymphoid structures in adults does not require Id2-dependent LTi cells, but depends on a program initiated by mature CD3+CD4+ T cells.
One reason that the development of ectopic lymphoid tissues may not require LTi cells is that these tissues typically form in adults in response to chronic inflammation or infection. Since adults have a wide variety of circulating mature lymphocytes that express LTαβ, particularly when activated [68,69], the role of LTi cells may be performed by activated lymphocytes. In fact, the presence of LTi cells in adults remains a controversial point [70]. Some investigators have found CD4+CD3− cells in adults and demonstrated that they provide help to follicular T cells in germinal centers [71]. Unlike embryonic LTi cells, adult CD4+CD3− cells express high levels of OX40L and CD30L [72]. However, CD4+CD3− cells in adults also express high levels of LTαβ and TNF. Moreover, the adoptive transfer of normal CD4+CD3− cells from either embryonic or adult tissues into LTα−/− mice leads to the segregation of B and T cell areas and the expression of CCL21 [73]. Despite these data however, the precise lineage relationship between embryonic LTi cells and adult CD4+CD3- cells is unclear and any potential role of these cells in the formation of ectopic follicles is unknown.
Another complication in the analysis of LTi cells and their role in the development of ectopic lymphoid tissues is that there are multiple populations of LTi cells. For example, RORγ-dependent LTi cells are not required for NALT development [74], whereas Id2-dependent LTi cells are essential for NALT development [56]. This may reflect a different tissue tropism of various populations of LTi cells or may reflect changing differentiation of LTi cells over time during embryogenesis. Given that NALT is one of the last secondary lymphoid organs to develop after birth [56], the LTi cells that home to NALT may have fundamentally different properties. If so, then it is possible that ectopic follicles may also require a particular subset of LTi cells or that ectopic follicles developing in different organs require different LTi cells. Thus, investigators should use caution when concluding that LTi cells are not required for ectopic lymphoid tissue development based only on the use of RORγ−/− or Id2−/− mice. In the meantime, the role of LTi cells in the development of ectopic follicles remains an open and important question.
5. The role of local stromal cells in the formation of ectopic follicles
The counterparts to LTi cells in the development of secondary lymphoid organs are lymph node organizer cells. These cells are mesenchymal in origin and form the stromal cell matrix of the developing lymphoid organ [19,22,23,75]. Just as there are multiple populations of LTi cells, there are also multiple types of LN organizer cells, with different expression patterns of adhesion molecules, cytokines and receptors [76]. In fact, different lymphoid tissue organizer cells are found at sites of mesenteric LN and Peyer’s patch development [76]. This may explain why different lymphoid organs have different requirements of their development. For example, IL-7R is essential for Peyer’s patch development [18,19,21], while RANK is required for LN development [20]. Although an organizer cell type that promotes the development of ectopic follicles has not yet been identified, it is clear that stromal cells are present in ectopic follicles [77], even though their precursors remain undefined. It is possible that local fibroblasts differentiate into stromal cells in situ due to interactions with LTαβ expressing lymphocytes [78]. However, it is also possible that circulating fibrocytes may be recruited by homeostatic chemokines to sites of ectopic follicle development from distal sites like the bone marrow [79,80]. This has clearly been shown in lung fibrosis [79], but has not been rigorously tested in the formation of ectopic follicles.
6. Autoimmunity and the development of lymphoid follicles
Tertiary lymphoid organs often develop in autoimmune diseases and tend to appear in the organs that are under autoimmune attack, such the joints and lungs of rheumatoid arthritis patients [38,81–84], the salivary glands in Sjogren’s syndrome [85–87], the thyroid in Hashimoto’s thyroiditis or Grave’s disease [51,88,89], the pancreas in diabetes [90,91] and the central nervous system in multiple sclerosis [92–95] (Table 1). In many cases, the formation of well-developed ectopic follicles in these sites correlates with increased severity of disease and the local production of auto-antibodies. For example, patients with rheumatoid arthritis can be divided into those with diffuse lymphoid aggregates in their synovium, those with B and T cell aggregates and those with highly developed germinal centers, FDCs and segregated B and T cell areas [59]. Those with the most developed ectopic follicles have more severe symptoms of disease [83]. However, other studies suggest that lymphoid neogenesis is not necessarily related to arthritis activity or severity, but does correlate with local autoantibody production and higher titers of anti-nuclear antibodies [81]. Increased local production of autoantibodies is also observed in patients with pulmonary manifestations of rheumatoid arthritis [82] and in patients with Sjogren’s syndrome [87].
Table 1.
Development of ectopic lymphoid follicles in inflammatory diseases.
| Disease | Tissues affected | Species | References |
|---|---|---|---|
| Arthritis | Joints, Lung | Human | [82,83,96,98,103,104] |
| Sjogren’s syndrome | Lung, Salivary gland | Human | [82,87] |
| Diabetes | Pancreas | Human, Mouse | [45,91] |
| Wegener’s granulomatosis | Lung | Human | [193] |
| Multiple sclerosis/EAE | CNS | Human, Mouse | [94,95,105,107] |
| Thyroiditis | Thyroid | Human, Mouse | [88,89,101] |
| Hypersensitivity Pneumonitis | Lung | Human | [190,191] |
| COPD | lung | Human, Mouse | [129,130,194] |
| Autoimmune gastritis | Gut | Human | [109] |
| Idiopathic pulmonary fibrosis | Lung | Human | [195] |
| Atherosclerosis | Arteries | Human | [196,197] |
| Inflammatory bowel disease | Gut | Human | [98,185,198,199] |
Increasing size and complexity of tertiary lymphoid tissues in autoimmune tissues also correlates with dramatically increased local expression of homeostatic cytokines and chemokines, including LTα, LTβ, CXCL13 and CCL21 [59,82,96], CCL20 [97] and CXCL12 [81]. Tissues with germinal centers tend to express much higher levels of LTα, CXCL13 and CCL21 than those with diffuse lymphoid infiltrates [59]. In fact, the expression level of CXCL13 and LTβ in synovial biopsies is highly predictive of the presence of germinal centers [59]. The expression of CXCL13 and LTβ also correlates with the presence of highly organized secondary B cell follicles in patients with pulmonary disease associated with rheumatoid arthritis [82] and in patients with Sjogren’s syndrome. [86].
CXCL13 is typically expressed within the B cell follicle in a reticular pattern consistent with FDCs [82]. Although CXCL13 expression is often associated with FDCs in mice, fibroblast-like synoviocytes also express homeostatic and inflammatory chemokines when stimulated by LTαβ, even though these cells do not differentiate into FDCs - at least in vitro [78]. Moreover, CXCL13 is also expressed by activated monocytes or macrophages, which may be located within FDC networks or even in B cell aggregates that lack FDCs [98,99]. Interestingly, CXCL13 and CCL21 can also be expressed in inflamed tissues that do not yet have organized lymphoid tissue [96], suggesting that these chemokines are induced by inflammation prior to the formation of segregated T and B cell areas, FDCs and germinal centers. This early expression of homeostatic chemokines may be independent of LTαβ and TNF, as seen in influenza infected lungs [100]. However, the pathways that lead to LTαβ-independent CXCL13 expression remain undefined.
Although B cells are known to be a potent source of surface LTαβ expression [30,68] and are important for maintaining the organization of B cell follicles and FDC networks in conventional lymphoid organs [28,101], B cells are also critical for T cell activation in rheumatoid arthritis [102]. Adoptive transfer experiments using human/mouse chimeras show that activation of CD4 T cells in rheumatoid synovium is strictly dependent on B cells and that non-B cell antigen-presenting cells can not maintain T cell activation on their own [102]. CD8 T cells are also activated in the ectopic lymphoid tissues in the joints of arthritis patients and often share common TCR V regions [103,104], suggesting that they are responding to particular antigens. Depletion of CD8 T cells in human/mouse chimeras causes loss of FDCs, disintegration of germinal centers and reductions in autoantibody secretion. Moreover, the expression of LTαβ is reduced in the absence of CD8 T cells, which express CD40 ligand [103,104]. A similar role for CD8 cells is observed in the development and function of ectopic follicles in transgenic mice that express the lymphocytic choriomeningitis virus glycoprotein in pancreatic islets [91]. These mice develop islet-associated lymphoid tissues and autoimmune diabetes upon immunization with dendritic cells that display the CTL epitope from the lymphocytic choriomeningitis virus glycoprotein [91]. Thus, there are surprising interactions between B cells and both CD4 and CD8 T cells in the induction and maintenance of tertiary lymphoid tissues in autoimmune disease.
B-cell follicles are also detected in the meninges of patients with secondary progressive multiple sclerosis, but not in patients with primary progressive multiple sclerosis [95]. These tertiary lymphoid tissues contain B and T cells, plasma cells and FDCs [95]. The presence of germinal centers along with memory B cells that express hyper-mutated immunoglobulin V genes corresponds to high expression of LTα and CXCL13 [105]. Those patients with follicles are more likely to experience onset of multiple sclerosis at a younger age, irreversible disability, more pronounced demyelination, microglia activation and loss of neurites in the cerebral cortex [94].
Experimental autoimmune encephalomyelitis (EAE) is widely used as an animal model of multiple sclerosis in which the availability of cytokines and chemokines can be experimentally manipulated. Many features of the mouse model are similar to those in human disease, such as the local upregulation of LTβ and LTβR [93], CXCL13 [106,107] and the two CCR7 ligands, CCL19 and CCL21 [92]. CXCL13 co-localizes with FDCs [107], whereas CCL21 is expressed by endothelial cells and CCL19 is expressed by astrocytes and microglia. [92,93] Consistent with the postulated role of the LT/homeostatic chemokine axis in the maintenance and function of tertiary lymphoid tissues in EAE and multiple sclerosis, the blockade of the LTβR and HVEM signaling pathways using LTβR-Ig disrupts the structure of tertiary lymphoid tissues, reduces B and T cell accumulation, decreases the local expression of CXCL10 and CXCL13 and reduces the clinical symptoms of EAE in affected mice [93]. Surprisingly, disease attenuation in this model is due to the blockade of LTβR rather than HVEM [108]. However, neither the treatment with LTβR-Ig nor the loss of LTα prevents EAE in a model that uses pertussis toxin as an adjuvant [43,108]. CXCL13−/−mice also have reduced clinical symptoms of EAE [106], which may be partly due to poor lymphocyte priming, since CXCL13−/− mice lack numerous LNs and have poorly organized secondary lymphoid organs [30]. However, CXCL13 also appears to be important for the effector phase of the disease as the adoptive transfer of effector cells from normal mice to CXCL13−/−recipients results in attenuated disease [106]. Thus, the maintenance and function of tertiary lymphoid tissues via the LT/homeostatic chemokine axis appears to be a part of the disease process in EAE and multiple sclerosis.
Homeostatic chemokines and molecules in the LT signaling pathway also correlate with the development of ectopic lymphoid tissues in numerous other autoimmune diseases. For example, CXCL13 and LTβ are highly expressed in the B cell follicles that develop in the stomachs of mice that have autoimmune gastritis [109]. The number and size of the cell clusters correlate with the age of the mice and the serum autoantibody titer [109]. LTαβ, CCL21, CXCL12 and CXCL13 are increased in thyroids from autoimmune patients [88], and CXCL12 and CXCL13 are significantly higher in autoimmune glands with ectopic secondary lymphoid follicles than in those without follicles [50,88,89]. However, despite clear demonstrations that tertiary lymphoid follicles are immunologically active in patients with these diseases, it is difficult to determine whether the formation of ectopic follicles leads to disease or whether autoimmunity promotes the development of ectopic follicles.
7. Infection often elicits the formation of ectopic lymphoid follicles
The development of ectopic lymphoid tissues probably evolved as a response to local infections, as numerous infectious agents trigger the development of tertiary lymphoid tissues in a variety of non-lymphoid organs (Table 2). For example, infection of the liver triggers the formation of ectopic lymphoid tissue in the portal area known as Portal tract-Associated Lymphoid Tissue or PALT [110–112]. Liver biopsies from patients with chronic hepatitis C infection exhibit PALT, with B cell follicles, FDCs and germinal centers surrounded by a T cell zone [113]. Germinal center B cells in the ectopic follicles of hepatitis infected patients exhibit antigen-driven oligoclonal expansions [114], which are associated with extrahepatic manifestations of hepatitis [112] and a high level of viral replication [113]. Infection with bacteria, such as Propionibacterium acnes, also promotes the development of PALT [111]. Interestingly, the granulomas that surround the bacteria and PALT are spatially separated. Inflammatory chemokines, such as MIP1α, are expressed in granulomas, whereas homeostatic chemokines, like CCL21 are expressed in PALT [115]. Anti-CCL21 antibody reduces the PALT expansion while exacerbating granuloma formation [115], suggesting that ectopic lymphoid follicles may result from the accumulation of dendritic cells that are activated locally and congregate at sites of lymphatic drainage [116].
Table 2.
Development of ectopic lymphoid tissues in infectious diseases.
| Infectious Disease | Tissues affected | Species | References |
|---|---|---|---|
| Influenza | Lung | Mouse | [77,100,137] |
| HIV and complications | Lung, Vagina, Brain | Human, Monkey | [131–133,200] |
| Hepatitis C | Liver | Human | [114,201–203] |
| Helicobacter pylori | Stomach | Human, Mouse, Pig | [110,204–207] |
| Propionbacterium acnes | Liver | Mouse | [111] |
| Mycobacterium tuberculosis | Lung | Mouse, human | [134–136,208] |
| Borrelia burgdorferi | Skin | Human | [127,209,210] |
| Klebsiella pneumoniae | Lung | Human | [190] |
| Psuedomonas aruginosa | Lung | Human | [190] |
| Haemophilus influenzae | Lung | Human | [190] |
| Other infection | Lung | Human | [211,212] |
Infection with Helicobacter pylori also triggers the development of tertiary lymphoid tissues in the stomach. CXCL13 is highly expressed in these areas, possibly by FDCs [117]. In contrast, CCL21 is expressed on endothelial cells in and around ectopic follicles [117]. PNAd is also expressed on HEVs, which increase in number during the course of disease. Lymphoid aggregates in gastric biopsies are nearly always associated with chronic active gastritis and concurrent Helicobacter pylori infection [118,119]. Moreover, eradication of Helicobacter pylori is associated with the disappearance of lymphoid follicles and HEV-like vessels in the gastric mucosa [120]. Interestingly, HSP60 is often expressed on FDCs in gastric MALT lymphoma tissues [121] and auto-antibodies that react with the HSP60 antigen are often increased in MALT lymphomas that result from Helicobacter pylori infection [122], suggesting that MALT lymphoma may be driven by antigen receptor stimulation.
In another example, infection with Borrelia burgdorferi often leads to Lyme arthritis [123], which like rheumatoid arthritis, is associated with the formation of tertiary lymphoid tissues in the joints. Although bacteria are sometimes visible surrounding the ectopic follicles, they are relatively few in number [123], and synovial inflammation may persist after the apparent eradication of the infection using antibiotics [124]. Interestingly, ectopic follicles in synovial biopsies from Lyme arthritis patients exhibit clonal clusters of isotype-switched B cells that have sequentially accumulated mutations in their V regions, suggesting antigen driven selection [125]. In fact, many of these B cells recognize cytokeratin 10, which is present in the synovial endothelium [126]. Furthermore, synovial B cells recognize both cytokeratin 10 and surface protein A of Borrelia burgdorferi [126]. Similar antigen driven B cell expansions are seen in the skin of Borrelia burgdorferi-infected patients, who often develop lymphocytoma cutis [127,128]. Thus, as with Helicobacter pylori infection, the formation of ectopic follicles in response to Borrelia infection is associated with both autoimmunity and malignant transformation of B cells.
The lung is also a common site for the formation of tertiary lymphoid tissue, which is sometimes known as inducible Bronchus Associated Lymphoid Tissue (iBALT). IBALT forms in patients with a wide variety of diseases, including chronic obstructive pulmonary disease (COPD) [129,130], AIDS [131–133], pulmonary fibrosis [79], hypersensitivity pneumonitis [82] and even lung cancer [82] (Table 2). However, iBALT formation is mostly linked with chronic or acute lung infection and the involvement of opportunistic infectious agents cannot be ruled out in many of the cases listed above. For example, infection of the lung with Mycobacterium tuberculosis promotes the development of iBALT in areas surrounding granuloma formation [134,135]. Germinal centers are also observed in the lungs of Mycobacterium tuberculosis-infected mice [136], suggesting that B cells are responding to local antigens. Consistent with the idea that B cells play some role in local resistance to infection, B cell-deficient mice exhibit exacerbated immunopathology and enhanced susceptibility to Mycobacterium tuberculosis [136].
IBALT formation is also triggered by acute infection with influenza [4,77,100,137] or murine γ-herpesvirus-68 in mice (Figure 1). In these cases, iBALT consists of B cell follicles with germinal centers and FDCs surrounded or interspersed with T cell areas that have CD11c+ DCs, PNAd-expressing HEVs and lymphatic vessels [77,100]. Like other ectopic lymphoid tissues, iBALT appears to participate in lymphocyte priming [100], recruitment of effector cells and maintenance of local memory cells [137]. Surprisingly, mice that lack all conventional lymphoid organs, but retain iBALT, survive higher inoculating doses of influenza than normal mice [100]. Thus, iBALT promotes local protective immunity in a way that reduces immunopathology.
Figure 1. Ectopic lymphoid tissue develops in the lungs of mice after viral infection.
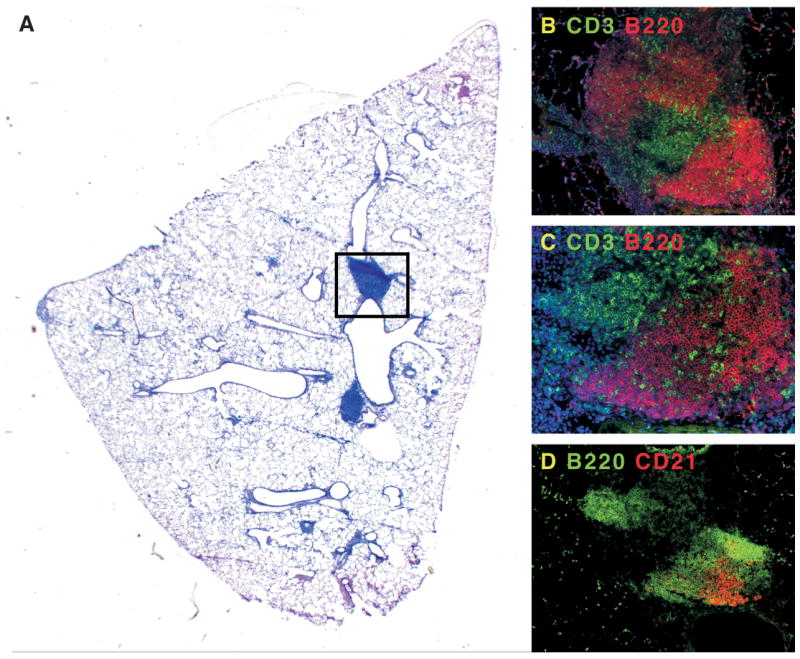
Mice were infected with murine γ-herpesvirus-68 and allowed to clear the lytic phase of the infection. A. Although the majority of the inflammatory response in the lung has resolved, areas of pulmonary lymphoid tissue remain next to major airways. B. The boxed area in panel A contains B220+ B cell areas (red), and CD3+ T cell areas (green). C. A magnification of the B cell areas shows infiltrating T cells. D. In a serial section of panel B, it is clear that some of the B cell follicles in iBALT (green) have CD21+ FDCs (red).
As in many tertiary lymphoid organs, CXCL13 is expressed on reticular cells in the B cell follicles of iBALT and CCL21 is expressed on HEVs that co-express PNAd [77,100]. Both CXCL13 and CCL21 are inducibly expressed in the lung after infection and interestingly, the influenza-induced expression of these chemokines is independent of both LTα and TNF [100]. Despite the LTα-independence of CXCL13 and CCL21 expression, iBALT is not organized properly in either LTα−/−or LTβR−/− mice [11]. This may be because the differentiation of HEVs or stromal cells remains dependent on LTα or that the expression of homeostatic chemokines is controlled differently in the lung during acute infection than it is during iBALT homeostasis after infection is resolved. Determining what controls chemokine expression during acute infection is probably one key to understanding how the formation of tertiary lymphoid tissues is initiated.
Despite the clear evidence that CXCL13 is highly expressed in iBALT as well as in spontaneously occurring ectopic lymphoid follicles in other tissues [77,100], CXCL13 is not necessary for the formation or the function of ectopic follicles in the lung [77]. For example, the basic structure of iBALT, with separated B and T cell areas, germinal centers and HEVs still forms in CXCL13−/−mice after influenza infection. The only apparent defect in these mice is that the B cell areas of iBALT do not form true follicles - the B cells are loosely aggregated and CD21+ FDCs are not observed [77]. However, these areas are functional and promote the normal expansion of influenza-specific B and T cells and lead to the normal production of influenza-specific IgG [77]. In contrast, the CCR7 ligands, CCL19 and CCL21 are very important for the formation and function of iBALT [77]. Both B and T cell responses are impaired in plt/plt mice, which lack CCL19 and CCL21, even though B cell follicle formation in iBALT appears intact [77]. These defects are even more pronounced in mice triply deficient in CXCL13, CCL19 and CCL21. Thus, the expression of homeostatic chemokines in pulmonary ectopic lymphoid tissues is important for both their development and function.
LTα is also important for the function of iBALT. LTα−/− mice exhibit impaired immune responses to influenza [138], particularly when splenectomized [100]. Although CXCL13 and CCL21 are expressed in the lungs of these mice and B and T cells accumulate in the lungs, organized follicles are not formed, FDCs do not appear, PNAd+ HEVs do not develop and the number of dendritic cells in the lungs is reduced. Nevertheless, T and B cell responses to influenza and murine γ-herpesvirus-68 can eventually be generated in these mice - albeit after a significant delay [138,139]. These data argue that the organization of ectopic lymphoid tissues makes local immune responses more efficient, but is not absolutely necessary. Even so, LT is important for more than just the organization of lymphoid tissues (reviewed in [43,140], including the homeostatic proliferation and survival of dendritic cells [69,141] and T cell priming [69]. Thus, impaired immune responses in the absence of LT are likely due to multiple factors, in addition to poor tertiary lymphoid organ development.
8. Some mucosal lymphoid tissues have properties of both conventional secondary lymphoid organs and tertiary lymphoid tissues
The studies cited above demonstrate that tertiary lymphoid tissues can form in numerous sites in a wide variety of non-lymphoid organs given the appropriate stimulation by pathogens or autoantigens. In contrast, LNs and Peyer’s patches form at predetermined sites in the absence of stimulation via pathogens or antigens. However, there are other types of lymphoid tissues that are formed at predetermined locations, but require some level of microbial stimulation for their development. For example, isolated lymphoid follicles (ILFs) are small clusters of lymphocytes that can be observed throughout the length of the anti-mesenteric wall of the mouse small intestine [142]. They are composed of a large B cell area, usually including a germinal center, and contain relatively few T cells. These tissues are clearly mucosal in nature, as the epithelium overlying the follicles contains M cells and the B cells within the follicles often switch to IgA [143]. Like conventional lymphoid organs, ILFs are absent in LTα−/− and aly/aly mice [142]. However, they reappear in adults after in utero treatment with LTβR-Ig, suggesting that their development is similar to that of Nasal Associated Lymphoid Tissue (NALT), which develops in adult LTα−/− mice that have been reconstituted with normal bone marrow [74]. Although the maintenance of ILFs absolutely requires LTα and signaling through the LTβR and TNFR1 [144], mice that were treated in utero with LTβR-Ig and allowed to recover as adults have enlarged ILFs [145]. This is similar to the exaggerated formation of iBALT in mice that lack conventional lymphoid organs [77,100]. On the other hand, treatment of adult mice with LTβR-Ig abolishes ILFs, even when the mice are immunized [146]. These results suggest that, like the formation of ectopic lymphoid follicles, the organogenesis of ILFs is not restricted to embryonic or neonatal development, even though LT signaling is important for maintaining the architecture of these tissues.
Another similarity of ILFs with ectopic lymphoid tissues is that germ free mice develop very few and atrophied ILF [144]. Other investigators find that ILFs (also known as Solitary Intestinal Lymphoid Tissue - SILT) are present in normal numbers in germfree mice, but are very small and immature and lack identifiable B cell follicles [147,148]. Moreover, c-kit+ cells outnumber B220+ cells in the ILFs of germfree mice. However, ILFs return to normal upon restoration of intestinal flora [144]. Importantly, infection with the enteric pathogen, Salmonella enterica, triggers the formation of inflammatory foci in the ILFs and promotes ILF maturation, but does not increase the number of ILFs [149]. These data argue that the location and number of ILFs is determined developmentally and cannot be altered by inflammation or infection. However, the maturation and organization of ILFs is dependent on microbial stimuli and can even be enhanced by infection with pathogenic bacteria.
Given that B cells are the predominant cell type in mature ILFs, it makes sense that they also play an important role in the development and maturation of ILFs. Interestingly, although LTα is required on B cells for the maturation of ILFs [150], B cells do not have to be antigen specific. In fact, B cells expressing a lysozyme-specific antigen receptor were incorporated into ILFs and expressed high levels of LT on their surface [150]. These B cells also induced high levels of CXCL13 in the ILFs [150]. In contrast, T cell derived LT is not necessary for ILF formation [150]. Consistent with the idea that mucosal phenotype B cells are important for ILF differentiation and function, both immature and mature ILFs are abolished in CCR6−/− mice [151]. When B cells are selectively deficient in CCR6, only a few immature ILFs are formed and no mature ILFs develop [151]. Finally, both ILFs and Peyer’s patches are hyperplastic in AID−/− mice [152] due to the inability of AID−/− B cells to terminally differentiate [153]. Together, these data argue that although B cells do not require BCR signaling to promote ILF development, B cell stimulation does lead to ILF maturation.
9. Relationship between the formation of tertiary lymphoid tissues and tumor progression and regression
Given the apparent immune function of ectopic lymphoid tissues in response to infectious disease, it seems that the development of functional tertiary lymphoid tissues surrounding tumors could be beneficial for tumor antigen recognition and tumor regression. In fact, tumor cell lines that are transfected with LTα promote the formation of tertiary lymphoid tissues, with B cell follicles, dendritic cells, HEVs and FDCs surrounding the tumors [154]. Interestingly, these LTα-expressing tumor lines still promote the development of HEVs and FDCs in the absence of B and T cells, suggesting that LTα by itself is directly promoting the differentiation of these cells. CD4+CD3− cells also infiltrate the tumor site, although it is unclear whether these cells are LTi cells or dendritic cells, or even whether they are required for the development of tertiary lymphoid tissue [154]. The engagement of the LT signaling pathway using an anti-tumor antibody fused to LTα also elicits the formation of tertiary lymphoid tissues surrounding the tumor [155] and promotes the activation and differentiation of tumor specific T cells - ultimately leading to tumor regression. Importantly, immune activation in this model occurs locally at the tumor site, as it still occurs in the absence of conventional LNs [156]. Thus, the development of tertiary lymphoid organs surrounding established tumors is clearly beneficial under some circumstances.
Activation of the LT signaling pathway is not the only way to achieve the formation of ectopic follicles and local T cell activation, since the local administration of CCL21-expressing dendritic cells also results in tumor-specific T cell activation and differentiation as well as the inhibition of tumor growth [157,158]. Targeting LIGHT to tumors also leads to tumor regression. For example, the inoculation of a LIGHT-expressing adenovirus directly into tumors elicits tumor-specific CD8 T cell responses and promotes tumor regression [159]. Although CCL21 expression in the tumor environment is increased by LIGHT signaling [159], immune activation seems to correlate more with direct T cell activation and recruitment, rather than the development of ectopic lymphoid tissues [160]. Consistent with this idea, the inoculation of primary tumors with LIGHT adenovirus also leads to the regression of metastases at distal sites [160]. Similar results are found using an attenuated strain of Salmonella typhimurium that expresses LIGHT [161], and an agonistic anti-LTβR monoclonal antibody [162], although the role of ectopic lymphoid tissues is not directly addressed in these experiments.
Given that both LIGHT and LTαβ bind to the LTβR [163] and that LIGHT can functionally replace LTα in some circumstances [42], it makes sense that these two molecules might exert similar anti-tumor effects. However, LIGHT also binds another receptor, HVEM [163]. LIGHT is expressed by dendritic cells and is important for T cell costimulation [164] and normal CD8 T cell responses to antigen [44,165]. Furthermore, HVEM signaling, rather than LTβR signaling, seems to be most important for T cell costimulation [166]. In part, this is due to the ability of HVEM signals to prevent apoptosis in T cells [166]. In contrast, LTβR signaling on DCs is important for T cell costimulation via dendritic cell activation [69]. Thus, LIGHT and LTαβ may promote local anti-tumor responses through mechanisms that are partly overlapping and partly distinct.
In addition to the therapeutically induced development of ectopic lymphoid tissues at sites of tumor growth, there are also examples of spontaneous ectopic follicles forming around some, but not the majority of tumors [82] (Figure 2). However, it is not clear whether these tissues are beneficial or promote tumor regression. For example, tertiary lymphoid tissues that develop around tumors could be responding to tumor antigens and inflammation and providing a beneficial effect. Alternatively, they could be developing in response to the local expression of homeostatic cytokines or chemokines and helping to promote tolerance to tumor antigens. In addition, there are examples in which the formation of ectopic follicles is actually associated with tumor development. For example, gastric carcinoma and MALT lymphoma develop at sites of Helicobacter pylori infection in the gastric mucosa of humans [167–169]. Similar effects are seen in the skin of Borrelia burgdorferi-infected patients, who often develop lymphocytoma cutis [127,128]. Although the malignant transformation of B cells may be driven by antigen receptor stimulation in some cases [121,122], the stromal cell microenvironment of ectopic and even conventional lymphoid tissues also plays an important role in the maintenance of some tumors [170]. These stromal cells seem to depend on TNF and LTαβ to differentiate and to support malignant B-cell survival [170]. As a result, the development of ectopic lymphoid tissue surrounding some tumors may promote tumorigenesis rather than facilitate anti-tumor immunity.
Figure 2. Ectopic lymphoid tissues spontaneously develop next to some tumors.
A. This panel shows an H&E stained section of a lung biopsy from a patient with bronchioalvaolar carcinoma. Numerous ectopic follicles (blue) can be observed. B and C. Some of the B cells in ectopic follicles express Proliferating Cell Nuclear Antigen (PCNA), suggesting that they are activated B cells that could contribute to germinal centers.
10. The role of Tregs in the formation of ectopic lymphoid follicles
Given that T regulatory cells (Tregs) are reduced in numbers or functionally impaired in many autoimmune diseases [171–175], it is not surprising that they might play a role in either the formation or maintenance of ectopic lymphoid tissues. However, it is difficult to distinguish the role of Tregs in the prevention of autoimmunity from their role in tertiary lymphoid organ development. For example, the impaired homing of Tregs to LNs is at least partly responsible for the formation of iBALT in CCR7−/− mice [176]. CCR7−/− mice have very few Tregs in their LNs and also develop iBALT shortly after birth [176]. Furthermore, the development of iBALT is prevented by the adoptive transfer of CCR7+/+ Tregs to CCR7−/− recipients. Surprisingly, CCR7−/− mice actually have higher numbers of Tregs in peripheral tissues, including the lung, suggesting that it is the activity of Tregs in the LNs rather than in the lungs that prevents iBALT formation [176].
If poor Treg activity or low Treg numbers results in the formation of ectopic lymphoid tissues, then we should see evidence of this in other autoimmune systems. However, NOD mice have normal numbers of Tregs [177] and still develop ectopic lymphoid tissues in the pancreas and salivary glands [90] as well as the lung [178]. Conversely, if the loss of CCR7 leads to reduced Treg activity, then CCR7 mice should manifest some aspects of autoimmunity. Although this has not been explicitly reported, it is clear that CCR7−/− mice have impaired tolerance induction to oral and inhaled antigens [179–181]. This is due in part to impaired migration of dendritic cells from the site of antigen deposition to the draining LNs [179,180] and may or may not have anything to do with the impaired Treg migration to the LNs.
Interestingly, other studies find that plt/plt mice, which are unable to express the ligands for CCR7 – CCL19 and CCL21 [182,183], do not spontaneously form iBALT [77]. Moreover, while these mice do form iBALT after influenza infection, the iBALT is not as functional and exhibits impaired germinal center formation and poor T cell activation [77]. This makes sense in the light of the expression of CCL21 on HEVs [77] and the expression of CCL19 in the T cell areas of ectopic lymphoid tissues. Interestingly, the number of Tregs in the lungs of influenza-infected plt/plt mice is dramatically increased and seems to play a role in dampening immune responsiveness to influenza (Rangel-Moreno unpublished). Thus, CCR7 signaling appears to play multiple and contradictory roles in the formation and function of ectopic lymphoid tissues.
11. Lymphocyte recirculation and the acquisition of antigen in ectopic follicles
CCR7 also plays a critical role in lymphocyte recirculation through secondary lymphoid organs [184] as well as tertiary lymphoid tissues. For example, PNAd-expressing HEVs that coexpress CCL21 are often observed in tertiary lymphoid tissues [49,77], suggesting that CCR7 and L-selectin may be the primary means that lymphocytes use to gain entry to these tissues from the blood. In fact, CCL21 is shown to trigger integrin-dependent adhesion of T cells to venules and to promote extravasation in tertiary lymphoid tissues [185]. Moreover, HEVs in areas of iBALT express relatively high levels of VCAM-1 and PNAd and lymphocyte homing to iBALT is dependent on L-selectin/PNAd, α4β1/VCAM and LFA-1 pathways [178]. On the other hand, entry of lymphocytes to ectopic lymphoid tissue in the thyroid is independent of L-selectin, even though it requires CCR7 [49]. These data point out that homing to ectopic lymphoid tissues does not follow the same rules as homing to conventional secondary lymphoid organs and needs to be further explored.
CCR7 is also important for the recovery of central memory cells from peripheral non-lymphoid tissues [186,187]. Although effector and memory T cells are attracted to non-lymphoid organs and sites of inflammation via inflammatory chemokines, those that express CCR7 can return to the circulation by entering lymphatic vessels, which often express CCL21 [186,187]. In the absence of CCL21 or CCR7, these cells are unable to return and may simply pile up in peripheral tissues. If enough T and B cells are stuck in these sites, they may begin to spontaneously form organized lymphoid tissues. This possibility is proposed by some investigators to explain the development of ectopic lymphoid tissues in CCR7−/− mice [188]. Although there are numerous immune abnormalities, such as altered Treg function or incipient autoimmunity in CCR7−/− mice, the poor recovery of activated B and T cells from peripheral non-lymphoid organs may play an important role in the development of ectopic lymphoid tissues in these animals.
A similar mechanism is proposed to explain the formation of tertiary lymphoid tissues at sites of inflammation or infection in normal individuals. In this model, infection or inflammation leads to rerouting of the lymphatic drainage towards newly formed tertiary lymphoid tissues [116]. As a result, the developing lymphoid tissue is maintained by the continuous influx of antigen and APCs, which leads to sustained local immune activation. Although there is no definitive evidence to support this model, it brings up the question of how tertiary lymphoid tissues acquire antigen. In cases where ectopic follicles are formed in response to autoantigens, such as in diabetes or rheumatoid arthritis, antigens could be expressed directly in the follicle - perhaps by dendritic cells. However, in cases where ectopic follicles are formed in response to infection, it is less clear how antigen may be acquired. When ectopic lymphoid tissues form around sites of infection, such as in granulomas, antigen may be present directly at the site of lymphoid tissue formation. However, there is often a spatial distinction between the granulomas that surround the bacteria and the tertiary lymphoid tissues [111,134,135,189]. In Propionibacterium acnes infection of the liver, dendritic cell precursors first migrate to the granuloma in response to MIP1α, where they mature and acquire antigen, and then migrate to the portal area in response to CCL21 [111,189]. This implies that there must either be lymphatic drainage between the granuloma and PALT or that dendritic cells directly migrate through the tissue. In addition, areas of iBALT in the lung seem to respond to exogenous inhaled antigens [190,191], suggesting that there is a mechanism to get antigen from the airways, across the respiratory epithelium and into iBALT. This may involve M cells [192], although the majority of iBALT areas seem to lack a true dome epithelium [4]. Thus, it is possible that tertiary lymphoid tissues such as iBALT develop afferent lymphatics that bring in antigens and dendritic cells from distal parts of the tissues. Lymphatic vessels surrounding the B cell areas of ectopic lymphoid tissues are found in both mouse and human tissues [50,82]. These lymphatics surround the B cell follicle and co-localize with the T cell area [50,82] (Figure 3). However, it is not clear whether these are afferent lymphatics that would bring antigens into the follicle or whether they are efferent lymphatics that would drain away from the follicle to downstream LNs. Understanding the lymphatic drainage of tertiary lymphoid tissues is another important issue that needs to be resolved.
Figure 3. Lymphangiogenesis and chemokine expression in ectopic lymphoid tissue.
Lungs from naïve mice (A) or mice that had recovered from influenza infection (B) were probed with antibodies to B cells (red) or lymphatic vessels (green). C. CCL21 (red) is expressed on vascular endothelium surrounding B cell follicles (green) in iBALT. D. CXCL13 (red) is expressed in a reticular pattern in the B cell follicle (green) of iBALT.
12. Concluding remarks
The development of ectopic or tertiary lymphoid tissues at sites of inflammation or infection is an important part of local immune responses. Depending on the type of immune responses, the development of these tissues can lead to detrimental or pathological immune responses (autoimmunity) or may help resolve local infection. In this respect, one might view ectopic lymphoid tissues much like other local lymphoid tissues that serve to initiate and amplify immune responses. However, there are hints that ectopic follicles may be controlling or imprinting particular characteristics on locally generated immune responses independently of the activities of conventional lymphoid organs. This means that it is even more important to understand the cellular and molecular mechanisms responsible for ectopic follicle development and to determine how these follicles function in both pathologic and protective immune responses.
Acknowledgments
This work was supported by the Trudeau Institute, NIH grants HL69409 and AI072689 and by the Sandler Program for Asthma Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goodnow CC. Chance encounters and organized rondezvous. Immunol Rev. 1997;156:5–10. doi: 10.1111/j.1600-065x.1997.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 2.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286(5447):2098–102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 3.Mowat AM, Viney JL. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156:145–166. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 4.Woodland DL, Randall TD. Anatomical features of anti-viral immunity in the respiratory tract. Semin Immunol. 2004;16(3):163–70. doi: 10.1016/j.smim.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gretz JE, Anderson AO, Shaw S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rev. 1997;156:11–24. doi: 10.1111/j.1600-065x.1997.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 6.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7(4):344–53. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 7.Nishikawa S, Honda K, Vieira P, Yoshida H. Organogenesis of peripheral lymphoid organs. Immunol Rev. 2003;195:72–80. doi: 10.1034/j.1600-065x.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 8.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6(3):205–17. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 9.Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, Bouley DM, Thomas J, Kanangat S, Mucenski ML. Lymphotoxin α deficient mice: effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155:1685–1693. [PubMed] [Google Scholar]
- 10.de Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 11.Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin b receptor controls organogenisis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 12.Miyawaki S, Nakamura Y, Suzuka H, Koba M, Yasumizu R, Ikehara S, Shibata Y. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur J Immunol. 1994;24(2):429–34. doi: 10.1002/eji.1830240224. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz ZB, Weih DS, Sivakumar V, Weih F. RelB is required for Peyer’s patch development: differential regulation of p52-RelB by lymphotoxin and TNF. Embo J. 2003;22(1):121–30. doi: 10.1093/emboj/cdg004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cupedo T, Mebius RE. Cellular interactions in lymph node development. J Immunol. 2005;174(1):21–5. doi: 10.4049/jimmunol.174.1.21. [DOI] [PubMed] [Google Scholar]
- 15.Cupedo T, Kraal G, Mebius RE. The role of CD45+CD4+CD3- cells in lymphoid organ development. Immunol Rev. 2002;189:41–50. doi: 10.1034/j.1600-065x.2002.18905.x. [DOI] [PubMed] [Google Scholar]
- 16.Akashi K, Kondo M, Cheshier S, Shizuru J, Gandy K, Domen J, Mebius R, Traver D, Weissman IL. Lymphoid development from stem cells and the common lymphocyte progenitors. Cold Spring Harb Symp Quant Biol. 1999;64:1–12. doi: 10.1101/sqb.1999.64.1. [DOI] [PubMed] [Google Scholar]
- 17.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3−LTb+ cells that can differentiate to APC, NK cells, and follicular cells, but notT or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 18.Adachi S, Yoshida H, Honda K, Maki K, Saijo K, Ikuta K, Saito T, Nishikawa SI. Essential role of IL-7 receptor alpha in the formation of Peyer’s patch anlage. Int Immunol. 1998;10(1):1–6. doi: 10.1093/intimm/10.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida H, Honda K, Shinkura R, Adachi S, Nishikawa S, Maki K, Ikuta K, Nishikawa SI. IL-7 receptor alpha+ CD3(−) cells in the embryonic intestine induces the organizing center of Peyer’s patches. Int Immunol. 1999;11(5):643–55. doi: 10.1093/intimm/11.5.643. [DOI] [PubMed] [Google Scholar]
- 20.Kim D, Mebius RE, MacMicking JD, Jung S, Cupedo T, Castellanos Y, Rho J, Wong BR, Josien R, Kim N, et al. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J Exp Med. 2000;192:1467–1478. doi: 10.1084/jem.192.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med. 2003;197(9):1191–8. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cupedo T, Vondenhoff MF, Heeregrave EJ, De Weerd AE, Jansen W, Jackson DG, Kraal G, Mebius RE. Presumptive lymph node organizers are differentially represented in developing mesenteric and peripheral nodes. J Immunol. 2004;173(5):2968–75. doi: 10.4049/jimmunol.173.5.2968. [DOI] [PubMed] [Google Scholar]
- 23.Honda K, Nakano H, Yoshida H, Nishikawa S, Rennert P, Ikuta K, Tamechika M, Yamaguchi K, Fukumoto T, Chiba T, et al. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer’s patch organogenesis. J Exp Med. 2001;193:621–630. doi: 10.1084/jem.193.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook MC, Korner H, Rininton DS, Lemkert FA, Hasbold J, Amesbury M, Hodgkin PD, Cyster JG, Sedgwick JD, Basten A. Generation of splenic follicular structure and B cell movement in tumor necrosis factor deficient mice. J Exp Med. 1998;188:1503–1510. doi: 10.1084/jem.188.8.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cyster JG. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J Exp Med. 1999;189:447–450. doi: 10.1084/jem.189.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169(1):424–33. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 27.Yu P, Wang Y, Chin RK, Martinez-Pomares L, Gordon S, Kosco-Vibois MH, Cyster J, Fu YX. B cells control the migration of a subset of dendritic cells into B cell follicles via CXC chemokine ligand 13 in a lymphotoxin-dependent fashion. J Immunol. 2002;168(10):5117–23. doi: 10.4049/jimmunol.168.10.5117. [DOI] [PubMed] [Google Scholar]
- 28.Endres R, Alimzhanov MB, Plitz T, Futterer A, Kosco-Vilbois MH, Nedospasov SA, Rajewski K, Pfeffer K. Mature follicular dendritic cell networks depend on expression of lymphotoxin β receptor by radioresistant stromal cells and of lymphotoxin β and tumor necrosis factor by B cells. J Exp Med. 1999;189:159–167. doi: 10.1084/jem.189.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez M, Mackay F, Browning JL, Kosco-Vilbois MH, Noelle RJ. The sequential role of lymphotoxin and B cells in the development of splenic follicles. J Exp Med. 1998;187:997–1007. doi: 10.1084/jem.187.7.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ansel KM, Ngo VN, Hayman PL, Luther SA, Forster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 31.Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A Putative Chemokine Receptor, BLR1, Directs B Cell Migration to Defines Lymphoid Organs and Specific Anatomic Compartments of the Spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 32.Cyster JG, Ansel KM, Rief K, Hyman PL, Tang HL, Luther SA, Ngo VN. Folicular stromal cells and lymphocyte homing to follicles. Immunol Rev. 2000;176:181–193. doi: 10.1034/j.1600-065x.2000.00618.x. [DOI] [PubMed] [Google Scholar]
- 33.Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, Browning JL, Sedgewick JD, Cyster JG. Lymphotoxin α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189:403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor RT, Patel SR, Lin E, Butler BR, Lake JG, Newberry RD, Williams IR. Lymphotoxin-independent expression of TNF-related activation-induced cytokine by stromal cells in cryptopatches, isolated lymphoid follicles, and Peyer’s patches. J Immunol. 2007;178(9):5659–67. doi: 10.4049/jimmunol.178.9.5659. [DOI] [PubMed] [Google Scholar]
- 35.Cuff CA, Schwartz J, Bergman CM, Russell KS, Bender JR, Ruddle NH. Lymphotoxin alpha3 induces chemokines and adhesion molecules: insight into the role of LT alpha in inflammation and lymphoid organ development. J Immunol. 1998;161(12):6853–60. [PubMed] [Google Scholar]
- 36.Cuff CA, Sacca R, Ruddle NH. Differential induction of adhesion molecule and chemokine expression by LTalpha3 and LTalphabeta in inflammation elucidates potential mechanisms of mesenteric and peripheral lymph node development. J Immunol. 1999;162(10):5965–72. [PubMed] [Google Scholar]
- 37.Browning JL, Allaire N, Ngam-Ek A, Notidis E, Hunt J, Perrin S, Fava RA. Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity. 2005;23(5):539–50. doi: 10.1016/j.immuni.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Pablos JL, Santiago B, Tsay D, Singer MS, Palao G, Galindo M, Rosen SD. A HEV-restricted sulfotransferase is expressed in rheumatoid arthritis synovium and is induced by lymphotoxin-alpha/beta and TNF-alpha in cultured endothelial cells. BMC Immunol. 2005;6(1):6. doi: 10.1186/1471-2172-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197(9):1153–63. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. 1996;183(4):1461–72. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hjelmstrom P, Fjell J, Nakagawa T, Sacca R, Cuff C, Ruddle N. Lymphoid Tissue Homing Chemokines Are Expressed in Chronic Inflammation. Am J Pathol. 2000;156:1133–1138. doi: 10.1016/S0002-9440(10)64981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Foster A, Chin R, Yu P, Sun Y, Wang Y, Pfeffer K, Fu YX. The complementation of lymphotoxin deficiency with LIGHT, a newly discovered TNF family member, for the restoration of secondary lymphoid structure and function. Eur J Immunol. 2002;32(7):1969–79. doi: 10.1002/1521-4141(200207)32:7<1969::AID-IMMU1969>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 43.Gommerman JL, Browning JL. Lymphotoxin/LIGHT, lymphoid microenvironments and autoimmune disease. Nat Rev Immunol. 2003;3(8):642–55. doi: 10.1038/nri1151. [DOI] [PubMed] [Google Scholar]
- 44.Scheu S, Alferink J, Potzel T, Barchet W, Kalinke U, Pfeffer K. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med. 2002;195(12):1613–24. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee Y, Chin RK, Christiansen P, Sun Y, Tumanov AV, Wang J, Chervonsky AV, Fu YX. Recruitment and activation of naive T cells in the islets by lymphotoxin beta receptor-dependent tertiary lymphoid structure. Immunity. 2006;25(3):499–509. doi: 10.1016/j.immuni.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 46.Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC Expression in Pancreatic Islets Causes B Cell Recruitment and Lymphotoxin-Dependent Lymphoid Neogenesis. Immunity. 2000;12:471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- 47.Chen SC, Vassileva G, Kinsley D, Holzmann S, Manfra D, Wiekowski MT, Romani N, Lira SA. Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node-like structures in pancreas, but not skin, of transgenic mice. J Immunol. 2002;168(3):1001–8. doi: 10.4049/jimmunol.168.3.1001. [DOI] [PubMed] [Google Scholar]
- 48.Fan L, Reilly CR, Luo Y, Dorf ME, Lo D. Cutting Edge: Ectopic Expression of the Chemokine TCA4/SLC Is Sufficient to Trigger Lymphoid Neogenesis. J Immunol. 2000;164:3955–3959. doi: 10.4049/jimmunol.164.8.3955. [DOI] [PubMed] [Google Scholar]
- 49.Martin AP, Coronel EC, Sano G, Chen SC, Vassileva G, Canasto-Chibuque C, Sedgwick JD, Frenette PS, Lipp M, Furtado GC, et al. A novel model for lymphocytic infiltration of the thyroid gland generated by transgenic expression of the CC chemokine CCL21. J Immunol. 2004;173(8):4791–8. doi: 10.4049/jimmunol.173.8.4791. [DOI] [PubMed] [Google Scholar]
- 50.Furtado GC, Marinkovic T, Martin AP, Garin A, Hoch B, Hubner W, Chen BK, Genden E, Skobe M, Lira SA. Lymphotoxin beta receptor signaling is required for inflammatory lymphangiogenesis in the thyroid. Proc Natl Acad Sci U S A. 2007;104(12):5026–31. doi: 10.1073/pnas.0606697104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lira SA, Martin AP, Marinkovic T, Furtado GC. Mechanisms regulating lymphocytic infiltration of the thyroid in murine models of thyroiditis. Crit Rev Immunol. 2005;25(4):251–62. doi: 10.1615/critrevimmunol.v25.i4.10. [DOI] [PubMed] [Google Scholar]
- 52.Goya S, Matsuoka H, Mori M, Morishita H, Kida H, Kobashi Y, Kato T, Taguchi Y, Osaki T, Tachibana I, et al. Sustained interleukin-6 signalling leads to the development of lymphoid organ-like structures in the lung. J Pathol. 2003;200(1):82–7. doi: 10.1002/path.1321. [DOI] [PubMed] [Google Scholar]
- 53.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185(12):2143–56. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun Z, Unutmaz D, Zou Y-R, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORγ in Thymocyte Survival and Lymphoid Organ Development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 55.Eberl G, Littman DR. The role of the nuclear hormone receptor RORgammat in the development of lymph nodes and Peyer’s patches. Immunol Rev. 2003;195:81–90. doi: 10.1034/j.1600-065x.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 56.Fukuyama S, Hiroi T, Yokota Y, Rennert PD, Yanagita M, Kinoshita N, Terawaki S, Shikina T, Yamamoto M, Kurono Y, et al. Initiation of NALT organogenesis is independent of the IL-7R, LTbetaR, and NIK signaling pathways but requires the Id2 gene and CD3(−)CD4(+)CD45(+) cells. Immunity. 2002;17(1):31–40. doi: 10.1016/s1074-7613(02)00339-4. [DOI] [PubMed] [Google Scholar]
- 57.Yokoto Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa SI, Gruss P. Development of peripheral lymphoid organs and natual killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 58.Meier D, Bornmann C, Chappaz S, Schmutz S, Otten LA, Ceredig R, Acha-Orbea H, Finke D. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity. 2007;26(5):643–54. doi: 10.1016/j.immuni.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O’Fallon WM, Goronzy JJ, Weyand CM. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167(2):1072–80. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- 60.van Roon JA, Verweij MC, Wijk MW, Jacobs KM, Bijlsma JW, Lafeber FP. Increased intraarticular interleukin-7 in rheumatoid arthritis patients stimulates cell contact-dependent activation of CD4(+) T cells and macrophages. Arthritis Rheum. 2005;52(6):1700–10. doi: 10.1002/art.21045. [DOI] [PubMed] [Google Scholar]
- 61.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181(4):1519–26. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chou YK, Bourdette DN, Barnes D, Finn TP, Murray S, Unsicker L, Robey I, Whitham RH, Buenafe AC, Allegretta M, et al. IL-7 enhances Ag-specific human T cell response by increasing expression of IL-2R alpha and gamma chains. J Neuroimmunol. 1999;96(1):101–11. doi: 10.1016/s0165-5728(99)00002-8. [DOI] [PubMed] [Google Scholar]
- 63.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98(15):8732–7. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rennert PD, Browning JL, Mebius RE, Mackay F, Hochman PS. Surface lymphotoxin a/b complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cupedo T. Regulation of Lymphoid Organogenesis. Amsterdam: Vrije Universiteit Amsterdam; 2003. p. 144. [Google Scholar]
- 66.Cupedo T, Jansen W, Kraal G, Mebius RE. Induction of secondary and tertiary lymphoid structures in the skin. Immunity. 2004;21(5):655–67. doi: 10.1016/j.immuni.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 67.Marinkovic T, Garin A, Yokota Y, Fu YX, Ruddle NH, Furtado GC, Lira SA. Interaction of mature CD3+CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J Clin Invest. 2006;116(10):2622–32. doi: 10.1172/JCI28993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Worm M, Geha RS. CD40-mediated lymphotoxin a expression in human B cells is tyrosine kinase dependent. Eur J Immunol. 1995;25:2438–2444. doi: 10.1002/eji.1830250905. [DOI] [PubMed] [Google Scholar]
- 69.Summers-DeLuca LE, McCarthy DD, Cosovic B, Ward LA, Lo CC, Scheu S, Pfeffer K, Gommerman JL. Expression of lymphotoxin-alphabeta on antigen-specific T cells is required for DC function. J Exp Med. 2007;204(5):1071–81. doi: 10.1084/jem.20061968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim MY, Toellner KM, White A, McConnell FM, Gaspal FM, Parnell SM, Jenkinson E, Anderson G, Lane PJ. Neonatal and adult CD4+ CD3- cells share similar gene expression profile, and neonatal cells up-regulate OX40 ligand in response to TL1A (TNFSF15) J Immunol. 2006;177(5):3074–81. doi: 10.4049/jimmunol.177.5.3074. [DOI] [PubMed] [Google Scholar]
- 71.Kim MY, Gaspal FM, Wiggett HE, McConnell FM, Gulbranson-Judge A, Raykundalia C, Walker LS, Goodall MD, Lane PJ. CD4(+)CD3(−) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18(5):643–54. doi: 10.1016/s1074-7613(03)00110-9. [DOI] [PubMed] [Google Scholar]
- 72.Kim MY, Anderson G, White A, Jenkinson E, Arlt W, Martensson IL, Erlandsson L, Lane PJ. OX40 ligand and CD30 ligand are expressed on adult but not neonatal CD4+CD3− inducer cells: evidence that IL-7 signals regulate CD30 ligand but not OX40 ligand expression. J Immunol. 2005;174(11):6686–91. doi: 10.4049/jimmunol.174.11.6686. [DOI] [PubMed] [Google Scholar]
- 73.Kim MY, McConnell FM, Gaspal FM, White A, Glanville SH, Bekiaris V, Walker LS, Caamano J, Jenkinson E, Anderson G, et al. Function of CD4+CD3− cells in relation to B- and T-zone stroma in spleen. Blood. 2007;109(4):1602–10. doi: 10.1182/blood-2006-04-018465. [DOI] [PubMed] [Google Scholar]
- 74.Harmsen A, Kusser K, Hartson L, Tighe M, Sunshine MJ, Sedgwick JD, Choi Y, Littman DR, Randall TD. Organogenesis of Nasal Associated Lymphoid Tissue (NALT) occurs independently of lymphotoxin-α (LTα) and retinoic acid receptor-related orphan receptor-γ, but the organization of NALT is LTα-dependent. J Immunol. 2002;168:986–990. doi: 10.4049/jimmunol.168.3.986. [DOI] [PubMed] [Google Scholar]
- 75.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3(4):292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 76.Okuda M, Togawa A, Wada H, Nishikawa S. Distinct activities of stromal cells involved in the organogenesis of lymph nodes and Peyer’s patches. J Immunol. 2007;179(2):804–11. doi: 10.4049/jimmunol.179.2.804. [DOI] [PubMed] [Google Scholar]
- 77.Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Kusser K, Randall TD. Pulmonary expression of CXC chemokine ligand 13, CC chemokine ligand 19, and CC chemokine ligand 21 is essential for local immunity to influenza. Proc Natl Acad Sci U S A. 2007;104(25):10577–82. doi: 10.1073/pnas.0700591104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Braun A, Takemura S, Vallejo AN, Goronzy JJ, Weyand CM. Lymphotoxin beta-mediated stimulation of synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2004;50(7):2140–50. doi: 10.1002/art.20356. [DOI] [PubMed] [Google Scholar]
- 79.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114(3):438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol. 2007 doi: 10.1189/jlb.0906587. [DOI] [PubMed] [Google Scholar]
- 81.Gregorio A, Gambini C, Gerloni V, Parafioriti A, Sormani MP, Gregorio S, De Marco G, Rossi F, Martini A, Gattorno M. Lymphoid neogenesis in juvenile idiopathic arthritis correlates with ANA positivity and plasma cells infiltration. Rheumatology (Oxford) 2007;46(2):308–13. doi: 10.1093/rheumatology/kel225. [DOI] [PubMed] [Google Scholar]
- 82.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006;116(12):3183–94. doi: 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weyand CM, Goronzy JJ. Ectopic germinal center formation in rheumatoid synovitis. Ann N Y Acad Sci. 2003;987:140–9. doi: 10.1111/j.1749-6632.2003.tb06042.x. [DOI] [PubMed] [Google Scholar]
- 84.Kim EA, Lee KS, Johkoh T, Kim TS, Suh GY, Kwon OJ, Han J. Interstitial lung diseases associated with collagen vascular diseases: radiologic and histopathologic findings. Radiographics. 2002;22 doi: 10.1148/radiographics.22.suppl_1.g02oc04s151. Spec No:S151–65. [DOI] [PubMed] [Google Scholar]
- 85.Ito I, Nagai S, Kitaichi M, Nicholson AG, Johkoh T, Noma S, Kim DS, Handa T, Izumi T, Mishima M. Pulmonary Manifestations of Primary Sjogren’s Syndrome: A Clinical, Radiologic, and Pathologic Study. Am J Respir Crit Care Med. 2004 doi: 10.1164/rccm.200403-417OC. [DOI] [PubMed] [Google Scholar]
- 86.Salomonsson S, Larsson P, Tengner P, Mellquist E, Hjelmstrom P, Wahren-Herlenius M. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjogren’s syndrome. Scand J Immunol. 2002;55(4):336–42. doi: 10.1046/j.1365-3083.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- 87.Salomonsson S, Jonsson MV, Skarstein K, Brokstad KA, Hjelmstrom P, Wahren-Herlenius M, Jonsson R. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjogren’s syndrome. Arthritis Rheum. 2003;48(11):3187–201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- 88.Armengol MP, Cardoso-Schmidt CB, Fernandez M, Ferrer X, Pujol-Borrell R, Juan M. Chemokines determine local lymphoneogenesis and a reduction of circulating CXCR4+ T and CCR7 B and T lymphocytes in thyroid autoimmune diseases. J Immunol. 2003;170(12):6320–8. doi: 10.4049/jimmunol.170.12.6320. [DOI] [PubMed] [Google Scholar]
- 89.Aust G, Sittig D, Becherer L, Anderegg U, Schutz A, Lamesch P, Schmucking E. The role of CXCR5 and its ligand CXCL13 in the compartmentalization of lymphocytes in thyroids affected by autoimmune thyroid diseases. Eur J Endocrinol. 2004;150(2):225–34. doi: 10.1530/eje.0.1500225. [DOI] [PubMed] [Google Scholar]
- 90.Faveeuw C, Gagnerault MC, Lepault F. Expression of homing and adhesion molecules in infiltrated islets of Langerhans and salivary glands of nonobese diabetic mice. J Immunol. 1994;152(12):5969–78. [PubMed] [Google Scholar]
- 91.Ludewig B, Odermatt B, Landmann S, Hengartner H, Zinkernagel RM. Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J Exp Med. 1998;188(8):1493–501. doi: 10.1084/jem.188.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alt C, Laschinger M, Engelhardt B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood-brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur J Immunol. 2002;32(8):2133–44. doi: 10.1002/1521-4141(200208)32:8<2133::AID-IMMU2133>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 93.Columba-Cabezas S, Griguoli M, Rosicarelli B, Magliozzi R, Ria F, Serafini B, Aloisi F. Suppression of established experimental autoimmune encephalomyelitis and formation of meningeal lymphoid follicles by lymphotoxin beta receptor-Ig fusion protein. J Neuroimmunol. 2006;179(1–2):76–86. doi: 10.1016/j.jneuroim.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 94.Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, Reynolds R, Aloisi F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130(Pt 4):1089–104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 95.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14(2):164–74. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Manzo A, Paoletti S, Carulli M, Blades MC, Barone F, Yanni G, Fitzgerald O, Bresnihan B, Caporali R, Montecucco C, et al. Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur J Immunol. 2005;35(5):1347–59. doi: 10.1002/eji.200425830. [DOI] [PubMed] [Google Scholar]
- 97.Page G, Miossec P. Paired synovium and lymph nodes from rheumatoid arthritis patients differ in dendritic cell and chemokine expression. J Pathol. 2004;204(1):28–38. doi: 10.1002/path.1607. [DOI] [PubMed] [Google Scholar]
- 98.Carlsen HS, Baekkevold ES, Morton HC, Haraldsen G, Brandtzaeg P. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood. 2004;104(10):3021–7. doi: 10.1182/blood-2004-02-0701. [DOI] [PubMed] [Google Scholar]
- 99.Perrier P, Martinez FO, Locati M, Bianchi G, Nebuloni M, Vago G, Bazzoni F, Sozzani S, Allavena P, Mantovani A. Distinct transcriptional programs activated by interleukin-10 with or without lipopolysaccharide in dendritic cells: induction of the B cell-activating chemokine, CXC chemokine ligand 13. J Immunol. 2004;172(11):7031–42. doi: 10.4049/jimmunol.172.11.7031. [DOI] [PubMed] [Google Scholar]
- 100.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10(9):927–34. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 101.Fu Y-X, Huang G, Wang Y, Chaplin DD. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin a dependent fashion. J Exp Med. 1998;187:1009–1018. doi: 10.1084/jem.187.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM. T cell activation in rheumatoid synovium is B cell dependent. J Immunol. 2001;167(8):4710–8. doi: 10.4049/jimmunol.167.8.4710. [DOI] [PubMed] [Google Scholar]
- 103.Wagner UG, Kurtin PJ, Wahner A, Brackertz M, Berry DJ, Goronzy JJ, Weyand CM. The role of CD8+ CD40L+ T cells in the formation of germinal centers in rheumatoid synovitis. J Immunol. 1998;161(11):6390–7. [PubMed] [Google Scholar]
- 104.Kang YM, Zhang X, Wagner UG, Yang H, Beckenbaugh RD, Kurtin PJ, Goronzy JJ, Weyand CM. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med. 2002;195(10):1325–36. doi: 10.1084/jem.20011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Corcione A, Casazza S, Ferretti E, Giunti D, Zappia E, Pistorio A, Gambini C, Mancardi GL, Uccelli A, Pistoia V. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101(30):11064–9. doi: 10.1073/pnas.0402455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bagaeva LV, Rao P, Powers JM, Segal BM. CXC chemokine ligand 13 plays a role in experimental autoimmune encephalomyelitis. J Immunol. 2006;176(12):7676–85. doi: 10.4049/jimmunol.176.12.7676. [DOI] [PubMed] [Google Scholar]
- 107.Magliozzi R, Columba-Cabezas S, Serafini B, Aloisi F. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148(1–2):11–23. doi: 10.1016/j.jneuroim.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 108.Gommerman JL, Giza K, Perper S, Sizing I, Ngam-Ek A, Nickerson-Nutter C, Browning JL. A role for surface lymphotoxin in experimental autoimmune encephalomyelitis independent of LIGHT. J Clin Invest. 2003;112(5):755–67. doi: 10.1172/JCI18648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Katakai T, Hara T, Sugai M, Gonda H, Shimizu A. Th1-biased tertiary lymphoid tissue supported by CXC chemokine ligand 13-producing stromal network in chronic lesions of autoimmune gastritis. J Immunol. 2003;171(8):4359–68. doi: 10.4049/jimmunol.171.8.4359. [DOI] [PubMed] [Google Scholar]
- 110.Shomer NH, Fox JG, Juedes AE, Ruddle NH. Helicobacter-induced chronic active lymphoid aggregates have characteristics of tertiary lymphoid tissue. Infect Immun. 2003;71(6):3572–7. doi: 10.1128/IAI.71.6.3572-3577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yoneyama H, Matsuno K, Zhang Y, Murai M, Itakura M, Ishikawa S, Hasegawa G, Naito M, Asakura H, Matsushima K. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J Exp Med. 2001;193(1):35–49. doi: 10.1084/jem.193.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sansonno D, Lauletta G, De Re V, Tucci FA, Gatti P, Racanelli V, Boiocchi M, Dammacco F. Intrahepatic B cell clonal expansions and extrahepatic manifestations of chronic HCV infection. Eur J Immunol. 2004;34(1):126–36. doi: 10.1002/eji.200324328. [DOI] [PubMed] [Google Scholar]
- 113.Mosnier JF, Degott C, Marcellin P, Henin D, Erlinger S, Benhamou JP. The intraportal lymphoid nodule and its environment in chronic active hepatitis C: an immunohistochemical study. Hepatology. 1993;17(3):366–71. [PubMed] [Google Scholar]
- 114.Murakami J, Shimizu Y, Kashii Y, Kato T, Minemura M, Okada K, Nambu S, Takahara T, Higuchi K, Maeda Y, et al. Functional B-cell response in intrahepatic lymphoid follicles in chronic hepatitis C. Hepatology. 1999;30(1):143–50. doi: 10.1002/hep.510300107. [DOI] [PubMed] [Google Scholar]
- 115.Itakura M, Tokuda A, Kimura H, Nagai S, Yoneyama H, Onai N, Ishikawa S, Kuriyama T, Matsushima K. Blockade of Secondary Lymphoid Tissue Chemokine Exacerbated Proionibacterium acnes-Induced Acute Lung Inflammation. J Immunol. 2001;166:2071–2079. doi: 10.4049/jimmunol.166.3.2071. [DOI] [PubMed] [Google Scholar]
- 116.Thaunat O, Kerjaschki D, Nicoletti A. Is defective lymphatic drainage a trigger for lymphoid neogenesis? Trends Immunol. 2006;27(10):441–5. doi: 10.1016/j.it.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 117.Mazzucchelli L, Blaser A, Kappeler A, Scharli P, Laissue JA, Baggiolini M, Uguccioni M. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J Clin Invest. 1999;104(10):R49–54. doi: 10.1172/JCI7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Genta RM, Hamner HW. The significance of lymphoid follicles in the interpretation of gastric biopsy specimens. Arch Pathol Lab Med. 1994;118(7):740–3. [PubMed] [Google Scholar]
- 119.Genta RM, Hamner HW, Graham DY. Gastric lymphoid follicles in Helicobacter pylori infection: frequency, distribution, and response to triple therapy. Hum Pathol. 1993;24(6):577–83. doi: 10.1016/0046-8177(93)90235-9. [DOI] [PubMed] [Google Scholar]
- 120.Kobayashi M, Mitoma J, Nakamura N, Katsuyama T, Nakayama J, Fukuda M. Induction of peripheral lymph node addressin in human gastric mucosa infected by Helicobacter pylori. Proc Natl Acad Sci U S A. 2004;101(51):17807–12. doi: 10.1073/pnas.0407503101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kobayashi K, Yokota K, Yoshino T, Kawahara Y, Dey A, Hirai Y, Oguma K, Akagi T. Detection of Helicobacter pylori associated antigen and heat shock protein 60 on follicular dendritic cells in the germinal centres of low grade B cell lymphoma of gastric mucosa associated lymphoid tissue (MALT) J Clin Pathol. 1998;51(5):396–8. doi: 10.1136/jcp.51.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kawahara Y, Yokota K, Mizuno M, Yunoki N, Uesu T, Okada H, Kobayashi K, Hirai Y, Oguma K, Tsuji T. Antibodies to human gastric epithelial cells and heat shock protein 60 in Helicobacter pylori positive mucosa associated lymphoid tissue lymphoma. Gut. 1999;45(1):20–3. doi: 10.1136/gut.45.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Steere AC, Duray PH, Butcher EC. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis. Comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 1988;31(4):487–95. doi: 10.1002/art.1780310405. [DOI] [PubMed] [Google Scholar]
- 124.Carlson D, Hernandez J, Bloom BJ, Coburn J, Aversa JM, Steere AC. Lack of Borrelia burgdorferi DNA in synovial samples from patients with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum. 1999;42(12):2705–9. doi: 10.1002/1529-0131(199912)42:12<2705::AID-ANR29>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 125.Ghosh S, Steere AC, Stollar BD, Huber BT. In situ diversification of the antibody repertoire in chronic Lyme arthritis synovium. J Immunol. 2005;174(5):2860–9. doi: 10.4049/jimmunol.174.5.2860. [DOI] [PubMed] [Google Scholar]
- 126.Ghosh S, Seward R, Costello CE, Stollar BD, Huber BT. Autoantibodies from synovial lesions in chronic, antibiotic treatment-resistant Lyme arthritis bind cytokeratin-10. J Immunol. 2006;177(4):2486–94. doi: 10.4049/jimmunol.177.4.2486. [DOI] [PubMed] [Google Scholar]
- 127.Leinweber B, Colli C, Chott A, Kerl H, Cerroni L. Differential diagnosis of cutaneous infiltrates of B lymphocytes with follicular growth pattern. Am J Dermatopathol. 2004;26(1):4–13. doi: 10.1097/00000372-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 128.Boudova L, Kazakov DV, Sima R, Vanecek T, Torlakovic E, Lamovec J, Kutzner H, Szepe P, Plank L, Bouda J, et al. Cutaneous lymphoid hyperplasia and other lymphoid infiltrates of the breast nipple: a retrospective clinicopathologic study of fifty-six patients. Am J Dermatopathol. 2005;27(5):375–86. doi: 10.1097/01.dad.0000179463.55129.8a. [DOI] [PubMed] [Google Scholar]
- 129.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 130.Vernooy JH, Dentener MA, van Suylen RJ, Buurman WA, Wouters EF. Long-term intratracheal lipopolysaccharide exposure in mice results in chronic lung inflammation and persistent pathology. Am J Respir Cell Mol Biol. 2002;26(1):152–9. doi: 10.1165/ajrcmb.26.1.4652. [DOI] [PubMed] [Google Scholar]
- 131.Brodie SJ, de la Rosa C, Howe JG, Crouch J, Travis WD, Diem K. Pediatric AIDS-associated lymphocytic interstitial pneumonia and pulmonary arterio-occlusive disease: role of VCAM-1/VLA-4 adhesion pathway and human herpesviruses. Am J Pathol. 1999;154(5):1453–64. doi: 10.1016/S0002-9440(10)65400-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 132.Marolda J, Pace B, Bonforte RJ, Kotin NM, Rabinowitz J, Kattan M. Pulmonary manifestations of HIV infection in children. Pediatr Pulmonol. 1991;10(4):231–5. doi: 10.1002/ppul.1950100402. [DOI] [PubMed] [Google Scholar]
- 133.Baskerville A, Ramsay AD, Addis BJ, Dennis MJ, Cook RW, Cranage MP, Greenaway PJ. Interstitial pneumonia in simian immunodeficiency virus infection. J Pathol. 1992;167(2):241–7. doi: 10.1002/path.1711670214. [DOI] [PubMed] [Google Scholar]
- 134.Kahnert A, Hopken UE, Stein M, Bandermann S, Lipp M, Kaufmann SH. Mycobacterium tuberculosis triggers formation of lymphoid structure in murine lungs. J Infect Dis. 2007;195(1):46–54. doi: 10.1086/508894. [DOI] [PubMed] [Google Scholar]
- 135.Ulrichs T, Kosmiadi GA, Trusov V, Jorg S, Pradl L, Titukhina M, Mishenko V, Gushina N, Kaufmann SH. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J Pathol. 2004;204(2):217–28. doi: 10.1002/path.1628. [DOI] [PubMed] [Google Scholar]
- 136.Maglione PJ, Xu J, Chan J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J Immunol. 2007;178(11):7222–34. doi: 10.4049/jimmunol.178.11.7222. [DOI] [PubMed] [Google Scholar]
- 137.Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD, Lefrancois L, Cauley LS, Harmsen AG, Lund FE, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25(4):643–54. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 138.Lund FE, Partida-Sanchez S, Lee BO, Kusser KL, Hartson L, Hogan RJ, Woodland DL, Randall TD. Lymphotoxin-alpha-Deficient Mice Make Delayed, But Effective, T and B Cell Responses to Influenza. J Immunol. 2002;169(9):5236–43. doi: 10.4049/jimmunol.169.9.5236. [DOI] [PubMed] [Google Scholar]
- 139.Lee BJ, Santee S, Von Gesjen S, Ware CF, Sarawar SR. Lymphotoxin-a-Deficient Mice Can Clear a Productive Infection with Murine Gammaherpesvirus 68 but Fail to Develop Spenomegaly or Lymphocytosis. J Virol. 2000;74:2786–2792. doi: 10.1128/jvi.74.6.2786-2792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Spahn TW, Eugster HP, Fontana A, Domschke W, Kucharzik T. Role of lymphotoxin in experimental models of infectious diseases: potential benefits and risks of a therapeutic inhibition of the lymphotoxin-beta receptor pathway. Infect Immun. 2005;73(11):7077–88. doi: 10.1128/IAI.73.11.7077-7088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG. Intrinsic Lymphotoxin-beta Receptor Requirement for Homeostasis of Lymphoid Tissue Dendritic Cells. Immunity. 2005;22(4):439–50. doi: 10.1016/j.immuni.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 142.Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168(1):57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- 143.Wang C, McDonald KG, McDonough JS, Newberry RD. Murine isolated lymphoid follicles contain follicular B lymphocytes with a mucosal phenotype. Am J Physiol Gastrointest Liver Physiol. 2006;291(4):G595–604. doi: 10.1152/ajpgi.00525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J Immunol. 2003;170(11):5475–82. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- 145.Kweon MN, Yamamoto M, Rennert PD, Park EJ, Lee AY, Chang SY, Hiroi T, Nanno M, Kiyono H. Prenatal blockage of lymphotoxin beta receptor and TNF receptor p55 signaling cascade resulted in the acceleration of tissue genesis for isolated lymphoid follicles in the large intestine. J Immunol. 2005;174(7):4365–72. doi: 10.4049/jimmunol.174.7.4365. [DOI] [PubMed] [Google Scholar]
- 146.Yamamoto M, Kweon MN, Rennert PD, Hiroi T, Fujihashi K, McGhee JR, Kiyono H. Role of gut-associated lymphoreticular tissues in antigen-specific intestinal IgA immunity. J Immunol. 2004;173(2):762–9. doi: 10.4049/jimmunol.173.2.762. [DOI] [PubMed] [Google Scholar]
- 147.Pabst O, Herbrand H, Friedrichsen M, Velaga S, Dorsch M, Berhardt G, Worbs T, Macpherson AJ, Forster R. Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J Immunol. 2006;177(10):6824–32. doi: 10.4049/jimmunol.177.10.6824. [DOI] [PubMed] [Google Scholar]
- 148.Pabst O, Herbrand H, Worbs T, Friedrichsen M, Yan S, Hoffmann MW, Korner H, Bernhardt G, Pabst R, Forster R. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur J Immunol. 2005;35(1):98–107. doi: 10.1002/eji.200425432. [DOI] [PubMed] [Google Scholar]
- 149.Halle S, Bumann D, Herbrand H, Willer Y, Dahne S, Forster R, Pabst O. Solitary intestinal lymphoid tissue provides a productive port of entry for Salmonella enterica serovar Typhimurium. Infect Immun. 2007;75(4):1577–85. doi: 10.1128/IAI.01392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McDonald KG, McDonough JS, Newberry RD. Adaptive immune responses are dispensable for isolated lymphoid follicle formation: antigen-naive, lymphotoxin-sufficient B lymphocytes drive the formation of mature isolated lymphoid follicles. J Immunol. 2005;174(9):5720–8. doi: 10.4049/jimmunol.174.9.5720. [DOI] [PubMed] [Google Scholar]
- 151.McDonald KG, McDonough JS, Wang C, Kucharzik T, Williams IR, Newberry RD. CC chemokine receptor 6 expression by B lymphocytes is essential for the development of isolated lymphoid follicles. Am J Pathol. 2007;170(4):1229–40. doi: 10.2353/ajpath.2007.060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298(5597):1424–7. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 153.Kumazaki K, Tirosh B, Maehr R, Boes M, Honjo T, Ploegh HL. AID−/−mus−/− mice are agammaglobulinemic and fail to maintain B220−CD138+ plasma cells. J Immunol. 2007;178(4):2192–203. doi: 10.4049/jimmunol.178.4.2192. [DOI] [PubMed] [Google Scholar]
- 154.Kim HJ, Kammertoens T, Janke M, Schmetzer O, Qin Z, Berek C, Blankenstein T. Establishment of early lymphoid organ infrastructure in transplanted tumors mediated by local production of lymphotoxin alpha and in the combined absence of functional B and T cells. J Immunol. 2004;172(7):4037–47. doi: 10.4049/jimmunol.172.7.4037. [DOI] [PubMed] [Google Scholar]
- 155.Schrama D, thor Straten P, Fischer WH, McLellan AD, Brocker EB, Reisfeld RA, Becker JC. Targeting of lymphotoxin-alpha to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity. 2001;14(2):111–21. doi: 10.1016/s1074-7613(01)00094-2. [DOI] [PubMed] [Google Scholar]
- 156.Schrama D, Voigt H, Eggert AO, Xiang R, Zhou H, Schumacher TN, Andersen MH, Thor Straten P, Reisfeld RA, Becker JC. Immunological tumor destruction in a murine melanoma model by targeted LTalpha independent of secondary lymphoid tissue. Cancer Immunol Immunother. 2007 doi: 10.1007/s00262-007-0352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kirk CJ, Hartigan-O’Connor D, Mule JJ. The dynamics of the T-cell antitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Res. 2001;61(24):8794–802. [PubMed] [Google Scholar]
- 158.Kirk CJ, Hartigan-O’Connor D, Nickoloff BJ, Chamberlain JS, Giedlin M, Aukerman L, Mule JJ. T cell-dependent antitumor immunity mediated by secondary lymphoid tissue chemokine: augmentation of dendritic cell-based immunotherapy. Cancer Res. 2001;61(5):2062–70. [PubMed] [Google Scholar]
- 159.Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y, Schietinger A, Philip M, Schreiber H, Fu YX. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5(2):141–9. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- 160.Yu P, Lee Y, Wang Y, Liu X, Auh S, Gajewski TF, Schreiber H, You Z, Kaynor C, Wang X, et al. Targeting the Primary Tumor to Generate CTL for the Effective Eradication of Spontaneous Metastases. J Immunol. 2007;179(3):1960–8. doi: 10.4049/jimmunol.179.3.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Loeffler M, Le’negrate G, Krajewska M, Reed JC. Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proc Natl Acad Sci U S A. 2007;104(31):12879–83. doi: 10.1073/pnas.0701959104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lukashev M, Lepage D, Wilson C, Bailly V, Garber E, Lukashin A, Ngam-Ek A, Zeng W, Allaire N, Perrin S, et al. Targeting the Lymphotoxin-{beta} Receptor with Agonist Antibodies as a Potential Cancer Therapy. Cancer Res. 2006;66(19):9617–24. doi: 10.1158/0008-5472.CAN-06-0217. [DOI] [PubMed] [Google Scholar]
- 163.Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu G-L, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, et al. LIGHT, a new member of the TNF superfamily and lymphotoxin a are ligands for Herpesvirus Entry Mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 164.Tamada K, Shimozaki K, Chapoval AI, Zhai Y, Su J, Chen SF, Hsieh SL, Nagata S, Ni J, Chen L. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol. 2000;164(8):4105–10. doi: 10.4049/jimmunol.164.8.4105. [DOI] [PubMed] [Google Scholar]
- 165.Tamada K, Ni J, Zhu G, Fiscella M, Teng B, van Deursen JM, Chen L. Cutting edge: selective impairment of CD8+ T cell function in mice lacking the TNF superfamily member LIGHT. J Immunol. 2002;168(10):4832–5. doi: 10.4049/jimmunol.168.10.4832. [DOI] [PubMed] [Google Scholar]
- 166.Xu Y, Flies AS, Flies DB, Zhu G, Anand S, Flies SJ, Xu H, Anders RA, Hancock WW, Chen L, et al. Selective targeting of the LIGHT-HVEM costimulatory system for the treatment of graft-versus-host disease. Blood. 2007;109(9):4097–104. doi: 10.1182/blood-2006-09-047332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Telford JL, Covacci A, Rappuoli R, Chiara P. Immunobiology of Helicobacter pylori infection. Curr Opin Immunol. 1997;9(4):498–503. doi: 10.1016/s0952-7915(97)80101-x. [DOI] [PubMed] [Google Scholar]
- 168.Go MF, Smoot DT. Helicobacter pylori, gastric MALT lymphoma, and adenocarcinoma of the stomach. Semin Gastrointest Dis. 2000;11(3):134–41. [PubMed] [Google Scholar]
- 169.Lochhead P, El-Omar EM. Helicobacter pylori infection and gastric cancer. Best Pract Res Clin Gastroenterol. 2007;21(2):281–97. doi: 10.1016/j.bpg.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 170.Ame-Thomas P, Maby-El Hajjami H, Monvoisin C, Jean R, Monnier D, Caulet-Maugendre S, Guillaudeux T, Lamy T, Fest T, Tarte K. Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: role of stromal cells in follicular lymphoma pathogenesis. Blood. 2007;109(2):693–702. doi: 10.1182/blood-2006-05-020800. [DOI] [PubMed] [Google Scholar]
- 171.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204(1):57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445(7129):766–70. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 173.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27(1):68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 174.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27(1):18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 175.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 176.Kocks JR, Davalos-Misslitz AC, Hintzen G, Ohl L, Forster R. Regulatory T cells interfere with the development of bronchus-associated lymphoid tissue. J Exp Med. 2007;204(4):723–34. doi: 10.1084/jem.20061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mellanby RJ, Thomas D, Phillips JM, Cooke A. Diabetes in non-obese diabetic mice is not associated with quantitative changes in CD4+ CD25+ Foxp3+ regulatory T cells. Immunology. 2007;121(1):15–28. doi: 10.1111/j.1365-2567.2007.02546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xu B, Wagner N, Pham LN, Magno V, Shan Z, Butcher EC, Michie SA. Lymphocyte homing to bronchus-associated lymphoid tissue (BALT) is mediated by L-selectin/PNAd, alpha4beta1 integrin/VCAM-1, and LFA-1 adhesion pathways. J Exp Med. 2003;197(10):1255–67. doi: 10.1084/jem.20010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Forster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203(3):519–27. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hintzen G, Ohl L, del Rio ML, Rodriguez-Barbosa JI, Pabst O, Kocks JR, Krege J, Hardtke S, Forster R. Induction of tolerance to innocuous inhaled antigen relies on a CCR7-dependent dendritic cell-mediated antigen transport to the bronchial lymph node. J Immunol. 2006;177(10):7346–54. doi: 10.4049/jimmunol.177.10.7346. [DOI] [PubMed] [Google Scholar]
- 181.Worbs T, Forster R. A key role for CCR7 in establishing central and peripheral tolerance. Trends Immunol. 2007;28(6):274–80. doi: 10.1016/j.it.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 182.Nakano H, Gunn MD. Gene duplications at the chemokine locus on mouse chromosome 4: multiple strain-specific haplotypes and the deletion of secondary lymphoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J Immunol. 2001;166(1):361–9. doi: 10.4049/jimmunol.166.1.361. [DOI] [PubMed] [Google Scholar]
- 183.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone Stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci. 2000;97:12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Okada T, Ngo VN, Ekland EH, Forster R, Lipp M, Littman DR, Cyster JG. Chemokine requirements for B cell entry to lymph nodes and Peyer’s patches. J Exp Med. 2002;196(1):65–75. doi: 10.1084/jem.20020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Weninger W, Carlsen HS, Goodarzi M, Moazed F, Crowley MA, Baekkevold ES, Cavanagh LL, von Andrian UH. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol. 2003;170(9):4638–48. doi: 10.4049/jimmunol.170.9.4638. [DOI] [PubMed] [Google Scholar]
- 186.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6(9):895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 187.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6(9):889–94. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hopken UE, Wengner AM, Loddenkemper C, Stein H, Heimesaat MM, Rehm A, Lipp M. CCR7 deficiency causes ectopic lymphoid neogenesis and disturbed mucosal tissue integrity. Blood. 2007;109(3):886–95. doi: 10.1182/blood-2006-03-013532. [DOI] [PubMed] [Google Scholar]
- 189.Grant AJ, Goddard S, Ahmed-Choudhury J, Reynolds G, Jackson DG, Briskin M, Wu L, Hubscher SG, Adams DH. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am J Pathol. 2002;160(4):1445–55. doi: 10.1016/S0002-9440(10)62570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Suda T, Chida K, Hayakawa H, Imokawa S, Iwata M, Nakamura H, Sato A. Development of bronchus-associated lymphoid tissue in chronic hypersensitivity pneumonitis. Chest. 1999;115(2):357–63. doi: 10.1378/chest.115.2.357. [DOI] [PubMed] [Google Scholar]
- 191.Charavaryamath C, Janardhan KS, Townsend HG, Willson P, Singh B. Multiple exposures to swine barn air induce lung inflammation and airway hyper-responsiveness. Respir Res. 2005;6:50. doi: 10.1186/1465-9921-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Neutra MR, Frey A, Kraehnenbuhl JP. Epithelial M cells: gateways of mucosal infection and immunization. Cell. 1996;86:345–348. doi: 10.1016/s0092-8674(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 193.Voswinkel J, Muller A, Lamprecht P. Is PR3-ANCA formation initiated in Wegener’s granulomatosis lesions? Granulomas as potential lymphoid tissue maintaining autoantibody production. Ann N Y Acad Sci. 2005;1051:12–9. doi: 10.1196/annals.1361.042. [DOI] [PubMed] [Google Scholar]
- 194.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59(7):574–80. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Marchal-Somme J, Uzunhan Y, Marchand-Adam S, Valeyre D, Soumelis V, Crestani B, Soler P. Cutting edge: nonproliferating mature immune cells form a novel type of organized lymphoid structure in idiopathic pulmonary fibrosis. J Immunol. 2006;176(10):5735–9. doi: 10.4049/jimmunol.176.10.5735. [DOI] [PubMed] [Google Scholar]
- 196.Ramshaw AL, Parums DV. Immunohistochemical characterization of inflammatory cells associated with advanced atherosclerosis. Histopathology. 1990;17(6):543–52. doi: 10.1111/j.1365-2559.1990.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 197.Houtkamp MA, de Boer OJ, van der Loos CM, van der Wal AC, Becker AE. Adventitial infiltrates associated with advanced atherosclerotic plaques: structural organization suggests generation of local humoral immune responses. J Pathol. 2001;193(2):263–9. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH774>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 198.Surawicz CM, Belic L. Rectal biopsy helps to distinguish acute self-limited colitis from idiopathic inflammatory bowel disease. Gastroenterology. 1984;86(1):104–13. [PubMed] [Google Scholar]
- 199.Kaiserling E. Newly-formed lymph nodes in the submucosa in chronic inflammatory bowel disease. Lymphology. 2001;34(1):22–9. [PubMed] [Google Scholar]
- 200.Baskerville A, Ramsay A, Cranage MP, Cook N, Cook RW, Dennis MJ, Greenaway PJ, Kitchin PA, Stott EJ. Histopathological changes in simian immunodeficiency virus infection. J Pathol. 1990;162(1):67–75. doi: 10.1002/path.1711620113. [DOI] [PubMed] [Google Scholar]
- 201.Racanelli V, Sansonno D, Piccoli C, D’Amore FP, Tucci FA, Dammacco F. Molecular characterization of B cell clonal expansions in the liver of chronically hepatitis C virus-infected patients. J Immunol. 2001;167(1):21–9. doi: 10.4049/jimmunol.167.1.21. [DOI] [PubMed] [Google Scholar]
- 202.Kumon I, Onji M, Nadano S, Kanaoka M, Ohta Y. Detection of hepatitis B surface antigen in lymphoid follicle in patients with chronic hepatitis type B. Gastroenterol Jpn. 1989;24(6):738. doi: 10.1007/BF02774178. [DOI] [PubMed] [Google Scholar]
- 203.Kumon I. In situ characterization of mononuclear cell phenotype in intrahepatic lymphoid follicles in patients with chronic viral hepatitis. Gastroenterol Jpn. 1992;27(5):638–45. doi: 10.1007/BF02774979. [DOI] [PubMed] [Google Scholar]
- 204.Oshima C, Okazaki K, Matsushima Y, Sawada M, Chiba T, Takahashi K, Hiai H, Katakai T, Kasakura S, Masuda T. Induction of follicular gastritis following postthymectomy autoimmune gastritis in Helicobacter pylori-infected BALB/c mice. Infect Immun. 2000;68(1):100–6. doi: 10.1128/iai.68.1.100-106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Uchida K, Okazaki K, Debrecceni A, Nishi T, Iwano H, Inai M, Uose S, Nakase H, Ohana M, Oshima C, et al. Analysis of cytokines in the early development of gastric secondary lymphoid follicles in Helicobacter pylori-infected BALB/c mice with neonatal thymectomy. Infect Immun. 2001;69(11):6749–54. doi: 10.1128/IAI.69.11.6749-6754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zaitoun AM. The prevalence of lymphoid follicles in Helicobacter pylori associated gastritis in patients with ulcers and non-ulcer dyspepsia. J Clin Pathol. 1995;48(4):325–9. doi: 10.1136/jcp.48.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Park JH, Seok SH, Cho SA, Baek MW, Lee HY, Kim DJ. The high prevalence of Helicobacter sp. in porcine pyloric mucosa and its histopathological and molecular characteristics. Vet Microbiol. 2004;104(3–4):219–25. doi: 10.1016/j.vetmic.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 208.Tsai MC, Chakravarty S, Zhu G, Xu J, Tanaka K, Koch C, Tufariello J, Flynn J, Chan J. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8(2):218–32. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 209.Colli C, Leinweber B, Mullegger R, Chott A, Kerl H, Cerroni L. Borrelia burgdorferi-associated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol. 2004;31(3):232–40. doi: 10.1111/j.0303-6987.2003.00167.x. [DOI] [PubMed] [Google Scholar]
- 210.Kerl H, Fink-Puches R, Cerroni L. Diagnostic criteria of primary cutaneous B-cell lymphomas and pseudolymphomas. Keio J Med. 2001;50(4):269–73. doi: 10.2302/kjm.50.269. [DOI] [PubMed] [Google Scholar]
- 211.Delventhal S, Hensel A, Petzoldt K, Pabst R. Effects of microbial stimulation on the number, size and activity of bronchus-associated lymphoid tissue (BALT) structures in the pig. Int J Exp Path. 1992;73:351–357. [PMC free article] [PubMed] [Google Scholar]
- 212.Gould SJ, Isaacson PG. Bronchus associated lymphoid tissue (BALT) in human fetal and infant lung. J Pathol. 1993;169:229–234. doi: 10.1002/path.1711690209. [DOI] [PubMed] [Google Scholar]




