Abstract
Derivative myelin associated glycoprotein (dMAG) results from proteolysis of transmembrane MAG and can inhibit axonal growth. We have tested the ability of certain matrix metalloproteinases (MMPs) elevated with inflammatory and demyelinating diseases to cleave MAG. We show MMP-2, MMP-7 and MMP-9, but not MMP-1, cleave recombinant human MAG. Cleavage by MMP-7 occurs at Leu 509, just distal to the transmembrane domain and, to a lesser extent, at Met 234. We also show that MMP-7 cleaves MAG expressed on the external surface of CHO cells, releasing fragments that accumulate in the medium over periods of up to 48 hours or more and that are able to inhibit outgrowth by dorsal root ganglion (DRG) neurons. We conclude that MMPs may have the potential both to disrupt MAG dependent axon-glia communication and to generate bioactive fragments that can inhibit neurite growth.
Introduction
Members of the matrix metalloproteinase (MMP) family have central roles in the degradation of many extracellular matrix (ECM) components and cell surface molecules. The general features of MMP expression in the CNS are reviewed elsewhere (Avolio et al., 2003; Gasche et al., 2006; Opdenakker et al., 2003). Various MMPs are expressed in the CNS and can be up-regulated and secreted into the extracellular space in response to growth factors, cytokines and a variety of other triggers. Excessive MMP activity can damage tissues and thus activity is usually tightly regulated, in part by naturally occurring tissue inhibitors of metalloproteinases (TIMPs). MMP and TIMP expression in the CNS is altered both in many normal physiological processes and in injury and disease.
In multiple sclerosis (MS) and various other myelin disorders, there is degradation of myelin sheathes surrounding nerve axons with potential damage to underlying nerves themselves. MMP levels are increased in MS (Anthony et al., 1997; Cossins et al., 1997; Gijbels et al., 1992; Maeda and Sobel, 1996; Ozenci et al., 2000; Ozenci et al., 1999; Rosenberg et al., 1996; Sellebjerg et al., 2000; Trojano et al., 1999; Waubant et al., 1999) and in animal models of MS including experimental allergic encephalomyelitis (EAE) (Clements et al., 1997; Gijbels et al., 1993; Graesser et al., 1998; Graesser et al., 2000; Jovanova-Nesic and Shoenfeld, 2006; Kieseier et al., 1998; Teesalu et al., 2001), and are very closely linked to pathogenesis and disease progression. Elevated serum MMP activity is detectable extremely early in the disease course, being present even in prodromal phases prior to development of clinically diagnosed MS (Correale and Bassani Molinas Mde, 2003). In relapsing-remitting MS, serum MMP activities are high during relapses and wax and wane in parallel with the disease course (Avolio et al., 2005; Kanesaka et al., 2006; Waubant et al., 1999). MMP-7 and -9 can be detected in active MS demyelinating lesions, with MMP-7 localized to microglia and invading macrophages (Cossins et al., 1997). In addition, levels of MMP-9 in serum and cerebrospinal fluid (CSF) are proposed to be a useful biomarker for the presence and progression of MS (Avolio et al., 2005; Fainardi et al., 2006; Waubant, 2006).
Important evidence for the involvement of MMPs in MS has also come from a range of studies in animal models such as EAE, where MMP expression has likewise been reported to be correlated with disease severity (Nygardas and Hinkkanen, 2002). Severe EAE in MMP-2 null mice is believed to be due to the elevated MMP-9 levels seen in these animals (Esparza et al., 2004). Conversely, young MMP-9 null mutant mice are resistant to EAE (Dubois et al., 1999) and it is possible to partially suppress EAE using MMP inhibitors (Gijbels et al., 1994; Hewson et al., 1995; Nygardas et al., 2004).
There is also evidence implicating TIMPs in MS, with alterations in MS serum (Waubant et al., 2001; Waubant et al., 1999) and CSF (Boz et al., 2006). In TIMP-1 knockout mice, EAE is more severe, suggesting TIMP-1 may have protective effects (Crocker et al., 2006), so the balance between MMPs and TIMPs may also be important. Comprehensive studies of changes in MMPs and TIMPs have shown that relative to control mice, there is up-regulation of TIMP-1 and various MMPs, including MMP-8, -9, -10 and -12, although not MMP-7, in EAE mice (Pagenstecher et al., 1998; Toft-Hansen et al., 2004).
It is now recognized that the MMPs also degrade other substrates in addition to ECM components, including, though not limited to, molecules expressed on the external surface of cells, as reviewed elsewhere (Avolio et al., 2003; Gasche et al., 2006; Opdenakker et al., 2003). It has been known for some time that MMP-9 is able to cleave myelin basic protein purified from human brain (Gijbels et al., 1993; Proost et al., 1993) and more recently it has also been reported that MMP-3 (stromelysin) can likewise cleave MBP (D’Souza and Moscarello, 2006). At least one of the peptides produced by MMP-9 cleavage corresponds to a major known encephalitogenic fragment of MBP, suggesting that increased MMP activity occurring in inflammation could contribute to autoantigen production. This could be one mechanism by which systemic infection increases MS relapse occurrence (Correale et al., 2006).
Myelin associated glycoprotein (MAG) is another important myelin-associated protein. It is believed to be instrumental in axon-glia communications (Li et al., 1998; Quarles, 2002) and has been shown to exert inhibitory effects on axonal growth in numerous studies, reviewed elsewhere (McKerracher and Winton, 2002). Studies in humans and other primates have demonstrated the existence in vivo of a cleavage derivative, dMAG, produced by one or more unknown proteases. This cleavage product is reported to retain the neurite inhibiting properties of full-length MAG (Tang et al., 2001; Tang et al., 1997b). We now report that MMP-7, MMP-9 and at least one other metalloproteinase, MMP-2, can cleave the extracellular domain of MAG. We also report data from investigations using a compartmentalized Campenot culture system suggesting that this MMP cleavage may modulate interactions between MAG and neurons.
Methods
Reagents
recombinant MAG (MAG-Fc, Cat. No. 538-MG) and recombinant active MMP-1 (Cat. No. 901 MP) were from R and D systems (Minneapolis, MN). Recombinant active MMP-7 (Cat. No. 444270) was from Calbiochem/EMD Biosciences unless otherwise indicated, as were recombinant active MMP-2 and -9 (Cat. No. PF023 and PF024). The MMP inhibitor (GM6001) was also from Calbiochem (Cat. No. 364205).
Western blotting
Western blots of cell lysates were performed using 20 μg of protein per lane, as determined by the Pierce BCA assay. Prior to analysis, samples were mixed with sample buffer containing 5% β-mercaptoethanol and boiled for 5 minutes at 95°C. Electrophoresis was performed on a 4-15% Tris-glycine polyacrylamide gradient gel (Biorad, Hercules, CA). Following electrophoretic transfer of the protein to a polyvinylidene difluoride (PVDF) membrane (Biorad, Hercules, CA), the membrane was blocked in 5% nonfat dry milk in TBST (150 mM NaCl, 100 mM Tris base, 0.1% Tween 20, pH 7.6) buffer for 1 hr. The blot was then probed with the indicated primary antibody, at a dilution recommended by the manufacturer, for 1.5 hr at room temperature. After washing the membrane 3 times (15 minutes each) in TBST buffer, it was incubated with an appropriate secondary antibody for 1/2 hour at room temperature. The membrane was then washed again in TBST buffer and immunoreactive bands were visualized using electrochemiluminescence (Amersham).
Cleavage of MAG
Western blot of MAG-Fc digests was performed similarly, except that the entire reaction, rather than 20 μg of total protein, was analyzed. In these experiments, 500 ng of MAG-Fc was incubated with enzyme at the indicated concentrations, and the digestion was carried out in 30 μl of buffer (50 mM Tris, 10 mM CaCl2, 150 mM NaCl, 1 mM ZnCl2, pH 7.5) at 37°C in the presence or absence of GM-6001 (10 μM). The construct consisted of Met1-Pro516 of rat MAG followed by a linker peptide DIEGRMD and then Pro100-Lys330 of human IgG1.
Peptide sequencing
The digestion products of MAG-Fc/ MMP-7 reactions were resolved by electrophoresis on a 4-15% Tris-glycine polyacrylamide gradient gel (Biorad, Hercules, CA), then transferred to a PVDF membrane (Biorad, Hercules, CA). Control lanes containing either MAG-Fc or recombinant MMPs alone were also run on the same gel and transferred to permit identification of bands unique to the digests. Following protein transfer, the PVDF membrane was stained with sequencing grade Coomassie blue (BioRad 1610436). Appropriate bands were identified and cut out for N terminal sequencing, which was performed with the Perkin-Elmer/Applied Biosystems Procise Protein Sequencing System. This method is compatible with samples that have been electroblotted onto PVDF.
Expression of MAG in CHO cells
A tetracycline repressible MAG system was generated in Chinese hamster ovary (CHO) cells. Rat L-MAG cDNA (a kind gift from Dr. Ronald Schnaar) was cloned into the tetracycline repressible pNIT vector (a kind gift from Dr. Hongjun Song). CHO cells were co-transfected using Lipofectamine™ 2000 with rat L-MAG and pIRES2-DsRed2 (Clontech Laboratories, Mountain View, CA). Stable lines were obtained by selection with 400ug/ml G418 and FACS sorted for DsRed2 positive cells. Several clones were derived and assessed for the following: simultaneous expression of MAG and DsRed2, tetracycline repressibility of MAG and membrane-bound expression of MAG. Addition of the tetracycline analog doxycycline at 1ug/ml for 3 days caused the complete inhibition of MAG transcription.
Culture of sensory neurons
Spinal dorsal root ganglia (DRG) were dissected from decapitated embryonic age day 15 rats and enzymatically dissociated with 0.25% trypsin in L-15 medium as described previously (Eldridge et al., 1987). Neurons were maintained in Neurobasal medium (Gibco Invitrogen, Grand Island, NY) supplemented with 1% fetal bovine serum, penicillin (1 U/L), streptomycin (1 U/L) and nerve growth factor (10 ng/ml). In vitro experiments were carried out in using a compartmentalized cell culture system, known as Campenot chambers (Campenot, 1977). Cells were plated into the middle chamber of collagen coated tissue culture dishes divided into three compartments as previously described (Campenot 1982). Briefly, Camp10 Teflon dividers (Tyler Research, Alberta, Canada) were carefully attached to 35 mm culture dishes using silicon vacuum grease. The integrity of the grease seals was assessed by placing medium into side chambers only and incubating the chambers overnight in a 37° C incubator. Only chambers that did not leak were used for the experiments.
Cell bodies were maintained from day 2-7 in growth medium containing cytosine arabinoside (10 uM) to eliminate Schwann cells and non neuronal, dividing cells. After 10-12 days in culture, which allows for maturation, axons had projected into the lateral compartments and were exposed to various test conditions as outlined above. The length of the axon segment in the lateral chamber was measured before treatment and after 48 hours of axonal growth, as previously described (Melli et al., 2006). Campenot experiments were performed twice with a representative shown. In that experiment, an average of 20 axons were measured in each condition with 6 replicates per condition.
Results
MMPs cleave recombinant MAG
We first tested the ability of various MMPs to cleave a recombinant MAGconstruct (MAG-Fc) in vitro.
Fig. 1A shows results from an experiment in which MAG-Fc was incubated with MMP-1, -2 or -7 and the PVDF membrane was probed with an antibody to the Fc portion of the construct. The intensity of the band representing the full length construct, which runs with an apparent molecular mass of 120 kDa, was observed to be diminished following incubation with MMP-2 or -7. With MMP-7, the 120 kDa band has almost disappeared. Of note, a low molecular weight band of approximately 35 kDa can be seen in the MMP-7 digest.
Fig. 1. Cleavage of MAG-Fc by MMPs.

Figs. 1A-C show Western blot results from an experiment in which 500 ng of recombinant human MAG (MAG-Fc) was incubated with or without 500 ng of the indicated MMP in 30 μl buffer (50 mM Tris, 10mM CaCl2, 150 mM NaCl, 1 mM ZnCl, ph7.5) for 30 min. at 37 degrees C. A control in which the MMP activity inhibitor GM-6001 was added to the MMP-7 reaction was also performed. Digestion products were resolved by Western blot and probed with anti-Fc, (Fig 1A), anti-MAG from Chemicon (Fig. 1B), or anti-MAG from R & D Systems (Fig. 1C). In separate experiments, MAG-Fc, MMP-7, or products of a 1h or 4h MMP-7/MAG-Fc digestion were resolved and transferred to PVDF, and then stained with Coomassie blue (Fig. 1dD). Select bands were then excised for N terminal sequencing. Major MAG cleavage products are indicated by arrows.
In subsequent experiments, blots from MAG/MMP digests were instead probed with anti MAG antibodies from two different commercial sources, Chemicon (Fig. 1B) or R & D Systems (Fig. 1C). As a control, we included the MMP activity inhibitor GM-6001 in one of the MMP-7/MAG digests as indicated. In separate digests in these experiments, we also included MMP-9, another MMP that may be elevated with demyelinating disease. Results suggest that like MMP-2 and -7, MMP-9 can cleave MAG-Fc. Results also suggest that cleavage occurs by all three MMPs so as to generate at least 2 immunoreactive fragments, one with an apparent mass of close to 90 kDa and the other with an apparent mass of approximately 35 kDa. Given that only the lower molecular weight fragment was detected by anti-Fc (Fig. 1A), results are consistent with MMP mediated cleavage of rMAG within the MAG-encoding region so as to generate an 80-90 kDa N terminal fragment containing only MAG-derived sequence and a ∼35 kDa C terminal fragment spanning the linker region and containing both MAG-derived and Fc-derived sequences.
Of note, it can be appreciated from some experiments that MAG breakdown with the generation of similarly sized fragments can occur to a small extent in the absence of added proteinase (e.g. leftmost lane, Fig. 1B). It is possible that the MAG sequence is vulnerable to spontaneous bond disruption at this site or that trace levels of proteolytic contaminants are present in the system. The degree of spontaneous breakdown is, however, small relative to the cleavage seen in the presence of added MMP-2, -7 or -9.
To determine where MMP mediated cleavage may occur, the resolved products of an MMP-7/MAG digestion were transferred to PVDF and stained with Coomassie blue. Select bands were then excised for N terminal sequencing. A representative Coomassie blue stained blot is shown in Fig. 1D. Major MAG cleavage products are indicated by arrows.
Sequencing results for the major low molecular weight product (∼35 kDa; lowermost arrow) were consistent with cleavage occurring at Leu 509, an extracellular residue in native MAG that is proximal to the transmembrane domain (see Fig 2). This band was sequenced because it corresponded to the ∼ 35 kD band in Fig. 1A that was recognized by the anti-Fc antibody, consistent with it being a C terminal fragment having a new N terminus. Sequencing results showed that cleavage may occur to a lesser extent at Met 234, the start site of the minor band noted by the asterix (Fig 1D).
Fig. 2. Location of putative cleavage sites in the MAG sequence.
Sequence information for MAG is shown. The RGD site, MMP-7 cleavage sites, and the transmembrane domain are in bold text. Potential glycosylation sites are indicated by an asterix.
Shown in Fig. 2 are the putative cleavage sites in the extracellular domain of MAG.
Cleavage of cell surface MAG in a CHO cell expression system
Cleavage of myelin proteins can differ between lipid-bound and lipid-free forms, reflecting conformational differences in hydrophobic and non-hydrophobic environments, as previously reported for MBP (Liuzzi et al., 1996). The same is also likely to hold for MAG, which is normally expressed as a transmembrane protein spanning the plasma membrane. It is therefore important to investigate cleavage of membrane-bound species in addition to cleavage of species in aqueous solution. In order to examine whether ectodomain cleavage still occurs when MAG, a transmembrane protein, is conformed across the hydrophobic membrane environment, we also tested the ability of MMP-7 to cleave transmembrane MAG expressed at the cell surface using a CHO cell expression system.
Cultures were incubated for 2h (Fig. 3) in the presence or absence of MMP-7 (50 nM). Cell lysates were then prepared and analyzed by Western blot (Fig.3).
Fig. 3. Cleavage of MAG in a CHO cell expression system.
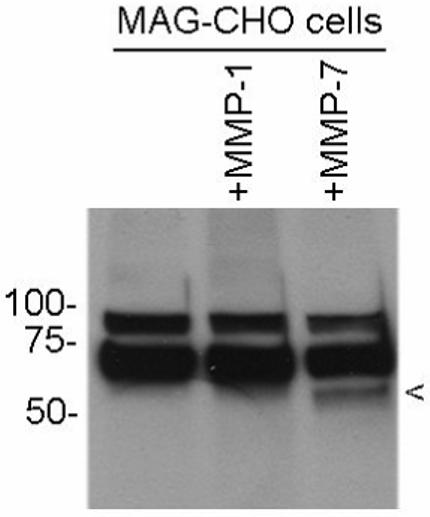
A cleavage fragment is generated by MMP-7 treatment of CHO cell cultures overexpressing MAG. CHO cell cultures were incubated for 2h in the presence or absence of 50 nM of the indicated MMP. Extracts were subsequently prepared and Western blot performed using an antibody to MAG from Chemicon. The putative cleavage fragment is indicated by the arrowhead. Higher molecular weight bands likely represent differentially glycosylated forms of full length MAG.
As shown in Fig. 3, a MAG-immunoreactive fragment of ∼60 kDa (arrowhead) was detected in the lysates of MAG expressing CHO cells that had been treated with MMP-7. In contrast, no similar fragment was observed in cells that had been treated with 50nM MMP-1. Retention of the fragment in MMP-7 treated cell extracts is likely secondary to its association with the cell surface or with extracellular matrix proteins following its cleavage.
The molecular weights of the two higher molecular weight bands are consistent with the reported molecular masses of 70-100 kDa for full-length rat L-MAG, dependent on the degree of glycosylation. This can vary considerably between species and cell types (Pedraza et al., 1990; Quarles, 2002).
The MAG extracellular domain comprises approximately 30% carbohydrate by weight and displays complex glycosylation patterns. There are eight potential N-glycosylation sites in the ectodomain and one potential cytoplasmic N-glycosylation site (Arquint et al., 1987). The predominant band of ∼70 kDa is observed in control and experimental samples. A second higher molecular weight band in all samples likely reflects a differentially glycosylated product. The cleavage product (arrowhead) is increased in amount by MMP-7, and migrates with a molecular weight 10-15 kDa less than that of the predominant band.
If cleavage from the cell surface occurs at Leu 509, and one assumes an average molecular weight per amino acid of 135 daltons, the putative N terminal fragment present in the MMP-7 treated sample would be expected to migrate with a molecular mass reduced by approximately 118 (the number of residues in the C terminal stub) multiplied by 135, or 15.9 kDa. This is consistent with results shown in Fig. 3.
The remnant stub comprising the transmembrane and C-terminal domain from residue 509 on the transmembrane and C-terminal domain would not be predicted to be secreted into the medium but would instead be expected to remain within the cell membrane until being taken up by endocytosis for degradation.
MMP-7 generated MAG fragments inhibit neuronal process outgrowth
Myelin inhibits axonal growth in the adult CNS. This is partly attributable to direct inhibitory effects exerted by MAG (McKerracher et al., 1994), which has been shown to inhibit neuronal process outgrowth in numerous studies, as reviewed elsewhere (McKerracher and Winton, 2002). It has also been reported that soluble dMAG species secreted from damaged white matter are able to inhibit axonal regeneration (Tang et al., 2001; Tang et al., 1997b). This inhibition appears to require both the presence of a sialic acid binding site at R118 within the first Ig domain as well as a second, unknown site (Tang et al., 1997a). The R118 site is present within an RGD sequence (Fig. 2), a motif important in interactions, often inhibitory in nature, with a restricted subset of integrins (Ivaska and Heino, 2000).
Cleavage at either Leu 509 or Met 234 would potentially release an N terminal fragment that includes the sialic-acid binding domain which is critical to the neurite outgrowth inhibitory effect of dMAG (Tang et al., 1997a). To assess the bioactivity of soluble MMP-generated MAG fragments, specifically the effects on neuronal process growth, we used Campenot chambers (Campenot, 1977).
In these experiments we focused on MMP-7, both because it is elevated in demyelinating disease (Clements et al., 1997; Cossins et al., 1997) and because the earlier experiments had demonstrated its relative potency in terms of its ability to cleave MAG.
We measured neuronal process growth under different conditions, as follows: 1. DRG neuronal cultures grown on uncoated tissue culture ware, 2. DRG neurons grown on a layer of wildtype CHO cells, 3. DRG neurons grown on a layer of wildtype CHO cells and treated with MMP-7, 4. DRG neurons grown on a layer of CHO cells over-expressing MAG, and 5. DRG neurons grown on a layer of CHO cells treated with supernatants from MMP-7 treated MAG expressing cells.
The results, shown in Fig. 4, demonstrate that MAG expressing CHO cells inhibit neurite outgrowth. ANOVA withTukey post hoc comparison showed significant differences between DRG cells grown on tissue culture ware or wildtype CHO cells and those grown on MAG expressing CHO cells (P < 0.001).
Fig. 4. dMAG fragments produced by MMP-7 cleavage inhibit neuronal outgrowth of DRG neurons grown in a Campenot compartmentalized culture system.

The figure shows the results of experiments in which sensory DRG neurons were grown on various substrates (tissue culture ware, CHO cells, or MAG expressing CHO cells) in a compartmentalized Campenot culture system, as described in Experimental Protocols. Neuronal outgrowth was measured and recorded as percent control. Shown are the mean and S.E.M. for an experiment with 6 replicates. As determined by ANOVA with Tukey’s post hoc analysis, the difference between either of the control experiments (cells grown on culture ware or wild type CHO cells) and cells grown on MAG CHO cells was significant at P < 0.001. The difference between either of the control experiments and cells grown on wild type CHO cells but treated with supernatants from MMP-7 treated MAG expressing CHO cells was also significant (P< 0.001).
To examine the effects of soluble dMAG fragments on neurite outgrowth, we also treated CHO cells over-expressing MAG for 3 hours with MMP-7 to release soluble dMAG fragments into the medium (Fig. 4, rightmost column). The conditioned medium was removed at the end of the 3 hr period and added to DRG cells grown on wildtype CHO cells that did not over-express MAG. Neurite outgrowth of DRG cells grown on wildtype CHO cells was significantly reduced by the supernatant from MMP-7 treated MAG expressing CHO cells, which presumably contained dMAG and could thus could inhibit axon growth in trans (P < 0.001). This suggests that MMP-7 generated cleavage products were stable and/or their effects long lasting and is consistent with previous findings that both soluble and cell surface associated MAG inhibits neuronal growth, reviewed elsewhere (McKerracher and Winton, 2002).
Of note, treatment with MMP-7 did not affect outgrowth of DRG cells grown on wildtype CHO cells (Fig. 4, middle column) relative to untreated cultures, showing MMP-7 itself has no effect on DRG outgrowth in this system in the absence of over-expressed MAG (P > 0.05).
In summary, we interpret these results to suggest that soluble dMAG retains neuronal growth inhibitory capabilities in this system but that if soluble dMAG generated by MMP-7 cleavage is removed, the remnant cell-bound MAG stubs remaining after cleavage and lacking the R118 sialic acid binding domain do not continue to exert inhibitory actions.
Discussion
Our data suggest that at least two different human MMPs can directly cleave free recombinant human MAG in vitro. Proteolysis of MAG-Fc occurs primarily at two main sites within the MAG ectodomain, one of which, Leu-509 near the transmembrane domain, is very close although not identical to the cleavage site between residues 512 and 513 that was previously reported for the soluble MAG fragment generated endogenously in myelin preparations in vitro. The two cleavage sites would generate products which are indistinguishable on standard Western blots. Our data also show that MMP-7 can cleave MAG expressed in a CHO cell system. In addition, the data show that the cleaved product retains its ability to inhibit neurite outgrowth in a DRG culture system. Of interest, neurite growth inhibition by MAG can differ according to cell type (Venkatesh et al., 2007).
Other studies have previously demonstrated a soluble MAG fragment, termed dMAG, in the CSF from healthy humans and patients with various neurological disorders (Yanagisawa et al., 1985). A similar soluble MAG fragment is released from CNS tissue in vitro (Stebbins et al., 1998). The dMAG fragment was proposed to be generated by a proteolytic activity endogenous to myelin suggested to be cathepsin-L, but more recently it has been reported that neither cathepsin-L nor calpain, another proposed candidate, are responsible for generating dMAG in vivo (Paivalainen et al., 2003).
The ability of MMPs to access MAG in vivo may be varied. MAG is expressed on the glial cell membrane as opposed to being a component of compact myelin per se, and localizes to the luminal surface of oligodendrocytes and Schwann cells, at the myelin periaxonal interface, as reviewed elsewhere (Quarles, 2002). Unless there has been axonal damage or myelin breakdown, MAG is likely to be primarily exposed to MMPs secreted by the axon or to autocrine effects of MMPs secreted by the parent oligodendrocyte or Schwann cell on which it is expressed. One possibility is that cleavage by an unknown enzyme at the previously reported native scissile bond between residues 512 and 513 may predominate under conditions where periaxonal MMPs are not high. However in pathological conditions such as inflammation or ischemia (Burke et al., 2003), up-regulation of MMP activity in the periaxonal space could lead to additional, MMP-catalyzed cleavage of MAG. More severe pathologies with myelin breakdown or axonal damage would be predicted to increase entry into the periaxonal space of MMPs from external sources such as astroglia, microglia and macrophage/monocytes, increasing MAG cleavage even more and further disrupting axon-glia interactions. It has previously been reported that affected white matter in MS has higher levels of MAG proteolysis and dMAG compared to control white matter (Moller et al., 1987).
Previously published studies suggest various MMPs are also likely to cleave other important myelin proteins (Chantry and Glynn, 1990; D’Souza and Moscarello, 2006; Gijbels et al., 1993; Proost et al., 1993). For example, MMP-3 (stromelysin) and MMP-9 can both cleave MBP (D’Souza and Moscarello, 2006; Gijbels et al., 1993; Proost et al., 1993). There are at least four MMP-9 cleavage sites on MBP, producing at least one encephalitogenic peptide.
Cleavage of MAG may also generate encephalitogenic peptides. Nonetheless, it should be noted that peptides derived from MAG have shown lesser encephalitogenic and immunogenic potential, with most failing to induce EAE in mice (Morris-Downes et al., 2002). Of interest, however, is the possibility that MAG cleavage plays a role in anti-MAG neuropathy, a condition of the peripheral nervous system in which antibodies to MAG are detected (Lunn et al., 2002).
Other potential effects of MAG cleavage include those on myelin structure/function and potentially related effects on signal transduction.
Studies with knockout mice show that while MAG is not essential for myelin formation per se (Li et al., 1994), it acts to ensure myelin is appropriately produced and structured to match the specific features of the axon being enveloped (Li et al., 1994; Montag et al., 1994). The MAG extracellular domain may act as both a glue and a spacer, maintaining the glial cell membrane and the axolemma in close apposition (Li et al., 1998). The MAG ectodomain is thought to interact with one or more gangliosides or molecules on the axonal surface.
Interactions between MAG and the axon are also thought to involve bidirectional signal transduction. By acting as a ligand, MAG likely influences axonal properties (Quarles, 2007). Indeed, such signaling appears essential for normal myelin maintenance and results in neurofilament phosphorylation with increased axon calibre (Quarles, 2002). It is tempting to speculate that MAG may promote neuronal/axonal survival via an effect similar to that of extracellular matrix molecules. By acting as a receptor, MAG may facilitate signal transduction within glia. Signal transduction into glia may occur via the intracellular portion of MAG to oligodendroglial or Schwann cell cytoplasmic molecules (Li et al., 1998; Quarles, 2002), including cytoskeletal components such as F-actin and spectrin (Trapp et al., 1989) as well as tubulin (Quarles, 2002). This signaling may enhance oligodendrocyte myelinating capacity (Quarles, 2002). MAG cleavage likely influences such signaling. For example, soluble and cell surface associated MAG may differ in terms of half life, and it is also possible that the two have differential signaling effects.
Overall, cleavage of the MAG ectodomain by MMPs would thus be predicted to have both extracellular and intracellular effects. The former would be likely to include disruption of the normal spatial and organizational relationships between the axolemma and the glial cell membrane, with subtle physical effects on myelin structure. The latter would be likely to include disruption of cytoplasmic signaling, affecting myelinating capacity in glia and axonal properties in neurons.
It is therefore possible to envisage two main forms of effects of MMPs involving MAG cleavage. Under physiological circumstances, any cleavage of MAG that does occur through actions of MMP-9 or other MMPs normally expressed in the nervous system might be expected to contribute to normal modulation of bi-directional glia-axon communication and signaling. Under pathological circumstances such as axon or myelin damage in disease, excessive or abnormal MMP cleavage of MAG as a result of higher levels of MMP-9, MMP-7 or other MMP activities could contribute to physical detachment of the myelin sheathe from the axon as well as disruption of normal axon-glia communication.
Depending on circumstances, this could add further to the nerve damage but could also be an essential step in removal of damaged nerve tissue that is necessary to allow regeneration to proceed later on when MMP levels return to normal. Axonal damage correlates with localized MMP expression in MS (Diaz-Sanchez et al.) but at least in some circumstances MMP-mediated degeneration of damaged axons may enhance the prospects for subsequent axonal regeneration (Krekoski et al., 2002).
While more studies are clearly required to confirm the relevance of these findings in vivo, it is concluded that at least three MMPs which are up-regulated in MS and other myelin disorders and inflammatory CNS conditions are able to cleave MAG and could contribute to both myelin degeneration and axonal damage.
Acknowledgements
We would like to thank Hyoje Ryu for technical assistance. This work was supported in part by the National Institutes of Health (NS052580) and NMSS TR-3760-A-3.
Footnotes
- CSF
- cerebrospinal fluid
- MAG
- myelin associated glycoprotein
- dMAG
- derivative MAG
- MBP
- myelin basic protein
- EAE
- experimental allergic ancephalomyelitis
- MS
- multiple sclerosis
- PVDF
- polyvinylidene difluoride
- MMP
- matrix metalloproteinase
- TIMPs
- tissue inhibitors of metalloproteinases
- ECM
- extracellular matrix
- CHO
- Chinese hamster ovary.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anthony DC, Ferguson B, Matyzak MK, Miller KM, Esiri MM, Perry VH. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23:406–415. [PubMed] [Google Scholar]
- Arquint M, Roder J, Chia LS, Down J, Wilkinson D, Bayley H, Braun P, Dunn R. Molecular cloning and primary structure of myelin-associated glycoprotein. Proc Natl Acad Sci U S A. 1987;84:600–604. doi: 10.1073/pnas.84.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avolio C, Filippi M, Tortorella C, Rocca MA, Ruggieri M, Agosta F, Tomassini V, Pozzilli C, Stecchi S, Giaquinto P, Livrea P, Trojano M. Serum MMP-9/TIMP-1 and MMP-2/TIMP-2 ratios in multiple sclerosis: relationships with different magnetic resonance imaging measures of disease activity during IFN-beta-1a treatment. Mult Scler. 2005;11:441–446. doi: 10.1191/1352458505ms1193oa. [DOI] [PubMed] [Google Scholar]
- Avolio C, Giuliani F, Liuzzi GM, Ruggieri M, Paolicelli D, Riccio P, Livrea P, Trojano M. Adhesion molecules and matrix metalloproteinases in Multiple Sclerosis: effects induced by Interferon-beta. Brain Res Bull. 2003;61:357–364. doi: 10.1016/s0361-9230(03)00098-4. [DOI] [PubMed] [Google Scholar]
- Boz C, Ozmenoglu M, Velioglu S, Kilinc K, Orem A, Alioglu Z, Altunayoglu V. Matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of matrix metalloproteinase (TIMP-1) in patients with relapsing-remitting multiple sclerosis treated with interferon beta. Clin Neurol Neurosurg. 2006;108:124–128. doi: 10.1016/j.clineuro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Burke B, Giannoudis A, Corke KP, Gill D, Wells M, Ziegler-Heitbrock L, Lewis CE. Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am J Pathol. 2003;163:1233–1243. doi: 10.1016/S0002-9440(10)63483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantry A, Glynn P. A novel metalloproteinase originally isolated from brain myelin membranes is present in many tissues. Biochem J. 1990;268:245–248. doi: 10.1042/bj2680245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JM, Cossins JA, Wells GM, Corkill DJ, Helfrich K, Wood LM, Pigott R, Stabler G, Ward GA, Gearing AJ, Miller KM. Matrix metalloproteinase expression during experimental autoimmune encephalomyelitis and effects of a combined matrix metalloproteinase and tumour necrosis factor-alpha inhibitor. J Neuroimmunol. 1997;74:85–94. doi: 10.1016/s0165-5728(96)00210-x. [DOI] [PubMed] [Google Scholar]
- Correale J, Bassani Molinas Mde L. Temporal variations of adhesion molecules and matrix metalloproteinases in the course of MS. J Neuroimmunol. 2003;140:198–209. doi: 10.1016/s0165-5728(03)00204-2. [DOI] [PubMed] [Google Scholar]
- Correale J, Fiol M, Gilmore W. The risk of relapses in multiple sclerosis during systemic infections. Neurology. 2006;67:652–659. doi: 10.1212/01.wnl.0000233834.09743.3b. [DOI] [PubMed] [Google Scholar]
- Cossins JA, Clements JM, Ford J, Miller KM, Pigott R, Vos W, Van der Valk P, De Groot CJ. Enhanced expression of MMP-7 and MMP-9 in demyelinating multiple sclerosis lesions. Acta Neuropathol (Berl) 1997;94:590–598. doi: 10.1007/s004010050754. [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Whitmire JK, Frausto RF, Chertboonmuang P, Soloway PD, Whitton JL, Campbell IL. Persistent macrophage/microglial activation and myelin disruption after experimental autoimmune encephalomyelitis in tissue inhibitor of metalloproteinase-1-deficient mice. Am J Pathol. 2006;169:2104–2116. doi: 10.2353/ajpath.2006.060626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza CA, Moscarello MA. Differences in susceptibility of MBP charge isomers to digestion by stromelysin-1 (MMP-3) and release of an immunodominant epitope. Neurochem Res. 2006;31:1045–1054. doi: 10.1007/s11064-006-9116-9. [DOI] [PubMed] [Google Scholar]
- Diaz-Sanchez M, Williams K, DeLuca GC, Esiri MM. Protein co-expression with axonal injury in multiple sclerosis plaques. Acta Neuropathol (Berl) 2006;111:289–299. doi: 10.1007/s00401-006-0045-0. [DOI] [PubMed] [Google Scholar]
- Dubois B, Masure S, Hurtenbach U, Paemen L, Heremans H, van den Oord J, Sciot R, Meinhardt T, Hammerling G, Opdenakker G, Arnold B. Resistance of young gelatinase B-deficient mice to experimental autoimmune encephalomyelitis and necrotizing tail lesions. J Clin Invest. 1999;104:1507–1515. doi: 10.1172/JCI6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge CF, Bunge MB, Bunge RP, Wood PM. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol. 1987;105:1023–1034. doi: 10.1083/jcb.105.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza J, Kruse M, Lee J, Michaud M, Madri JA. MMP-2 null mice exhibit an early onset and severe experimental autoimmune encephalomyelitis due to an increase in MMP-9 expression and activity. Faseb J. 2004;18:1682–1691. doi: 10.1096/fj.04-2445com. [DOI] [PubMed] [Google Scholar]
- Fainardi E, Castellazzi M, Bellini T, Manfrinato MC, Baldi E, Casetta I, Paolino E, Granieri E, Dallocchio F. Cerebrospinal fluid and serum levels and intrathecal production of active matrix metalloproteinase-9 (MMP-9) as markers of disease activity in patients with multiple sclerosis. Mult Scler. 2006;12:294–301. doi: 10.1191/135248506ms1274oa. [DOI] [PubMed] [Google Scholar]
- Gasche Y, Soccal PM, Kanemitsu M, Copin JC. Matrix metalloproteinases and diseases of the central nervous system with a special emphasis on ischemic brain. Front Biosci. 2006;11:1289–1301. doi: 10.2741/1883. [DOI] [PubMed] [Google Scholar]
- Gijbels K, Galardy RE, Steinman L. Reversal of experimental autoimmune encephalomyelitis with a hydroxamate inhibitor of matrix metalloproteases. J Clin Invest. 1994;94:2177–2182. doi: 10.1172/JCI117578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijbels K, Masure S, Carton H, Opdenakker G. Gelatinase in the cerebrospinal fluid of patients with multiple sclerosis and other inflammatory neurological disorders. J Neuroimmunol. 1992;41:29–34. doi: 10.1016/0165-5728(92)90192-n. [DOI] [PubMed] [Google Scholar]
- Gijbels K, Proost P, Masure S, Carton H, Billiau A, Opdenakker G. Gelatinase B is present in the cerebrospinal fluid during experimental autoimmune encephalomyelitis and cleaves myelin basic protein. J Neurosci Res. 1993;36:432–440. doi: 10.1002/jnr.490360409. [DOI] [PubMed] [Google Scholar]
- Graesser D, Mahooti S, Haas T, Davis S, Clark RB, Madri JA. The interrelationship of alpha4 integrin and matrix metalloproteinase-2 in the pathogenesis of experimental autoimmune encephalomyelitis. Lab Invest. 1998;78:1445–1458. [PubMed] [Google Scholar]
- Graesser D, Mahooti S, Madri JA. Distinct roles for matrix metalloproteinase-2 and alpha4 integrin in autoimmune T cell extravasation and residency in brain parenchyma during experimental autoimmune encephalomyelitis. J Neuroimmunol. 2000;109:121–131. doi: 10.1016/s0165-5728(00)00275-7. [DOI] [PubMed] [Google Scholar]
- Hewson AK, Smith T, Leonard JP, Cuzner ML. Suppression of experimental allergic encephalomyelitis in the Lewis rat by the matrix metalloproteinase inhibitor Ro31-9790. Inflamm Res. 1995;44:345–349. doi: 10.1007/BF01796266. [DOI] [PubMed] [Google Scholar]
- Ivaska J, Heino J. Adhesion receptors and cell invasion: mechanisms of integrin-guided degradation of extracellular matrix. Cell Mol Life Sci. 2000;57:16–24. doi: 10.1007/s000180050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanova-Nesic K, Shoenfeld Y. MMP-2, VCAM-1 and NCAM-1 expression in the brain of rats with experimental autoimmune encephalomyelitis as a trigger mechanism for synaptic plasticity and pathology. J Neuroimmunol. 2006;181:112–121. doi: 10.1016/j.jneuroim.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Kanesaka T, Mori M, Hattori T, Oki T, Kuwabara S. Serum matrix metalloproteinase-3 levels correlate with disease activity in relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2006;77:185–188. doi: 10.1136/jnnp.2005.068619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieseier BC, Kiefer R, Clements JM, Miller K, Wells GM, Schweitzer T, Gearing AJ, Hartung HP. Matrix metalloproteinase-9 and -7 are regulated in experimental autoimmune encephalomyelitis. Brain. 1998;121(Pt 1):159–166. doi: 10.1093/brain/121.1.159. [DOI] [PubMed] [Google Scholar]
- Krekoski CA, Neubauer D, Graham JB, Muir D. Metalloproteinase-dependent predegeneration in vitro enhances axonal regeneration within acellular peripheral nerve grafts. J Neurosci. 2002;22:10408–10415. doi: 10.1523/JNEUROSCI.22-23-10408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Trapp B, Ludwin S, Peterson A, Roder J. Myelin associated glycoprotein modulates glia-axon contact in vivo. J Neurosci Res. 1998;51:210–217. doi: 10.1002/(SICI)1097-4547(19980115)51:2<210::AID-JNR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Li C, Tropak MB, Gerlai R, Clapoff S, Abramow-Newerly W, Trapp B, Peterson A, Roder J. Myelination in the absence of myelin-associated glycoprotein. Nature. 1994;369:747–750. doi: 10.1038/369747a0. [DOI] [PubMed] [Google Scholar]
- Liuzzi GM, Tamborra R, Ventola A, Bisaccia F, Quagliariello E, Riccio P. Different recognition by clostripain of myelin basic protein in the lipid-free and lipid-bound forms. Biochem Biophys Res Commun. 1996;226:566–571. doi: 10.1006/bbrc.1996.1395. [DOI] [PubMed] [Google Scholar]
- Lunn MP, Crawford TO, Hughes RA, Griffin JW, Sheikh KA. Anti-myelin-associated glycoprotein antibodies alter neurofilament spacing. Brain. 2002;125:904–911. doi: 10.1093/brain/awf072. [DOI] [PubMed] [Google Scholar]
- Maeda A, Sobel RA. Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J Neuropathol Exp Neurol. 1996;55:300–309. doi: 10.1097/00005072-199603000-00005. [DOI] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- McKerracher L, Winton MJ. Nogo on the go. Neuron. 2002;36:345–348. doi: 10.1016/s0896-6273(02)01018-8. [DOI] [PubMed] [Google Scholar]
- Melli G, Keswani SC, Fischer A, Chen W, Hoke A. Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated neuropathy. Brain. 2006;129:1330–1338. doi: 10.1093/brain/awl058. [DOI] [PubMed] [Google Scholar]
- Moller JR, Yanagisawa K, Brady RO, Tourtellotte WW, Quarles RH. Myelin-associated glycoprotein in multiple sclerosis lesions: a quantitative and qualitative analysis. Ann Neurol. 1987;22:469–474. doi: 10.1002/ana.410220405. [DOI] [PubMed] [Google Scholar]
- Montag D, Giese KP, Bartsch U, Martini R, Lang Y, Bluthmann H, Karthgasan J, Kirschner DA, Wintergerst ES, Nave K-A, Zielasek J, Toyka KV, Lipp HP, Schachner M. Mice deficient for the myelin-associated glycoprotein show subtle abnormalities in myelin. Neuron. 1994;13:229–246. doi: 10.1016/0896-6273(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Morris-Downes MM, McCormack K, Baker D, Sivaprasad D, Natkunarajah J, Amor S. Encephalitogenic and immunogenic potential of myelin-associated glycoprotein (MAG), oligodendrocyte-specific glycoprotein (OSP) and 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) in ABH and SJL mice. J Neuroimmunol. 2002;122:20–33. doi: 10.1016/s0165-5728(01)00460-x. [DOI] [PubMed] [Google Scholar]
- Nygardas PT, Gronberg SA, Heikkila J, Joronen K, Sorsa T, Hinkkanen AE. Treatment of experimental autoimmune encephalomyelitis with a neurotropic alphavirus vector expressing tissue inhibitor of metalloproteinase-2. Scand J Immunol. 2004;60:372–381. doi: 10.1111/j.0300-9475.2004.01491.x. [DOI] [PubMed] [Google Scholar]
- Nygardas PT, Hinkkanen AE. Up-regulation of MMP-8 and MMP-9 activity in the BALB/c mouse spinal cord correlates with the severity of experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2002;128:245–254. doi: 10.1046/j.1365-2249.2002.01855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdenakker G, Nelissen I, Van Damme J. Functional roles and therapeutic targeting of gelatinase B and chemokines in multiple sclerosis. Lancet Neurol. 2003;2:747–756. doi: 10.1016/s1474-4422(03)00587-8. [DOI] [PubMed] [Google Scholar]
- Ozenci V, Kouwenhoven M, Teleshova N, Pashenkov M, Fredrikson S, Link H. Multiple sclerosis: pro- and anti-inflammatory cytokines and metalloproteinases are affected differentially by treatment with IFN-beta. J Neuroimmunol. 2000;108:236–243. doi: 10.1016/s0165-5728(00)00281-2. [DOI] [PubMed] [Google Scholar]
- Ozenci V, Rinaldi L, Teleshova N, Matusevicius D, Kivisakk P, Kouwenhoven M, Link H. Metalloproteinases and their tissue inhibitors in multiple sclerosis. J Autoimmun. 1999;12:297–303. doi: 10.1006/jaut.1999.0285. [DOI] [PubMed] [Google Scholar]
- Pagenstecher A, Stalder AK, Kincaid CL, Shapiro SD, Campbell IL. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am J Pathol. 1998;152:729–741. [PMC free article] [PubMed] [Google Scholar]
- Paivalainen S, Suokas M, Lahti O, Heape A. Degraded myelin-associated glycoprotein (dMAG) formation from pure human brain myelin-associated glycoprotein (MAG) is not mediated by calpain or cathepsin L-like activities. J Neurochem. 2003;84:533–545. doi: 10.1046/j.1471-4159.2003.01539.x. [DOI] [PubMed] [Google Scholar]
- Pedraza L, Owens GC, Green LA, Salzer JL. The myelin-associated glycoproteins: membrane disposition, evidence of a novel disulfide linkage between immunoglobulin-like domains, and posttranslational palmitylation. J Cell Biol. 1990;111:2651–2661. doi: 10.1083/jcb.111.6.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proost P, Van Damme J, Opdenakker G. Leukocyte gelatinase B cleavage releases encephalitogens from human myelin basic protein. Biochem Biophys Res Commun. 1993;192:1175–1181. doi: 10.1006/bbrc.1993.1540. [DOI] [PubMed] [Google Scholar]
- Quarles RH. Myelin sheaths: glycoproteins involved in their formation, maintenance and degeneration. Cell Mol Life Sci. 2002;59:1851–1871. doi: 10.1007/PL00012510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles RH. Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem. 2007;100:1431–1448. doi: 10.1111/j.1471-4159.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Dencoff JE, Correa N, Jr., Reiners M, Ford CC. Effect of steroids on CSF matrix metalloproteinases in multiple sclerosis: relation to blood-brain barrier injury. Neurology. 1996;46:1626–1632. doi: 10.1212/wnl.46.6.1626. [DOI] [PubMed] [Google Scholar]
- Sellebjerg F, Madsen HO, Jensen CV, Jensen J, Garred P. CCR5 delta32, matrix metalloproteinase-9 and disease activity in multiple sclerosis. J Neuroimmunol. 2000;102:98–106. doi: 10.1016/s0165-5728(99)00166-6. [DOI] [PubMed] [Google Scholar]
- Stebbins JW, Jaffe H, Moller JR. Characterization of myelin-associated glycoprotein (MAG) proteolysis in the human central nervous system. Neurochem Res. 1998;23:1005–1010. doi: 10.1023/a:1021092624046. [DOI] [PubMed] [Google Scholar]
- Tang S, Qiu J, Nikulina E, Filbin MT. Soluble myelin-associated glycoprotein released from damaged white matter inhibits axonal regeneration. Mol Cell Neurosci. 2001;18:259–269. doi: 10.1006/mcne.2001.1020. [DOI] [PubMed] [Google Scholar]
- Tang S, Shen YJ, De Bellard ME, Mukhopadhyay G, Salzer JL, Crocker PR, Filbin MT. Myelin-associated glycoprotein interacts with neurons via a sialic acid binding site at ARG118 and a distinct neurite inhibition site. J Cell Biol. 1997a;138:1355–1366. doi: 10.1083/jcb.138.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Woodhall RW, Shen YJ, deBellard ME, Saffell JL, Doherty P, Walsh FS, Filbin MT. Soluble myelin-associated glycoprotein (MAG) found in vivo inhibits axonal regeneration. Mol Cell Neurosci. 1997b;9:333–346. doi: 10.1006/mcne.1997.0633. [DOI] [PubMed] [Google Scholar]
- Teesalu T, Hinkkanen AE, Vaheri A. Coordinated induction of extracellular proteolysis systems during experimental autoimmune encephalomyelitis in mice. Am J Pathol. 2001;159:2227–2237. doi: 10.1016/S0002-9440(10)63073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft-Hansen H, Nuttall RK, Edwards DR, Owens T. Key metalloproteinases are expressed by specific cell types in experimental autoimmune encephalomyelitis. J Immunol. 2004;173:5209–5218. doi: 10.4049/jimmunol.173.8.5209. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Andrews B, Wong A, O’Connell M, Griffin JW. Co-localization of the myelin-associated glycoprotein and the microfilament components, F-actin and spectrin, in Schwann cells of myelinated nerve fibres. J Neurocytol. 1989;18:47–60. doi: 10.1007/BF01188423. [DOI] [PubMed] [Google Scholar]
- Trojano M, Avolio C, Liuzzi GM, Ruggieri M, Defazio G, Liguori M, Santacroce MP, Paolicelli D, Giuliani F, Riccio P, Livrea P. Changes of serum sICAM-1 and MMP-9 induced by rIFNbeta-1b treatment in relapsing-remitting MS. Neurology. 1999;53:1402–1408. doi: 10.1212/wnl.53.7.1402. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Chivatakarn O, Sheu SS, Giger RJ. Molecular dissection of the myelin-associated glycoprotein receptor complex reveals cell type-specific mechanisms for neurite outgrowth inhibition. J Cell Biol. 2007;177:393–399. doi: 10.1083/jcb.200702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waubant E. Biomarkers indicative of blood-brain barrier disruption in multiple sclerosis. Dis Markers. 2006;22:235–244. doi: 10.1155/2006/709869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waubant E, Gee L, Miller K, Stabler G, Goodkin D. IFN-beta1a may increase serum levels of TIMP-1 in patients with relapsing-remitting multiple sclerosis. J Interferon Cytokine Res. 2001;21:181–185. doi: 10.1089/107999001750133230. [DOI] [PubMed] [Google Scholar]
- Waubant E, Goodkin DE, Gee L, Bacchetti P, Sloan R, Stewart T, Andersson PB, Stabler G, Miller K. Serum MMP-9 and TIMP-1 levels are related to MRI activity in relapsing multiple sclerosis. Neurology. 1999;53:1397–1401. doi: 10.1212/wnl.53.7.1397. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, Quarles RH, Johnson D, Brady RO, Whitaker JN. A derivative of myelin-associated glycoprotein in cerebrospinal fluid of normal subjects and patients with neurological disease. Ann Neurol. 1985;18:464–469. doi: 10.1002/ana.410180409. [DOI] [PubMed] [Google Scholar]



