Abstract
Levels of the soluble form of the interleukin-1 receptor like 1 protein (IL-1RL-1 / ST2) are elevated in the serum of patients with diseases characterized by an inflammatory response. The objective of this study was to determine the concentration of soluble ST2 (sST2) in dengue infected patients during the course of the disease. Twenty four patients with confirmed dengue infection, classified as dengue fever, and eleven patients with other febrile illness (OFI) were evaluated. Levels of sST2 in serum and laboratory variables usually altered during dengue infections were measured. Dengue infected patients had higher serum sST2 levels than OFI at the end of the febrile stage and at defervescence (p=0.0088 and p=0.0004 respectively). Patients with secondary dengue infections had higher serum sST2 levels compared with patients with primary dengue infections (p=0.047 at last day of fever and p=0.030 at defervescence). Furthermore, in dengue infected patients, we found a significant negative correlation of sST2 with platelet and WBC counts, and positive correlation with thrombin time and transaminases activity. We suggest that sST2 could be a potential marker of dengue infection, could be associated with severity or could play a role in the immune response in secondary dengue virus infection.
Keywords: soluble ST2, dengue, inflammation, Th1/Th2
1. INTRODUCTION
Dengue virus is a single-stranded RNA mosquito-borne virus that belongs to the Flaviviridae family. It infects humans and produces a disease with a broad spectrum of clinical manifestations that ranges from an acute self-limiting febrile illness (Dengue Fever, DF) to various grades of a severe disease (Dengue Hemorrhagic Fever, DHF) that could result in a life-threatening syndrome (Dengue Shock Syndrome, DSS) [1]. Symptoms include high fever, headache, myalgias, skin rash, thrombocytopenia, coagulation alterations, hepatic inflammation and hemorrhagic manifestations. Increased vascular permeability that results in vascular leakage is the characteristic event that occurs and defines DHF [2].
Dengue virus can be classified into four antigenically distinct serotypes: D1V, D2V, D3V, and D4V, and each one of them can cause DF or DHF [3]. Infection with one of the serotypes imparts immunity to the infecting serotype. Multiple infections with different (heterologous) serotypes can occur during the lifetime and DHF/DSS is usually associated with secondary infections [4, 5]. When a secondary infection occurs, the immune response could be dominated by the pre-existing cross-reactive memory cells from a previous dengue infection rather than by the naïve pool of high-affinity specific cells for the infecting serotype (“original antigenic sin” [6]). These low-affinity memory clones are rapidly activated and undergo clonal expansion. This results in the production of antibodies that bind to the heterologous serotype at non-neutralizing epitopes, which could lead to antibody-mediated immune enhancement instead of blocking viral infectivity [7, 8]. Cross reactivity also generates a dysfunctional T cell response that results in suboptimal clearance of the virus and an uncontrolled production of soluble mediators [9]. Elevated circulating levels of both type 1 (Th1) and type 2 (Th2) cytokines and various chemokines including gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin (IL)-1beta (IL-1β), IL-6, IL-10, IL-13, IL-8, macrophage chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein 1 beta (MIP-1β) have been detected in dengue infected patients [10–18], and the kinetics and persistence of some of these mediators seem to be related to the severity of the disease.
The Interleukin-1 receptor like-1 protein (IL-1RL-1, ST2) is a member of the interleukin-1 receptor (IL-1R) family of proteins. It was originally detected as a primary response gene in murine fibroblasts [19, 20] and as a HA-ras oncogen-responsive gene [21]. Alternative splicing of the gene generates three mRNAs, corresponding to a longer membrane-anchored form (ST2L), a shorter released form (sST2) and a membrane bound variant form (ST2V) [22–24]. The expression of the three forms has been detected in various human tissues and cells, including hematopoietic and endothelial cells [25]. ST2L has been proposed as a marker for Th2 CD4+ T cells since it is selectively expressed on Th2 but not on Th1 CD4+ T cells [26, 27] and might be involved in the effector phase of Th2 immune responses [28]. Expression of sST2 protein can be induced in vitro by pro-inflammatory stimuli, like lipo-polysaccaride (LPS), IL-1β, TNF-α and IL-6 in human and murine inflammatory models [25, 29]. In mice, the production of pro-inflammatory cytokines precedes sST2 expression [30]. Elevated levels of sST2 have been found in patients with inflammatory disorders associated with abnormal Th2 mediated responses, including autoimmune diseases [31], asthma [32, 33], idiopathic pulmonary fibrosis [29], and sepsis [34]; sST2 levels are also found elevated in patients with other inflammatory conditions, like LPS induced inflammation [35] and myocardial infarction [36]. Additionally, sST2 has been proposed as a biomarker for heart failure [37].
Dengue virus infection is an acute infection which involves over-production of pro-inflammatory molecules. Here we report elevated levels of sST2 protein in serum of dengue infected patients. Furthermore, secondary dengue virus infections show significantly higher serum sST2 protein levels as compared to primary dengue virus infection.
2. RESULTS
2.1. Clinical and laboratory characteristic of patients
The characteristics of the patients enrolled in the study protocol are shown in Table 1. Patients were classified based on detection of dengue virus specific IgM and genomic dengue RNA in serum. Patients positive for IgM or genomic RNA were classified as “dengue” and patients that did not meet these criteria were classified as “other febrile illness (OFI)”. Dengue patients were further sub-classified as primary or secondary infections based on hemmaglutination inhibition assay (HI) titer. Our study included a group of eleven OFI and twenty four dengue patients; ten dengue patients had primary infections and thirteen had secondary infections and one was not sub-classified. All dengue patients were classified as DF according to the World Health Organization case definition [38]. The frequency of petechiae (p<0.001), edema (p=0.045) and rash (p=0.002) were higher in dengue patients compared with OFI patients. Significant differences in the minimum white blood cell (WBC) count (p=0.011), minimum platelets count (p<0.001), maximum aspartate aminotransferase (AST) (p<0.001), maximum alanine transaminase (ALT) (p=0.004), maximum prolonged thrombin time difference (ΔTT) (p<0.001) and minimum fibrinogen levels (p<0.001) were observed between OFI and dengue patients. Further, when primary and secondary infected patients were compared, we found significant differences in the minimum platelets count (p=0.018), maximum AST (p=0.042) and maximum ALT (p=0.049).
Table 1.
Clinical profile of patients enrolled in the study protocol
| Classification a | OFI (N=11) | Dengue |
||
|---|---|---|---|---|
| All (N=24) | Primary (N=10) | Secondary (N=13) | ||
| Age b | 33 (13–56) | 22 (9–55) | 22 (9–33) | 25 (11–55) |
| Sex (F : M) | (8 : 3) | (8 : 16) | (5 : 5) | (2 : 11) |
| Clinical Sign and Symptoms c | ||||
| Petechiae d | 0 | 18 | 7 | 10 |
| Hemorrhage e | 3 | 6 | 2 | 4 |
| Vascular Leakage (ultrasound) | 0 | 2 | 0 | 2 |
| Edema | 0 | 7 | 3 | 3 |
| Laboratory Parameters f | ||||
| Minimum platelet count (×103/µl) | 187 ± 18 (147–228) | 90 ± 10 (69–111) | 118 ± 11 (92–144) | 69 ± 14 (39–98) |
| Minimum WBC (×103/µl) | 4.4 ± 0.7 (3.0–5.9) | 2.6 ± 0.2 (2.1–3.1) | 2.6 ± 0.3 (1.8–3.3) | 2.7 ± 0.3 (2.0–3.5) |
| Maximum AST (U/ml) | 69.4 ± 37.6 (14.3–153.1) | 186.5 ± 33.5 (117.2–255.8) | 93.4 ± 10.6 (69.4–117.3) | 249.0 ± 54.1 (131.1–367.0) |
| Maximum ALT (U/ml) | 61.2 ± 29.7 (4.9–127.3) | 137.9 ± 25.0 (86.1–189.7) | 70.1 ± 8.2 (51.6–88.6) | 189.0 ± 40.8 (100.1–277.9) |
| Maximum thrombin time difference (ΔTT, s) | 1.1 ± 0.5 (0.1–2.3) | 12.0 ± 2.9 (6.0–17.9) | 5.2 ± 1.0 (2.8–7.5) | 17.2 ± 4.8 (6.7–29.6) |
| Minimum fibrinogen (mg/dL) | 387 ± 27 (327–446) | 283 ± 12 (257–309) | 299 ± 20 (255–344) | 277 ± 16 (243–312) |
Patients were classified according to dengue viral RNA and IgM detection. Dengue patients were positive for both dengue viral RNA and IgM; OFI patients were negative for both parameters. Primary patients had HI titers ≤1:1280 and secondary patients had HI titers > 1280. One dengue patient could not be classified as primary or secondary.
Age in years (median and range).
Frequency of patients with each sign / symptoms during the period of study.
Petechiae: positive tourniquet test and / or spontaneous petechiae.
Types of Hemorrhages: OFI (epistaxis, hematoma); primary (gum bleeding); secondary (epistaxis, gum bleeding, hematoma, hematuria).
Average value ± standard deviation and 95% confidence interval.
2.2. Soluble ST2 levels in dengue patients
We assayed serum from healthy donors, OFI and dengue patients for sST2 protein. The sST2 levels (pg/ml) in serum of healthy donors were significant lower than levels in patients during the acute stage of the disease. The sST2 levels in serum of OFI and dengue infected patients during the course of the disease are shown in Figure 1A. The sST2 levels were elevated during late febrile days of the disease, reaching maximum values on fever days −1 and 0 (see Methods), followed by decrease in sST2 protein levels close to healthy donors values by the convalescent day (at least 15 days after onset of the disease) to levels similar to those of healthy donors. The increase in sST2 protein levels were statistically significant for all dengue patients (p<0.001) but not for the OFI group, indicating a specific increase in sST2 protein levels during acute stage of dengue virus infections. sST2 protein levels were significantly higher on fever days −1 and 0 (p<0.001) as compared to convalescence in all dengue patients while for OFI there were no statistically significant differences in sST2 levels between stages. We also found statistically higher sST2 levels in all dengue patients compared to OFI at fever days −1 (p=0.0088) and 0 (p=0.0004) suggesting that sST2 protein levels are preferentially increased in dengue virus infections. When we analyzed the dengue patients sub-classified as primary or secondary infections, we found statistically significant higher sST2 levels in secondary infections on fever days −1 (p<0.001) and 0 (p<0.01) as compared to the sST2 levels on convalescence (Figure 1B), a result that was not observed in primary infections. We also found statistically significant higher sST2 levels in secondary infections compared to primary infections at fever days −1 (p=0.047) and 0 (p=0.030). We could not statistically compare dengue and OFI patients at day −2 because 10 out of 11 OFI patients were enrolled at day −1.
Figure 1.
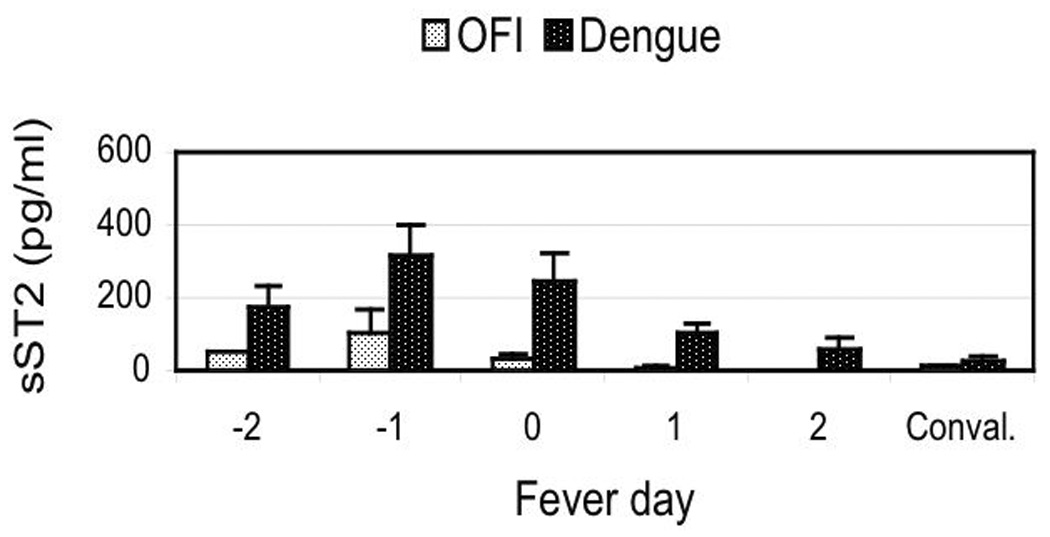
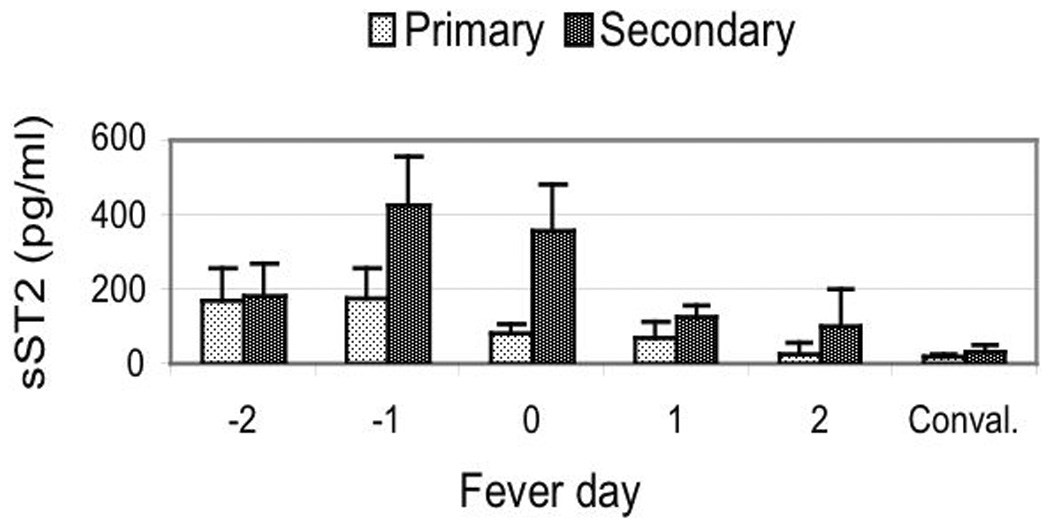
Levels of serum soluble ST2 protein in dengue patients. (A) Soluble ST2 protein levels in serum from OFI and dengue virus infected patients. (B) Soluble ST2 protein levels in primary and secondary dengue virus infections. Results are expressed as sST2 mean values (pg/ml) ± standard error of mean for each patient group and each disease day. Mean sST2 levels for healthy donors was: 15.9 ± 4.4 (N=14) pg/ml. Mann-Whitney statistical analysis between OFI and dengue (except for day −2) or between primary and secondary dengue virus infections at each disease day: significant differences between OFI and dengue at days −1 (p=0.0088) and day 0 (p=0.0004) and between primary and secondary infections at days −1 (p=0.0470) and day 0 (p=0.0300). Conval.: convalescence.
2.3. Correlations between sST2 and laboratory parameters
We correlated sST2 protein levels with laboratory parameters associated with disease severity in dengue virus infections. Correlations were assessed between the sST2 value (pg/ml) and the corresponding value for each laboratory parameter for the same day of the disease. In all dengue virus infected patients we found a negative correlation between sST2 protein levels and WBC (r=−0.357; p<0.01) and platelet counts (r=−0.504; p<0.01) and positive correlation between sST2 protein levels and ΔTT (r=0.366; p<0.01), AST (r=0.462; p<0.01) and ALT (r=0.237; p<0.05). In the secondary infected patients we found a negative correlation between sST2 protein levels and WBC (r=−0.505; p<0.01) and platelet counts (r=−0.553; p<0.01) as well as a positive correlation between sST2 and AST (r=0.496; p<0.01) and ΔTT (r=0.306; p<0.05). Finally, in the primary infected patients, we only found a positive correlation between sST2 and AST (r=0.312; p<0.05) and ΔTT (r=0.356; p<0.05).
3. DISCUSSION
In this study, we found higher levels of sST2 protein in serum from dengue virus infected patients as compared to OFI patients. Serum levels of sST2 protein were found to be elevated at the end of the febrile stage of the disease, reaching a peak between fever days −1 and 0 followed by a decrease of the levels to normal values in convalescence. The viremia tendency is to rapidly decrease at late febrile stage of the disease through defervescence [16]. The levels of sST2 tend to increase at late febrile stage and at defervescence. Therefore, levels of sST2 seem to be inversely correlated with the viremia. As we found higher levels of sST2 in dengue infected patients comparing to OFI patients at days −1 and 0 (p=0.0088 and p=0.0004) but also at day +1 (p=0.069), we propose sST2 has a potential use as a marker for differential dengue diagnosis at a late febrile stage or even during defervescence or immediately after, when most of the virus has been cleared. Maximum sST2 levels were also associated to the final antibody titer, as we found higher levels of sST2 protein in patients who had higher HI titer (secondary infections). Finally, we correlated sST2 protein levels with laboratory parameters associated with severity; even thought all our patients were classified as “dengue fever”, we found different degrees of alterations of various laboratory parameters usually associated with severity in dengue infection. We found that sST2 had a negative correlation with platelet count and WBC count and a positive correlation with the prolonged thrombin time and AST/ALT activity. Dengue virus infections are characterized by thrombocytopenia [39], and we found that sST2 protein levels were higher as the platelet counts were lower. We found the same tendency for WBC count. Dengue virus infected patients also have coagulation alterations, like prolonged thrombin time [40], and sST2 levels were higher when thrombin time was longer. Prolonged thrombin time reflects alterations in fibrinogen levels and / or fibrinogen / fibrin degradation products [41]. Dengue virus infected patients frequently develop hepatic inflammation that is reflected by higher activity of hepatic transaminases AST/ALT [42], and sST2 levels were higher in patients with higher AST/ALT activity. We do not know which cells are responsible for the production of the sST2 protein in vivo during dengue virus infections; preliminary results using quantitative RT-PCR (Low Density Arrays) have shown increased expression of ST2 mRNA in peripheral blood mononuclear cells from dengue infected patients compared to OFI (data not shown).
The function of sST2 is not completely clear. Recent reports have suggested that sST2 protein could be involved in the inflammatory response as well as in Th2 immune responses [43, 44]. Some evidence suggests that sST2 could act as an anti-inflammatory mediator, through a mechanism that involves the inhibition of Toll-like receptor signaling by sequestration of MyD88 and Mal adapter proteins [45, 46] or inhibition of I-κB degradation [47] resulting in down-regulation of NF-κB. In vitro and in vivo experiments have shown that sST2 protein or an ST2-fusion protein is able to attenuate the production of pro-inflammatory cytokines IL-1β, TNF-α, IL-6, and IL-12 [30, 45, 48]. In two mouse models of ischemia/reperfusion, pre-treatment with an sST2-Fc fusion protein decreased the inflammatory response [49, 50]. Some evidence suggests IL-10 as a possible mediator of this effect [50]. High levels of pro-inflammatory cytokines like TNF-α and IL-6 have been found in dengue patients [10, 11, 17] and these cytokines are known to induce sST2 [25, 29]. Hence, we suggest that elevated sST2 levels found in dengue patients could be an indication of the immune hyperactivation, and / or a mechanism to down-regulate inflammation.
Other evidence suggests that sST2 could act as a negative regulator of the Th2 response [43, 51]. Recently, it has been suggested that sST2 could be acting as a decoy receptor for IL-33 regulating its biological function; in myocardium IL-33/ST2L interactions are cardioprotective and sST2 seems to have a role blocking the anti-hypertrophic effect of IL-33 [52]. IL-33 was identified as a ligand for ST2L, a marker of Th2 T lymphocytes [51]. ST2L is involved in the regulation of the Th2 associated immune response at the effector stage and in Th2 driven immunopathology. The interaction of IL-33 with ST2L leads to the induction of the Th2 cytokines IL-4, IL-5 and IL-13 through a signaling mechanism that involves the activation of NF-κB and MAP kinases [51]. In dengue virus infections a shift from a predominant Th1 response to a Th2 response around the time of defervescence appears to correlate with disease severity [14, 53] and higher levels of IL-10 and IL-13 have been found in DHF compared to DF patients [13, 14, 54]. Therefore, elevated levels of sST2 protein could be part of a down-regulation mechanism triggered to attenuate the Th2 response that occurs in dengue patients.
Overall, the findings in this study show a transient elevation of sST2 protein levels in the serum of dengue virus-infected patients around the time of defervescence, and higher sST2 protein levels correlate with markers of severity in dengue virus infections. Frequently, severe manifestations correlate with secondary infections, and our results show that in our sample population, levels of sST2 protein in serum were not only higher in patients with secondary infections but also in patients with more severe manifestations. Levels of sST2 protein in serum could be a potential differential marker for dengue infections at late febrile and / or defervescence stage of the disease. This may be useful in cases where the virus had been cleared. Understanding the molecular mechanism involved in the regulation and biological effect of sST2 protein in dengue virus infections deserves further investigation.
4. METHODS
4.1. Patients
Thirty five Venezuelan patients with suspected dengue virus infection were included in this study. All patients were enrolled in a study protocol conducted by the University of Massachusetts Medical School (UMMS), Worcester, MA, USA and Banco Municipal de Sangre del Distrito Capital (BMS), Caracas, Venezuela, between 2001 and 2005 [55]. Written informed consent was obtained from all subjects. Criteria for enrollment included presence of a febrile illness (corporal temperature >38.5°C), with no evidence of other defined infections. Enrolled febrile patients attended the consult daily until 2 days after the fever resolved. A final consult was performed at least 2 weeks after the onset of symptoms (convalescence). Blood samples for hematology, coagulation tests, serology and biochemical analysis were obtained daily. Serum and plasma samples were separated in aliquots and stored at −70°C for analysis. Fourteen healthy donors from BMS and UMMS were used as controls for normal sST2 protein levels.
4.2. Clinical record and laboratory analysis
Complete clinical exam and routine laboratory tests were performed each day, starting at the day of enrollment and until 2 days after defervescence and finally at the day of convalescence. Based on corporal temperature, we defined “fever day zero (0)” as the day of defervescence (corporal temperature <38°C); days before defervescence were numbered as fever days −1, −2 and days after defervescence were numbered +1, +2. A thorax/abdomen ultrasound study was performed on day +1. Peripheral blood studies were performed using Gen-S autoanalyzer (Beckman-Coulter). Thrombin time (TT) was measured in plasma samples in a STA Compact automated coagulation analyzer (Diagnostica Stago) and compared against control TT obtained from healthy donors (ΔTT= TTpatient − TTcontrol). Fibrinogen levels were measured in plasma samples by Clauss method (Diagnostica Stago). Aspartate aminotransferase (AST) and alanine transaminase (ALT) were measured in serum samples using Sigma-transaminase kit (Sigma-Aldrich).
4.3. Dengue Diagnosis
Dengue diagnosis was done through the detection of dengue genomic RNA and serological test to detect dengue specific antibodies. Paired serum samples were obtained from each enrolled patient: S1 or acute sample (obtained at enrollment) and S2 or convalescent sample (obtained at convalescence), with a minimum of 7 days interval between both samples. Dengue RNA was isolated from S1 (acute serum) samples using the QIAmp Viral RNA kit (QIAGEN). Dengue virus serotype specific reverse transcription and polymerase chain reaction (RT-PCR) was performed using the One-step PCR kit (QIAGEN) and primers described in Lanciotti et al. [56] adapted to a one-step RT-PCR using reverse primer and serotype specific forward primers. Dengue antibodies were measured in both S1 (acute serum) and S2 (convalesecent serum) samples by ELISA (IgM) and hemagglutination inhibition assay (HI), at the Instituto Nacional de Higiene Rafael Rangel (National Reference Laboratory), Caracas, Venezuela. Patients were classified as Dengue or as an Other Febrile Illness (OFI) based on the detection of genomic dengue RNA, presence of IgM antibodies and / or a four-fold or greater increase in titer in S2 (convalescent sample) compared to the S1 (acute sample). The maximum HI titers in the confirmed dengue cases were used to further classify dengue patients as a primary infection (HI titer ≤ 1:1280) or secondary infection (HI titer > 1:1280) [38].
4.4. Quantification of Soluble ST2 Protein
Serum levels of sST2 were measured by ELISA (MBL Int.) following the manufacturer’s instructions. Serum samples during febrile (fever days −2 and −1), defervescence (fever day 0), post-febrile (fever days +1 and +2) and convalescence (at least 2 weeks after the onset) stages were tested for soluble ST2 protein levels. For each healthy donor a single serum sample was analyzed, to generate basal levels of sST2.
4.5. Statistical analysis
The Mann-Whitney U or Kruskal-Wallis tests were used for comparisons between groups for continuous variables not normally distributed. X2 was used to compare categorical data. Spearman’s correlation was used to examine correlations between continuous variables. We used the software SPSS 14.0 for Windows (Copyright SPSS Inc. 1989–2005) for the statistical analysis.
ACKNOWLEDGMENTS
This study was funded by NIAID grants # U01 AI45440. We thank the personnel at Banco Municipal de Sangre, Caracas, Venezuela for the clinical evaluation of the patients and laboratory tests. We thank Katherine Martin for analysis of gene expression of patient blood cells. To Jonathan Dinsmore, for critical reading of the manuscript. We declare that we have no financial conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaturvedi U, Nagar R, Shrivastava R. Dengue and dengue haemorrhagic fever: implications of host genetics. FEMS Immunol Med Microbiol. 2006;47:155–166. doi: 10.1111/j.1574-695X.2006.00058.x. [DOI] [PubMed] [Google Scholar]
- 2.Rothman AL, Ennis FA. Immunopathogenesis of Dengue hemorrhagic fever. Virology. 1999;257:1–6. doi: 10.1006/viro.1999.9656. [DOI] [PubMed] [Google Scholar]
- 3.Monath TP. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci U S A. 1994;91:2395–2400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halstead SB, Nimmannitya S, Cohen SN. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J Biol Med. 1970;42:311–328. [PMC free article] [PubMed] [Google Scholar]
- 5.Guzman MG, Kouri GP, Bravo J, Soler M, Vazquez S, Morier L. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am J Trop Med Hyg. 1990;42:179–184. doi: 10.4269/ajtmh.1990.42.179. [DOI] [PubMed] [Google Scholar]
- 6.Rothman AL. Dengue: defining protective versus pathologic immunity. J Clin Invest. 2004;113:946–951. doi: 10.1172/JCI21512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halstead SB. Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev Infect Dis. 1989;11 Suppl 4:S830–S839. doi: 10.1093/clinids/11.supplement_4.s830. [DOI] [PubMed] [Google Scholar]
- 8.Morens DM. Antibody-dependent enhancement of infection and the pathogenesis of viral disease. Clin Infect Dis. 1994;19:500–512. doi: 10.1093/clinids/19.3.500. [DOI] [PubMed] [Google Scholar]
- 9.Welsh RM, Rothman AL. Dengue immune response: low affinity, high febrility. Nat Med. 2003;9:820–822. doi: 10.1038/nm0703-820. [DOI] [PubMed] [Google Scholar]
- 10.Hober D, Poli L, Roblin B, Gestas P, Chungue E, Granic G, Imbert P, Pecarere JL, Vergez-Pascal R, Wattre P, et al. Serum levels of tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), and interleukin-1 beta (IL-1 beta) in dengue-infected patients. Am J Trop Med Hyg. 1993;48:324–331. doi: 10.4269/ajtmh.1993.48.324. [DOI] [PubMed] [Google Scholar]
- 11.Hober D, Delannoy AS, Benyoucef S, De Groote D, Wattre P. High levels of sTNFR p75 and TNF alpha in dengue-infected patients. Microbiol Immunol. 1996;40:569–573. doi: 10.1111/j.1348-0421.1996.tb01110.x. [DOI] [PubMed] [Google Scholar]
- 12.Raghupathy R, Chaturvedi UC, Al-Sayer H, Elbishbishi EA, Agarwal R, Nagar R, Kapoor S, Misra A, Mathur A, Nusrat H, Azizieh F, Khan MA, Mustafa AS. Elevated levels of IL-8 in dengue hemorrhagic fever. J Med Virol. 1998;56:280–285. doi: 10.1002/(sici)1096-9071(199811)56:3<280::aid-jmv18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Green S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, Rothman AL, Ennis FA. Elevated plasma interleukin-10 levels in acute dengue correlate with disease severity. J Med Virol. 1999;59:329–334. [PubMed] [Google Scholar]
- 14.Mustafa AS, Elbishbishi EA, Agarwal R, Chaturvedi UC. Elevated levels of interleukin-13 and IL-18 in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol. 2001;30:229–233. doi: 10.1111/j.1574-695X.2001.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 15.Spain-Santana TA, Marglin S, Ennis FA, Rothman AL. MIP-1 alpha and MIP-1 beta induction by dengue virus. J Med Virol. 2001;65:324–330. doi: 10.1002/jmv.2037. [DOI] [PubMed] [Google Scholar]
- 16.Libraty DH, Endy TP, Houng HS, Green S, Kalayanarooj S, Suntayakorn S, Chansiriwongs W, Vaughn DW, Nisalak A, Ennis FA, Rothman AL. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis. 2002;185:1213–1221. doi: 10.1086/340365. [DOI] [PubMed] [Google Scholar]
- 17.Avila-Aguero ML, Avila-Aguero CR, Um SL, Soriano-Fallas A, Canas-Coto A, Yan SB. Systemic host inflammatory and coagulation response in the Dengue virus primo-infection. Cytokine. 2004;27:173–179. doi: 10.1016/j.cyto.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Lee YR, Liu MT, Lei HY, Liu CC, Wu JM, Tung YC, Lin YS, Yeh TM, Chen SH, Liu HS. MCP-1, a highly expressed chemokine in dengue haemorrhagic fever/dengue shock syndrome patients, may cause permeability change, possibly through reduced tight junctions of vascular endothelium cells. J Gen Virol. 2006;87:3623–3630. doi: 10.1099/vir.0.82093-0. [DOI] [PubMed] [Google Scholar]
- 19.Tominaga S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett. 1989;258:301–304. doi: 10.1016/0014-5793(89)81679-5. [DOI] [PubMed] [Google Scholar]
- 20.Yanagisawa K, Tsukamoto T, Takagi T, Tominaga S. Murine ST2 gene is a member of the primary response gene family induced by growth factors. FEBS Lett. 1992;302:51–53. doi: 10.1016/0014-5793(92)80282-l. [DOI] [PubMed] [Google Scholar]
- 21.Werenskiold AK, Hoffmann S, Klemenz R. Induction of a mitogen-responsive gene after expression of the Ha-ras oncogene in NIH 3T3 fibroblasts. Mol Cell Biol. 1989;9:5207–5214. doi: 10.1128/mcb.9.11.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanagisawa K, Takagi T, Tsukamoto T, Tetsuka T, Tominaga S. Presence of a novel primary response gene ST2L, encoding a product highly similar to the interleukin 1 receptor type 1. FEBS Lett. 1993;318:83–87. doi: 10.1016/0014-5793(93)81333-u. [DOI] [PubMed] [Google Scholar]
- 23.Bergers G, Reikerstorfer A, Braselmann S, Graninger P, Busslinger M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. Embo J. 1994;13:1176–1188. doi: 10.1002/j.1460-2075.1994.tb06367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tominaga S, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Komatsu N. Presence and expression of a novel variant form of ST2 gene product in human leukemic cell line UT-7/GM. Biochem Biophys Res Commun. 1999;264:14–18. doi: 10.1006/bbrc.1999.1469. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Tzimas MN, Griswold DE, Young PR. Expression of ST2, an interleukin-1 receptor homologue, is induced by proinflammatory stimuli. Biochem Biophys Res Commun. 1997;235:474–478. doi: 10.1006/bbrc.1997.6810. [DOI] [PubMed] [Google Scholar]
- 26.Yanagisawa K, Naito Y, Kuroiwa K, Arai T, Furukawa Y, Tomizuka H, Miura Y, Kasahara T, Tetsuka T, Tominaga S. The expression of ST2 gene in helper T cells and the binding of ST2 protein to myeloma-derived RPMI8226 cells. J Biochem (Tokyo) 1997;121:95–103. doi: 10.1093/oxfordjournals.jbchem.a021577. [DOI] [PubMed] [Google Scholar]
- 27.Xu D, Chan WL, Leung BP, Huang F, Wheeler R, Piedrafita D, Robinson JH, Liew FY. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med. 1998;187:787–794. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trajkovic V, Sweet MJ, Xu D. T1/ST2--an IL-1 receptor-like modulator of immune responses. Cytokine Growth Factor Rev. 2004;15:87–95. doi: 10.1016/j.cytogfr.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Tajima S, Oshikawa K, Tominaga S, Sugiyama Y. The increase in serum soluble ST2 protein upon acute exacerbation of idiopathic pulmonary fibrosis. Chest. 2003;124:1206–1214. doi: 10.1378/chest.124.4.1206. [DOI] [PubMed] [Google Scholar]
- 30.Oshikawa K, Yanagisawa K, Tominaga S, Sugiyama Y. ST2 protein induced by inflammatory stimuli can modulate acute lung inflammation. Biochem Biophys Res Commun. 2002;299:18–24. doi: 10.1016/s0006-291x(02)02578-0. [DOI] [PubMed] [Google Scholar]
- 31.Kuroiwa K, Arai T, Okazaki H, Minota S, Tominaga S. Identification of human ST2 protein in the sera of patients with autoimmune diseases. Biochem Biophys Res Commun. 2001;284:1104–1108. doi: 10.1006/bbrc.2001.5090. [DOI] [PubMed] [Google Scholar]
- 32.Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, Tominaga SI, Sugiyama Y. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001;164:277–281. doi: 10.1164/ajrccm.164.2.2008120. [DOI] [PubMed] [Google Scholar]
- 33.Oshikawa K, Yanagisawa K, Tominaga S, Sugiyama Y. Expression and function of the ST2 gene in a murine model of allergic airway inflammation. Clin Exp Allergy. 2002;32:1520–1526. doi: 10.1046/j.1365-2745.2002.01494.x. [DOI] [PubMed] [Google Scholar]
- 34.Brunner M, Krenn C, Roth G, Moser B, Dworschak M, Jensen-Jarolim E, Spittler A, Sautner T, Bonaros N, Wolner E, Boltz-Nitulescu G, Ankersmit HJ. Increased levels of soluble ST2 protein and IgG1 production in patients with sepsis and trauma. Intensive Care Med. 2004;30:1468–1473. doi: 10.1007/s00134-004-2184-x. [DOI] [PubMed] [Google Scholar]
- 35.Oshikawa K, Yanagisawa K, Ohno S, Tominaga S, Sugiyama Y. Expression of ST2 in helper T lymphocytes of malignant pleural effusions. Am J Respir Crit Care Med. 2002;165:1005–1009. doi: 10.1164/ajrccm.165.7.2105109. [DOI] [PubMed] [Google Scholar]
- 36.Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, Lee RT. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109:2186–2190. doi: 10.1161/01.CIR.0000127958.21003.5A. [DOI] [PubMed] [Google Scholar]
- 37.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–726. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]
- 38.Joint WHO HQ/SEAROP/WPRO meeting on DengueNet implementation in South-East Asia and the Western Pacific, Kuala Lumpur, 11–13 December 2003. Wkly Epidemiol Rec. 2003;78:346–347. [PubMed] [Google Scholar]
- 39.Srichaikul T, Nimmannitya S. Haematology in dengue and dengue haemorrhagic fever. Baillieres Best Pract Res Clin Haematol. 2000;13:261–276. doi: 10.1053/beha.2000.0073. [DOI] [PubMed] [Google Scholar]
- 40.Krishnamurti C, Kalayanarooj S, Cutting MA, Peat RA, Rothwell SW, Reid TJ, Green S, Nisalak A, Endy TP, Vaughn DW, Nimmannitya S, Innis BL. Mechanisms of hemorrhage in dengue without circulatory collapse. Am J Trop Med Hyg. 2001;65:840–847. doi: 10.4269/ajtmh.2001.65.840. [DOI] [PubMed] [Google Scholar]
- 41.Sosothikul D, Seksarn P, Pongsewalak S, Thisyakorn U, Lusher J. Activation of endothelial cells, coagulation and fibrinolysis in children with Dengue virus infection. Thromb Haemost. 2007;97:627–634. [PubMed] [Google Scholar]
- 42.Kalayanarooj S, Vaughn DW, Nimmannitya S, Green S, Suntayakorn S, Kunentrasai N, Viramitrachai W, Ratanachu-eke S, Kiatpolpoj S, Innis BL, Rothman AL, Nisalak A, Ennis FA. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176:313–321. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- 43.Amatucci A, Novobrantseva T, Gilbride K, Brickelmaier M, Hochman P, Ibraghimov A. Recombinant ST2 boosts hepatic Th2 response in vivo. J Leukoc Biol. 2007 doi: 10.1189/jlb.1006625. [DOI] [PubMed] [Google Scholar]
- 44.Tajima S, Bando M, Ohno S, Sugiyama Y, Oshikawa K, Tominaga S, Itoh K, Takada T, Suzuki E, Gejyo F. ST2 gene induced by type 2 helper T cell (Th2) and proinflammatory cytokine stimuli may modulate lung injury and fibrosis. Exp Lung Res. 2007;33:81–97. doi: 10.1080/01902140701198583. [DOI] [PubMed] [Google Scholar]
- 45.Sweet MJ, Leung BP, Kang D, Sogaard M, Schulz K, Trajkovic V, Campbell CC, Xu D, Liew FY. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J Immunol. 2001;166:6633–6639. doi: 10.4049/jimmunol.166.11.6633. [DOI] [PubMed] [Google Scholar]
- 46.Brint EK, Xu D, Liu H, Dunne A, McKenzie AN, O'Neill LA, Liew FY. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–379. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 47.Takezako N, Hayakawa M, Hayakawa H, Aoki S, Yanagisawa K, Endo H, Tominaga S. ST2 suppresses IL-6 production via the inhibition of IkappaB degradation induced by the LPS signal in THP-1 cells. Biochem Biophys Res Commun. 2006;341:425–432. doi: 10.1016/j.bbrc.2005.12.206. [DOI] [PubMed] [Google Scholar]
- 48.Leung BP, Xu D, Culshaw S, McInnes IB, Liew FY. A novel therapy of murine collagen-induced arthritis with soluble T1/ST2. J Immunol. 2004;173:145–150. doi: 10.4049/jimmunol.173.1.145. [DOI] [PubMed] [Google Scholar]
- 49.Yin H, Huang BJ, Yang H, Huang YF, Xiong P, Zheng F, Chen XP, Chen YF, Gong FL. Pretreatment with soluble ST2 reduces warm hepatic ischemia/reperfusion injury. Biochem Biophys Res Commun. 2006;351:940–946. doi: 10.1016/j.bbrc.2006.10.166. [DOI] [PubMed] [Google Scholar]
- 50.Fagundes CT, Amaral FA, Souza AL, Vieira AT, Xu D, Liew FY, Souza DG, Teixeira MM. ST2, an IL-1R family member, attenuates inflammation and lethality after intestinal ischemia and reperfusion. J Leukoc Biol. 2007;81:492–499. doi: 10.1189/jlb.0606422. [DOI] [PubMed] [Google Scholar]
- 51.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaturvedi UC, Agarwal R, Elbishbishi EA, Mustafa AS. Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol Med Microbiol. 2000;28:183–188. doi: 10.1111/j.1574-695X.2000.tb01474.x. [DOI] [PubMed] [Google Scholar]
- 54.Chen RF, Liu JW, Yeh WT, Wang L, Chang JC, Yu HR, Cheng JT, Yang KD. Altered T helper 1 reaction but not increase of virus load in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol. 2005;44:43–50. doi: 10.1016/j.femsim.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 55.Cardier JE, Marino E, Romano E, Taylor P, Liprandi F, Bosch N, Rothman AL. Proinflammatory factors present in sera from patients with acute dengue infection induce activation and apoptosis of human microvascular endothelial cells: possible role of TNF-alpha in endothelial cell damage in dengue. Cytokine. 2005;30:359–365. doi: 10.1016/j.cyto.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 56.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


