Abstract
Objectives:
To detail clinical and polysomnographic characteristics in patients affected with Trypanosoma brucei gambiense (T.b.g.) human African trypanosomiasis (HAT) at different stages of evolution and to measure and compare cerebrospinal fluid (CSF) levels of hypocretin-1 with narcoleptic patients and neurologic controls.
Methods:
Twenty-five untreated patients affected with T.b.g. HAT were included. The patients were evaluated using a standardized clinical evaluation and a specific interview on sleep complaints. Diagnosis of stages I and II and intermediate stage was performed by CSF cell count and/or presence of trypanosomes: 4 patients were classified as stage II, 13 stage I, and 8 “intermediate” stage. Seventeen untreated patients completed continuous 24-hour polysomnography. We measured CSF levels of hypocretin-1 in all patients at different stages and evolutions, and we compared the results with 26 patients with narcolepsy-cataplexy and 53 neurologic controls.
Results:
CSF hypocretin-1 levels were significantly higher in T.b.g. HAT (423.2 ± 119.7 pg/mL) than in narcoleptic patients (40.16 ± 60.18 pg/mL) but lower than in neurologic controls (517.32 ± 194.5 pg/mL). One stage I patient had undetectable hypocretin levels and 1 stage II patient showed intermediate levels, both patients (out of three patients) reporting excessive daytime sleepiness but without evidence for an association with narcolepsy. No differences were found in CSF hypocretin levels between patients with HAT stages; however, the presence of major sleep-wake cycle disruptions was significantly associated with lower CSF hypocretin-1 level with a same tendency for the number of sleep-onset rapid eye movement periods.
Conclusion:
The present investigation is not in favor of a unique implication of the hypocretin system in T.b.g. HAT. However, we propose that dysfunction of the hypothalamic hypocretin region may participate in sleep disturbances observed in African trypanosomiasis.
Citation:
Dauvilliers Y; Bisser S; Chapotot F; Vatunga G; Cespuglio R; Josenando T; Buguet A. Hypocretin and human African trypanosomiasis. SLEEP 2008;31(3):348-354.
Keywords: Trypanosome, sleeping sickness, hypocretin, narcolepsy, hypersomnia, REM sleep
HUMAN AFRICAN TRYPANOSOMIASIS (HAT), SLEEPING SICKNESS, IS A REEMERGENT ENDEMIC PARASITIC DISEASE IN INTERTROPICAL AFRICA, ALTHOUGH dramatic figures of 450,000 cases in 1997 have dropped to 60 to 80,000.1 Two trypanosome groups (Trypanosoma brucei, T.b., gambiense [T.b.g.] and rhodesiense) are transmitted to humans by tsetse flies in 2 geographic areas (Western and Central Africa humid forest, and Eastern Africa arborous savannah), provoking a slowly or rapidly evolutive disease, respectively. The course of the disease evolves in 2 different stages. The hemolymphatic “Stage I” occurs 1 to 3 weeks after the bite and the formation of a nodule (trypanome) at the inoculation site, with intermittent fever, lymph-node swelling, splenomegaly, skin eruption, and headaches as the main features. Stage I ends when trypanosomes cross the blood-brain barrier to penetrate the central nervous system (CNS), leading insidiously to the meningoencephalitic “Stage II.” Stage II is characterized by CNS infection with neurologic (pyramidal, extrapyramidal, and cerebellar signs) and psychiatric (anxiety, aggressiveness, confusion, etc.) symptoms, including especially sleep disturbances.
Stage determination is crucial because treatment procedures differ between Stage I and Stage II: well-tolerated pentamidine at Stage I versus toxic medications at Stage II. For example, treatment with melarsoprol, an arseniate used since 1949, may lead to the development of a reactive arsenical encephalopathy with lethal outcome in approximately half the patients.2 However, there are no highly specific clinical signs nor biologic tests for stage determination. Cerebrospinal fluid (CSF) examination remains the centerpiece in assessing the evolutive stage. Presence of trypanosomes in the CSF indicates Stage II, as trypanosomes have entered the CNS. The main problem resides in that trypanosome detection is not sensitive. Cell count in the CSF is not specific and cut-offs have been arbitrarily proposed.3–5 Recent meetings of experts organized jointly between the World Health Organization (WHO) and FIND (Foundation for Innovative New Diagnostics) were held in Geneva in 2006 (Joint Meeting for the launching of the new FIND/WHO Initiative for development of diagnostic tests to support the control of human African trypanosomiasis, WHO, Geneva, Switzerland, February 6, 2006; Meeting on HAT Staging Markers, FIND, Geneva, November 30, 2006). Recommendations were that 3 cases were to be considered: fewer than 5 cells/μL of CSF without any trypanosome in the sample (Stage I); more than 20 cells/μL of CSF and/or presence of trypanosome (Stage II); and an intermediate stage between those cell-count limits, the sample being free of any trypanosome.6,7
Considering the difficulty of stage determination, the international community has recently stressed the importance of reenforcing specific and sensitive diagnostic methods.6–7 Polysomnography has been proposed as a potential diagnosis and follow-up tool in T.b.g. HAT.8 Polysomnographic features in Stage II comprise major sleep disturbances with alterations in the 24-hour sleep-wake distribution and alterations of sleep structure with the occurrence of sleep-onset rapid eye movement (REM) periods (SOREMPs).9
T.b.g. HAT shares some features with narcolepsy, such as severe excessive daytime sleepiness (EDS), sleep fragmentation, and especially the occurrence of SOREMPs.8,9 Cataplexy has not been individualized in T.b.g. HAT because advanced cases of the disease are rare nowadays, due to the efforts sustained to detect and treat patients at the earliest stage. However, descriptions of cataplexy-like events such as sudden loss of muscle tone have been reported in the literature, especially in the well-documented cases described by Lhermitte (1910),10 and have been seen regularly on amateur films. Because neuropathologic studies have shown severe and selective loss of hypocretin neurons in the posterior hypothalamus in narcoleptic brains,11,12 the hypothesis that sleep disturbances in T.b.g. HAT may result from hypothalamic damage to the hypocretin system was explored.
The aims of the present study were (1) to compare CSF levels of hypocretin-1 to clinical and polysomnographic characteristics of patients at different stages of T.b.g. HAT and (2) to compare the results with those of narcoleptic patients and neurologic controls.
PATIENTS AND METHODS
Patients
Patients were recruited during a field survey in Angola in 2003. The investigation was conducted in the hilly (1100–1200 m altitude) Kwanza Norte province (Samba-Cajù and Camabatela). The protocol was approved by the public health authorities of Angola (Ministry of Health), and all included patients gave their informed consent to participate.
Patients' interview and clinical signs were collected and are summarized in Table 1. Briefly, EDS (defined as the inability to stay awake and alert during the major waking episodes of the day, resulting in unintended lapses into sleep), insomnia (defined by a repeated difficulty with sleep initiation, duration, consolidation, or quality, resulting in daytime impairment), fatigue (defined as lack of energy, listlessness, or weariness as from labor), sensory and gait disturbances, presence of primitive reflexes (palmomental reflex, sucking reflex), modified tendon reflexes (abolition or exaggerated with a possibility of a Babinski sign), psychiatric disorders (confusion, mood swings, agitation, aggressive behavior, euphoria, absent gaze, mutism, and indifference), anorexia (defined as lack of appetite, the patient does not look for his food by himself or herself), fever (defined as a feeling of being hot, hot flashes), cephalalgia (any kind of headache), abnormal movements such as tremor (resting, postural, or kinetic tremor, on left and/or right side and on upper or lower limbs), or myoclonic jerks were noted.
Table 1.
Clinical, Biological and Polysomnographic Data Obtained in 25 Patients Affected with T.b.g. Human African Trypanosomiasis
| Stage I n = 13 | Intermediate stage n = 8 | Stage II n = 4 | P | |
|---|---|---|---|---|
| Clinical symptoms | ||||
| Sex ratio (M/F) | 3/10 | 2/6 | 2/2 | ns |
| Age, mean ± SD | 29.4 ± 14.4 | 43.0 ± 15.8 | 29.8 ± 13.3 | ns |
| BMI, mean ± SD | 20.5 ± 3.5 | 19.7 ± 3.2 | 17.1 ± 1.6 | ns |
| Worsening of condition,* n | 0 | 4 | 1 | 0.028 |
| Fever, n | 10 | 6 | 4 | ns |
| Fatigue, n | 3 | 3 | 3 | ns |
| Daytime sleepiness, n | 1 | 0 | 2 | 0.03 |
| Insomnia, n | 1 | 1 | 2 | ns |
| Anorexia, n | 1 | 2 | 1 | ns |
| Cephalalgia, n | 11 | 7 | 3 | ns |
| CSF findings | ||||
| Hypocretin-1 level, mean ± SD | 424.1± 142.6 | 467.8 ± 68.5 | 320.5 ± 101.2 | ns |
| Abnormal hypocretin-1 level, n | 1** | 0 | 1 | ns |
Worsening of clinical symptoms, i.e., severely affected in term of general, neurological and/or psychiatric status, with a clear alteration in quality of life.
abnormal polysomnography
Out of 32 patients with parasitologically confirmed and untreated T.b.g. HAT, 25 patients (7 men and 18 women; mean age, 33.8 ± 15.5 years; body mass index, 19.7 ± 3.3 kg/m2) underwent CSF examination. Stage determination was obtained following the recommendations from the 2006 expert meetings cited above. Among the 25 patients, 13 were classified as being at Stage I, 8 at the intermediate stage, and 4 at Stage II (Table 1).
Blood Samples
Blood and CSF samples were taken from each patient between 09:00 and 17:00 in the course of conventional diagnostic procedures. Samples were stored immediately in liquid nitrogen for transportation, then at −80°C until use.
Polysomnographic Recordings
The continuous 24-hour polysomnographic recordings were collected on Vitaport 2 ambulatory systems (Temec, Kerkrade, The Netherlands). Traces were visually analyzed in 20-second epochs according to standard criteria13 using the PRANA software (PhiTools, Strasbourg, France). Because 5 patients had been previously treated and 1 patient had died from cerebral malaria before examination, polysomnographic recordings were considered in 19 patients, but only 17 traces were readable (Table 2). Sleep-wake disruptions were analyzed as previously proposed 8,9: (0) undisturbed sleep-wake distribution, sleeping at night and staying awake during the day; (1) fragmented sleep with normal day-night (nychthemeral) distribution, with conserved higher nighttime sleep than daytime sleep; and (2) major disturbance of the sleep-wake cycle (circadian dysrhythmia: sleep and wake episodes alternating throughout the 24-hour cycle, with nearly the same amount of sleep time during daytime and nighttime). The number of SOREMPs (refer to any sleep onset in which REM sleep occurs within a lapse of 0 to 15 minutes after the onset of sleep) in each sleep episode was noted. REM sleep latency was determined to be the time of the first epoch of sleep in the sleep episode (2 different sleep episodes separated by at least 15 minutes of uninterrupted wakefulness) to the beginning of the first epoch of REM sleep (defined as more than 15 seconds of REM sleep in a 30-second epoch) regardless of the intervening stages of sleep or wakefulness.14
Table 2.
Individual Data Obtained in the 17 Patients with Completed Polysomnography
| Patient | CSF cells/μL | CSF T+ | Stage from CSF | EDS | Clinical stage | CSF hypocretin-1 pg/mL | Sleep-wake fragmentation | SOREMP (number) |
|---|---|---|---|---|---|---|---|---|
| SC12 | 1 | No | I | Yes | I | 0 | 2 | 3 |
| SC3 | 179 | Yes | II | Yes | II | 194 | 2 | 4 |
| SC6 | 490 | Yes | II | Yes | II | 379 | 2 | 5 |
| SC7 | 0 | No | I | No | I | 402 | 2 | 5 |
| SC18 | 1 | No | I | No | I | 548 | 2 | 3 |
| SC2 | 4 | Yes | II | No | I | 287 | 2 | 3 |
| SC1 | 7 | No | IS | No | I | 499 | 1 | 2 |
| SC5 | 1 | No | I | No | I | 468 | 1 | 1 |
| SC9 | 11 | No | IS | No | IS | 492 | 1 | 1 |
| SC28 | 2 | No | I | No | IS | 351 | 1 | 1 |
| SC4 | 22 | Yes | II | No | II | 422 | 0 | 0 |
| SC11 | 2 | No | I | No | I | 420 | 0 | 0 |
| SC14 | 3 | No | I | No | I | 489 | 0 | 0 |
| SC21 | 1 | No | I | No | I | 508 | 1 | 0 |
| SC27 | 18 | No | IS | No | IS | 388 | 0 | 0 |
| SC29 | 9 | No | IS | No | IS | 532 | 1 | 0 |
| SC30 | 1 | No | I | No | I | 488 | 1 | 0 |
CSF: cerebrospinal fluid; T+: presence of trypanosomes in the CSF; EDS: excessive daytime sleepiness; IS intermediate stage; Sleep-wake fragmentation: 0, non fragmented sleep with or without daytime nap; 1, fragmented sleep with normal day/night (nychthemeral) distribution; 2: major disturbance of the sleep-wake cycle (polyphasic sleep).
Hypocretin Radioimmunoassay
CSF hypocretin-1 (orexin-A) level was determined in duplicate from CSF samples in all patients without prior extraction using [125I] radioimmunoassay kits (Phoenix Peptide, Phoenix Pharmaceuticals, Inc., Belmont, CA) in accordance with the manufacturer's instructions. The detection limit was 40 pg/mL, and intraassay variability was less than 10%. CSF hypocretin-1 levels below 110 pg/mL were considered as low, intermediate between 110 and 200 pg/mL and normal over 200 pg/mL, as already reported in Mignot et al.15 Results were compared to available samples from our Reference centre from narcoleptic patients with cataplexy (n=26) but also to normal values obtained from CSF from patients with neurological diseases known not to affect the hypocretin system (neurodegenerative, inflammatory and vascular disorders; n= 53), after duplicating the measure with the Leiden reference centre. All values were finally back-referenced to Stanford reference samples.
Statistical Analysis
Statistical analyses were performed using one-way analysis of variance (ANOVA) or χ2 test, depending on data distributions to compare CSF hypocretin levels among T.b.g. HAT patients at different stages, and between HAT patients, narcoleptic patients and other non infectious neurological disorders. Contrast analyses were carried out with Tukey HSD test. Data are reported as mean ± standard deviation (SD). Statistical significance was set at P < 0.05.
RESULTS
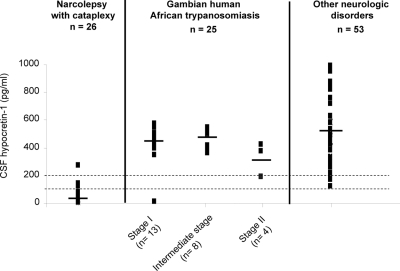
Mean (± SD) CSF hypocretin-1 level in T.b.g. HAT was 421.5 ± 123.4 pg/mL (n= 25, extreme 0 to 579 pg/mL), without statistical differences between stage evolution (Fig. 1, Table 1). CSF hypocretin-1 levels in HAT patients were significantly different from narcolepsy with cataplexy patients, 40.16 ± 60.18 pg/mL (n= 26, extreme 0 to 271 pg/mL) and to other various neurological conditions, 517.32 ± 194.5 pg/mL (n= 53, extreme 123 to 996) (P < 0.001) (Fig. 1). Tukey HSD post-hoc analysis revealed that, as a group, HAT patients had higher CSF hypocretin-1 levels than narcoleptic patients (P <0.0001) but lower levels than neurological controls (P < 0.03).
Figure 1.
CSF hypocretin-1 levels among narcolepsy with cataplexy, Trypanosoma brucei gambiense human African trypanosomiasis patients at stage I, intermediate stage and stage II, and other neurological disorders. Each data point represents the crude concentration of CSF hypocretin-1 in one individual patient. The cut-offs for normal (>200 pg/mL) and low (<110pg/mL) hypocretin-1 levels are represented. Mean values are shown as a horizontal bar for each group.
Table 2 gives hypocretin-1 values in 17 patients in whom polysomnographic recordings were readable, as well as a summary of biological, clinical and sleep data for each patient. Only two HAT patients showed abnormal levels of CSF hypocretin-1 (Table 2). A 15 year-old female (SC12) was found with undetectable CSF hypocretin-1 levels. She reported fever, cephalalgia and EDS without any nocturnal insomnia, sleep attacks, clear-cut cataplexy, hypnagogic hallucinations or sleep paralysis. Clinical examination failed to report any pyramidal, extrapyramidal and cerebellar signs, psychiatric symptoms, or episodes of anorexia or bulimia. She was classified as being at stage I according to the presence of only 1 cell/μL in CSF and absence of any trypanosome. The continuous 24-h polysomnography revealed a complete disruption of the circadian occurrence of sleep and wakefulness episodes, and the occurrence of three SOREMPs. Patient SC3, a 27-year old man, showed intermediate CSF hypocretin-1 levels (194 pg/mL). This patient was affected with stage II (179 cells/μL and presence of trypanosomes in the CSF), accompanied by an alteration of general state, fever, cephalalgia, EDS and nocturnal insomnia, sensory and gait disturbances, and confusion. The 24-h polysomnography revealed a severe disruption of the circadian sleep-wake cycle, and the occurrence of four SOREMPs.
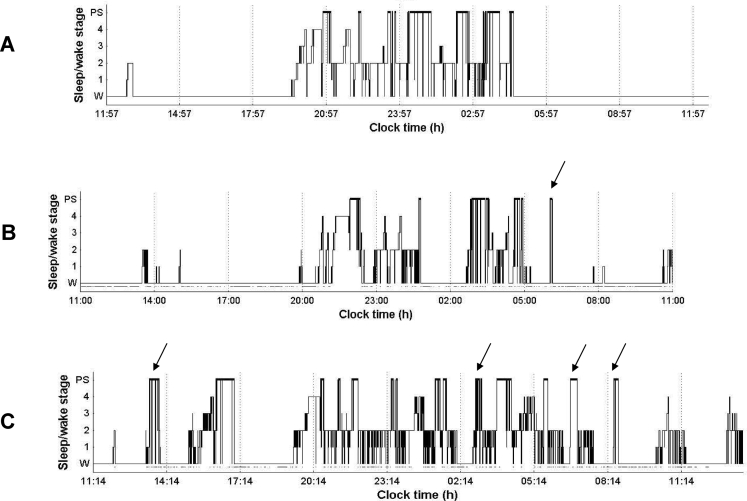
The complaint of EDS occurred in only three patients (Table 2: SC3, SC6, SC12), two of them having low or intermediate CSF hypocretin-1 level, and two being at stage II. The continuous 24-h polysomnographic recordings revealed high sleep fragmentation with nychthemeral disruption in six patients including three patients at stage I and three at stage II (Table 2). Figure 2 represents examples of hypnograms obtained from 24-hour polysomnography of three patients before treatment at three different stages of evolution. Comparisons between sleep-wake pattern groups revealed that patients with severe sleep-wake cycle disruptions (score at 2) had significantly lower CSF hypocretin-1 levels when compared to patients with normal or only mild (score at 0 or 1) sleep-wake cycle abnormalities (mean ± SD at 301.7 ± 77.3 vs 459.8 ± 17.0 pg/mL, P= 0.0068) (Table 2). However, this difference did not hold when patients with abnormal CSF hypocretin-1 levels (SC3 and SC12) were excepted from the statistic.
Figure 2.
Hypnograms obtained from 24-hour polysomnographic recordings in three patients before treatment (W: wakefulness; 1, 2, 3, 4: Stages 1, 2, 3, 4, PS: paradoxical sleep or rapid eye movement sleep, REM sleep): A. Patient S27 diagnosed as being at intermediate stage of HAT with normal sleep patterns. CSF hypocretin-1 level was 388 pg/mL B. Patient SC9 diagnosed as being at intermediate stage with disturbed night sleep patterns, but with a normal nychthemeral distribution. Arrow indicates the presence of one SOREMP. CSF hypocretin-1 level was 492 pg/mL C. Patient SC3 diagnosed as being at stage II with nychthemeral dysrhythmia, abnormal nighttime sleep, and frequent occurrences of sleep onset REM periods among the 24-hour polysomnography. Arrows indicate the presence of SOREMPs. CSF hypocretin-1 level was 194 pg/mL
The occurrence of at least one SOREMP among sleep episodes was noted in 10 patients and at least two SOREMPs in seven. Among the latter patients, two exhibited abnormal CSF hypocretin levels. Although non significant, there was a tendency for CSF hypocretin-1 differences between patients with at least two SOREMPS and those with none or only one SOREMP (mean ± SD at 329.9 ± 188.4 vs 455.8 ± 57.9, respectively, P=0.083).
Considering specifically the occurrence of SOREMP and their relation to CSF cell count staging including stage II (>20 cells/μL of CSF) and stage I (<5 cells/μL), we noted that stage II patients differed significantly from the stage I group, with 3.0 ± 2.2 SOREMPs per 24-h for the former group vs. 1.2 ± 1.6 for the latter group (P=0.039). In this case, CSF hypocretin-1 values were lower even non significant in the stage II group (320.5 ± 101.2 pg/mL) than in stage I patients (424.1 ± 142.6 pg/mL). When patients with abnormal CSF hypocretin-1 results (SC3 and SC12) were excluded, mean values ± SD gave 465.4 ± 61.2 pg/mL in stage I patients vs. 362.7 ± 69.0 pg/mL in stage II patients, the difference becoming significant (P=0.036).
DISCUSSION
As previously hypothesized, polysomnography may represent a diagnostic tool to distinguish the two stages of T.b. gambiense HAT.8 The occurrence of SOREMP and/or sleep disruptions are thought to be important to diagnose the CNS involvement, especially in misclassified intermediate or stage I patients, in whom clinical and laboratory findings often fail to determine accurately the passage from stage I to stage II. Despite an extensive immune reaction during the initial hemolymphatic stage, EDS remains classically absent. In addition, only two patients at stage II did report EDS. However, one patient classed as stage I from CSF counts (SC12) complained of EDS and had undetectable CSF hypocretin-1. As this patient had also a highly disturbed polysomnography, it may be that the case could have been misclassified. One may hypothesize that patient SC12 was affected with both T.b. gambiense HAT and narcolepsy. However, the occurrence of both conditions concomitantly remains improbable, due to the rarity of the narcolepsy disorder, the absence of positive history of EDS before the HAT onset, and the absence of other features of narcolepsy such as sleep attacks, clear-cut cataplexy, hypnagogic hallucinations, sleep paralysis, obesity or parasomnias. In addition, no clear clinical or polysomnographic differences except the severe EDS allows to distinguish patient SC12 from other HAT patients of the present study. Despite the absence of HLA typing and the absence of possibility to interview the patient after treatment, we may finally assume that the risk of this patient to be concomitantly affected with HAT and hypocretin deficient narcolepsy was low. Another patient reporting EDS (SC3), who was at stage II and showed an intermediate value in CSF hypocretin-1 level, also had a disturbed polysomnography. However, the other stage II patient reporting EDS (SC6) and with disturbed polysomnography did not have any impairment in hypocretin-1 secretion.
Since the description of SOREMPs in patients with narcolepsy, narcolepsy has often been regarded as a disorder of REM sleep generation.11,16, However, SOREMPs also exist in other rare sleep disorders and in normal adults as recently reported in a large community-based study with high body mass index (BMI) and frequent antidepressants intake.17 In the present study, BMI was quite low and none of the patients was treated with antidepressants. In the present study, SOREMPs and sleep fragmentation were observed especially in stage II patients, although one of them (SC4) had no abnormal sleep patterns. Furthermore, three patients among 13 at stage I or intermediate stage showed disturbed sleep patterns with one SOREMP, and four patients more than 1 SOREMP (Table 2). The present data seem to indicate that the occurrence of one SOREMP may represent a threshold indicating an involvement of CNS dysfunction.
A dramatic decrease in the number of hypocretin-containing neurons of the hypothalamus and undetectable CSF hypocretin-1 level have been reported in most patients with narcolepsy-cataplexy.12,15,18 In addition, a low CSF hypocretin-1 level was highly specific (99.1%) and sensitive (88.5%) for narcolepsy with cataplexy.15,18 Only rare other neurological conditions as encephalopathy, Guillain-Barré in a large proportion of Asian patients and traumatic brain injury are associated with low CSF hypocretin levels.15,18–20 We reported in the present study that CSF hypocretin-1 levels were significantly higher in T.b.g. HAT patients than in narcoleptic patients with cataplexy but lower than in the patients with other neurological conditions. We may note that our neurological controls but also T.b.g. HAT patients present higher CSF hypocretin-1 levels when compared to values of normal healthy subjects,15 findings in relationship with the use of different CSF samples and with the high interassay variability of the RIA kit. However, we measured the CSF samples at different concentrations for the correct determination of CSF hypocretin-1 levels and all CSF hypocretin values reported in the present study were transformed to the Stanford reference values. A dysfunction of the hypothalamic hypocretin region may be involved in T.b.g. HAT especially in cases of sleep disturbances. Hence, two of the three patients with a complaint of EDS had abnormal CSF hypocretin-1 level, including one patient with undetectable level being an exceptional condition in absence of narcolepsy-cataplexy. We also found that the presence of major sleep-wake cycle disruptions was significantly associated with lower CSF hypocretin-1 level, with a same tendency for the presence of SOREMPs. One may also hypothesize that nocturnal sleep-wake disturbances were caused by other non-specific symptoms frequently reported in HAT patients such as pain and headache, and not by the disease process per se. However, our results do not favor this hypothesis and previous studies in HAT revealed that nocturnal sleep-wake disturbances were correlated to HAT stages evolution.8,9 Furthermore, previous reports observed no differences in sleep patterns of stage II HAT patients whether they were recorded with or without an intravenous catheter during two consecutive randomly assigned twenty-four hour polysomnographic recordings.21 Overall, the present investigation is not in favor of the direct and/or unique implication of the hypocretin system in T.b.g. HAT.
Localizations of CNS lesions are unclear in HAT while CT-scan, MRI and/or autopsy have not reported any systematized neuropathological alterations so far.22,23 Recent MRI findings in the meningoencephalitic stage show unspecific and wide lesions, involving bilateral and central lesions in the deep white of the internal and external capsule, cerebellum, splenium of the corpus callosum but also in the gray matter of basal ganglia and cerebral cortex.22–23 Autopsy studies in patients who have died from stage II HAT revealed a meningoencephalitis with a diffuse white matter infiltration with perivascular cuffing of lymphocytes.24 None of those studies have reported an infiltration of the hypothalamus region but this region has not been extensively studied in HAT.
One question addressing the presence of cataplexy in our population remains uncertain. In our experience, an abrupt loss of cervical muscle tone in stage II HAT patients is often observed. However, the triggering of such abrupt events by emotional factors has not been established mainly due to language difficulties and culture differences, and the potential association of cataplexy to the “narcolepsies of sleeping sickness”10 remains unclear. Old literature in the field noted the existence of a cataplectic form of HAT with atonia of striated muscles in late-stage of the disorder.10 Furthermore, no HLA DQB1*0602 typing, associated to narcolepsy-cataplexy has ever been analyzed from DNA samples of HAT patients.
As stage II patients are generally treated with melarsoprol, an arsenate that may provoke a deadly reactive encephalopathy,2,9 a precise determination of the HAT evolution stage is therefore crucial. The reversibility of the sleep–wake cycle and sleep structure alterations after appropriate treatment constitutes the basis of an evaluation of the healing process.9 However, if the association of polysomnography to help avoiding misdiagnosed stages and/or post-treatment follow-up in HAT patients is of interest, there is no clear indication to date that hypocretin-1 analysis would help in this context even in the presence of EDS and/or major sleep-wake cycle disruptions. In addition, normal CSF hypocretin levels do not necessarily imply a normal hypocretin function/cell count 25,26 and dysfunction of other aspects of the hypocretin system such as hypocretin receptor 1 or 2, or involving another neuromodulating system needs to be elucidated. Hence, the hypothalamic involvement in the occurrence of SOREMPs and sleep disruptions may result from a complex interaction between hypocretin and prolactin neurons in HAT, as hypoprolactinemic rats present SOREMPs.27 An increased CSF prostaglandin D2 levels has also been reported in HAT patients and may be another possible candidate for sleep-wake disturbances in this disorder.28 Another hypothesis linking the hypothalamic dysfunction to that of serotoninergic pontine networks, which are deeply involved in sleep mechanisms especially regarding REM sleep triggering, has been forwarded.29 Hence, a previous study has also shown a degeneration of serotonin-specific neurons in experimental infection of mice by Trypanosoma brucei rhodesiense.30
In conclusion, the present investigation is not in favor of an unique implication of the hypocretin system in T.b.g. HAT, but dysfunction of the hypothalamic hypocretin region cannot be discarded regarding the observed sleep disturbances. Further studies that focus on sleep, SOREMPs, the presence of clear-cut cataplexy, HLA DQ typing and CSF hypocretin-1 and prolactin measurements before and after melarsoprol treatment in HAT patients are warranted.
ACKNOWLEDGMENTS
This investigation received financial support from the UNICEF / UNDP / World Bank / WHO Special Programme for Research and Training in Tropical Diseases (TDR, Collaborative Research Project N° A50468 “Polysomnography, electrochemistry, immunology – neuroanatomy to the diagnosis of human African trypanosomiasis”, 2006) and World Health Organization Technical Services Agreement # T7/83/2 Support in research on “human African trypanosomiasis (HAT) polysomnographic syndrome, of orexins and nitric oxide (NO) as potential diagnostic tools” (2005). We are grateful to G. L. Lammers for his precious help in the CSF hypocretin-1 measurements.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no conflicts of interest.
REFERENCES
- 1.Barrett MP. The rise and fall of sleeping sickness. Lancet. 2006;367:1377–8. doi: 10.1016/S0140-6736(06)68591-7. [DOI] [PubMed] [Google Scholar]
- 2.Bouteille B, Oukem O, Bisser S, Dumas M. Treatment perspectives for human African trypanosomiasis. Fundam Clin Pharmacol. 2003;17:171–81. doi: 10.1046/j.1472-8206.2003.00167.x. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Report of a WHO Expert Committee. Technical Report Series n° 881. Geneva, Switzerland: 1998. Control and Surveillance of African Trypanosomiasis; p. 114. [PubMed] [Google Scholar]
- 4.Simarro PP, Ruiz JA, Franco JR, Josenando T. Attitude towards CATT-positive individuals without parasitological confirmation in the African trypanosomiasis (T.b. gambiense) focus pf Quicama (Angola) Trop Med Int Health. 1999;4:858–61. doi: 10.1046/j.1365-3156.1999.00494.x. [DOI] [PubMed] [Google Scholar]
- 5.Bisser S, Lejon V, Preux PM, Bouteille B, Stanghellini A, Jauberteau MO, B̈uscher P, Dumas M. Blood-cerebrospinal fluid barrier and intrathecal immunoglobulins compared to field diagnosis of central nervous system involvement in sleeping sickness. J Neurol Sci. 2002;193:127–35. doi: 10.1016/s0022-510x(01)00655-4. [DOI] [PubMed] [Google Scholar]
- 6.Anonymous. Development and evaluation of new diagnostic tests for human African trypanosomiasis. Wkly Epidemiol Rec. 2006;81:59–60. [PubMed] [Google Scholar]
- 7.Steverding D. A new initiative for the development of new diagnostic tests for human African trypanosomiasis. Kinetoplastid Biol Dis. 2006;5:1–3. doi: 10.1186/1475-9292-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buguet A, Bisser S, Josenando T, Chapotot F, Cespuglio R. Sleep structure: a new diagnostic tool for stage determination in sleeping sickness. Acta Trop. 2005;93:107–17. doi: 10.1016/j.actatropica.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Buguet A, Bourdon L, Bouteille B, Cespuglio R, Vincendeau P, Radomski MW, Dumas M. The duality of sleeping sickness: focusing on sleep. Sleep Med Rev. 2001;5:139–53. doi: 10.1053/smrv.2000.0130. [DOI] [PubMed] [Google Scholar]
- 10.Lhermitte J. XX° congrès des médecins aliénistes et neurologistes de France et des pays de langue française. Bruxelles; 1910. La maladie du sommeil et les narcolepsies; pp. 6–31. [Google Scholar]
- 11.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369:499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 12.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 13.Rechtschaffen A, Kales A. A Manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, D.C.: NIH publications n°204, Public Health Service, U.S. Govt. P.O; 1968. [DOI] [PubMed] [Google Scholar]
- 14.Littner MR, Kushida C, Wise M, et al. Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 15.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 16.Dauvilliers Y, Billiard M, Montplaisir J. Clinical aspects and pathophysiology of narcolepsy. Clin Neurophysiol. 2003;114:2000–17. doi: 10.1016/s1388-2457(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 17.Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the Multiple Sleep Latency Test in community adults. Brain. 2006;129:1609–23. doi: 10.1093/brain/awl079. [DOI] [PubMed] [Google Scholar]
- 18.Dauvilliers Y, Baumann CR, Carlander B, et al. CSF hypocretin-1 levels in narcolepsy, Kleine-Levin syndrome, and other hypersomnias and neurological conditions. J Neurol Neurosurg Psychiatry. 2003;74:1667–73. doi: 10.1136/jnnp.74.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishino S, Kanbayashi T. Symptomatic narcolepsy, cataplexy and hypersomnia, and their implications in the hypothalamic hypocretin/orexin system. Sleep Med Rev. 2005;9:269–310. doi: 10.1016/j.smrv.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti CL. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007;130:1873–83. doi: 10.1093/brain/awm109. [DOI] [PubMed] [Google Scholar]
- 21.Buguet A, Bert J, Tapie P, et al. Sleep-wake cycle in human African trypanosomiasis. J Clin Neurophysiol. 1993;10:190–6. doi: 10.1097/00004691-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Sabbah P, Brosset C, Imbert P, Bonardel G, Jeandel P, Briant JF. Human African trypanosomiasis: MRI. Neuroradiology. 1997;39:708–10. doi: 10.1007/s002340050491. [DOI] [PubMed] [Google Scholar]
- 23.Braakman HM, van de Molengraft FJ, Hubert WW, Boerman DH. Lethal African trypanosomiasis in a traveller: MRI and neuropathology. Neurology. 2006;66:1094–6. doi: 10.1212/01.wnl.0000209306.41647.13. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy PG. Diagnostic and neuropathogenesis issues in human African trypanosomiasis. Int J Parasitol. 2006;36:505–12. doi: 10.1016/j.ijpara.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Gerashchenko D, Murillo-Rodriguez E, Lin L, et al. Relationship between CSF hypocretin levels and hypocretin neuronal loss. Exp Neurol. 2003;184:1010–6. doi: 10.1016/S0014-4886(03)00388-1. [DOI] [PubMed] [Google Scholar]
- 26.Fronczek R, Overeem S, Lee SY, et al. Hypocretin (orexin) loss in Parkinson's disease. Brain. 2007;130:1577–85. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- 27.Roky R, Obal F, Valatx JL, Bredow S, Fang J, Pagano L, Krueger JM. Prolactin and rapide eye movement sleep regulation. Sleep. 1995;18:536–42. [PubMed] [Google Scholar]
- 28.Pentreath VW, Rees K, Owolabi OA, Philip KA, Doua F. The somnogenic T lymphocyte suppressor prostaglandin D2 is selectively elevated in cerebrospinal fluid of advanced sleeping sickness patients. Trans R Soc Trop Med Hyg. 1990;84:795–9. doi: 10.1016/0035-9203(90)90085-s. [DOI] [PubMed] [Google Scholar]
- 29.Buguet A. Is sleeping sickness a circadian disorder? The serotonergic hypothesis. Chronobiol Int. 1999;16:477–89. doi: 10.3109/07420529908998722. [DOI] [PubMed] [Google Scholar]
- 30.Ormerod WE, Hussein MSA. The ventricular ependyma of mice infected with Trypanosoma brucei. Trans Roy Soc Trop Med Hyg. 1986;80:626–33. doi: 10.1016/0035-9203(86)90161-6. [DOI] [PubMed] [Google Scholar]