Abstract
Study Objectives:
To examine (1) the prevalence of home-monitored sleep-disordered breathing (SDB) and obstructive sleep apnea syndrome in a Japanese working population and (2) whether home monitoring with a type 3 portable monitor and actigraphy can produce reliable data to analyze SDB in usual lifestyles.
Methods:
A cross-sectional survey using a self-administered questionnaire was conducted on a group of employees at a wholesale company in Osaka, Japan. Examinations by physicians and by sleep monitoring were also performed. Unattended home cardiorespiratory (type 3) sleep studies with actigraphy were conducted for 2 nights to diagnose SDB in 322 subjects. From the baseline questionnaires and sleep diaries, participants were assessed to follow their usual lifestyles during the study (e.g., time in bed, alcohol intake).
Results:
Of 466 Japanese male employees, 396 responded to the questionnaire survey (85.0%). Results from 322 male employees aged 23 to 59 (43.8 ± 8.4 years) were analyzed. Respiratory disturbance index (RDI), calculated from the type 3 portable monitors and actigraphy, was highly reliable with an intraclass correlation of 0.98 for interscorer reliability and with an intraclass correlation of 0.95 for night-to-night reliability. Prevalence of mild (5 ≤ RDI < 15), moderate (15 ≤ RDI < 30) and severe (RDI ≥ 30) SDB in this population were 37.4%, 15.7%, and 6.6%, respectively. The prevalence of obstructive sleep apnea syndrome (RDI ≥ 5 and Epworth Sleepiness Scale score > 10) was 17.6%.
Conclusions:
The prevalence of moderate to severe SDB (RDI ≥ 15) was 22.3% in this Japanese male working population aged 23 to 59, measured in participant's usual life settings. Unattended home monitoring with type 3 portable monitors and actigraphy was highly reliable and may be suitable for analyzing SDB in the usual lifestyle setting.
Citation:
Nakayama-Ashida Y; Takegami M; Chin K; Sumi K; Nakamura T; Takahashi K; Wakamura T; Horita S; Oka Y; Minami I; Fukuhara S; Kadotani H. Sleep-disordered breathing in the usual lifestyle setting as detected with home monitoring in a population of working men in Japan. SLEEP 2008;31(3):419-425.
Keywords: Sleep apnea, lifestyle, portable monitor, alcohol, body mass index, interscorer reliability, night-to-night variability
SLEEP-RELATED HEALTH PROBLEMS AND THEIR IMPACT ON SOCIETY AND ON INDIVIDUALS ARE A MAJOR SOCIAL ISSUE. PEOPLE WITH SLEEP-DISORDERED breathing (SDB) are at increased risk for hypertension,1,2 cardiovascular diseases,3 and mortality.3,4 However, SDB and obstructive sleep apnea syndrome (OSAS) are prevalent but largely undiagnosed in adults.5 In spite of this wide population of undiagnosed patients, in-laboratory sleep-recording capacity has remained limited. This current situation presents an urgent need to move toward the use of portable monitors in home settings.
Epidemiologic studies for SDB and OSAS have been conducted not only in Western countries,1–5 but also in Asian countries.6,7 However, sound estimates of SDB prevalence in Japan are lacking: previous Japanese studies have been limited by use of nonprobability samples, low response rates, small samples, and other methodologic problems. All previous Japanese epidemiologic studies on SDB and OSAS were performed with type 4 portable monitors.8–10
In our current study, a cardiorespiratory monitoring device (also referred to as a type 3 portable monitoring device) was used together with actigraphy at home to analyze SDB. Type 3 portable monitors are defined as devices with a minimum of 4 channels monitored,11 including ventilation or airflow (at least 2 channels of respiratory movement or respiratory movement and airflow), heart rate or electrocardiogram, and oxygen saturation, whereas portable monitors that do not meet these criteria are designated as type 4.11 Attended type 3 sleep studies are thought to be a standard method and an alternative for polysomnography in the diagnosis and follow-up for SDB and OSAS.12 On the other hand, type 4 portable monitors are not recommended for diagnosis because they have a higher percentage of false-positive results and high rates of false negatives or conflicting results.11
Most epidemiologic studies have been performed with polysomnography in laboratory settings. Using in-laboratory polysomnography as the gold standard has limitations in validity, partly because subjects tend to sleep more poorly in the laboratory than at home.11 In this study, participants were asked to follow their usual lifestyles, and sleep recordings were performed at home. Home monitoring that more closely reflects the subjects' usual drinking and sleeping habits may present a different prevalence or different outcomes than those from previous reports in the laboratory settings.
Recently, obesity has become a major social problem in Japan. The prevalence of obesity (body mass index: [BMI] ≥ 25) in 1983, 1993, and 2003 was 26.7%, 24.5% and 34.4%, respectively, among the Japanese male general population aged 40 to 49 years.13 Obesity and weight gain are positively associated with SDB.14 Thus, SDB may become more prevalent than has been previously reported in Japanese.
The primary goal of this paper was to examine the prevalence of home-monitored SDB and OSAS in a Japanese working population. The secondary goal was to test the hypotheses that home monitoring with a type 3 portable monitor and actigraphy can produce reliable data to analyze SDB in usual lifestyles. Here, we report the first epidemiologic study in Japan with a type 3 portable monitor and actigraphy at home to examine the prevalence and related factors of SDB.
METHODS
Subjects and Study Design
The subjects of this cross-sectional study were employees of a wholesale company. There was a wide range of jobs within this employee group. All the employees who worked in 11 branches of the company spreading over Osaka prefecture in Japan from January 26, 2004, to December 19, 2005, were invited. We visited each workplace and distributed questionnaires to the employees. Out of 476 male employees, 10 subjects were excluded because they changed their workplaces. Out of responders to the questionnaires, those who provided written informed consent underwent sleep tests. Subjects were asked to keep their usual lifestyles during a 1-week survey period. BMI was calculated as weight (minus 1 kg for measured weight of the participants to correct for clothing) divided by the square of height in meters. The study protocol was approved by the Kyoto University Graduate School and Faculty of Medicine Ethics Committee.
Questionnaire
The questionnaires included demographics, the Epworth Sleepiness Scale (ESS),15 and the Pittsburgh Sleep Quality Index (PSQI).16,17 The ESS score was adjusted to compare with the current Japanese version of the ESS.18
Alcohol use was asked in the questionnaire. Frequency of alcohol consumption during the previous year was asked (almost every day, once or twice a week, once or twice a month, only on special occasions, or do not drink). Subjects who answered almost everyday were considered to be habitual drinkers. All the subjects were also asked if they consumed alcohol during the past week. Those consuming alcoholic beverages during the previous week were asked about the types of drinks and the total consumption of each type.
Current smoking habits were asked about in the questionnaire. Smokers were asked their smoking history in years and the number of cigarettes smoked per day. Previous smoking habits were similarly asked about.
Total sleep time and sleep latency data during the previous month were obtained from the PSQI. Subjects who answered snoring every night or often were considered to be habitual snorers. A brief sleep diary was also filled out during the survey period. The diary was used to quantify the participant's daily lifestyle and to know the alcohol use within 2 to 3 hours before bed time. These diary data were used to compare changes in alcohol use and sleep duration between usual lifestyle from the baseline questionnaire and lifestyle during the 1-week survey period.
Examinations and Home Monitoring
Specialists in respiratory medicine, sleep medicine, or both interviewed and examined all the participants at their workplaces. Each subject was asked to wear an actigraph (Actiwatch AW-Light: Mini-Mitter, Bend, Ore.) for 7 days to estimate sleep-wake time19 and a type 3 portable monitor (Morpheus: Teijin, Tokyo, Japan, which is the same as Somté: Compumedics, Victoria, Australia) for 2 nights at home. About half of the participants wore the portable monitors for 2 consecutive nights, and the others wore it 1 night apart. The portable monitors recorded chest and abdominal respiratory movements, nasal pressure, oxygen saturation, heart rate, and body position. The sampling rate for the oxygen saturation was 1 Hz. The portable monitors recorded and stored signals for about 24 hours in a CompactFlash memory card, and the data were transferred to a PC and analyzed later. After being trained twice by specialists in respiratory medicine and sleep medicine, each participant followed written step-by-step instructions and hooked up the monitor by himself at home before going to bed.
Actigraph and Portable Monitor Data Analysis
Sleep duration was estimated from analysis of the wrist actigraph tracing19 in conjunction with a sleep diary. The time at which the participant went to bed and the time he got out of bed were obtained from the diary. Sleep onset was estimated by noting sustained cessation of wrist movement on the actigraphy tracing. The time of awakening was noted by the appearance of wrist movements on the actigraphy tracing. Sleep durations, used for portable-monitor analysis, were the estimated length of time between the sleep onset and the final awakening. Main bouts of comprehensible portable-monitor recordings within the estimated sleep durations were used for portable-monitor analyses, and the length of the comprehensible portable-monitor data was called the “analyzed time length.”
The respiratory disturbance index ([RDI] the number of apneas and hypopneas per hour of the analyzed time length) was calculated from the data of both the actigraphy and the portable monitors. The portable-monitor records were visually inspected and scored by at least 2 medical doctors who specialize in respiratory medicine. Apneas (cessation of breathing for at least 10 seconds) and hypopneas (more than 50% reduction in the amplitude of nasal pressure or respiratory effort associated with a more than 3% reduction in oxyhemoglobin saturation for at least 10 seconds)11 were scored blindly to other information except sleep-wake time estimated by actigraphy. Data without oxygen-saturation signals or incomprehensible recordings were excluded from analysis. Data recorded for less than 2 hours were also excluded because Medicare guidelines require at least 2 hours of documented sleep time.11 When data from both of the 2 recorded nights were available, records from the second night were further analyzed. Subjects with an RDI of 5 to 14.9, 15 to 29.9 and 30 or higher were considered to have mild, moderate, and severe SDB, respectively.
The night-to-night variability was assessed for all of the subjects whose portable-monitor recordings were available for both nights. To evaluate interscorer reliability, recordings from the first half (the first 139 consecutive participants) of the studies were manually scored by another physician who specialized in sleep medicine blinded to RDI scores by the respiratory medicine specialists. These scored results from another physician were used only in the assessment of the interscorer reliability.
Statistical Analysis
Categorical data are presented as proportions, and continuous data are presented as means and standard deviations (SD). The night-to-night variability and interscorer reliability were estimated using the intraclass correlation coefficient (ICC). A Bland-Altman plot was drawn to present the relationship between the mean of 2 individual values and the difference between those values. We analyzed the joint occurrence of SDB and habitual snoring, daytime sleepiness detected by ESS, age, BMI, alcohol use, and current smoking habits. We compared proportions between groups using the χ2 test for trend, and analyzed continuous data between groups with analysis of variance (ANOVA) for trend. A multivariate logistic regression model was constructed to examine the independent associations of the important covariates with moderate to severe SDB. The model included terms for habitual snoring (yes vs no), age (1-year increment), BMI (second, third, or fourth quartile vs first quartile), alcohol use in the recorded nights (drink vs not drink), and current smoker (yes vs no). Statistical significance was defined as a P value less than 0.05. The Bland-Altman plots were drawn using MedCalc version 9.3.1 (MedCalc Software, Mariakerke, Belgium). The other statistical analyses were performed using SAS for Windows release 8.02 (SAS Institute Inc., Cary, NC).
RESULTS
Characteristics of the Subjects
Out of 466 male subjects invited to participate in our survey, 396 subjects answered the baseline questionnaire (85.0%: eligible subjects). Of the questionnaire responders, 322 subjects (69.1%: participants) underwent monitoring with the home-based portable monitors. Participants designated themselves as clerks (4.0%), managers (9.0%), technical engineers (1.2%), salespersons (55.9%), service persons (4.7%), shipping agents or corresponding clerks (11.5%), manufacturers or manual laborers (8.7%), others (1.6%), and not ascertained (3.4%). Amount of self-reported annual income was 1 to 3 million yen (2.2%), 3 to 5 million yen (14.3%), 5 to 7 million yen (53.7%), 7 to 10 million yen (25.5%), more than 10 million yen (2.2%), and not ascertained (2.2%). Characteristics of the participants were presented in Table 1.
Table 1.
Characteristics of the Subjects
| Variable | Participants (n = 322) | Entire eligible sample (n = 396) |
|---|---|---|
| Age | 43.8 ± 8.4 | 44.1 ± 8.4 |
| BMI, kg/m2 | 23.7 ± 3.1 | 23.6 ± 3.0 |
| Current smoker | 175 (54.9) | 218 (55.1) |
| Habitual snorer, everyday or often | 156 (48.9) | 180 (45.5) |
| Habitual drinker, almost everyday | 177 (56.1) | 222 (56.1) |
| Alcohol consumption, g·d−1·kg−1 | 0.5 ± 0.5 | 0.5 ± 0.6 |
| Hypertension | 50 (16.1) | 61 (15.4) |
| Daytime sleepiness, ESS > 10 | 86 (27.6) | 105 (26.5) |
| ESS score | 8.1 ± 4.3 | 8.0 ± 4.3 |
| Total sleep time, h | 5.9 ± 0.9 | 5.9 ± 0.9 |
| Sleep latency, min | 10.8 ± 15.7 | 10.8 ± 14.8 |
| Sleeping pill use, yes | 8 (2.5) | 9 (2.3) |
Data are expressed as mean ± standard deviation or number of subjects (%).
BMI refers to body mass index; ESS, Epworth Sleepiness Scale.
Portable-Monitor Recordings
All of the subjects were asked to wear the portable monitors for 2 nights. Out of 644 sleep recordings, 63 could not be analyzed. One subject refused to perform the second recording. Data from 26 recordings were lost, probably due to technical or mechanical problems. No oxygen-saturation data were obtained in 17 recordings. No comprehensible data were available from in 14 recordings. Five recordings were shorter than 2 hours. Data from only 1 night were analyzed in 29 subjects, and data from both nights were available for analyses in 276 subjects. As a result, data from 305 subjects (94.7%) were used for further analysis; 95.7% (292/305) of these recordings were carried out during the weekday. The mean RDI was 10.6 ± 11.4. The mean analyzed portable-monitor time was 5.4 ± 1.2 hours, and 34.8% of portable-monitor recordings had less than 5 hours of analyzable time. The mean RDI scored by a second physician was 11.1 ± 12.7 in the first consecutive 139 subjects. The interscorer reliability of RDI was excellent between scorers, with an ICC of 0.98 (95% confidence interval [95%CI]: 0.98–0.99).
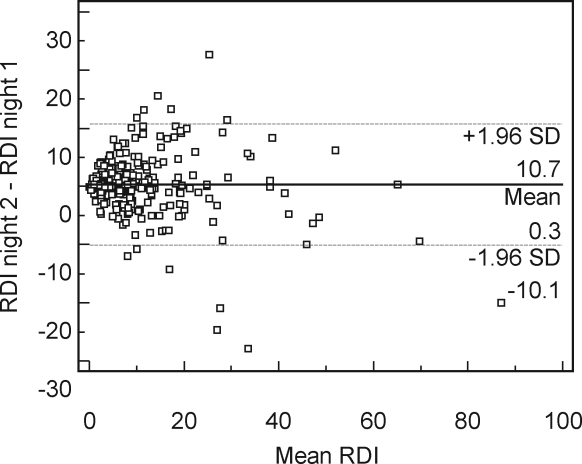
In the 276 subjects whose portable-monitor records were available in 2 nights, mean RDIs of the first and second night were 10.3 ± 12.5 and 10.7 ± 11.5 (P = 0.326, paired t-test), and the ICC was 0.95 (95%CI: 0.94–0.96). Visual inspection of the Bland-Altman plot revealed a low percentage of data points outside of the 95%CI (Figure 1).
Figure 1.
Bland-Altman plot between 2 recorded nights. The difference in Respiratory Disturbance Index (RDI) between first and second recorded nights (RDI in the second night minus RDI in the first night) plotted against the mean of the 2 nights (n = 276). A solid horizontal line indicates the mean value of RDI difference, and the 2 dotted lines indicate the 95% confidence interval (mean difference ± 1.96 SD).
Lifestyles During the 1-Week Survey Period
Participants were asked to follow their usual lifestyles. We used the baseline questionnaires to detect usual lifestyles and the diaries to analyze behaviors during the 1-week survey period. The usual length of time in bed (TIB) calculated from the baseline PSQI questionnaire was 6.0 ± 0.9 hours. The average TIB during the survey period detected from the sleep diary was 6.0 ± 1.1 hours. No significant difference was found in these TIB durations (P = 0.905, paired t-test).
Mean alcohol consumption during the previous week was associated with alcohol-consumption frequency during the previous year. Mean alcohol consumption per body weight among participants who consumed alcohol almost everyday (n = 168), once or twice a week (n = 50), once or twice a month, or only in special occasions (n = 55) and do not drink (n = 19) was 0.743 ± 0.575, 0.200 ± 0.203, 0.069 ± 0.131 and 0.001 ± 0.003 g·day−1·kg−1, respectively (P < 0.001, ANOVA for trend).
Whether or not participants consumed alcohol on the nights of the portable-monitors recording was associated with their usual alcohol habits; 84.7% (144/170), 26.9% (14/52), 5.4% (3/56), and 5.3% (1/19) of participants who consumed alcohol almost everyday, once or twice a week, once or twice a month or only in special occasions, and do not drink took alcohol in the nights of the portable-monitor recording, respectively (P < 0.001, χ2 test for trend); 63.9% (159/249) of participants who consumed and 5.8% (3/52) of those who did not consume alcohol during the previous week had alcohol on the nights of the portable-monitor recording (P < 0.001, χ2 test). Mean alcohol consumption was 0.732 ± 0.595 g·day−1·kg−1 among those who consumed alcohol on the portable-monitor recorded nights and 0.158 ± 0.238 g·day−1·kg−1 among those who did not (P < 0.001, t-test).
SDB Prevalence
The prevalence of mild, moderate, and severe SDB was 37.4%, 15.7%, and 6.6%, respectively (Table 2). Prevalence of OSAS, as defined by an RDI of 5 or higher and an ESS score above 10, was 17.6%.
Table 2.
Prevalence of Sleep-Disordered Breathing by Selected Sleep and Demographic Factors
| Characteristic | Overall | Respiratory Disturbance Index Category |
P value for trend | |||
|---|---|---|---|---|---|---|
| 0–4.9 | 5–14.9 | 15–29.9 | ≥ 30 | |||
| Participants, n (%) | 305 | 123 (40.3) | 114 (37.4) | 48 (15.7) | 20 (6.6) | |
| Snoring, n (%)a | ||||||
| Habitual | 148 | 41 (27.7) | 58 (39.2) | 31 (21.0) | 18 (12.2) | <0.001 |
| Nonhabitual | 154 | 80 (52.0) | 55 (35.7) | 17 (11.0) | 2 (1.3) | |
| ESS score, n (%) * | ||||||
| >10 | 82 | 30 (36.6) | 30 (36.6) | 14 (17.1) | 8 (9.8) | 0.189 |
| ≤10 | 214 | 89 (41.6) | 81 (37.9) | 32 (15.0) | 12 (5.6) | |
| ESS score, mean ± SD | 8.2 ± 4.3 | 8.0 ± 4.1 | 8.1 ± 4.4 | 7.9 ± 4.2 | 9.7 ± 4.8 | 0.406 |
| Age, y, n (%) | ||||||
| 20–39 | 96 | 57 (59.4) | 26 (27.1) | 8 (8.3) | 5 (5.2) | <0.001 |
| 40–49 | 124 | 44 (35.5) | 51 (41.1) | 24 (19.4) | 5 (4.0) | |
| 50–59 | 85 | 22 (25.9) | 37 (43.5) | 16 (18.8) | 10 (11.8) | |
| Age, y, mean ± SD | 44.0 ± 7.9 | 41.1 ± 8.1 | 45.6 ± 8.1 | 46.3 ± 7.0 | 46.7 ± 7.4 | <0.001 |
| BMI quartile, n (%)b | ||||||
| I | 76 | 38 (50.0) | 33 (43.4) | 4 (5.3) | 1 (1.3) | <0.001 |
| II | 76 | 38 (50.0) | 29 (38.2) | 8 (10.5) | 1 (1.3) | |
| III | 76 | 31 (40.8) | 30 (39.5) | 12 (15.8) | 3 (4.0) | |
| IV | 77 | 16 (20.8) | 22 (28.6) | 24 (31.2) | 15 (19.5) | |
| BMI, kg/m2, mean ± SD | 23.7 ± 2.8 | 22.8 ± 2.7 | 23.3 ± 2.9 | 25.7 ± 2.9 | 27.1 ± 2.4 | <0.001 |
| Alcohol use, n (%)c | ||||||
| Yes | 165 | 66 (40.0) | 66 (40.0) | 23 (13.9) | 10 (6.1) | 0.604 |
| No | 140 | 57 (40.7) | 48 (34.3) | 25 (17.9) | 10 (7.1) | |
| Smoking, n (%)a | ||||||
| Current | 169 | 75 (44.4) | 61 (36.1) | 24 (14.2) | 9 (5.3) | 0.063 |
| Never or former | 133 | 46 (34.6) | 52 (39.1) | 24 (18.1) | 11 (8.3) | |
BMI refers to body mass index; ESS, Epworth Sleepiness Scale. Joint occurrence of sleep-disordered breathing (SDB) was analyzed with habitual snoring, daytime sleepiness detected by ESS, age, body mass index (BMI), alcohol use, and current smoking habits. We compared proportions between groups using the χ2 test for trend, and analyzed continuous data between groups with analysis of variance for trend.
Analysis includes 3, 9, and 4 participants with missing snoring, ESS, and smoking information, respectively.
BMI quartiles: I, 16.9–21.4; II, 21.5–23.7; III, 23.8–25.9; IV, 25.9–32.6 kg/m2
Whether or not taking alcohol on the recorded night.
The percentage of subjects with a BMI of 18.5 to 24.9, 25 to 29.9, and 30 kg/m2 or more was 61.0%, 32.8%, and 2.0%, respectively. Six subjects had a BMI of more than 30 kg/m2, but none of them had severe SDB. To further investigate the association between SDB and BMI, we compared SDB severity with BMI in quartiles. As shown in Table 2, subjects with moderate or severe SDB aggregated into the fourth BMI quartile.
SDB severity was significantly associated with habitual snoring, age, and BMI. However, the proportion of subjects with daytime sleepiness was slightly but not significantly increased with SDB severity.
Adjusted odds ratios are presented in Table 3 to show the relationship of potential risk factors with moderate to severe SDB. Snoring, age, and BMI were found to be closely associated with an increased risk of moderate to severe SDB. Results from the unadjusted logistic regression model were basically the same (data not shown).
Table 3.
Independent Associations of Potential Risk Factors with Sleep-Disordered Breathing Estimated by Multivariable Logistic Regression Model
| Model Terms | Odds ratios (95%CI) |
|
|---|---|---|
| Habitual snoring | ||
| No | 1.00 | (reference) |
| Yes | 3.16 | (1.63–6.10) |
| Age | ||
| 1-year increment | 1.06 | (1.02–1.11) |
| BMI quartile | ||
| First | 1.00 | (reference) |
| Second | 2.32 | (0.72–7.51) |
| Third | 4.09 | (1.35–12.38) |
| Fourth | 14.43 | (5.05–41.23) |
| Alcohol usea | ||
| No | 1.00 | (reference) |
| Yes | 1.38 | (0.72–2.63) |
| Current smoker | ||
| No | 1.00 | (reference) |
| Yes | 0.66 | (0.34–1.28) |
Whether or not consuming alcohol on the recorded night. CI refers to confidence interval; BMI, body mass index.
DISCUSSION
This study demonstrates that SDB is prevalent in a Japanese male working population younger than 60 years of age. This is the first report on the prevalence of SDB and OSAS in a Japanese working population detected by a type 3 portable monitor.
Considering the high participation rate of 69.1% (322/466) and no apparent differences in characteristics from the entire eligible samples, our sample does not appear to have self-selection bias and our estimate of the prevalence may well represent that of the whole subject population (i.e., all of the male employees of this company in Osaka prefecture).
From the analysis of sleep time and alcohol use, our subjects seemed to follow their usual lifestyles during the survey. Quantity of alcohol consumption during the previous week and frequency of alcohol intake during the previous year were both significantly associated with alcohol use on the nights on which portable monitoring was recorded. No significant difference was found between the usual duration of the TIB detected by the baseline PSQI questionnaire and the TIB length during the survey periods.
This study demonstrates a high degree of reliability in RDI. The interscorer reliability of RDI in our study is excellent (ICC = 0.98), which is similar to that reported in the Sleep Heart Health Study (SHHS) with home polysomnography (ICC = 0.97 in RDI with 3% desaturation).20 It appears that the manual scoring of RDI with an associated oxygen desaturation can yield highly reliable measures, even for data collected in an unattended setting with a type 3 portable monitors. Night-to-night reliability of RDI was also excellent (ICC = 0.95), which is similar to the previous report with a type 3 portable monitor (ICC = 0.90 in RDI with 4% desaturation).21 Thus, the first-night effect, which was seen in polysomnography, may be small or not present in home recordings using type 3 portable monitors.
The association between severity of SDB and sleepiness was slightly but not significantly stronger among subjects with more severe SDB (Table 2). Sleepiness and SDB have been reported to correlate only weakly, and often inconsistently.12
In this study, alcohol use on the recorded night and current smoking habits were not associated with SDB severity (Table 2). Thus, results from this study are generalizable to other populations of employed Japanese men, regardless of smoking and drinking habits. Our results might be typical of a Japanese male working population, rather than only the specific company.
Some epidemiologic studies have used only records that were more than 4 to 5 hours in length for examination.22,23 However, the mean length of time analyzed by portable monitors in this study was 5.4 ± 1.2 hours, and more than one third of the subjects had less than 5 hours of analyzed time. If sleep monitoring records of less than 5 hours in duration had been discarded, remaining records might not have been representative of this population.
We compared the effects of BMI and obesity on SDB. When Caucasians and East Asians were compared under the same BMI cutoffs, East Asians were found to have a higher prevalence of SDB in nonobese subjects.24 Recently, BMI has been reported to be closely associated with SDB in East Asians6 as well as in Caucasians.14 However, this association has not been well studied in the nonclinical population in Japan.
In 2003, the general Japanese male population in the following age ranges of 20 to 29, 30 to 39, 40 to 49, and 50 to 59 years was reported to have a mean BMI of 22.10 ± 3.09, 23.76 ± 3.72, 23.95 ± 3.10, and 23.81 ± 3.05 kg/m2, and an obesity percentage (BMI ≥ 25) of 14.8%, 32.7%, 34.4%, and 30.9%, respectively.13 Mean BMI and age in this study were 23.7 ± 3.1 kg/m2 and 43.8 ± 8.4 years. The proportion of BMI of 18.5 to 24.9, 25 to 29.9, and 30 kg/m2 or more in this population was 61.0%, 32.8% and 2.0%, respectively. Thus, there seemed to be not much of a difference in the mean BMI and the prevalence of obesity between our population and the general Japanese male population.
In the Sleep Heart Health Study in the United States, the proportion of subjects with a BMI of 18 to 24.9, 25 to 29.9 and 30 kg/m2 or more was 26.5%, 40.8% and 32.8%, respectively.2 The mean BMI in the Sleep Heart Health Study was 28.5 kg/m2. This suggests that there is an apparent difference in BMI distribution between these populations.
To analyze the effects of BMI distribution on SDB, we compared SDB severity with BMI quartiles (Table 2). The prevalence of moderate to severe SDB was 6.6%, 11.8%, 19.7%, and 50.6% in the first, second, third and fourth BMI quartiles in our population, whereas that of the Sleep Heart Health Study was 10%, 13%, 17%, and 32%,14 respectively. Significantly more subjects with moderate to severe SDB were found in the fourth quartile than in the first quartile, especially in our population (Table 3). Not only craniofacial anatomy,24 but also capacity to gain weight, may be different between these populations. Once weight is gained to a certain percentile, subjects may have an increased risk of SDB independent of their ethnicity.
It was reported that SDB and OSAS are prevalent but largely undiagnosed in adults.5 In fact, from the baseline questionnaire, only 1 subject had been diagnosed with OSAS or SDB prior to our study. However, the prevalence of moderate to severe SDB was more than 20%. Considering the mortality rate elevated by severe SDB in male subjects younger than 50 years old25 and the high mortality rate in populations with untreated or insufficiently treated severe SDB,3, 4 the screening and diagnosis for SDB and OSAS is urgently needed.
Although the unattended setting has not been recommended for clinical diagnoses, the unattended type 3 portable monitors are probably most widely used in settings in which polysomnography is not available.11 An unattended type 3 portable monitor has been reported to provide a valid RDI when manually scored.26 We have not performed polysomnography to verify this portable monitoring device, but this device has been reported to be capable of accurately measuring a wide range of AHI,27 to have a high specificity and sensitivity in comparison with polysomnography,28 and to provide sufficient quality data to diagnose SDB in unattended home settings.29 This device has been used as an alternative to polysomnography in both clinical30 and research circumstances.31
Considering our high participation rate (69.1%: 322/466), low failure rate (5.3%: 17/322) in sleep monitoring, and excellent interscorer and night-to-night reliability (ICC = 0.98 and 0.95, respectively), the setting of home monitoring with a type 3 portable monitor and actigraphy is suitable for screening SDB.
The study company provided an ideal site to collect data: it had a sufficient number of full-time non–shift-working male employees working at the same industry and employer, which allowed us to control for work-environment factors such as occupational participation, employment sector, and employment policy. It has been reported that 58.5% of the Japanese population lives in the 7 largest metropolitan areas of Japan32 and that 67.2% of Japanese workers are employed in the tertiary sector of industry (also known as the service sector or the service industry).33 Thus, approximately 40% of Japanese workers may be in the tertiary sector of industry in the metropolitan areas, in which our study population was included. The mean time interval between completion of the baseline questionnaire and portable-monitor recordings was 19.0 ± 17.0 days. The purpose of this study is to monitor SDB status in usual lifestyles, and so we decided to choose the unattended setting and to use actigraphy for estimating sleep-wake time.
In summary, we have performed the first Japanese epidemiologic study of sleep using a type 3 portable monitor and actigraphy. The prevalence of moderate to severe SDB (RDI ≥ 15) was 22.3% in this Japanese male working population aged 23 to 59 years, measured in participants' usual life settings. Considering the alcohol use and sleep durations during the survey period, participants followed their usual lifestyles. Our study had excellent interscorer reliability, excellent night-to-night reliability, a high participation rate, and a low failure rate. Thus, unattended home monitoring with a type 3 portable monitor and an actigraph may be suitable for analyzing SDB in the usual lifestyle setting and for screening for SDB.
ACKNOWLEDGMENTS
This work was supported by Special Coordination Funds for Promoting Science and Technology; grants in aid from Ministry of Health, Labor and Welfare of Japan; and research grants from PRESTO JST, Suzuken Memorial Foundation, Takeda Science Foundation, Mitsui Life Social Welfare Foundation, Chiyoda Kenko Kaihatsu Jigyodan Foundation, and Health Science Center Foundation. We are grateful to the participants, their family, and their company. We thank Dr. Ryan K. Louie and Dr. Go Ashida for helpful comments with this manuscript and Dr. Satoshi Morita and Dr. Kanako Arai for their help in this study.
This work was performed at Horizontal Medical Research Organization, Graduate School of Medicine, Kyoto University, Kyoto, Japan.
This was not an industry-supported study. Dr. Minami has received a research grant from Suzuken memorial Foundation. Ms. Nakayama-Ashida, Ms. Takegami, Drs. Chin, Sumi, Nakamura, Takahashi, and Wakamura, Ms. Horita, Drs. Oka, Fukuhara, and Kadotani have indicated no financial conflicts of interest.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no conflicts of interest.
REFERENCES
- 1.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 2.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 4.He J, Kryger MH, Zorick FJ, et al. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest. 1988;94:9–14. [PubMed] [Google Scholar]
- 5.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, In K, Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170:1108–13. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 7.Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119:62–9. doi: 10.1378/chest.119.1.62. [DOI] [PubMed] [Google Scholar]
- 8.Kayukawa Y, Okada T. Prevalence, age and gender of obstructive sleep apnea syndrome. Biomed Therap. 1996;30:55–8. [Google Scholar]
- 9.Hida W, Shindoh C, Miki H, et al. Prevalence of sleep apnea among Japanese industrial workers determined by a portable sleep monitoring system. Respiration. 1993;60:332–7. doi: 10.1159/000196231. [DOI] [PubMed] [Google Scholar]
- 10.Tanigawa T, Tachibana N, Yamagishi K, et al. Usual alcohol consumption and arterial oxygen desaturation during sleep. JAMA. 2004;292:923–5. doi: 10.1001/jama.292.8.923-b. [DOI] [PubMed] [Google Scholar]
- 11.Chesson AL, Jr, Berry RB, Pack A. Practice parameters for the use of portable monitoring devices in the investigation of suspected obstructive sleep apnea in adults. Sleep. 2003;26:907–13. doi: 10.1093/sleep/26.7.907. [DOI] [PubMed] [Google Scholar]
- 12.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 13.Ministry of Health, Labour and Welfare. Results of physical examination pp 149–184. [Accessed on: October 17, 2007];Report of National Health and Nutrition Survey 2003. Available at: http://www.mhlw.go.jp/bunya/kenkou/eiyou-chosa2-01/index.html.
- 14.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: The Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 15.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 16.Buysse DJ, Reynolds CF, Monk TH, 3rd, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 17.Doi Y, Minowa M, Uchiyama M, et al. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000;97:165–172. doi: 10.1016/s0165-1781(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 18.Fukuhara S, Takegami M, Suzukamo Y, et al. The Japanese version of the Epworth Sleepiness Scale (JESS) J Jap Respir Soc. 2006;44:896–8. [Google Scholar]
- 19.Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–41. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 20.Whitney CW, Gottlieb DJ, Redline S, et al. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21:749–57. doi: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 21.Stepnowsky CJ, Jr, Orr WC, Davidson TM. Nightly variability of sleep-disordered breathing measured over 3 nights. Otolaryngol Head Neck Surg. 2004;131:837–43. doi: 10.1016/j.otohns.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]
- 23.Kadotani H, Kadotani T, Young T, et al. Association between apolipoprotein E epsilon4 and sleep-disordered breathing in adults. JAMA. 2001;285:2888–90. doi: 10.1001/jama.285.22.2888. [DOI] [PubMed] [Google Scholar]
- 24.Li KK, Kushida C, Powell NB, et al. Obstructive sleep apnea syndrome: a comparison between far-east Asian and white men. Laryngoscope. 2000;110:1689–93. doi: 10.1097/00005537-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Lavie P, Lavie L, Herer P. All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J. 2005;25:514–20. doi: 10.1183/09031936.05.00051504. [DOI] [PubMed] [Google Scholar]
- 26.Quintana-Gallego E, Villa-Gil M, Carmona-Bernal C, et al. Home respiratory polygraphy for diagnosis of sleep-disordered breathing in heart failure. Eur Respir J. 2004;24:443–8. doi: 10.1183/09031936.04.00140603. [DOI] [PubMed] [Google Scholar]
- 27.Cunnington D, Menagh J, Cherry G, et al. Comparison of full in laboratory polysomnography to a portable sleep data acquisition device. Am J Respir Crit Care Med. 2003;167:A405. [Google Scholar]
- 28.Kondo T, Fukushima K, Iga T, et al. Sensitivity and specificity of automated portable device for diagnosing sleep apnea syndrome (Morphase) Respir Circ. 2004;52:427–31. [Google Scholar]
- 29.Menagh J, Cherry G, Cunnington D, et al. The utility of unattended, at home diagnostic sleep studies using a new portable sleep data acquisition device. Am J Respir Crit Care Med. 2003;167:A406. [Google Scholar]
- 30.Ng DK, Kwok KL, Chow PY. Diagnostic Access for Sleep Apnea in Hong Kong. Am J Respir Crit Care Med. 2004;170:196. doi: 10.1164/ajrccm.170.2.952. [DOI] [PubMed] [Google Scholar]
- 31.Sasayama S, Izumi T, Seino Y, et al. Effects of nocturnal oxygen therapy on outcome measures in patients with chronic heart failure and cheyne-stokes respiration. Circ J. 2006;70:1–7. doi: 10.1253/circj.70.1. [DOI] [PubMed] [Google Scholar]
- 32.Kanemoto Y, Tokuoka K. Proposal for the Standards of Metropolitan Areas of Japan. J Appl Regional Sci. 2002;7:1–15. [Google Scholar]
- 33.Ministry of Internal Affairs and Communication. The 2005 Population Census. [Accessed on: October 17, 2007]; Available at: http://www.stat.go.jp/english/data/kokusei/e_cen_en.htm.