Abstract
Study Objectives:
To determine if systemic administration of selected sedative-hypnotics that modulate the function of the γ-amino-butyric acid-A (GABAA) receptor can: (i) delay arousal thereby allowing genioglossus (GG) activity to increase more in response to respiratory stimulation during sleep, (ii) also cause the robust increase in GG activity during undisturbed sleep recently observed with barbiturates. We also determined effects on GG activity with local application to the hypoglossal motor nucleus (HMN).
Design, Participants, and Interventions:
Sleep-wake states, GG and diaphragm activities were recorded in freely-behaving rats after systemic administration of lorazepam (0.5 mg/kg and 1 mg/kg, n = 9 and 5 mg/kg, n = 7), zolpidem (5 mg/kg and 10 mg/kg, n = 6) and the antihistamine diphenhydramine (20 mg/kg, n = 9). Rats were also exposed to ramp increases in inspired CO2 in NREM sleep. The effects of lorazepam and zolpidem applied directly to the HMN were also determined in 37 anesthetized rats.
Measurements and Results:
Lorazepam, zolpidem and diphenhydramine all increased arousal threshold, consistent with their sedative action. GG activity before arousal in response to hypercapnia was increased with lorazepam and zolpidem only, an effect mainly due to increased baseline activity before CO2 stimulation. Lorazepam and zolpidem applied directly to the HMN, however, decreased GG activity.
Conclusions:
Lorazepam and zolpidem have an inhibitory effect on GG activity via local effects at the HMN. Following systemic administration, however, this inhibitory effect can be outweighed both by a delay in arousal (allowing greater CO2-mediated respiratory stimulation in sleep) and excitatory influences on baseline GG activity via mechanisms operating outside the HMN.
Citation:
Park E; Younes M; Liu H; Liu X; Horner RL. Systemic vs. Central administration of common hypnotics reveals opposing effects on genioglossus muscle activity in rats. SLEEP 2008;31(3):355-365.
Keywords: Respiratory control, genioglossus muscle, sedatives, GABA-A receptor, pharyngeal muscles, obstructive sleep apnea, control of breathing
APPROXIMATELY 2.5% OF NORTH AMERICAN ADULTS USE PRESCRIPTION HYPNOTICS, AND 3% USE OVER-THE-COUNTER SLEEP-PROMOTING AGENTS, PRIMARILY antihistamines.1 Understanding the effects of such commonly administered agents on the mechanisms controlling respiratory muscle activity is important, given the prevalence of obstructive sleep apnea (OSA) in the general population and the serious public health impact of this disorder.2 Some evidence from case reports3 and studies in unselected OSA patients4 suggest that benzodiazepines may promote OSA in susceptible individuals, but this is not a consistent finding.5–8 Recent reviews question the commonly held notion that sedative-hypnotics are universally contraindicated in all OSA patients,9,10 and this likely has a physiological basis as the mechanisms contributing to OSA pathogenesis vary between individuals.11
The concept that suppression of arousal from sleep may not be detrimental in all OSA patients arises because arousals can promote ventilatory instability and recurrence of obstructions.12 Moreover, during OSA most patients are capable of mounting an effective compensatory upper airway muscle response to the accumulating respiratory stimulation and can initiate airway reopening, but arousal from sleep interferes with this orderly response.12,13 Accordingly, the use of certain sedatives in selected OSA patients with low arousal threshold may promote stable breathing and reduce OSA severity, but only if the sedative does not suppress (as much) the reflex pharyngeal dilator muscle responses to respiratory stimulation.
We recently showed that sedative doses of pentobarbital caused a large increase in baseline GG activity during undisturbed sleep in freely behaving rats.14 This finding, however, was paradoxical because barbiturates are known to enhance the chloride-mediated inhibition produced by γ-amino-butyric acid (GABA) binding at GABAA receptors, with these receptors being inhibitory at the hypoglossal motor nucleus in vivo15 and in vitro.16 Pentobarbital is no longer available for use as an orally administered hypnotic. Accordingly, the first aim of the present study was to determine if the more commonly prescribed hypnotics lorazepam (a benzodiazepine) and zolpidem (an imidazopyridine compound) have the same effects on GG activity when administered systemically. Lorazepam and zolpidem also enhance GABA-mediated neuronal inhibition, but via interactions with binding sites on GABAA receptors different from barbiturates. Therefore, the first hypothesis to be tested was that sedative doses of lorazepam and zolpidem would also delay arousal from sleep and allow GG activity to increase more in response to respiratory stimulation, and would also cause a robust increase in GG activity during undisturbed sleep. Importantly, we also determined if these GG augmenting effects were specific to these agents that bind to GABAA receptors, or whether they also occurred with the sedative antihistamine diphenhydramine.
Despite the commonly-held notion that sedative-hypnotics are contraindicated in all OSA patients, there have been no studies characterizing the effect of such agents when applied directly to respiratory motoneurons. Accordingly, in separate experiments in anesthetized rats we tested the second hypothesis that local application of lorazepam and zolpidem to the hypoglossal motor nucleus would suppress GG activity via their modulating effects on GABAA receptor-mediated inhibitory neurotransmission.
Overall, the present study is the first to show local inhibitory influences on hypoglossal motor activity following application of common sedative hypnotics to the hypoglossal motor nucleus, and is also important in revealing a novel excitatory influence on GG activity that is specific to sedatives that modulate the function of the GABAA receptor when these agents are administered systemically. These observations of dual opposing influences on hypoglossal motor output to GG muscle are contrary to the prevailing notion of only inhibitory influences, and reveal novel mechanisms of integrative respiratory motor control operating in vivo.
METHODS
All procedures conformed to the recommendations of the Canadian Council on Animal Care and the University of Toronto Animal Care Committee approved the experimental protocols. Rats were housed individually, maintained on a 12/12 hr light/dark cycle (lights on 07:00), and had free access to food and water.
1. Studies in Freely Behaving Rats
Anesthesia and Surgical Procedures
Experiments were performed on 31 male Wistar rats (Charles River, mean body weight = 286.4g, range = 240–360g). Sterile surgery was performed under general anesthesia as previously described.17 Electroencephalogram (EEG) and neck electromyogram (EMG) electrodes were implanted for determination of sleep-wake states, and GG and diaphragm wires to record respiratory muscle activity. For the placement of the GG electrodes, the ventral surface of GG muscle was exposed via a submental incision and dissection of the overlying geniohyoid and mylohyoid muscles. Two insulated, multistranded stainless steel wires (AS631; Cooner Wire, Chatsworth, CA, USA) were implanted bilaterally into GG and secured with sutures and tissue glue. Sections of the medial branches of the hypoglossal nerves has shown that GG activity is recorded with such electrode placements.18 To record diaphragm activity, two insulated, multistranded stainless steel wires (AS636: Cooner Wire) were sutured onto the costal diaphragm via an abdominal approach. The size, configuration, and placement of the GG and diaphragm electrodes were consistent across experiments.
To confirm respiratory-related activity, both the GG and diaphragm signals were monitored on loudspeaker (AM8 Audio Amplifier, Grass) during surgery. All electrode leads were tunnelled subcutaneously and connected to pins inserted into a miniature plug (STC-89PI-220ABS, Carleton University, Ottawa, ON) that was affixed to the skull with dental acrylic and anchor screws. Tests for the accurate placement of the GG electrodes and their function throughout the experiments included the recording of respiratory-related GG activity recording during surgery and observing tongue movements during electrical stimulation of the GG wires (0.4–0.8 V) both during surgery and at the end of the experiments. The rats were given soft food for the first day after surgery and recovered for an average of 8.4 days (range, 6–15 days).
Recording Procedures
On the days of the experiments, rats were placed in the recording chamber (PLY 3223, Buxco Electronics, Wilmington, NC, USA), and a lightweight shielded cable was connected to the plug on the rat's head. The cable was attached to a counterbalanced swivel that permitted free movement. For habituation, the rats were connected to the apparatus for several hours the day before the experiments, and were also exposed to the transient CO2 stimuli (see below).
The electrical signals were amplified and filtered (Super-Z head-stage amplifiers and BMA-400 amplifiers/filters, CWE Inc., Ardmore, PA, USA). The GG and diaphragm signals were recorded at the same amplification across all experiments. The EEG was filtered between 1 and 100 Hz, whereas the neck, GG, and diaphragm EMGs were filtered between 100 and 1000 Hz.17 The electrocardiogram was removed from the diaphragm EMG using an oscilloscope and an electronic blanker (Model SB-1, CWE Inc.). The moving-time averages of the neck EMG (time constant = 50 ms), and the GG and diaphragm EMGs (time constant = 100 ms) were also obtained (Model MA 821, CWE Inc.). The level of CO2 in the animal chamber was measured continuously (Beckman LB-2) at a flow rate of 500 cc·min−1 with the sampled air returned to the rat chamber. All signals were recorded on a computer (Spike 2 software, 1401 interface, CED Ltd, Cambridge, UK).
Protocol
The experiments began at approximately 09:00, i.e., during the normal sleeping period of the rats. Prior to monitoring, the rats were administered either a dose of the chosen sedative or the vehicle control. Doses of sedatives and vehicle were administered in random order, and on separate days, with a wash-out period of ≥ 48 h between drugs. Data collection started ≥20 min after injection, corresponding to time of peak plasma and brain tissue concentrations for each of these chosen agents.19–21 Experiments continued for approximately 3 h, because after this time the sedative effects were reduced (the plasma half-life of each sedative being < 2 h).19–21
Periodically when the rat was in NREM sleep for ≥ 20 s, hypercapnic respiratory challenges were produced using a custom-designed solenoid-triggered CO2 delivery system (Model RCFC-1001, YRT Ltd, Winnipeg, MB) that increased the inspired CO2 levels by approximately 0.2% per s (Figure 1), with the stimuli being highly reproducible between interventions. The flow of CO2 was maintained until the rat aroused from sleep or chamber CO2 reached 10%, at which time the CO2 was terminated and the chamber was flushed with air.
Figure 1.
Example showing the effects of lorazepam (1 mg/kg) on arousal threshold and respiratory muscle activities. The traces show the electroencephalogram (EEG), neck electromyogram (EMG), inspired CO2 level, and the genioglossus (GG) and diaphragm (DIA) muscle activities. The GG and DIA signals are displayed as their moving-time averages (MTA) in arbitrary units (AU). The baseline of the integrator (i.e., electrical zero) is also shown. The arrows on the MTA signals denote an increase in EMG activity. The points of arousal from sleep with vehicle and lorazepam are indicated by the vertical black dashed lines. The time to arousal from the onset of the CO2 stimuli with vehicle is also superimposed on the lorazepam trace to further highlight the delay in arousal and the corresponding increase in CO2 level at arousal. Note that GG activity was higher in the presence of lorazepam before arousal from sleep (A2 vs. A1), and even at baseline before application of the CO2 (B2 vs. B1). The large increase in GG activity following arousal typically occurred in association with the increased motor activity also observed in the neck, and we did not observe any consistent effect of sedation on that relationship.
The effects of 3 clinically relevant sedatives on arousal threshold and GG activity were investigated.
Lorazepam:
In the first set of experiments, 9 rats were administered 0.5 mg/kg and 1 mg/kg of lorazepam (Sabex, Boucherville, PQ) via intraperitoneal (IP) injection with 0.9% saline used as the vehicle control. Additional experiments with 5 mg/kg lorazepam were also performed in a separate group of 7 rats. Lorazepam is a short-acting benzodiazepine that binds to the benzodiazepine site on GABAA receptors and accentuates GABA-mediated inhibitory neurotransmission; lorazepam binds with high affinity to both type I and II benzodiazepine sites.22
Zolpidem:
Six rats were administered 5 mg/kg and 10 mg/kg of zolpidem (Tocris Bioscience, Bristol, UK). Due to the insolubility of zolpidem in saline, the vehicle control was a mixed solution of 5% ethanol, 85% polyethylene glycol, and 10% saline. Zolpidem is a short-acting non-benzodiazepine hypnotic that also accentuates GABA-mediated inhibitory neurotransmission, but binds selectively to type I benzodiazepine sites.22
Diphenhydramine:
Nine rats were administered 20 mg/kg diphenhydramine (type 1 histamine receptor antagonist, diphenhydramine chlorohydrate, Sabex, Boucherville, PQ) with 0.9% saline used as the control.
Doses of sedatives:
Doses were chosen based on their NREM sleep promoting effects established from multiple preclinical rodent studies in vivo23–25 and our preliminary experiments. Indeed, the rats continued to arouse spontaneously and in response to CO2 and behave normally i.e., the doses were adequate simply to promote sleep. After completion of the studies, each rat was re-anesthetized, and tests for GG electrode function were performed before the rats were euthanised with urethane (2–3 mL of 0.5 g/mL, IP).
Data Analysis
Sleep-wake states were identified visually and classified using standard criteria.17 Sleep periods that terminated spontaneously and without CO2 interventions (i.e., undisturbed sleep) were used to calculate sleep time. The EMGs were analyzed from the respective moving-time average signals (above electrical zero) and were quantified in arbitrary units. The GG signal was quantified as mean tonic activity (i.e., basal activity in expiration) and respiratory-related activity (i.e., peak inspiratory–tonic activity). Diaphragm amplitude, respiratory rate, and diaphragm minute activity (i.e., diaphragm amplitude*respiratory rate [the neural surrogate for ventilation]) were also quantified. These parameters were analyzed breath-by-breath for the 20 s prior to the onset of CO2, and the last 5 breaths immediately before arousal.
As in all our previous studies in behaving rats we chose not to report GG activity as some percentage of a maximum level recorded during the experiments and the data are reported in arbitrary units.14,15,26–28 We considered expressing GG activity as a percent of maximum, but such maximal activity always occurred in association with tongue movements engaged during active behaviors. Importantly, unlike humans where behavioral tongue activity can be standardized (e.g., maximum voluntary protrusion), maximum behavioral activity in this animal model cannot be controlled, and may itself be affected by sedation. Furthermore, behavioral motor activity may originate from different motoneurons than those generating the phasic respiratory-related activity.29 Some investigators who do not work in behaving animals have quantified maximal GG activity as that obtained during CO2 stimulation when the GG activity plateaus. Although such plateaus are easily obtained under reduced conditions (e.g., anesthesia), studies in conscious animals are severely complicated by ongoing behaviors in wakefulness, while the interventions in sleep are interrupted by arousal. Overall, therefore, maximum GG activity is not a robust reference for these studies. It is relevant to note, however, that when the results of GG activity derived from measurements in arbitrary units have been compared to the results obtained from the percent of maximum, the results and conclusions are the same.26–28 Nevertheless, given that (i) maximum respiratory activity can not be reliably measured in these behaving preparations (see above), (ii) that each animal serves as its own control, and (iii) that the order of vehicle or drug studies were randomized to account for any potential time-dependent changes in muscle-electrode coupling, the quantification of GG activity in arbitrary units is suitably robust.14,15,26–28
2. Studies in Anesthetized Rats
Surgical Preparation
Thirty-seven rats (mean body weight = 270g, range = 240–310g) were anesthetized with urethane (1 g/kg, IP). Rats were also given atropine (1 mg/kg) and dexamethosone (0.2 mg) to minimize airway secretions and brain edema respectively. Following the onset of surgical anesthesia the rats were tracheotomized and the femoral artery and vein were cannulated. Sixteen of the 37 rats were studied with the vagus nerves intact, and 21 were studied after bilateral cervical vagotomy, in order to investigate the effects of lorazepam or zolpidem on GG activity under conditions of baseline and enhanced GG muscle activity respectively.17 The rats spontaneously breathed a 50:50 mixture of room air and oxygen with any additional anesthesia (halothane, typically 0.2%–1%) administered by inhalation. When halothane was initiated within an animal typically no, or only minor, adjustments were necessary across the experiment to maintain stable EEG and respiratory muscle activities. We routinely use this anaesthetic regime because it provides highly reliable preparations with stable respiratory and EEG activities over the course of the experiments18 that is not possible, for example, with periodic bolus doses of supplemental urethane which can transiently suppress GG activity, or even for prolonged periods. Core body temperature was monitored with a rectal probe and maintained between 36–38°C with a water pump and heating pad (T/Pump-Heat Therapy System, Gaymar, NY, USA). The rats received continuous intravenous fluid (0.4 mL/hr) containing 7.6 mL saline, 2 mL 5% dextrose and 0.4 mL of 1M NaHCO3. Bipolar electrodes were inserted into the GG and costal diaphragm for EMG recordings.17 The rats were then placed in a stereotaxic apparatus (Kopf Model 962, Tujunga, CA) and 2 stainless steel screws attached to insulated wire were implanted in the skull over the frontal-parietal cortex to record the cortical EEG.17
Microdialysis and Recordings
Microdialysis probes (CMA/11 14/01, CSC, St. Laurent, QC) were targeted into the hypoglossal motor nucleus to infuse artificial cerebrospinal fluid (ACSF) followed by either lorazepam or zolpidem. The probes were placed 13.6 ± 0.06 (SEM) mm posterior to bregma (range, 13.0–14.0 mm), 0.11 ± 0.03 mm lateral to the midline (range −0.1 to 0.4 mm) and 9.9 ± 0.08 mm ventral to bregma (range 9.1–10.7 mm). The rats stabilized for at least 30 min before any interventions. The microdialysis probes were 240 μm in diameter with a 1 mm cuprophane membrane and a 6000 Dalton cut-off. The probes were connected to FEP Teflon tubing (inside diameter = 0.12 mm) in turn connected to 1.0 mL syringes via a zero dead space switch (Uniswitch, B.A.S. West Lafayette, IN). The probes were continually flushed with at a flow rate of 2.1 μL.min−1 using a syringe pump and controller (MD-1001 and MD-1020, B.A.S., West Lafayette, IN). The composition of ACSF (mM) was NaCl (125), KCl (3), KH2PO4 (1), CaCl2 (2), MgSO4 (1), NaHCO3 (25) and D-glucose (30). The electrical signals were amplified and filtered as for the sleeping experiments above. Each signal, along with blood pressure (DT-XX transducer, Ohmeda, Madison, WI and PM-1000 Amplifier, CWE Inc.) was also recorded on computer (as above).
Protocol and Data Analyses
Interventions were performed during steady-state periods with predominantly high-voltage and low-frequency EEG activity. The microdialysis probes were perfused with vehicle for ≥30 min followed by 0.001, 0.01, 0.1, and 1 mM of lorazepam or zolpidem, each for at least 30 min. A total of 21 rats were studied with lorazepam (10 rats with the vagus nerves intact and 11 after vagotomy), and 16 rats were studied with zolpidem (6 rats with the vagus nerves intact and 10 after vagotomy). In a subset of 5 vagotomized rats studied with lorazepam and 6 vagotomized rats with zolpidem, 100 μM bicuculline (GABAA receptor antagonist) was also applied to the hypoglossal motor nucleus after the highest dose of the respective drug to show that any GG suppression was mediated by GABAA receptor mechanisms and not deterioration of the GG signal per se. Previous studies have shown that this dose of bicuculline is an effective antagonist of GABAA receptors at the hypoglossal motor nucleus.15 GG and diaphragm responses were measured from the moving average signals above electrical zero, as described in previous experiments17 and for the above experiments in sleep. In practice there was no tonic GG activity under anesthesia, so data is only reported for respiratory-related activity.
Statistical Analyses
For all comparisons, differences were considered significant if the null hypothesis was rejected at P < 0.05 using a two-tailed test. Where only one drug dose was tested in each animal (e.g. lorazepam 5 mg/kg or diphenhydramine) the results with drug were compared to the vehicle control using the paired t-test. Where two or more doses of a drug were tested, statistics were performed using analysis of variance with repeated measures (ANOVA-RM), and post-hoc t-tests were performed using Bonferroni corrected P values. All data are expressed as mean ± SEM. Analyses were performed using Sigmastat (Jandel Scientific, San Rafael, CA).
RESULTS
1. Studies in Freely Behaving Rats
Effects of Sedatives on Arousal Threshold in Response to CO2
Table 1 shows, for each drug regimen, the average recording time, duration of NREM sleep periods that terminated spontaneously (i.e., without CO2 challenge), and the number of CO2 challenges per animal. Overall the rats were monitored for ≈3 h and received ≈10 CO2 challenges per study, with no differences in these values among drugs. Lorazepam and zolpidem increased the duration of NREM sleep periods that terminated spontaneously, with the increase being statistically significant for all doses except lorazepam at 0.5 mg/kg. By contrast, NREM sleep period duration did not significantly increase with diphenhydramine (Table 1).
Table 1.
Effect of Sedatives on Spontaneous Sleep Duration and Arousal Threshold in Response to CO2
| Dose (mg/kg) | Lorazepam |
Zolpidem |
Diphenhydramine |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 0 | 5.0 | 0 | 5.0 | 10.0 | 0 | 20.0 | |
| Recording time (hr) | 3.22 | 2.89 | 3.02 | 3.17 | 2.90 | 3.18 | 3.17 | 3.06 | 3.75 | 3.70 |
| (0.13) | (0.08) | (0.09) | (0.23) | (0.19) | (0.08) | (0.08) | (0.11) | (0.29) | (0.24) | |
| NREM sleep period duration (min)1 | 1.26 | 1.52 | 2.00** | 1.33 | 2.09*** | 1.07 | 1.48* | 1.51* | 1.47 | 1.66 |
| (0.12) | (0.07) | (0.22) | (0.12) | (0.13) | (0.05) | (0.08) | (0.14) | (0.11) | (0.11) | |
| CO2 stimuli (per animal) | 10.7 | 12.4 | 12.8 | 10.3 | 11.6 | 8.8 | 11.8 | 10.6 | 9.5 | 9.6 |
| (1.3) | (1.2) | (0.9) | (1.9) | (1.3) | (1.3) | (2.2) | (1.4) | (1.1) | (1.1) | |
All data are shown as mean ± SEM.
Sleep period duration is the average duration of NREM sleep periods that terminated spontaneously (i.e., without CO2 challenge). Abbreviations: *, P < 0.025; **, P < 0.01; ***, P < 0.001; all comparisons are relative to the respective vehicle control by paired t-test (where only one dose was tested) or post hoc paired t-test after analysis of variance with repeated measures (where 2 doses of drug were used). All data are shown as mean ± SEM.
Figure 1 shows an example of the effects of 1 mg/kg lorazepam on arousal threshold and respiratory muscle activities in response to CO2 stimulation. This figure shows that compared to vehicle control, lorazepam delayed arousal from sleep and allowed CO2 levels to increase to higher levels before arousal occurred. Note that respiratory-related GG activity was also higher during NREM sleep before arousal in the presence of lorazepam compared to vehicle control (i.e., A2 vs. A1 in Figure 1). Figure 2 shows that consistent with the sedative action of the drugs used, both the latency to arousal and the chamber CO2 levels at arousal were significantly increased with all doses of each sedative compared to the respective vehicle controls (each P < 0.05).
Figure 2.
Group mean data showing the effects of lorazepam, zolpidem and diphenhydramine on the latency to arousal from sleep following the onset of the CO2 stimuli, and the chamber CO2 level at arousal from sleep. * indicates P < 0.05 compared to the respective vehicle controls (black bars). See text for further details. All data are shown as mean ± SEM.
Effects of Sedatives on GG Activity Immediately before Arousal from Sleep
Lorazepam and Zolpidem:
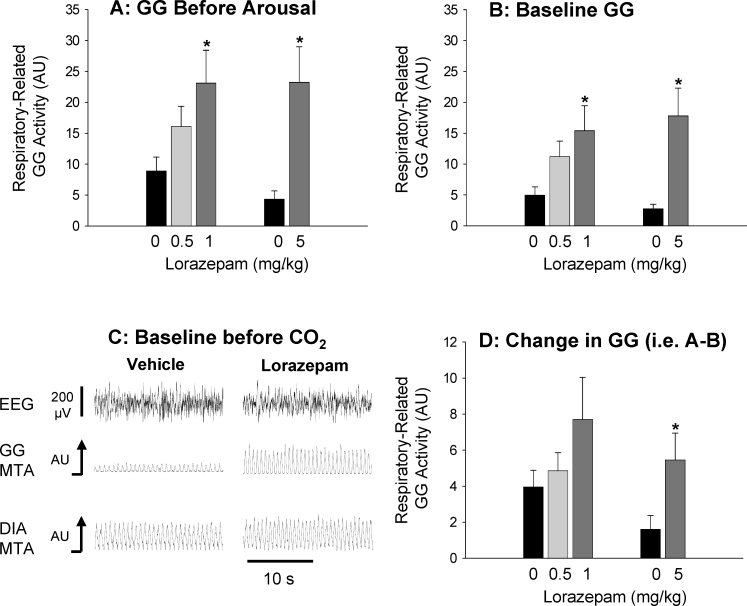
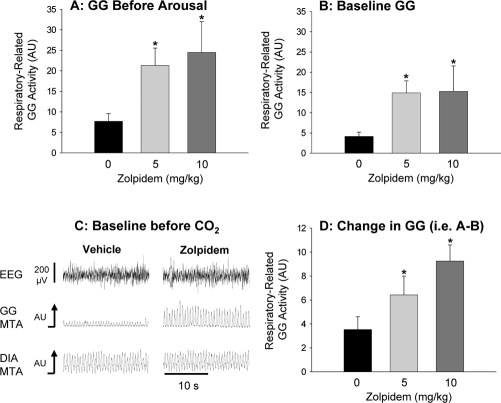
Figures 3 and 4 show that lorazepam and zolpidem had similar modifying effects on GG activity. With both drugs, the respiratory-related GG activity reached prior to CO2-mediated arousal from sleep (i.e., corresponding to period A in Figure 1) was significantly higher compared to the interventions with vehicle (Figures 3A and 4A, P < 0.012 for 1 mg/kg and 5 mg/kg lorazepam, and P < 0.014 for 5 mg/kg and 10 mg/kg zolpidem). This increase in GG activity was only partly due to the delayed arousal from sleep allowing higher levels of CO2–mediated respiratory stimulation (see Figure 2 for reference). Indeed, both lorazepam and zolpidem caused a clear increase in baseline GG activity during NREM sleep before application of the CO2 stimuli, i.e., during undisturbed sleep (see Figure 3B–C and Figure 4B–C, P < 0.018 for 1 mg/kg and 5 mg/kg lorazepam vs. control, and P < 0.025 for 5 mg/kg and 10 mg/kg zolpidem vs. control). Tonic GG activity was minimal in NREM sleep and did not change significantly with either lorazepam or zolpidem compared to the vehicle controls (all P > 0.271).
Figure 3.
This figure shows (A) the group mean level of GG activity measured immediately before arousal from sleep elicited by the CO2 stimuli, in the presence of vehicle control and the different doses of systemically applied lorazepam. The robust increases in baseline GG activity (i.e., before application of the CO2 stimuli) in the presence of lorazepam is also shown for the group data (B) and from the original signals from one rat (C, 1 mg/kg lorazepam). The changes in GG activity elicited by application of the CO2 stimuli (i.e., GG activity immediately before arousal–GG activity at baseline) are also shown (D) * indicates P < 0.05 compared to the respective vehicle controls (black bars). See text for further details. All data are shown as mean ± SEM.
Figure 4.
This figure shows (A) the group mean level of GG activity measured immediately before arousal from sleep elicited by the CO2 stimuli, in the presence of vehicle control and the different doses of systemically applied zolpidem. The robust increases in baseline GG activity (i.e., before application of the CO2 stimuli) in the presence of zolpidem is also shown for the group data (B) and from the original signals from one rat (C, 10 mg/kg zolpidem). The changes in GG activity elicited by application of the CO2 stimuli (i.e., GG activity immediately before arousal–GG activity at baseline) are also shown (D) * indicates P<0.05 compared to the respective vehicle controls (black bars). See text for further details. All data are shown as mean ± SEM.
This clear increase in baseline respiratory-related GG activity in NREM sleep after lorazepam and zolpidem was not solely responsible, however, for the overall increase in respiratory-related GG activity before CO2-mediated arousal from sleep. Further analysis showed that the increase in GG activity in response to CO2 stimulation (i.e., GG activity before arousal–baseline GG activity) was significantly increased with 5 mg/kg lorazepam and both doses of zolpidem compared to the vehicle controls (Figures 3D and 4D, each P < 0.04). This overall result showed that with lorazepam and zolpidem, a component of the increase in GG activity in response to CO2 was due to the delayed arousal from sleep and the concomitant increase in the level of CO2 stimulation.
Diphenhydramine:
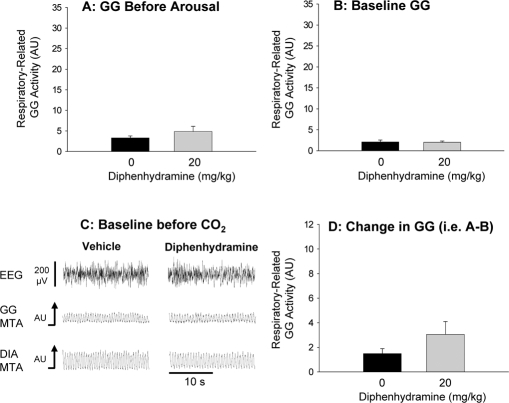
Figure 5 illustrates the effects of the antihistamine diphenhydramine on GG activity. In contrast to the GABAA receptor modulating agents lorazepam and zolpidem, there was no significant increase in pre-arousal GG activity (Figure 5A, P = 0.282), baseline GG activity in NREM sleep (Figure 5B–C, P = 0.910), or the change in GG activity in response to CO2 stimulation (Figure 5D, P = 0.140). Tonic GG activity was minimal in NREM sleep and also did not change with diphenhydramine (P = 0.963).
Figure 5.
This figure shows (A) the group mean level of GG activity measured immediately before arousal from sleep elicited by the CO2 stimuli, in the presence of vehicle control and 20 mg/kg of systemically applied diphenhydramine. There was no effect of diphenhydramine on the levels of GG activity measured immediately before arousal from sleep (A), nor on baseline GG activity before CO2 stimulation (B and C) and the change in activity elicited by application of the CO2 stimuli (D). The data are plotted on the same scales as for lorazepam and zolpidem (Figures 3 and 4 respectively) to facilitate visual comparisons. See text for further details. All data are shown as mean ± SEM.
Effects of Sedatives on Diaphragm Activation
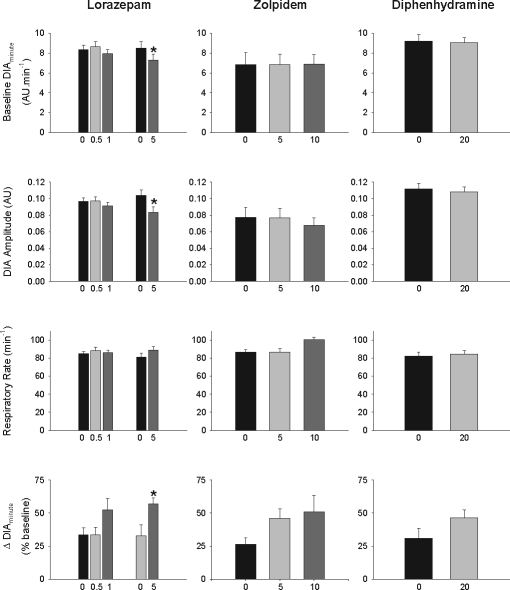
Figure 6 shows that none of the drugs significantly affected baseline diaphragm activity or respiratory rate at any dose, with the exception of a small reduction in baseline diaphragm minute activity with lorazepam at 5 mg/kg (P = 0.023, paired t-test) due to reduced diaphragm amplitude (P = 0.022). Figure 6 also includes the average increases in diaphragm minute activity from before CO2 stimulation to immediately before arousal, with each dose of sedative and vehicle. The increase in diaphragm minute activity in response to CO2 was statistically significant with lorazepam at 5 mg/kg (P = 0.019, paired t-test), but only tended to be greater with zolpidem, diphenhydramine, and the lower doses of lorazepam compared to the respective vehicle controls (P = 0.100, 0.150, and 0.165 respectively). Overall, these results showed that any potential suppression of the diaphragm response to CO2 in the presence of the sedative was more than offset by the delay in arousal, thereby allowing a higher level of respiratory stimulation to be reached before arousal occurred (see Figure 1 for reference).
Figure 6.
Group mean data showing the effects of lorazepam, zolpidem and diphenhydramine on diaphragm minute activity (DIAminute), diaphragm amplitude and respiratory rate during baseline breathing before application of the CO2 stimuli. Also shown are the average increases in diaphragm minute activity (Δ DIAminute) from before CO2 stimulation to immediately before arousal with each dose of sedative and vehicle, expressed as a percent (%) of activity at baseline. * indicates P < 0.05 compared to the respective vehicle controls (black bars). See text for further details. All data are shown as mean ± SEM. Abbreviations: AU, arbitrary units.
2. Effects of Lorazepam and Zolpidem at the Hypoglossal Motor Nucleus
Sites of Microdialysis
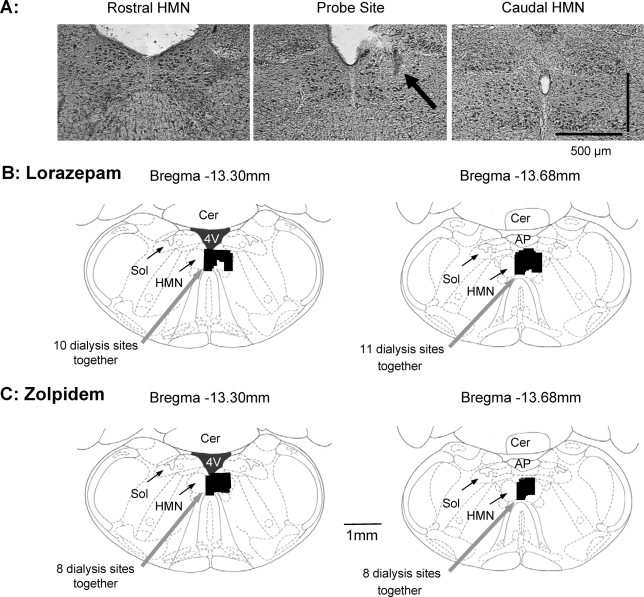
Figure 7 shows an example of the lesion site made by the microdialysis probe in the hypoglossal motor nucleus, and the distribution of microdialysis sites from all experiments in the anesthetized rats. Microdialysis probes were successfully implanted into, or immediately adjacent to, the hypoglossal motor nucleus in all animals.
Figure 7.
Example and group data showing the location of the microdialysis probes in the anesthetized rats undergoing local application of lorazepam and zolpidem to the hypoglossal motor nucleus (HMN). (A) Histological section showing an example of a lesion site made by the microdialysis probe in the HMN. Also shown are the distribution of individual microdialysis sites from all rats administered lorazepam (B) and zolpidem (C). The sizes of the bars represent the apparent size of the lesions from the histological sections. Abbreviations: AP, area postrema; Cer, cerebellum; Sol, nucleus of the tractus solitarius; 4V, fourth ventricle.
Effects of Lorazepam and Zolpidem at the Hypoglossal Motor Nucleus on GG Activity
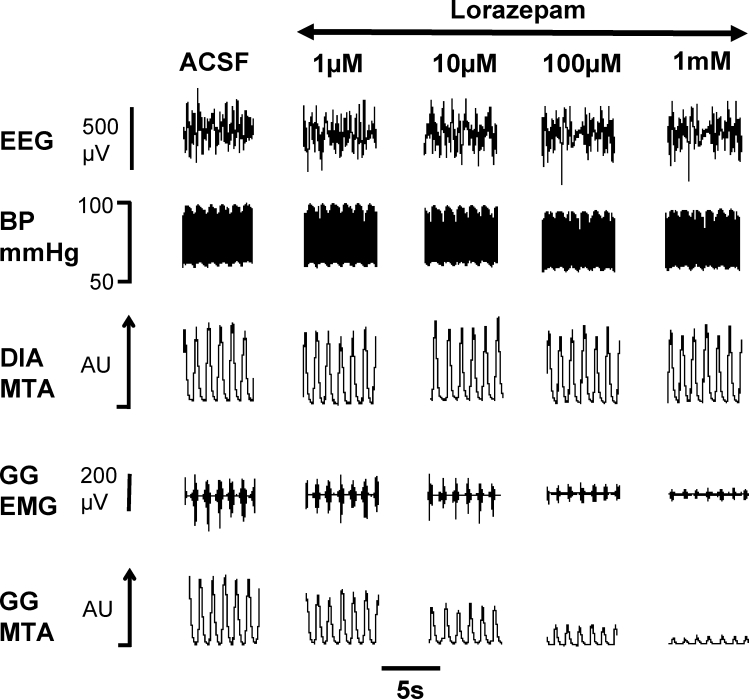
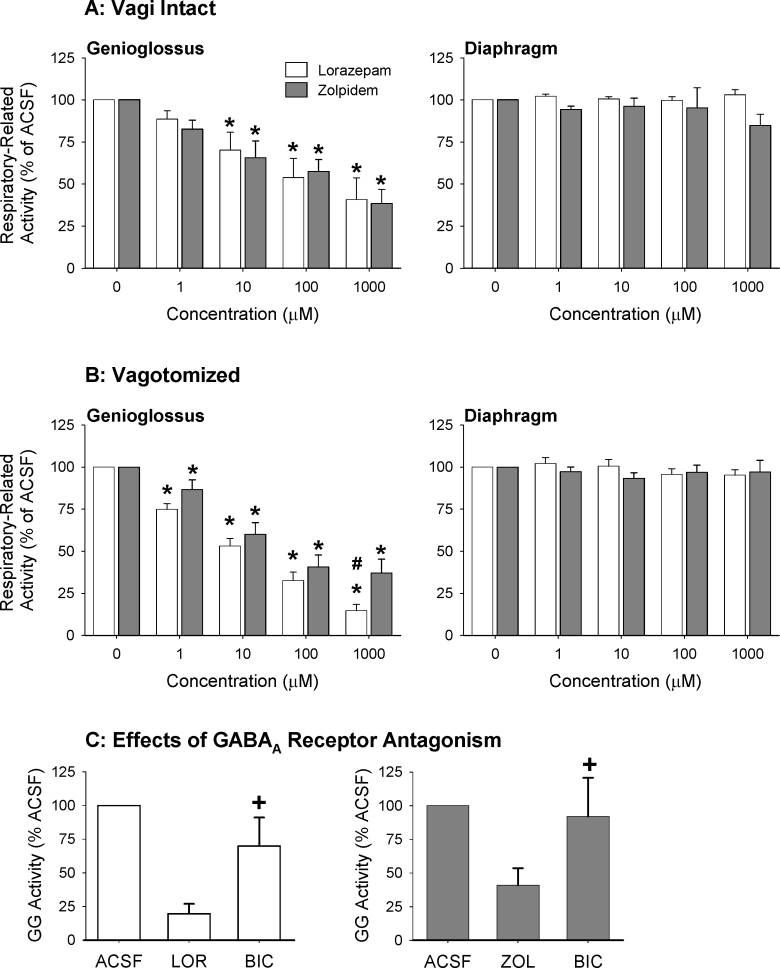
Figure 8 shows an example of the progressive decreases in GG muscle activity with increasing lorazepam at the hypoglossal motor nucleus. The group data in Figure 9 show that both lorazepam and zolpidem at the hypoglossal motor nucleus resulted in a dose-dependent suppression of GG activity, in both the rats with intact vagus nerves and vagotomized rats (P < 0.001, 2-way ANOVA-RM). For both lorazepam and zolpidem this suppression of GG activity became statistically significant at 10 μM and 1 μM in the vagi-intact and vagotomized rats respectively (see Figure 9, each P < 0.001 from post hoc paired t-tests).
Figure 8.
Example showing progressive suppression of GG muscle activity with increasing lorazepam at the hypoglossal motor nucleus. Abbreviations are as for Figure 1 with the addition of the electroencephalogram (EEG) and blood pressure (BP).
Figure 9.
Group data to compare the suppression of GG activity with increasing lorazepam and zolpidem at the hypoglossal motor nucleus in the vagus nerve intact (A) and vagotomized (B) rats. GG activity is quantified as the percent (%) of activity in the presence of the respective artificial cerebrospinal fluid (ACSF) controls. * indicates P < 0.05 compared to these ACSF controls, and # indicates significant difference between lorazepam and zolpidem. As expected there were no effects on diaphragm activity with these local interventions at the hypoglossal motor nucleus. Also shown (C) is the suppression of GG activity with 1000 μM lorazepam (LOR) and zolpidem (ZOL) from the ACSF controls, and the subsequent increase following application of the GABAA receptor antagonist bicuculline (BIC). + indicates a significant increase (P < 0.05) with bicuculline compared to lorazepam or zolpidem. All data are shown as mean ± SEM.
In the vagus nerve intact rats, the magnitude of GG suppression did not depend on whether lorazepam or zolpidem was applied to the hypoglossal motor nucleus (P = 0.94, 2-way ANOVA-RM). In the vagotomized rats, however, the magnitude of GG suppression was dependant on the presence of lorazepam or zolpidem (P = 0.020, 2-way ANOVA-RM); GG activity was significantly lower with lorazepam compared with zolpidem at the highest doses applied (see Figure 9, P = 0.003, post hoc t-test).
Figure 9C shows that following application of these highest doses of lorazepam and zolpidem in a subset of the vagotomized rats, application of the GABAA receptor antagonist bicuculline was able to increase GG activity, i.e., indicating that the prior GG suppression was mediated by GABAA receptor mechanisms and not deterioration of the GG signal per se. This increase in GG activity was statistically significant with bicuculline after zolpidem and lorazepam (both P < 0.05, post hoc paired t-tests after 1-way ANOVA-RM showed a significant effect of applied drug with both P < 0.009, see Figure 9C).
In contrast to the effects of lorazepam and zolpidem on GG activity, there was no effect on diaphragm activity across the range of doses tested in either the vagus nerve intact or vagotomized animals (both P > 0.44, 2-way ANOVA-RM; see Figure 9).
DISCUSSION
The major findings of this study are: (1) Systemic administration of the commonly used sedative-hypnotics lorazepam and zolpidem led to significant increases in baseline respiratory-related GG activity during undisturbed sleep without effects on diaphragm activity. (2) Lorazepam and zolpidem also increased the level of GG activity immediately before arousal from sleep elicited by hypercapnic respiratory stimulation. This latter effect on GG activity was mediated, at least in part, by a sedative-induced increase in arousal threshold which allowed CO2-mediated chemical respiratory drive to increase more before arousal occurred. (3) Both the effects on baseline GG activity during undisturbed sleep, and the activity immediately preceding CO2-mediated arousal, were specific to the agents that bind to sites on the GABAA-receptor because these effects did not occur with the antihistamine diphenhydramine. (4) In contrast to the GG augmentation following systemic administration of lorazepam and zolpidem, direct application of these agents to the hypoglossal motor nucleus led to significant suppression of GG activity. This latter result is also the first characterization of the direct effect of sedative-hypnotics on respiratory motoneuron activity, and is particularly relevant because hypoglossal motoneurons innervate the GG muscle which is importantly involved in the pathogenesis of OSA.
This combination of results also highlights that the significant increases in baseline respiratory-related GG activity after systemic administration of lorazepam and zolpidem can only be attributed to mechanisms operating outside the hypoglossal motor nucleus. Overall, therefore, this study reveals for the first time that the net effect of systemically applied GABAA-receptor modulating sedatives on GG activity is a balance between (i) inhibitory effects acting locally at the hypoglossal motor nucleus and (ii) GG augmenting effects acting via pre-motor inputs, with the balance in favour of the latter in rodents. These observations of dual opposing influences on hypoglossal motor output to GG muscle are contrary to the prevailing notion of only inhibitory influences, and reveal novel mechanisms of integrative respiratory motor control operating in vivo.
GG Motor Suppression with Lorazepam and Zolpidem at the Hypoglossal Motor Nucleus
Local application of lorazepam and zolpidem to the hypoglossal motor nucleus caused a significant suppression of GG activity, an effect observed under conditions of normal respiratory drive as well as during augmented respiratory input to the hypoglossal motor nucleus following vagotomy (Figure 9). This clear suppression of respiratory-related GG activity with local application of lorazepam and zolpidem to the hypoglossal motor nucleus is best explained by effects at the GABAA receptor. The GABAA receptor is comprised of 3 functional units: a benzodiazepine recognition site, a GABA recognition site, and a chloride ionophore.22 Binding of lorazepam and zolpidem to the benzodiazepine receptor site on the GABAA receptor complex enhances the postsynaptic effects of GABA and leads to enhanced chloride-mediated inhibition.22 GABAA receptor stimulation at the hypoglossal motor nucleus is inhibitory both in vivo15 and in vitro.16 Since inspiratory hypoglossal motoneurons receive synaptic inhibition toward the end of inspiration and at the transition to expiration30 with GABA contributing to this inhibition,16 then any augmentation of this ongoing GABAA receptor-mediated inhibition by lorazepam and zolpidem would explain the suppression of respiratory-related GG activity.
At the ultrastructural level, the GABAA receptor is composed of at least five subunits and each subunit can have multiple isoforms, although two specific combinations (types I and II) are the most common in the central nervous system.22 Lorazepam is a benzodiazepine with affinity for both the type I and type II GABAA-benzodiazepine receptor configurations, whereas zolpidem is an imidazopyridine compound with selectivity for type I.22 There was more suppression of GG activity with lorazepam than with zolpidem, but this effect was only revealed under conditions of enhanced drive to the hypoglossal motor nucleus produced by vagotomy (Figure 9B). This result may be explained (at least in part) by regional variations in GABAA receptor subunit composition, as labelling of α1 subunits comprising the type I but not type II configuration is modest in the adult rat hypoglossal motor nucleus,31 and zolpidem is selective for the type I configuration. Whether this difference between lorazepam and zolpidem persists under other conditions that enhance GG activity (e.g., hypoxia or hypercapnia) remains to be determined. Of physiological relevance, however, is that airway occlusion is accompanied by neural inspiration without phasic lung-volume related afferent vagal feedback, i.e., effects akin to vagotomy.
Augmentation of Baseline GG Activity with Systemic Lorazepam and Zolpidem
In contrast to the local inhibitory effects of lorazepam and zolpidem on hypoglossal motor outflow, systemic administration of these agents led to clear augmentation of GG activity during undisturbed sleep. This GG augmenting effect was specific to these sedatives that bind to sites on the GABAA-receptor because baseline GG activity did not change with the antihistamine diphenhydramine. The specificity of the responses to agents modulating the GABAA-receptor complex is also indicated by our previous observation of increased baseline GG activity following systemic administration of sedative doses of pentobarbital14; barbiturates influence the chloride ionophore of the GABAA-receptor to enhance the inhibitory post-synaptic effects of GABA.22 These observations of dual opposing influences on hypoglossal motor output to GG muscle are contrary to the commonly held view that only inhibitory influences are present.
The central neural networks underlying the increase in baseline GG activity after systemic administration of lorazepam and zolpidem remain to be determined. However, this augmentation of respiratory motor activity was specific to the GG muscle as no alterations (increases or decreases) in baseline diaphragm activity were observed (Figs 3–5 and Table 1). Indeed, only at the highest dose of lorazepam was any suppression of diaphragm activity observed in these studies. In contrast, GG activity was increased at the lower doses of lorazepam, and across all doses of zolpidem, when diaphragm activity did not change (Figures 3–4 and Table 1). It is also possible that sedative-induced decreases in blood pressure may have contributed to the increased GG activity via baroreceptor mechanisms. However, this mechanism is unlikely as blood pressure does not decrease with zolpidem or benzodiazepines in rats.32 The doses of the hypnotics used in this study were chosen based on their NREM sleep promoting effects established from multiple preclinical rodent studies in vivo23–25 and preliminary experiments. We did not measure arterial blood gases in this study because we wanted to avoid chronic arterial catheterization and repeated blood withdrawal in these sleeping animals. Nevertheless, the lack of an increase in diaphragm activity is relevant, because it makes it unlikely that the increased GG activity was related to an increase in upper airway resistance and reflex activation via the negative pressure reflex.33 Apart from the fact that the negative pressure reflex is fairly weak during sleep,33 such an increase in loading in sleep would inevitably decrease ventilation resulting in subsequent diaphragm activation via chemical feedback.34–36
We have previously shown that a component of the increased GG activity following sedative (i.e., nonanesthetic) doses of pentobarbital developed after sleep onset, an effect that was increased with pentobarbital because the sleep period durations were prolonged.14 Interestingly, similar observations have been made for expiratory intercostal muscle activity in sleeping cats.37 The present data support these observations because baseline GG activity was also significantly increased during undisturbed sleep, but only at those drug concentrations that increased the durations of NREM sleep episodes (i.e., 1 mg/kg and 5 mg/kg for lorazepam, 5 mg/kg and 10 mg/kg for zolpidem). In the case of diphenhydramine, the durations of NREM sleep episodes were not increased and baseline GG activity did not change (Fig. 5B). We have previously speculated that the progressive augmentation of GG activity in sleep (prolonged by sedatives) was due to selective augmentation of activating inputs to the hypoglossal motor nucleus,14 which are distinct from the phrenic motor nucleus.30 This hypothesized mechanism remains to be tested and is beyond the scope of the present report. Indeed, the ultimate source(s) of the rhythmic respiratory drive to respiratory neurons and motoneurons, specifically to the pharyngeal vs. the respiratory pump muscles, has not yet been fully clarified.
The concept that lorazepam and zolpidem can lead to increased GG activity is novel and potentially of high clinical relevance if this response can be manipulated. However, this excitatory mechanism may at first seem paradoxical given the commonly held notion that benzodiazepine hypnotics are contraindicated in all OSA patients. As reviewed in the Introduction, however, this notion was based on case-reports3 and early studies in unselected OSA patients,4 whereas recent reviews question this common view and GG activity is not always decreased with sedatives.9,10,38 Moreover, anaesthetic doses of halothane and pentobarbital lead to increased c-Fos expression in specific brainstem hypoglossal premotor neurons, and produced increased GG activity.39 Significantly, the region showing marked neuronal activation following pentobarbital was the Kölliker-Fuse nucleus in the pons39 which projects to and excites hypoglossal motoneurons.40
It is noteworthy, however, that the robust increase in GG activity observed during sleep with pentobarbital,14 zolpidem and lorazepam (Figures 1, 3, and 4) does not agree with the hypoglossal suppression observed in decerebrate cats after systemic administration of barbiturates and benzodiazepines.41,42 Indeed, it was these initial observations in decerebrate animals that, in large part, were the basis for the notion that benzodiazepines and barbiturates were detrimental to mechanisms maintaining upper airway muscle tone. In these latter studies, however, the act of decerebration likely removed the suprabulbar hypoglossal premotor neurons activated by anesthesia or sedation that are responsible for the increased GG activity,39 such that only the suppressant action of these drugs remained via their local inhibitory effects at the hypoglossal motor nucleus (Figures 7 and 8).
GG Activity Reached Before Arousal from Sleep with Systemic Lorazepam and Zolpidem
A consistent finding of the present study was also that the level of GG activity reached immediately before CO2-induced arousal from sleep was higher with the GABAA receptor modulating sedative-hypnotics compared to the vehicle controls. Although a major component of this increase in GG activity was the robust increase in baseline GG activity prior to CO2 stimulation, a component was also due to a sedative-induced increase in the latency to arousal from sleep which allowed CO2-mediated chemical respiratory drive to increase more before arousal occurred (Figure 1). Interestingly, this effect on the latency and CO2 levels at arousal were qualitatively similar between all three drugs, but only with lorazepam and zolpidem was the change in GG activity during respiratory stimulation significantly increased (compare Figures 3D and 4D with 5D). Overall, this result agrees with the concept that certain sedatives may confer beneficial effects to the mechanisms maintaining upper airway patency by delaying arousal from sleep and allowing progressive recruitment of neuromuscular compensatory responses, particularly in those individuals with low arousal threshold in whom repeated arousal promotes respiratory instability and recurrence of obstructions.12,13 However, a formal test of the effects of specific sedatives on breathing during sleep in selected OSA patients has yet to be performed (i.e., those subjects with effective neuromuscular compensatory responses to airway obstruction but low arousal threshold—using standard techniques to identify such individuals12,13). Nevertheless, in unselected patients with OSA improvements in apnea index occurred in half the OSA patients studied with a hypnotic,5 and there was a lack a statistically significant adverse group effects in other studies.6–8,43 These results suggest that the net response to a particular sedative (i.e., a positive or negative effect on sleep disordered breathing) depends on the relative balance between a suppression of the destabilizing effects of arousal from sleep on ventilatory control, and effects on the ability to mount an effective pharyngeal muscle response to respiratory stimuli by reflex mechanisms. In support of this overall concept, a further study in unselected patients with severe OSA showed that zolpidem did not impair the efficacy of an effective level continuous positive airway pressure (CPAP).44 Moreover, in five of the OSA patients in that study who had sub-optimal CPAP and an apnea-hypopnea index >5, zolpidem reduced the apnea index in each individual suggesting an overall beneficial effect of zolpidem on the integrative mechanisms maintaining airway patency.44
Summary
The results of this study show for the first time that the net effect on GG activity of sedatives that modulate the function of the GABAA receptor is a balance between inhibitory effects acting locally at the hypoglossal motor nucleus and GG augmenting effects acting via premotor inputs. In addition, delayed arousal may also confer beneficial effects to the mechanisms maintaining upper airway patency by allowing progressive recruitment of reflex compensatory responses, which would otherwise be preempted and interrupted by the destabilising influence of arousals, i.e., before the beneficial effects on airway patency can be realized.
ACKNOWLEDGMENTS
This work was supported by funds from the Canadian Institutes of Health Research. RLH is supported by a Tier 1 Canada Research Chair in Sleep and Respiratory Neurobiology.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Younes is owner and president of YRT Limited, a Winnepeg, Canada research and development company and has consulted for Respironics and Nellcore Puritan Bennett. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Wysowski DK, Baum C. Outpatient use of prescription sedative-hypnotic drugs in the United States, 1970 through 1989. Arch Int Med. 1991;151:1779–83. [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Mendelson WB, Garnett D, Gillin JC. Flurazepam-induced sleep apnea syndrome in a patient with insomnia and mild sleep-related respiratory changes. J Nerv Ment Dis. 1981;169:261–64. doi: 10.1097/00005053-198104000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Dolly FR, Block AJ. Effect of flurazepam on sleep-disordered breathing and nocturnal oxygen desaturation in asymptomatic subjects. Am J Med. 1982;73:239–243. doi: 10.1016/0002-9343(82)90185-1. [DOI] [PubMed] [Google Scholar]
- 5.Hoijer U, Hedner J, Ejnell H, Grunstein R, Odelberg E, Elam M. Nitrazepam in patients with sleep apnoea: a double-blind placebo-controlled study. Eur Respir J. 1994;7:2011–15. [PubMed] [Google Scholar]
- 6.Camacho ME, Morin CM. The effect of temazepam on respiration in elderly insomniacs with mild sleep apnea. Sleep. 1995;18:644–645. doi: 10.1093/sleep/18.8.644. [DOI] [PubMed] [Google Scholar]
- 7.Carskadon MA, Seidel WF, Greenblatt DJ, Dement WC. Daytime carryover of triazolam and flurazepam in elderly insomniacs. Sleep. 1982;5:361–371. doi: 10.1093/sleep/5.4.361. [DOI] [PubMed] [Google Scholar]
- 8.Berry RB, Kouchi K, Bower J, Prosise G, Light RW. Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:450–4. doi: 10.1164/ajrccm.151.2.7842205. [DOI] [PubMed] [Google Scholar]
- 9.Robinson RW, Zwillich CW. Medications, sleep and breathing. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 3rd ed. Philadelphia: WB Saunders; 2000. pp. 797–812. [Google Scholar]
- 10.Douglas NJ. Respiratory physiology: control of ventilation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 3rd ed. Philadelphia: WB Saunders; 2000. pp. 221–41. [Google Scholar]
- 11.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–70. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 12.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–33. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 13.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–58. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 14.Younes Y, Park E, Horner RL. Pentobarbital sedation increases genioglossus respiratory activity in sleeping rats. Sleep. 2007;30:478–88. doi: 10.1093/sleep/30.4.478. [DOI] [PubMed] [Google Scholar]
- 15.Morrison JL, Sood S, Liu H, Park E, Nolan P, Horner RL. GABA-A receptor antagonism at the hypoglossal motor nucleus increases genioglossus muscle activity in NREM but not REM sleep. J Physiol. 2003;548:569–83. doi: 10.1113/jphysiol.2002.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Brien JA, Berger AJ. Cotransmission of GABA and glycine to brain stem motoneurons. J Neurophysiol. 1999;82:1638–41. doi: 10.1152/jn.1999.82.3.1638. [DOI] [PubMed] [Google Scholar]
- 17.Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs. diaphragm muscle response to CO2 in rats. J Appl Physiol. 2002;92:878–87. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- 18.Morrison JL, Sood S, Liu X, et al. Glycine at the hypoglossal motor nucleus: genioglossus activity, CO2 responses and the additive effects of GABA. J Appl Physiol. 2002;93:1786–96. doi: 10.1152/japplphysiol.00464.2002. [DOI] [PubMed] [Google Scholar]
- 19.Durand A, Thenot JP, Bianchetti G, Morselli PL. Comparative pharmacokinetic profile of two imidazopyridine drugs: zolpidem and alpidem. Drug Metab Rev. 1992;24:239–66. doi: 10.3109/03602539208996294. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg MJ, Spector R, Chiang CK. Transport of diphenhydramine in the central nervous system. J Pharmacol Exp Ther. 1987;240:717–22. [PubMed] [Google Scholar]
- 21.Schillings RT, Sisenwine SF, Ruelius HW. Disposition and metabolism of lorazepam in the male rat. Drug Metab Dispos. 1977;5:425–35. [PubMed] [Google Scholar]
- 22.Mendelson WB. Hypnotics: basic mechanisms and pharmacology. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 3rd ed. Philadelphia: WB Saunders; 2000. pp. 407–13. [Google Scholar]
- 23.Bonavita CD, Bisagno V, Bonelli CG, Acosta GB, Rubio MC, Wikinski SI. Tolerance to the sedative effect of lorazepam correlates with a diminution in cortical release and affinity for glutamate. Neuropharmacology. 2002;42:619–25. doi: 10.1016/s0028-3908(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 24.Depoortere H, Francon D, van Luijtelaar EL, Drinkenburg WH, Coenen AM. Differential effects of midazolam and zolpidem on sleep-wake states and epileptic activity in WAG/Rij rats. Pharmacol Biochem Behav. 1995;51:571–76. doi: 10.1016/0091-3057(95)00091-a. [DOI] [PubMed] [Google Scholar]
- 25.Kaneko Y, Shimada K, Saitou K, Sugimoto Y, Kamei C. The mechanism responsible for the drowsiness caused by first generation H1 antagonists on the EEG pattern. Methods Find Exp Clin Pharmacol. 2000;22:163–68. [PubMed] [Google Scholar]
- 26.Sood S, Liu X, Liu H, Horner RL. Genioglossus muscle activity and serotonergic modulation of hypoglossal motor output in obese Zucker rats. J Appl Physiol. 2007;102:2240–50. doi: 10.1152/japplphysiol.01229.2006. [DOI] [PubMed] [Google Scholar]
- 27.Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med. 2005;172:1338–47. doi: 10.1164/rccm.200502-258OC. [DOI] [PubMed] [Google Scholar]
- 28.Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med. 2006;174:1264–73. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- 29.Gestreau C, Dutschmann M, Obled S, Bianchi AL. Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respir Physiol Neurobiol. 2005;147:159–76. doi: 10.1016/j.resp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Peever JH, Mateika JH, Duffin J. Respiratory control of hypoglossal motoneurones in the rat. Pflugers Archives – Eur J Physiol. 2001;442:78–86. doi: 10.1007/s004240000502. [DOI] [PubMed] [Google Scholar]
- 31.O'Brien JA, Berger AJ. The nonuniform distribution of the GABA(A) receptor alpha 1 subunit influences inhibitory synaptic transmission to motoneurons within a motor nucleus. J Neurosci. 2001;21:8482–94. doi: 10.1523/JNEUROSCI.21-21-08482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mailliet F, Galloux P, Poisson D. Comparative effects of melatonin, zolpidem and diazepam on sleep, body temperature, blood pressure and heart rate measured by radiotelemetry in Wistar rats. Psychopharmacology. 2001;156:417–26. doi: 10.1007/s002130100769. [DOI] [PubMed] [Google Scholar]
- 33.Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep. 1996;19:827–53. doi: 10.1093/sleep/19.10.827. [DOI] [PubMed] [Google Scholar]
- 34.Hudgel DW, Mulholland M, Hendricks C. Neuromuscular and mechanical responses to inspiratory resistive loading during sleep. J Appl Physiol. 1987;63:603–8. doi: 10.1152/jappl.1987.63.2.603. [DOI] [PubMed] [Google Scholar]
- 35.Wiegand L, Zwillich CW, White DP. Sleep and the ventilatory response to resistive loading in normal men. J Appl Physiol. 1988;64:1186–95. doi: 10.1152/jappl.1988.64.3.1186. [DOI] [PubMed] [Google Scholar]
- 36.Badr MS, Skatrud JB, Dempsey JA, Begle RL. Effect of mechanical loading on expiratory and inspiratory muscle activity during NREM sleep. J Appl Physiol. 1990;68:1195–1202. doi: 10.1152/jappl.1990.68.3.1195. [DOI] [PubMed] [Google Scholar]
- 37.Dick TE, Parmeggiani PL, Orem J. Intercostal muscle activity during sleep in the cat: an augmentation of expiratory activity. Respiration Physiol. 1982;50:255–65. doi: 10.1016/0034-5687(82)90022-6. [DOI] [PubMed] [Google Scholar]
- 38.Leiter JC, Knuth SL, Krol RC, Bartlett D., Jr. The effect of diazepam on genioglossal muscle activity in normal human subjects. Am Rev Respir Dis. 1985;132:216–19. doi: 10.1164/arrd.1985.132.2.216. [DOI] [PubMed] [Google Scholar]
- 39.Roda F, Pio J, Bianchi AL, Gestreau C. Effects of anesthetics on hypoglossal nerve discharge and c-Fos expression in brainstem hypoglossal premotor neurons. J Comp Neurol. 2004;468:571–86. doi: 10.1002/cne.10974. [DOI] [PubMed] [Google Scholar]
- 40.Kuna ST, Remmers JE. Premotor input to hypoglossal motoneurons from Kolliker-Fuse neurons in decerebrate cats. Respir Physiol. 1999;117:85–95. doi: 10.1016/s0034-5687(99)00058-4. [DOI] [PubMed] [Google Scholar]
- 41.Bonora M, St John WM, Bledsoe TA. Differential elevation by protriptyline and depression by diazepam of upper airway respiratory motor activity. Am Rev Respir Dis. 1985;131:41–5. doi: 10.1164/arrd.1985.131.1.41. [DOI] [PubMed] [Google Scholar]
- 42.Hwang JC, St John WM, Bartlett D. Respiratory-related hypoglossal nerve activity: influence of anesthetics. J Appl Physiol. 1983;55:785–92. doi: 10.1152/jappl.1983.55.3.785. [DOI] [PubMed] [Google Scholar]
- 43.Cirignotta F, Mondini S, Zucconi M, Gerardi R, Farolfi A, Lugaresi E. Zolpidem-polysomnographic study of the effect of a new hypnotic drug in sleep apnea syndrome. Pharmacol Biochem Behav. 1988;29:807–9. doi: 10.1016/0091-3057(88)90212-2. [DOI] [PubMed] [Google Scholar]
- 44.Berry RB, Patel PB. Effect of zolpidem on the efficacy of continuous positive airway pressure as treatment for obstructive sleep apnea. Sleep. 2006;29:1052–56. doi: 10.1093/sleep/29.8.1052. [DOI] [PubMed] [Google Scholar]