Abstract
Study Objectives:
To explore absolute concentrations of brain metabolites including gamma amino-butyric acid (GABA) in the medial prefrontal cortex and basal ganglia of young adults with narcolepsy.
Design:
Proton magnetic resonance (MR) spectroscopy centered on the medial prefrontal cortex and the basal ganglia was acquired. The absolute concentrations of brain metabolites including GABA and glutamate were assessed and compared between narcoleptic patients and healthy comparison subjects.
Setting:
Sleep and Chronobiology Center at Seoul National University Hospital; A high strength 3.0 Tesla MR scanner in the Department of Radiology at Seoul National University Hospital.
Patients or Participants:
Seventeen young adults with a sole diagnosis of HLA DQB1 0602 positive narcolepsy with cataplexy (25.1 ± 4.6 years old) and 17 healthy comparison subjects (26.8 ± 4.8 years old).
Interventions:
N/A.
Measurements and Results:
Relative to comparison subjects, narcoleptic patients had higher GABA concentration in the medial prefrontal cortex (t = 4.10, P <0.001). Narcoleptic patients with nocturnal sleep disturbance had higher GABA concentration in the medial prefrontal cortex than those without nocturnal sleep disturbance (t = 2.45, P= 0.03), but had lower GABA concentration than comparison subjects (t = 2.30, P = 0.03).
Conclusions:
The current study reports that young adults with narcolepsy had a higher GABA concentration in the medial prefrontal cortex, which was more prominent in patients without nocturnal sleep disturbance. Our findings suggest that the medial prefrontal GABA level may be increased in narcolepsy, and the increased medial prefrontal GABA might be a compensatory mechanism to reduce nocturnal sleep disturbances in narcolepsy.
Citation:
Kim SJ; Lyoo IK; Lee YS; Sung YH; Kim HJ; Kim JH; Kim KH; Jeong DU. Increased GABA levels in medial prefrontal cortex of young adults with narcolepsy. SLEEP 2008;31(3):342-347.
Keywords: Narcolepsy, GABA, proton MR spectroscopy, medial prefrontal cortex
NARCOLEPSY IS A SLEEP DISORDER CHARACTERIZED BY EXCESSIVE DAYTIME SLEEPINESS AND CATAPLEXY.1 EXCESSIVE DAYTIME SLEEPINESS represents an abnormal sleep control in narcolepsy. Cataplexy has been considered to be a decoupling of two REM sleep phenomena, i.e., cortical activation and atonia. Recently, narcolepsy has strongly been suggested to be caused by hypocretin deficiency,1 as narcoleptic patients have shown a markedly decreased hypocretin concentration in the cerebrospinal fluid (CSF).2,3
However, hypocretin cannot solely regulate the sleep-wake or REM-NREM sleep state. A number of neurotransmitters work in concert with hypocretin for the modulation of the sleep-wake state.4 Among sleep-related neurotransmitters, gamma amino-butyric acid (GABA) and glutamate are major inhibitory and excitatory neurotransmitters, respectively.5,6 Hypocretin neurons have positive feedback mechanism with glutamate and negative feedback mechanism with GABA.4 Therefore, narcolepsy may accompany changes of GABA and/or glutamate.
For in vivo measurement of metabolites including GABA and glutamate in brain disorders, proton magnetic resonance spectroscopy (MRS) has been used.7 There have also been proton MRS studies on narcolepsy. In ventral pontine area or occipito-parietal cortex, no significant metabolite changes have been reported in narcolepsy.8,9 In contrast, in the hypothalamus of narcoleptic patients with cataplexy, decreased N-acetyl-aspartate (NAA)/creatine (Cr) ratio has been reported.9
However, to the best of our knowledge, GABA or glutamate changes in narcoleptic brains has not been studied by proton MRS until now. This can be attributable to difficulties in differentiating GABA, glutamate, and glutamine at lower Tesla scanners, as these metabolites have largely overlapped resonances on proton MR spectra.8,10 As previous MRS studies on narcolepsy used a 1.5 Tesla magnetic resonance (MR) scanner, it was difficult to accurately measure the level of GABA and glutamate. In the present study, a 3.0 Tesla MR scanner was used to detect in vivo changes of GABA and glutamate levels.
In addition, previous MRS studies have calculated relative metabolite ratios, typically the ratio over Cr, rather than measuring absolute concentrations. Relative metabolites ratios can be substantially affected by even small changes of criteria metabolites. In the present study, absolute metabolite concentrations were reliably measured from proton MRS.11
Previous proton MRS studies on narcolepsy have investigated brainstem, hypothalamus and occipital cortex. Other brain areas such as medial prefrontal cortex or basal ganglia have not been explored by MRS yet, although various other brain imaging methods have reported abnormalities of these brain areas in narcoleptic patients.12–14 Therefore, we intended to investigate GABA and glutamate levels in the medial prefrontal cortex and the basal ganglia of narcoleptic patients.
We planned to explore absolute concentrations of brain metabolites including GABA and glutamates in the medial prefrontal cortex and basal ganglia of young adults with a sole diagnosis of DQB1 0602 positive narcolepsy with cataplexy, using a high strength 3.0 Tesla MR scanner. We hypothesized that narcoleptic patients would have an altered concentration of GABA or glutamate in the medial prefrontal cortex or the basal ganglia, relative to healthy comparison subjects.
METHODS
Subjects
Seventeen young narcoleptic patients (14 men, 3 women, 25.1 ± 4.6 years of age) were recruited from the Sleep and Chronobiology Center at the Seoul National University Hospital. Inclusion criteria for narcoleptic patients were (1) ages: 17–35 years, (2) subjective excessive daytime sleepiness, (3) presence of cataplexy, (4) DQB1 0602 positive on human leukocyte antigen typing (HLA) typing, (5) two or more sleep onset REM periods on the multiple sleep latency test (MSLT), (6) mean sleep latency under 5 minutes on MSLT.
Exclusion criteria were (1) current or past significant medical or neurological illness including hypertension, hepatitis, and diabetes mellitus; (2) sleep disorders potentially related to daytime sleepiness such as obstructive sleep apnea syndrome, periodic limb movement disorder, and REM sleep behavior disorder; (3) current or lifetime Axis I psychiatric disorders, as identified by the Structured Clinical Interview for DSM-IV (SCID-IV); (4) antisocial or borderline personality disorders, as identified by the Personality Disorder Questionnaire-4; (5) lifetime exposure to any other DSM-IV dependence- or abuse-related drugs, except nicotine, caffeine, social drinking of alcohol; (6) previous history of attention deficit hyperactivity disorder during childhood or I.Q. <71; and (7) contraindications to MR scans.
Seventeen healthy comparison subjects (12 men and 5 women, 26.8 ± 4.8 years of age, with a range of 17–35 years) were recruited through advertisements at local newspapers. Same exclusion criteria for narcoleptic patients were also applied to healthy comparison subjects.
Study protocol was approved by the Institutional Review Board at the Seoul National University Hospital. After complete description of the study to the subjects, written informed consent was obtained.
Magnetic Resonance Imaging and Proton Magnetic Resonance Spectroscopy
Brain magnetic resonance imaging (MRI) was performed using a 3.0 Tesla General Electric (GE) whole body imaging system (GE VH/i, USA). A three-dimensional spoiled gradient echo pulse sequence was used to produce 248 0.7-mm-thick contiguous sagittal images (echo time [TE] = 14 ms; repetition time [TR] = 5.7 ms; inversion time [TI] = 400 ms; 256 x 256 matrix; field of view [FOV] = 22 cm; flip angle = 20°; 1 number of excitation [NEX]).
Axial T-2 weighted images (TE = 118 ms; TR = 3,500 ms; 256 x 192 matrix; FOV = 22 cm; flip angle = 90°; 3 NEX, 5-mm-thick slices, 1.5 mm skip) as well as fluid attenuated inversion recovery (FLAIR) axial images (TE = 145 ms; TR = 9,900 ms; TI = 2,250 ms; 256 x 192 matrix; FOV = 22 cm; flip angle = 90°; 1 NEX; 5-mm-thick slices, 1.5 mm skip) were obtained to screen for brain structural abnormalities. No brain structural abnormalities were noted for either group of subjects when read by an experienced neuroradiologist.
Localized point resolved spectroscopy (PRESS) pulse sequence was performed using a single-voxel proton MRS obtained by a quadrature head coil on a 3 Tesla whole body MR unit (GE VH/i, USA). Measurement parameters were as follows; TR/TE = 2,000/35 msec, phase-cycling = 8, voxel of interest (VOI) = 15x15x15 mm, acquisition time=128 x 2 sec, bandwidth = 2,500 Hz. Concentrations in institutional units of each metabolite were calculated by using the pulse sequence according to values in the literature.11,15 All data were processed by using automated routines by a trained research assistant (S.Y.H.) blinded to the clinical information of study subjects, including diagnosis. All narcoleptic patients and comparison subjects were assessed within the same one-year period. In addition, magnet characteristics have been stable during this period with regular maintenance and phantom checkups. Phantoms containing specific concentrations of common metabolites were measured in order to assess the variation of the magnet stability.
All subjects were asked to refrain from any prescribed medications including those for narcolepsy for at least 7 days before scanning, and caffeine- or alcohol-related beverages for more than 24 hours before scanning. Smokers were asked not to smoke for 2 hours before scanning. Twelve narcoleptic patients and 6 healthy comparison subjects reported sleep during scans.
Voxel Placement
Medial prefrontal cortex voxels was chosen to maximally include both left and right frontal gray matter (Figure 1). In the axial slice, which showed the most anterior boundary of the genu of corpus callosum, voxel (15×15×15 mm3) was placed closest to the genu of the corpus callosum and centered on the interhemispheric fissure. This voxel, which contains predominantly the bilateral anterior cingulate gray matter as previously described,16 was segmented into gray, white, and CSF using Analyze 5 (BIR). Compositions of the brain tissue and CSF were 94.7 ± 3.1 and 4.3% ± 1.9% respectively for narcoleptic patients and 96.1 ± 3.5 and 4.5% ± 2.4% respectively for healthy comparison subjects. There was no significant difference between tissue compositions between groups (t-test, df = 32, t = 1.23, P = 0.23; df = 32, t = 0.27, P = 0.79, respectively).
Figure 1.
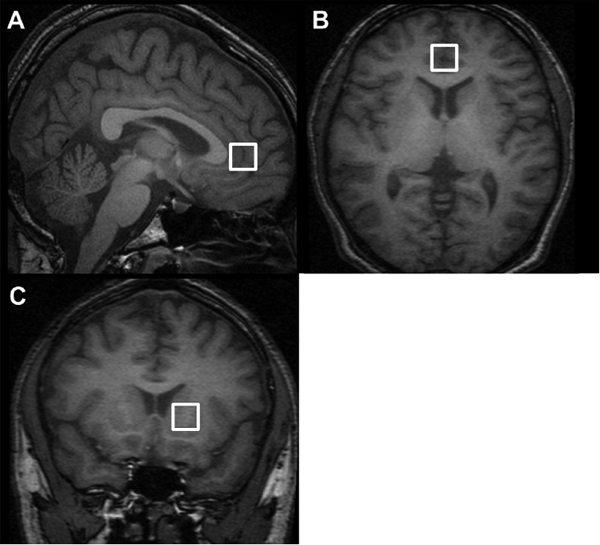
Voxels placed in brains of narcoleptic patients and healthy comparison subjects A) and B): medial prefrontal cortex C) basal ganglia
Basal ganglia voxel included left side caudate and putamen. Voxel was placed to include only tissue avoiding tissue-air interfaces (figure 1).
Absolute metabolite concentrations, in mM/kg concentration (institutional unit), were estimated with LCModel software,11 using the unsuppressed water signal as an internal concentration reference. The standard GE libraries of model metabolite spectra provided with the LCModel were used in a basis set. The LCModel is fully automatic and user independent.
For details of the LCModel procedure, it analyzes in vivo spectrum as a linear combination of a basis set of in vitro spectrum. This utilizes complete model spectra of metabolite solution in vitro in order to incorporate maximum information and uniqueness into analysis. Nearly model-free constrained regularization method is used for convolution and baseline.
Statistical Analysis
Group differences in demographic variables involving continuous data and categorical data were evaluated using an independent t-test and Fisher's exact test for the 2 x k table, respectively.
Multiple regression analysis was also used to test differences in metabolite concentrations between groups, controlling for demographic and clinical variables. Correlation between metabolite concentrations and clinical variables were tested using Pearson correlation analysis. Statistical significance was defined at an alpha of < 0.05 and two-tailed. All error ranges were expressed as standard deviations. Statistica 6.1 for Windows was used for all computations.
RESULTS
Demographic and clinical characteristics of study subjects, including data from polysomnography and sleep questionnaires, are presented in Table 1. There was no significant difference in age, sex, prevalence of social alcohol drinking or smoking, or handedness between narcoleptic patients and healthy comparison subjects. Among 17 narcoleptic patients, 11 had been medicated for narcolepsy; 6 were drug-naïve, as they were newly diagnosed during the enrollment periods for the current study. Nine narcoleptic patients suffered from nocturnal sleep disturbance more than 3 days a week while 8 narcoleptic patients did not.
Table 1.
Demographic and Clinical Characteristics of Narcoleptic Patients and Healthy Comparison Subjects
| Narcoleptic patients (n=17) Mean ± SD | Healthy comparison subjects (n=17) Mean ± SD | |
|---|---|---|
| Demographic variables | N (%) | N (%) |
| Age (yr) | 25.1 ± 4.6 | 26.8 ± 4.8 |
| Sex (men) | 14 (82.4 %) | 12 (70.6 %) |
| Handedness (right) | 17 (100 %) | 15 (88.2 %) |
| Social alcohol drinker | 11 (76.5 %) | 14 (82.4 %) |
| Current smoker | 8 (47.1 %) | 8 (47.1 %) |
| Sleep questionnaire | ||
| Ullanlinna Narcolepsy Scale | 27.9 ± 6.7 | - |
| Epworth Sleepiness Scale | 16.9 ± 3.5 | - |
| Duration of narcoleptic symptom | ||
| Daytime sleepiness (yr) | 11.0 ± 6.8 | - |
| Cataplexy (yr) | 9.0 ± 6.5 | - |
| Nocturnal PSG | - | |
| TIB (min) | 482.8 ± 38.5 | - |
| Sleep latency (min) | 2.5 ± 4.0 | - |
| SPT (min) | 479.3 ± 38.7 | - |
| TST (min) | 439.3 ± 52.2 | - |
| Sleep efficiency (%) | 91.0 ± 8.0 | - |
| TSWS (%) | 10.3 ± 8.4 | - |
| TREM (%) | 16.8 ± 6.4 | - |
| REM latency (min) | 51.9 ± 70.2 | - |
| MSLT | - | |
| SOREMP (number) | 4.5 ± 0.9 | - |
| Sleep latency (min) | 1.5 ± 1.5 | - |
Abbreviation: SD: standard deviation; PSG: polysomnography; TIB: time in bed; SPT: sleep period time; TST: total sleep time; TSWS: total slow wave sleep; TREM: total rapid eye movement sleep; REM: rapid eye movement; MSLT: multiple sleep latency test; SOREMP: sleep onset rapid eye movement periods; -: not applicable
Relative to comparison subjects, narcoleptic patients had higher GABA concentration in the medial prefrontal cortex (narcoleptic patients: 1.15 ± 0.36 mM/kg; comparison group: 0.62 ± 0.39 mM/kg; independent t-test, t = 4.10, df = 32, P <0.001). Concentrations of other brain metabolites in the medial prefrontal cortex were not significantly different between narcoleptic patients and healthy comparison subjects. In addition, there were no significant differences in any brain metabolites of the basal ganglia between two groups. Representative spectra from a subject with narcolepsy and a healthy comparison subject are demonstrated in Figure 2. Details of the measurement are presented in Table 2.
Figure 2.
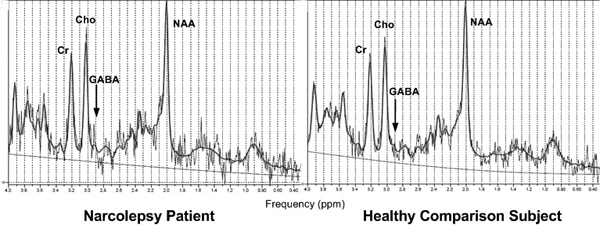
Representative 1H-MR spectrum for the medial prefrontal cortex. The spectrum was performed using a localized point resolved spectroscopy (PRESS) pulse sequence. Abbreviations: GABA: gamma-aminobutyric acid; NAA: N-acetyl Aspartate; Cr: creatine; Cho: Choline.
Table 2.
Concentrations of Brain Metabolites in Narcoleptic Patients and Healthy Comparison Subjects
| Metabolites (mM/kg) | Narcoleptic patients (n=17) Mean ± SD | Healthy comparison subjects (n=17) Mean ± SD | t | P |
|---|---|---|---|---|
| Medial prefrontal cortex | ||||
| GABA | 1.15 ± 0.36 | 0.62 ± 0.39 | 4.10 | <0.001* |
| NAA | 10.69 ± 0.90 | 10.31 ± 0.94 | 1.20 | 0.24 |
| Cr | 9.18 ± 0.86 | 8.99 ± 1.00 | 0.61 | 0.54 |
| Cho | 3.00 ± 0.46 | 2.90 ± 0.41 | 0.83 | 0.51 |
| mI | 8.20 ± 1.58 | 7.63 ± 1.73 | 1.01 | 0.32 |
| lactate | 2.43 ± 1.68 | 1.58 ± 1.38 | 0.55 | 0.59 |
| Glx | 22.29 ± 2.91 | 19.83 ± 4.01 | 2.04 | 0.0496* |
| glutamate | 13.82 ± 2.61 | 12.44 ± 3.75 | 1.32 | 0.20 |
| glutamine | 8.48 ± 2.91 | 7.39 ± 3.78 | 0.97 | 0.34 |
| Basal ganglia | ||||
| GABA | 1.25 ± 0.58 | 1.11 ± 0.57 | 0.68 | 0.50 |
| NAA | 10.57 ± 1.57 | 10.30 ± 1.33 | 0.55 | 0.58 |
| Cr | 9.22 ± 1.14 | 9.52 ± 0.89 | 0.85 | 0.40 |
| Cho | 2.66 ± 0.44 | 2.56 ± 0.28 | 0.83 | 0.41 |
| mI | 5.63 ± 1.83 | 5.01 ± 1.90 | 0.96 | 0.34 |
| lactate | 1.83 ± 1.47 | 1.59 ± 1.03 | 0.30 | 0.77 |
| Glx | 21.42 ± 3.15 | 20.85 ± 4.39 | 0.44 | 0.66 |
| glutamate | 13.14 ± 2.91 | 13.03 ± 2.94 | 0.12 | 0.90 |
| glutamine | 8.28 ± 3.01 | 7.82 ± 3.00 | 0.44 | 0.66 |
Abbreviations: SD: standard deviation; GABA: gamma-aminobutyric acid; NAA: N-acetyl Aspartate; Cr: creatine; Cho: Choline; mI: Myo-inositol; Glx: glutamate/glutamine/GABA
The influences by potential confounders including age, sex, and voxel tissue composition were tested in additional analyses. After controlling for age and sex, between-group differences remained similar and significant. When between-group comparisons were repeated controlling for voxel tissue composition, differences in above metabolites remained significant. In addition, results remained similar and significant after controlling for smoking or social drinking.
There were no significant correlations between medial prefrontal GABA concentration and clinical variables including Ullanlinna Narcolepsy Scale score, Epworth Sleepiness Scale, and polysomnographic measure within the narcoleptic patients.
We compared narcoleptic patients with nocturnal sleep disturbance (n=9) and those without nocturnal sleep disturbance (n=8). Relative to narcoleptic patients with nocturnal sleep disturbance, those without had higher GABA concentration in the medial prefrontal cortex (narcoleptic patients with nocturnal sleep disturbance: 0.97 ± 0.33 mM/kg; narcoleptic patients without nocturnal sleep disturbance: 1.34 ± 0.30 mM/kg; independent t-test, t = 2.45, df = 16, P = 0.03). However, when compared with comparison subjects, GABA concentration in the medial prefrontal cortex of the narcoleptic patients with nocturnal sleep disturbance were remained high (independent t-test, t = 2.30, df = 24, P = 0.03).
In addition, we compared drug-naïve narcoleptic patients (n=6) with narcoleptic patients who had been exposed to medication for narcolepsy (n=11). There were no differences in brain metabolites between drug-naïve and drug-exposed narcoleptic patients in either the medial prefrontal cortex or the basal ganglia. In addition, there were no significant differences in brain metabolites between narcoleptic patients who slept during scan (n=12) and narcoleptic patients who did not (n=5).
DISCUSSION
In the current study, young adults with narcolepsy had a higher GABA concentration in the medial prefrontal cortex. This was more prominent in a subgroup of narcoleptic patients without nocturnal sleep disturbance. To the best of our knowledge, the present report constitutes the first MRS study investigating GABA concentrations in subjects with narcolepsy.
As hypothesized, narcoleptic patients had increased GABA concentrations in the medial prefrontal cortex. Our findings are in accord with previous imaging studies that report lower glucose metabolism and blood flow in the same areas of narcoleptic patients,13,14 with regard to the fact that GABA is the most profuse inhibitory neurotransmitter in the brain.5
Unexpectedly, the frontal GABA increase was more salient in narcoleptic patients without nocturnal sleep disturbance. Our finding indicates that the frontal GABA increase in narcolepsy might be more related to nocturnal symptoms rather than to other typical REM-related daytime symptoms. This finding corresponds with the previous report that slow wave sleep (SWS), but not REM sleep, was increased by chronic exposure to excessive GABA.17 In addition, gamma-hydroybutyrate (GHB), a metabolite of GABA, is known to promote nocturnal SWS and improve the nocturnal sleep disturbance in narcolepsy, while other stimulants for narcolepsy do not.18 Therefore, it can be suggested that the GABA increase in narcoleptic patients might attenuate nocturnal sleep disturbance by enhancing SWS.
Recently, it has become established that narcolepsy is caused by hypocretin deficiency. GABA increase under the hypocretin deficiency of narcolepsy may seem counterintuitive, as hypocretin has usually been reported to excite GABA neurons.4 Paradoxically higher GABA level in narcolepsy may arise from the unknown multifaceted interplay between neurotransmitters,4,5 the compensatory GABA activation to protect hypocretin neuron against narcolepsy-related excitotoxic injury,19 or the different modes of hypocretin action on GABA neuron depending on exposure time20 or brain regions.21–25 However, the reason of GABA increase in narcolepsy cannot be affirmed by our study design.
In the current study, higher GABA concentration in narcolepsy was observed only in the medial prefrontal cortex. This observation implies that GABA levels of the medial prefrontal cortex may more easily be influenced by narcolepsy than those of basal ganglia. However, the reason why the GABA increase of narcolepsy can be found only in the medial prefrontal cortex still remains unclear.
Limitations of the present study may include a relatively small sample size. Our strict inclusion/exclusion criteria for recruiting homogeneous subjects may be in part responsible for this issue. Therefore, the result of the current study should be considered as a preliminary finding. Future studies with a lager sample size are necessary for confirming our results. In addition, the GABA signal has rather low signal-to-noise ratio (SNR) in the proton MRS, with potentially less-than-desirable reliability and validity of measurement compared to high SNR metabolites such as creatine and NAA.11 Readers should consider this limitation while interpreting the results.
Another limitation is the fact that hypocretin level in the CSF was not measured in the present study. Consequently, narcoleptic patients without hypocretin deficiency cannot be excluded in the present study. The fact that only six narcoleptic patients were drug-naïve may be a potential limitation. Although all narcoleptic patients discontinued prescribed medications before MR scans, long-term effects of narcoleptic medications on brain metabolites cannot be excluded. However, there were no differences in metabolite levels between drug-naïve and drug-exposed narcoleptic patients.
In contrast, our study has several points of strength. Absolute concentrations, rather than the ratio of metabolites, were utilized, thus minimizing the potential variance caused by metabolite changes in denominators. Moreover, we used a high strength 3.0 T scanner, which has 1.41-fold SNR increase over the 1.5 T scanner at the same condition. Highly homogeneous sample may be another strength. Narcoleptic patients commonly have comorbid psychiatric or sleep disorders,26 in which brain metabolite changes have been reported in a number of prior proton MRS studies.7, 27,28 As psychiatric and sleep disorders are difficult to diagnose solely based on past history, we excluded subjects with these disorders by structured interview and nocturnal polysomnography. Moreover, we confined the age of our subjects to under 35 years old in order to minimize potential confounding effects of aging on the brain metabolite levels.
In conclusion, we report GABA increases in the medial prefrontal cortex of narcoleptic patients. The increase in brain GABA concentrations was greater in narcoleptic patients without nocturnal sleep disturbance than those with it. High medial prefrontal GABA levels in might be associated with the adaptation mechanisms of narcoleptic patients, which may explain their nocturnal sleep disturbances.
ACKNOWLDEGMENTS
This research was supported by a grant (M103KV010021-07K2201-02110, LyooIK) from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, the Republic of Korea.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no conflicts of interest.
REFERENCES
- 1.Siegel JM. Narcolepsy: a key role for hypocretins (orexins) Cell. 1999;98:409–12. doi: 10.1016/s0092-8674(00)81969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 3.Overeem S, Scammell TE, Lammers GJ. Hypocretin/orexin and sleep: implications for the pathophysiology and diagnosis of narcolepsy. Curr Opin Neurol. 2002;15:739–45. doi: 10.1097/01.wco.0000044800.53746.5a. [DOI] [PubMed] [Google Scholar]
- 4.Hajak G, Geisler P. Orchestrating sleep-wake functions in the brain. Nat Med. 2003;9:170–1. doi: 10.1038/nm0203-170. [DOI] [PubMed] [Google Scholar]
- 5.Gottesmann C. GABA mechanisms and sleep. Neuroscience. 2002;111:231–9. doi: 10.1016/s0306-4522(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 6.Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–86. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Lyoo IK, Renshaw PF. Magnetic resonance spectroscopy: current and future applications in psychiatric research. Biol Psychiatry. 2002;51:195–207. doi: 10.1016/s0006-3223(01)01313-0. [DOI] [PubMed] [Google Scholar]
- 8.Ellis CM, Simmons A, Lemmens G, Williams SC, Parkes JD. Proton spectroscopy in the narcoleptic syndrome. Is there evidence of a brainstem lesion? Neurology. 1998;50:S23–6. doi: 10.1212/wnl.50.2_suppl_1.s23. [DOI] [PubMed] [Google Scholar]
- 9.Lodi R, Tonon C, Vignatelli L, et al. In vivo evidence of neuronal loss in the hypothalamus of narcoleptic patients. Neurology. 2004;63:1513–5. doi: 10.1212/01.wnl.0000142259.94107.4c. [DOI] [PubMed] [Google Scholar]
- 10.Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–13. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- 11.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann C, Schuld A, Pollmacher T, Auer DP. Reduced cortical gray matter in narcolepsy: preliminary findings with voxel–based morphometry. Neurology. 2002;58:1852–5. doi: 10.1212/wnl.58.12.1852. [DOI] [PubMed] [Google Scholar]
- 13.Joo EY, Tae WS, Kim JH, Kim BT, Hong SB. Glucose hypometabolism of hypothalamus and thalamus in narcolepsy. Ann Neurol. 2004;56:437–40. doi: 10.1002/ana.20212. [DOI] [PubMed] [Google Scholar]
- 14.Joo EY, Hong SB, Tae WS, et al. Cerebral perfusion abnormality in narcolepsy with cataplexy. Neuroimage. 2005;28:410–6. doi: 10.1016/j.neuroimage.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci USA. 1993;90:5662–6. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamasue H, Fukui T, Fukuda R, et al. 1H-MR spectroscopy and gray matter volume of the anterior cingulate cortex in schizophrenia. Neuroreport. 2002;13:2133–7. doi: 10.1097/00001756-200211150-00029. [DOI] [PubMed] [Google Scholar]
- 17.Arnulf I, Konofal E, Gibson KM, et al. Effect of genetically caused excess of brain gamma-hydroxybutyric acid and GABA on sleep. Sleep. 2005;28:418–24. doi: 10.1093/sleep/28.4.418. [DOI] [PubMed] [Google Scholar]
- 18.Mamelak M, Black J, Montplaisir J, Ristanovic R. A pilot study on the effects of sodium oxybate on sleep architecture and daytime alertness in narcolepsy. Sleep. 2004;27:1327–34. doi: 10.1093/sleep/27.7.1327. [DOI] [PubMed] [Google Scholar]
- 19.Katsuki H, Akaike A. Quinolinic acid toxicity on orexin neurons blocked by gamma aminobutyric acid type A receptor stimulation. Neuroreport. 2005;16:1157–61. doi: 10.1097/00001756-200508010-00005. [DOI] [PubMed] [Google Scholar]
- 20.Viggiano A, Monda M, Viggiano A, Fuccio F, De Luca B. Extracellular GABA in the medial hypothalamus is increased following hypocretin-1 administration. Acta Physiol Scand. 2004;182:89–94. doi: 10.1111/j.1365-201X.2004.01298.x. [DOI] [PubMed] [Google Scholar]
- 21.Wu M, Zhang Z, Leranth C, Xu C, van den Pol AN, Alreja M. Hypocretin increases impulse flow in the septohippocampal GABAergic pathway: implications for arousal via a mechanism of hippocampal disinhibition. J Neurosci. 2002;22:7754–65. doi: 10.1523/JNEUROSCI.22-17-07754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korotkova TM, Eriksson KS, Haas HL, Brown RE. Selective excitation of GABAergic neurons in the substantia nigra of the rat by orexin/hypocretin in vitro. Regul Pept. 2002;104:83–9. doi: 10.1016/s0167-0115(01)00323-8. [DOI] [PubMed] [Google Scholar]
- 23.van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–71. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burdakov D, Liss B, Ashcroft FM. Orexin excites GABAergic neurons of the arcuate nucleus by activating the sodium—calcium exchanger. J Neurosci. 2003;23:4951–7. doi: 10.1523/JNEUROSCI.23-12-04951.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.John J, Wu MF, Kodama T, Siegel JM. Intravenously administered hypocretin-1 alters brain amino acid release: an in vivo microdialysis study in rats. J Physiol. 2003;548:557–62. doi: 10.1113/jphysiol.2002.038729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniels E, King MA, Smith IE, Shneerson JM. Health-related quality of life in narcolepsy. J Sleep Res. 2001;10:75–81. doi: 10.1046/j.1365-2869.2001.00234.x. [DOI] [PubMed] [Google Scholar]
- 27.Ham BJ, Chey J, Yoon SJ, et al. Decreased N-acetyl-aspartate levels in anterior cingulate and hippocampus in subjects with post-traumatic stress disorder: a proton magnetic resonance spectroscopy study. Eur J Neurosci. 2007;25:324–9. doi: 10.1111/j.1460-9568.2006.05253.x. [DOI] [PubMed] [Google Scholar]
- 28.Kamba M, Inoue Y, Higami S, Suto Y. Age-related changes in cerebral lactate metabolism in sleep-disordered breathing. Neurobiol Aging. 2003;24:753–60. doi: 10.1016/s0197-4580(02)00191-4. [DOI] [PubMed] [Google Scholar]


