Abstract
Study Objectives:
To provide guidelines for collecting and analyzing urinary, salivary, and plasma melatonin, thereby assisting clinicians and researchers in determining which method of measuring melatonin is most appropriate for their particular needs and facilitating the comparison of data between laboratories.
Methods:
A modified RAND process was utilized to derive recommendations for methods of measuring melatonin in humans.
Results:
Consensus-based guidelines are presented for collecting and analyzing melatonin for studies that are conducted in the natural living environment, the clinical setting, and in-patient research facilities under controlled conditions.
Conclusions:
The benefits and disadvantages of current methods of collecting and analyzing melatonin are summarized. Although a single method of analysis would be the most effective way to compare studies, limitations of current methods preclude this possibility. Given that the best analysis method for use under multiple conditions is not established, it is recommended to include, in any published report, one of the established low threshold measures of dim light melatonin onset to facilitate comparison between studies.
Citation:
Benloucif S; Burgess HJ; Klerman EB; Lewy AJ; Middleton B; Murphy PJ; Parry BL; Revell VL. Measuring melatonin in humans. J Clin Sleep Med 2008;4(1):66-69.
Keywords: Plasma melatonin, salivary melatonin, 6-sulphatoxymelatonin, DLMO
Melatonin synthesis from the pineal gland is regulated by the circadian pacemaker located in the suprachiasmatic nuclei and by ocular light exposure. Melatonin has a circadian rhythm that peaks during the night in normally entrained individuals. In the absence of light and other synchronizing signals, the rhythm of melatonin production persists with an elevation that occurs during the subjective, as opposed to the actual, night. There is a relatively direct anatomic pathway between the suprachiasmatic nuclei and the pineal gland, and comparatively few exogenous factors are known to affect melatonin concentrations (see Table 1 from Arendt, 2005, for a summary of these factors).1 As a result, the rhythm of melatonin production has been shown to reflect both the phase and, if collected over more than 1 cycle, the period of the endogenous circadian oscillator, thus providing a reliable means to estimate the timing of the internal circadian clock located in the suprachiasmatic nuclei.
The circadian melatonin rhythm is currently the most commonly used circadian phase marker in humans.2–4 A variety of methods for sampling and analyzing melatonin have been described, but there are no established guidelines on when and how these various methods should be used. The multitude of assessment methods described can be confusing to those seeking to assess circadian rhythms in their own patients. Furthermore, the variety of melatonin phase markers reported in the literature increases the difficulty of comparing results from different studies, and the lack of comparable normative values impedes evaluation of results from clinical populations.
This workgroup was formed following an Associated Professional Sleep Societies workshop on the use of melatonin as a circadian phase marker. Our goal was to achieve consensus for collecting and analyzing melatonin, thereby assisting clinicians and researchers in determining which method of measuring melatonin is most appropriate for their particular needs, as well as facilitating the comparison of data among researchers and clinicians. We present here a consensus statement on the preferred uses of urine, saliva, and blood samples and discuss the advantages and disadvantages of each approach. Also discussed are factors that may affect the measurement of melatonin and the different circadian phase markers that might be derived from these methods.
METHODS
This workgroup was formed by the discussion leaders of a 2005 Associated Professional Sleep Societies workshop on the use of melatonin as a circadian phase marker. Initial discussions, conducted by email, identified a number of areas of disagreement. We utilized a modified RAND process to determine the level of consensus for each method of collecting or analyzing melatonin under specified conditions.5 Briefly, this consisted of voting independently to assess the acceptability of each of 54 separate items. The “acceptability” of a particular item was based on reliability, validity, and practical utility. A conference call was then held to assess the level of agreement and disagreement for each item, to discuss reasons for disagreement, and to determine areas of consensus. A consensus-based document was drafted and recirculated for comments and revisions. The draft document was finalized upon approval of all of the workgroup members.
RESULTS
The workgroup's consensus-based summary and recommendations for collection and analysis of urinary, salivary, and plasma melatonin are detailed below. The utility of these methods for studies conducted outside of the clinic or inpatient facility in the natural living environment (“field studies”), studies conducted primarily for phase assessment in a clinical setting (“clinical studies”), and research studies conducted in an inpatient facility under controlled conditions (“research studies”) are described.
Sampling
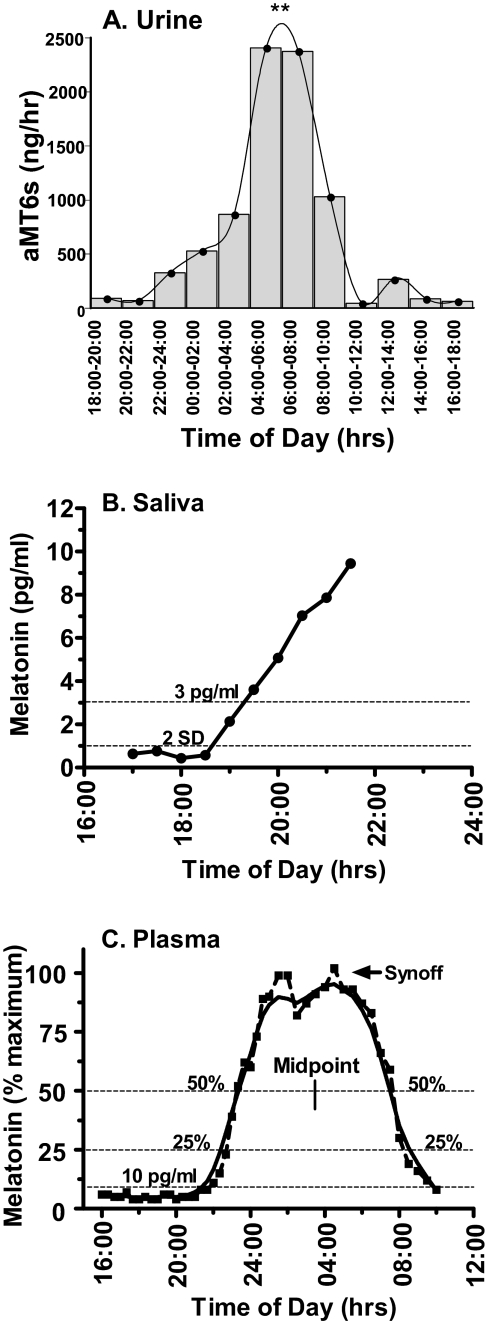
The primary urinary metabolite of melatonin, 6-sulphatoxymelatonin (aMT6s), collected every 2 to 8 hours over a 24- to 48-hour period, is a practical method to estimate the global timing and amount of melatonin production (Figure 1A). Urine sampling is an established method for field, clinical, and research studies. In special populations such as infants and older adults with dementia, collection of urine by a caretaker may be the most practical approach. Overnight aMT6s excretion rates or levels can be calculated from the first morning void. Thus, this method does not require any disruption of sleep. The phase of the rhythm is typically estimated from the timing of the acrophase (time of fitted peak) of a cosine fitted curve (see Figure 1A). For many field studies, urine collection is the most feasible method of circadian phase assessment. However, the practicality of this method should be weighed against the possibility that measurement of the phase of the circadian aMT6s rhythm with overnight or 2- to 8-hour bins may be less precise than the phase measured with more frequent sampling. The frequency of aMT6s measurement is limited by ability to void and compliance with collection. Frequent sampling, typically conducted with blood or saliva samples as described below, may be required if small phase changes are of interest.
Figure 1.
Illustrations of 3 melatonin sample types and their associated phase estimates. (A) 24-hour rhythm of the primary urinary melatonin metabolite 6-sulphatoxymelatonin (aMT6s) derived from urine samples collected in 2-h bins under dim light. The fitted curve reveals a significant 24-hour rhythm with maximum levels observed between 04:00 and 08:00 (**p < 0.01). (B) Salivary melatonin profile collected under dim-light conditions. The low-threshold dim-light melatonin onset (DLMO) was defined as either the first sample to exceed and remain above a threshold of 3 pg/mL or that was 2 SD above the mean of the first 3 baseline samples (2 SD). (C) Overnight plasma melatonin profile, plotted as a percentage of maximum (dashed line) and smoothed with a Lowess curve fit to the raw data (solid line). Some frequently used phase markers are shown: DLMO at 10 pg/mL, DLMO or dim-light melatonin offset (DLMOff) at 25% or 50% of maximum levels, the midpoint, and the termination of melatonin synthesis (Synoff).
Saliva sampling is a relatively practical and reliable method for field, clinical, and research trials, provided that samples are taken every 30 to 60 minutes under dim light (< 30 lux) for at least 1 hour prior to and throughout the expected rise in melatonin (Figure 1B). Saliva collection can be conducted at home (as part of a field study) if the subjects remain in dim light and follow instructions to avoid contamination of samples with food particles, food dye, or blood. However, the disruption or complete deprivation of sleep that occurs during frequent salivary sample collection limits its overnight use. Most assays require a volume of at least 0.4 mL per tube or a minimum of 1 mL for duplicates; young children and older adults may require monitoring or assistance to ensure compliance with the protocol and to obtain a sufficient quantity of sample for analysis.
Blood is typically sampled at frequent intervals through the use of intravenous catheters. It is a common practice to insert the intravenous catheter at least 2 hours before sampling to ensure that any increase in adrenergic levels during catheter insertion does not affect melatonin levels. The addition of long-line tubing can allow frequent sampling during the evening and throughout the night without major disturbance of sleep. Sleep may be disrupted, however, if there is discomfort or pain associated with the intravenous line or if there is a need to replace the catheter during the night.
Plasma melatonin levels are about 3 times greater than levels obtained from saliva.6 Thus, when comparing results between studies, a 3 pg/mL salivary dim-light melatonin onset (DLMO) would be comparable to a plasma DLMO of approximately 10 pg/mL. The higher melatonin levels present in plasma allow greater resolution and sensitivity than sampling by urine or saliva, particularly for individuals with low melatonin concentrations.
Afternoon-through-overnight or 24-hour plasma melatonin profiles can provide accurate measures of circadian phase, duration, and amplitude (Figure 1C). Therefore, if blood is sampled at frequent intervals (e.g., every 20 to 30 minutes) throughout the night, plasma melatonin can be the most informative of these 3 sample types. However, the loss of a viable vein may become problematic in some subjects, especially those who are overweight, elderly, or female. Because plasma sampling is via an intravenous catheter, there are several potential problems: it is invasive and associated with a slight health risk, there may be data loss due to technical problems related to the intravenous catheter and long-line tubing, and it requires trained medical personnel. Therefore, plasma sampling is not recommended for field studies or routine clinical use.
Analysis
A variety of methods are currently used to determine circadian phase from the nocturnal melatonin profile, including absolute thresholds, relative thresholds (percentage of maximum or 24-hour mean), and curve-fitting techniques (Figure 1). If only a partial melatonin profile is available, e.g., from sampling for a portion of the night, phase can only be determined by the time of the rise or the fall in melatonin levels. When sampled under dim light, these phase markers are commonly referred to as the DLMO or dim-light melatonin offset (DLMOff).2,4 DLMO is an approximation of the onset of synthesis and secretion. Although there is no standard definition of this measure, it is currently the most commonly reported phase marker of the melatonin rhythm. If the individual's circadian phase is approximately normal, then their DLMO will be observed about 2 to 3 hours prior to habitual sleep. It should be kept in mind that, although the absence of a detectable level of melatonin at the time of the expected rise may indicate low melatonin synthesis, very low levels might also result from active melatonin production at another time (e.g., during midday), reflecting an abnormal circadian phase.
Three of the most frequently used ways of determining DLMO (or DLMOff) from a partial melatonin profile are (1) an absolute threshold in the range of 2 to 10 pg/mL, (2) a threshold calculated at 2 SD above the average baseline (i.e., 3 or more pre-rise) samples, and (3) a visual estimate of the point of change from baseline to rising (or declining) levels (Figure 1B). The choice of DLMO may depend on the level of melatonin produced and the sensitivity of the assay used. Although a low-threshold DLMO may reduce variations in phase estimates resulting from individual differences in amplitude, there is limited evidence to support the use of any one low-threshold DLMO over another.2,4
The termination of melatonin synthesis (Synoff) is also likely to represent a unique and important aspect of melatonin physiology, although there is no consensus on its best method of measurement.2,4 Synoff represents the transition from maximum nocturnal melatonin production to the morning decline (synthesis off); DLMOff provides a measure of the return to daytime melatonin levels (Figure 1C). Thus, although some phase markers are thought to reflect the onset and offset of melatonin synthesis, others may reflect the timing and duration (night to morning) of physiologically effective levels of circulating melatonin. Results should be interpreted cautiously with disease states or medication use that affects the liver or kidney, since the morning decline can be influenced by alterations in melatonin metabolism or excretion.
Under most conditions the relationship between the onset and offset of melatonin is invariant. However, endogenous and environmental perturbations can alter the magnitude and direction of the onset and offset of melatonin secretion differently. These results have been interpreted as support for a 2-oscillator model of melatonin secretion in which the onset and offset of melatonin synthesis, although coupled, are differentially regulated. The 2-oscillator concept remains controversial. In addition, the physiologic significance of a change in the duration or amplitude of melatonin synthesis in nonphotoperiodic species (i.e., those that do not undergo seasonal physiologic changes such as reproductive status or coat color) is not well understood.
Relative thresholds may be determined when an overnight or 24-hour melatonin profile is available (Figure 1C). Relative thresholds normalize differences in amplitude, thereby facilitating comparisons between individuals and groups. These methods might include a percentage of maximum levels (e.g., 20%, 25%, or 50% of maximum) on the rising or declining phase, the midpoint between the rising and declining phases, or a point (such as the acrophase) determined from a cosine curve that has been fit to the raw melatonin data. A recently described modified cosine function that adjusts for fixed low baseline levels during the day may improve the fit of the curve at the abrupt transition from baseline to the rising phase.7 A limitation of the relative phase markers is that they require sample collection throughout the night. Many curve-fitting functions, including the simple, 2-harmonic, and baseline-adjusted cosine curves, require close to 24 hours of data.
Intraindividual melatonin profiles (both timing and amplitude) are, in general, very stable. In contrast, there are often large interindividual differences in the amount of melatonin synthesized. As discussed above, several strategies have been developed to minimize the influence of this variability on the measurement of phase, including the use of a low-threshold DLMO, a change in slope, or relative thresholds that are based on a percentage of maximum levels.2, 4 An alternative approach calculates individual secretion and clearance rates to estimate the onset and offset of melatonin secretion.8 This differential equation-based method can also be used to estimate onset if only the rising portion of the profile is available. Whatever method of analysis used, it is critical to visually inspect the data for artifacts and not rely solely on digital processing.
Lighting and Posture Caveats
It is well known that melatonin synthesis is acutely suppressed by light.9 Therefore, to accurately measure the timing of the underlying circadian clock it is necessary to maintain light at a level that does not suppress melatonin production. Collection of samples in dim light of less than 30 lux is considered acceptable for most purposes. Some investigators prefer maintaining light levels at less than 10 lux to ensure measurement of a DLMO that is not acutely suppressed or masked by light exposure. Light that is very dim in comparison with a subject's habitual light/dark cycle may serve as a dark pulse, potentially altering the timing of the melatonin onset or offset,.
It is not necessary to keep subjects under dim light during urine collection, allowing the continuous monitoring of circadian melatonin rhythms in natural “field” conditions over many days. However, if samples are collected under natural lighting, the observed rhythm of aMT6s may be of lower amplitude. Natural lighting also raises the possibility of masking of the acrophase, the likelihood of which depends on the timing of the nocturnal melatonin rhythm relative to the external light cycle. Phase estimates determined in the field may, therefore, require confirmation with more frequent sampling under dim light.
Prior light-exposure history has also been shown to influence the amount of melatonin suppression following exposure to light.10 Thus, when examining the effect of light on the melatonin rhythm (e.g., for suppression or phase shifting), the subject's light exposure prior to sampling may affect the magnitude of the response. Measuring and reporting the subject's light exposure during the week (or at least the day) prior to sample collection will facilitate the comparison of results from different studies.
Evidence to date suggests no difference in the time of the DLMO if a subject is recumbent, reclining, or sitting. However, posture, particularly standing, may affect the concentration of circulating melatonin. Frequent changes between postural positions could therefore result in changes in amplitude that would complicate determination of phase.11 Given that standing-induced increases in plasma melatonin concentration can be reversed within 10 minutes, it is recommended that activity and postural changes be minimized just prior to and during collection of plasma or saliva samples.12
DISCUSSION
This consensus document describes available methods for collecting and analyzing melatonin. Although it would be desirable to have a single method of analysis by which to compare studies, limitations of the collection methods preclude this possibility. For example, collection of saliva samples during the evening hours necessitates the use of a low threshold; this approach would not be satisfactory for 2- to 8-hour urine samples, which are analyzed by the acrophase of a cosine fit.
The most diversity in published methods occurs with determination of the onset of melatonin secretion following overnight or 24-hour sampling of blood or saliva, as DLMO can be assessed by many different methods (i.e., visual inspection of a change in slope, a low-threshold change from baseline, the fit of a cosine curve, a threshold based on a change from average baseline levels or a threshold based on a percentage of maximum levels). Therefore, to facilitate comparison of results between published partial and overnight or 24-hour melatonin profiles, we suggest including in the report (when feasible from the data available) an established low-threshold DLMO (e.g. 2 SD, or < 10 pg/mL for plasma or < 3 pg/mL for saliva) along with any other method used. Development and comparative testing of other objective measurements, such as computerized assessment of changes in slope or improved curve-fitting functions, could potentially standardize the measurement of both the onset and offset of the melatonin profile.
ACKNOWLEDGMENTS
Our appreciation to Drs. Josephine Arendt and Mary Cars-kadon for comments on this manuscript. Supported by Public Health Service grants NIH HL 67604 (SB); NIH HL072408, NR007677, and HL086934 (HJB); NIH K02-HD045459 (EBK); R56 AG15370 and R01 AG12112 (PM); NIMH MH-59919, MH-063462, MH-070788 and NIH Clinical Research Center (CRC) Grant No. M01-RR-00827 (BLP); and 6th Framework project EUCLOCK (No. 018741) (VR).
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Arendt J. Melatonin: characteristics, concerns, and prospects. J Biol Rhythms. 2005;20:291–303. doi: 10.1177/0748730405277492. [DOI] [PubMed] [Google Scholar]
- 2.Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L'Hermite-Balériaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005;20:178–188. doi: 10.1177/0748730404273983. [DOI] [PubMed] [Google Scholar]
- 3.Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 4.Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 1999;14:227–236. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- 5.Fitch K, Berstein SJ, Aguilar MD, et al. The RAND/UCLA Appropriateness Method User's Manual. Santa Monica: RAND Corporation; 2001. [Google Scholar]
- 6.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 7.Van Someren E, Nagtegaal E. Improving melatonin circadian phase estimates. Sleep Med. 2007;8:590–601. doi: 10.1016/j.sleep.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 8.St Hilaire MA, Gronfier C, Zeitzer JM, Klerman EB. A physiologically based mathematical model of melatonin including ocular light suppression and interactions with the circadian pacemaker. J Pineal Res. 2007;43:294–304. doi: 10.1111/j.1600-079X.2007.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 10.Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cajochen C, Jewett ME, Dijk DJ. Human circadian melatonin rhythm phase delay during a fixed sleep-wake schedule interspersed with nights of sleep deprivation. J Pineal Res. 2003;35:149–157. doi: 10.1034/j.1600-079x.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 12.Deacon S, Arendt J. Posture influences melatonin concentrations in plasma and saliva in humans. Neurosci Lett. 1994;167:191–194. doi: 10.1016/0304-3940(94)91059-6. [DOI] [PubMed] [Google Scholar]