Abstract
Clear adverse effects of blood lead levels ≥ 10 μg/dL have been documented in children. Given that the majority of US children have levels below 10 μg/dL, clarification of adverse effects below this cutoff value is needed. Our study evaluated the associations between blood lead levels < 10 μg/dL and a broad spectrum of children’s cognitive abilities. Data were analyzed from 534 children aged 6-10, enrolled in the New England Children’s Amalgam Trial (NECAT) from the urban area of Boston, Massachusetts and rural Farmington, Maine. Adjusting for covariates (age, race, socioeconomic status, and primary caregiver IQ), children with 5-10 μg/dL had 5.0 (s.d. 2.3) points lower IQ scores compared to children with blood lead levels of 1-2 μg/dL (p=0.03). Verbal IQ was more negatively affected than performance IQ, with the most prominent decrement occurring in children’s vocabulary. Wechsler Individual Achievement Test scores were strongly negatively associated with blood lead levels of 5-10 μg/dL. In adjusted analyses, children with levels of 5-10 μg/dL scored 7.8 (s.d. 2.4) and 6.9 (s.d 2.2) points lower on reading and math composite scores respectively, compared to children with levels of 1-2 μg/dL (p<0.01). Finally, levels of 5-10 μg/dL were associated with decreased attention and working memory. Other than associations of lead exposure with achievement, which even persisted after adjustment for child IQ, the most pronounced deficits were in the areas of spatial attention and executive function. Overall, our analyses support prior research that children’s blood levels < 10 μg/dL are related to compromised cognition and highlight that these may especially be related to academic achievement.
Keywords: Lead, children, cognition
INTRODUCTION
It is well known that high levels of lead exposure can result in adverse neurocognitive and behavioral consequences in children (Juberg et al., 1997; Schwartz, 1994; Wakefield, 2002). However, given the pervasiveness of high lead levels before regulatory measures were taken, opportunities to study low lead levels in the US have been limited until recently. Due to increasing evidence of cognitive effects at lower exposures, the Centers for Disease Control’s (CDC) guidelines regarding the lowest adverse level of lead have continued to shift downward, resulting in a redefinition of an “elevated” blood lead level four times since the early 1970’s (Koller et al., 2004). Although the CDC’s current definition is 10 μg/dL, lead levels below 10 μg/dL have recently been associated with neurocognitive deficits in children (Canfield et al., 2003; Lanphear et al., 2000; Tellez-Rojo et al., 2006) and no “safe” level has yet been established (Wigle and Lanphear, 2005).
Determination of the lowest lead level that poses a threat to children has also been a subject of intense political debate (Ferber, 2002; Gilbert and Weiss, 2006; Juberg et al., 1997). The debate has been complicated by the finding that the impact of increased exposure at lower levels of lead might be greater than the proportional impact at higher levels (i.e., the dose-response curve is non-linear). That is, the effects of an increase in blood lead concentration of 1 μg/dL on IQ are greater in children with blood lead levels < 10 μg/dL compared to the effects in children with blood lead levels > 10 μg/dL (Lanphear et al., 2005).
National data from the Third National Health and Nutrition Examination Survey (NHANES III) indicates that the majority of children exposed to lead in the US have blood lead levels below 10 μg/dL (Brody et al., 1994), and levels continue to decrease (CDC, 2005). The purpose of this study is to assess the associations between blood lead levels < 10 μg/dL and a broad spectrum of children’s neurocognitive abilities.
METHODS
Data used in this study were originally gathered for the New England Children’s Amalgam Trial (NECAT) (Bellinger et al., 2006), the aim of which was to assess the effect of amalgam dental fillings on children’s neurodevelopment. NECAT recruited a cohort of 6 to 10 year old children from the urban area of Boston, Massachusetts and rural Farmington, Maine. Children were eligible if they were English-speaking, had no prior or existing amalgam restorations, had two or more posterior teeth with dental caries, and, by parent report, had no physician-diagnosed psychological, behavioral, neurological immunosuppressive, or renal disease. Of 598 children found to be eligible, parental consent and child assent were obtained for the 534 who participated (291 from Boston, 243 from Maine). The institutional review boards of all participating sites approved this study.
At baseline (before placement of amalgam fillings), children were given an extensive battery of neuropsychological tests including tests of memory, learning, visual-motor ability, reading, reaction time, and IQ (see Children’s Amalgam Trial (2003) for details). At this same time, sociodemographic information was collected from parents/guardians and blood lead level was measured.
Blood Lead Determination
Blood samples were obtained at baseline from 515 of the 534 children. Three of the 515 children had a blood lead level > 10 μg/dL (range 11-13 μg/dL) and were excluded from this analysis.
Blood lead level was measured at the Clinical Chemistry Laboratory at Rochester General Hospital in Rochester, New York by an electrothermal process using an atomic absorption spectrometer with Zeeman background correction. Analysis was done on single samples using a Perkin Elmer AA 600 Atomic Absorption Spectrometer. A monthly quality check was done by WSLH PT (Wisconsin) and by the state in Albany. Blood samples, blood-based quality control materials and aqueous standards were diluted 1:9 with a matrix modifier solution containing nitric acid, Triton X-100 and ammonium dihydrogen phosphate.
Neuropsychological Outcome Measures
We selected the Full-Scale IQ on the Wechsler Intelligence Scale for Children -Third Edition (WISC-III) as our primary outcome measure. This is an apical score that integrates a child’s performance over a diversity of cognitive domains and for which scores frequently have been found to be inversely related to children’s lead burden. Secondary outcomes included the Wechsler Individual Achievement Test (WIAT), the Behavior Assessment System for Children, and a battery of additional neuropsychological tests of memory, executive functions, fine motor skill, visual-motor integration, attention. The tests in the battery included the Wide Range Assessment of Visual Motor Ability (WRAVMA), the Wide Range Assessment of Memory and Learning (WRAML), the Stroop Color-Word Interference Test, the Wisconsin Card Sorting Test (WCST), the Trail-Making Test, a verbal cancellation task, tests of verbal fluency, finger tapping, and reaction time. Quality of the neuropsychological assessments was maintained by standardized training and certification of all examiners in both the Boston and Maine sites. Additionally, completed test protocols were rescored by a second individual, with internal consistency checked by computerized algorithms. Caregiver IQ was measured at baseline using the Kaufman-Brief Intelligence Test (K-BIT). The Life Stress score was measured with the Parenting Stress Index (PSI).
Statistical Analyses
Analysis of covariance was used to model the association between neuropsychological test scores and blood lead level, adjusting for relevant covariates.
Bar graphs were created to show average IQ and other test scores versus blood lead by 1 μg/dL increments to provide a sense of the shape of the dose-response relationships. After examination of these graphs and available sample sizes, we grouped blood lead into three categories: 1-2, 3-4, and 5-10 μg/dL. Blood lead of 1-2 μg/dL was used as the reference category.
We used a common set of covariates in the analyses performed on the different cognitive outcomes. Using WISC III Full-Scale IQ as our primary outcome, we evaluated bivariate associations, with linear regression or analysis of variance, between thirteen sociodemographic variables and IQ: site (Boston or Maine), age, gender, race (non-Hispanic White, non-Hispanic Black, Hispanic, other), primary caregiver education (< high school, high school, college+), socioeconomic status (calculated using the method of Green 1970), marital status of the primary caregiver, birth order, birth weight, parenting stress, maternal utilization of annual health care, maternal utilization of prenatal care, and primary caregiver IQ. The reason for age adjustment was to take into account the wide range of ages when blood lead was assessed. Variables found to be significantly associated with IQ in bivariate analyses (p<0.2) were included in a multivariate analysis of covariance model. Variables then found statistically significant (p<0.2) in multivariate analysis were included as covariates in all models for the association between IQ/other neuropsychological tests and blood lead level. Due to missing data on primary caregiver IQ, the sample size was reduced from 512 to 408.
Two sensitivity analyses were conducted by: (1) including all covariates significant in bivariate analysis at the level of <0.2 (N=385 due to missing data), and (2) omitting primary caregiver IQ, for which considerable data were missing (N=512). All analyses were conducted using SAS version 9.1 (SAS Institute Inc., Cary, N.C).
Further analyses were carried out to determine if lead-associated deficits were evident on the Wechsler Achievement Test adjusting for child full-scale IQ, along with the other covariates (adult IQ, age, socioeconomic status, race, and birthweight).
Results
Because of missing data, in our analyses we used subsets of the 534 children. Table 1 shows baseline characteristics of the samples of 512 and 408 children respectively. The 512 children are those with non-missing WISC III Full-Scale IQ and baseline blood lead level ≤ 10 μg/dL, and the 408 children are those who for whom baseline primary caregiver IQ was measured.
Table 1.
Sample characteristics
| Children with IQ data and blood lead ≤ 10 μg/dL
N=512* |
Children with IQ data, primary caregiver IQ data, and blood lead ≤ 10 μg/dL
N=408** |
|
|---|---|---|
| Site (N %) | ||
| Boston | 272 53.1% | 169 41.4% |
| Maine | 240 46.9% | 239 58.6% |
| Age (mean, SD, range) | 8.0, (1.4), 6.0-11.5 | 7.9, (1.4), 6.0-11.5 |
| Gender (N %) | ||
| Female | 277 54.1% | 218 53.4% |
| Male | 235 45.9% | 190 46.6% |
| Race (N %)‡ | ||
| Non-Hispanic White | 320 62.5% | 303 74.3% |
| Non-Hispanic Black | 89 17.4% | 65 15.9% |
| Hispanic | 37 7.2% | 11 2.7% |
| Other | 66 12.9% | 29 7.1% |
| Household income (N %) | ||
| ≤$20,000 | 156 31.3% | 108 26.7% |
| $20,001 - $40,000 | 213 42.8% | 181 44.8% |
| > $40,000 | 129 25.9% | 115 28.5% |
| Education of primary caregiver (N %) | ||
| < High school | 69 13.7% | 41 10.1% |
| High school graduate | 383 76.1% | 329 80.6% |
| College graduate | 51 10.1% | 38 9.3% |
| Socioeconomic status (mean, SD) | 52.4, (6.7), 29.4-75.8 | 52.8, (6.2), 30.6-75.8 |
| Primary caregiver married (N %) | 315 63.0% | 252 62.4% |
| Birth order (mean, SD, range) | 1.6, (0.8), 1.0-5.0 | 1.6, (0.8), 1.0-5.0 |
| Birth weight, grams (mean, SD, range) | 3343, (542.3), 1600-4848 | 3368, (538.6), 1843-4848 |
| Life stress (mean, SD)† | 9.6, (9.2), 0-49 | 9.7, (9.4), 0-49 |
| Maternal utilization of annual health care (yes) (N %) | 433 73.2% | 281 72.6% |
| Maternal utilization of prenatal care (yes) (N %) | 441 96.3% | 383 98.5% |
| Adult IQ (mean, SD, range) | 97.3, (12.8), 52-157 | 97.3, (12.8), 52-157 |
| Child WISC-III‡‡ full scale IQ (mean, SD, range) | 95.7, (13.5), 62-141 | 97.2, (13.0), 62-141 |
| Child blood lead level (μg/dL) (mean, SD range) | 2.3, (1.6), 1-10 | 2.2, (1.6), 1-10 |
N=512 for site, age, gender, race, socioeconomic status, and Wechsler Intelligence Scale for Children - 3rd Edition (WISC-III). N=498 for income. N=503 for education. N=500 for marital status. N=504 for birth order. N=455 for birth weight. N=408 for adult IQ composite. N=440 for family life stress. N=458 for prenatal visit. N=455 for annual health care visit.
N=408 for site, age, gender, race, education, socioeconomic status, birth order, WISC-III, and adult IQ composite. N=404 for income and marital status. N=389 for birth weight and prenatal visit. N=400 for family life stress. N=387 for annual health care visit.
Race was self-reported by the parents of the children.
This was part of the Parenting Stress Index.
Wechsler Intelligence Scale for Children - 3rd Edition
Children in the two samples were similar in terms of most baseline characteristics. However, the sub-sample with IQ data for the primary caregiver IQ (N=408) included proportionally fewer children from the Boston site, and children had a higher mean IQ.
Of the thirteen potential covariates, bivariate analyses indicated that seven of them were significantly associated with IQ: site, age, race, SES, marital status of the primary caregiver, birth weight, and primary caregiver IQ (data not shown). Being from Boston (vs. Maine), being of Black or Hispanic race (versus non-Hispanic White), having an unmarried primary caregiver, or having a caregiver with a high school education or less (vs. a college education) were related to lower child IQ (WISC-III) (p<0.001). Greater age, lower SES, lower birth weight, and lower primary caregiver IQ were also related to lower IQ scores in the children (p<0.001). Of these seven variables, four remained significant in multivariate analysis – age, race, SES, and primary caregiver IQ – and were included in all subsequent outcome models. Along with these variables, birthweight was included in subsequent models both because it met our inclusion criterion of <0.2 as a cutoff for statistical significance.
Figure 1 shows plots of average Full Scale WISC-III IQ, WIAT Reading Composite, WIAT Math Composite, and Wisconsin Card Sorting perseveration errors versus blood lead level for the 408 children for whom primary caregiver IQ scores were available. All four outcomes tend to decrease with increasing blood lead level.
Figures 1.
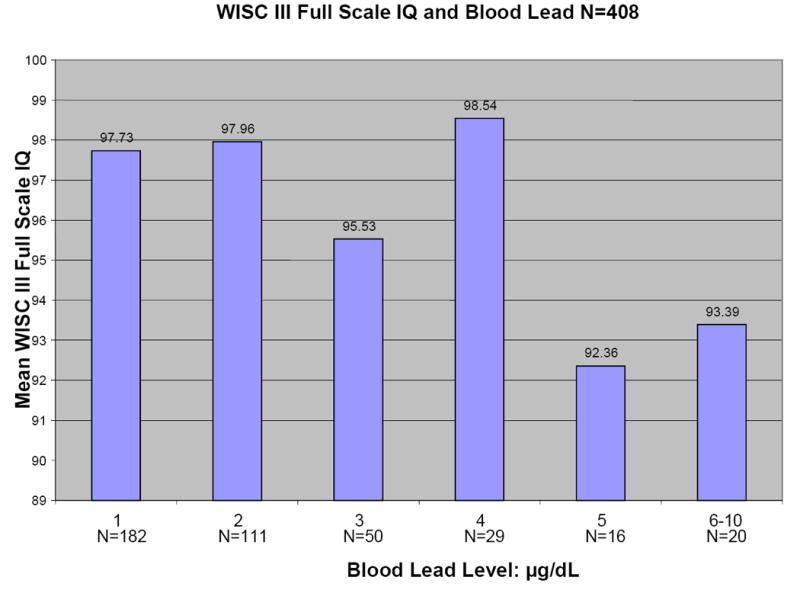
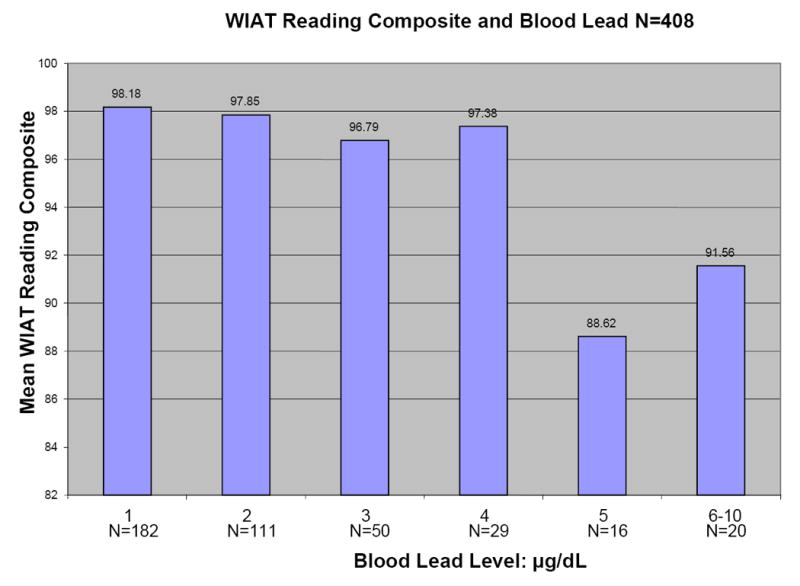
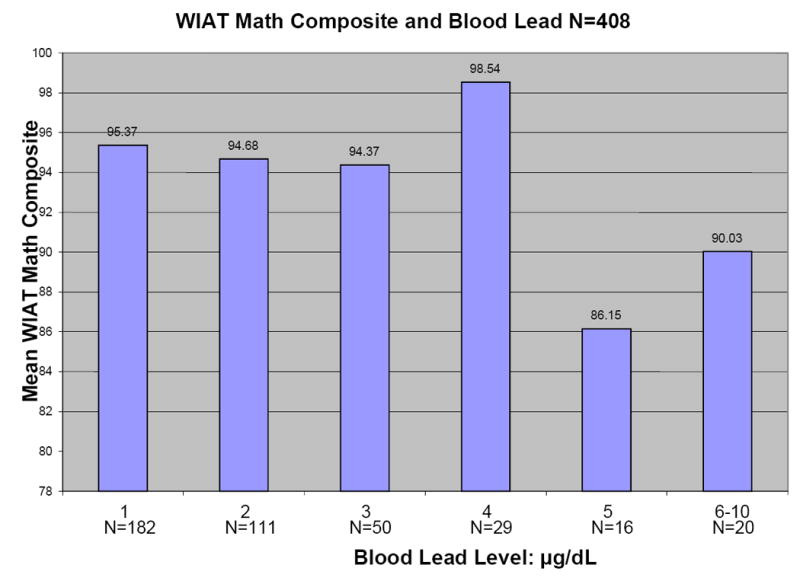
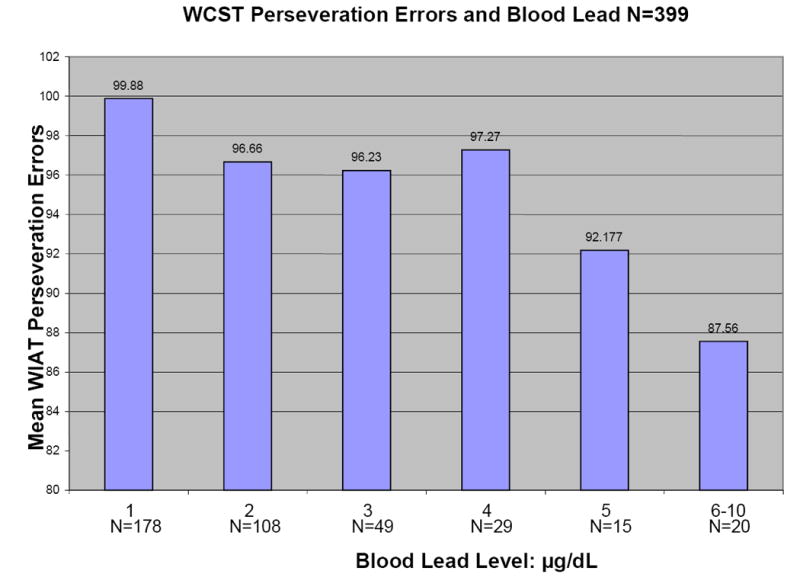
a-d: Relationships between selected neuropsychological tests and blood lead levels adjusted for age, adult IQ, SES, and race
Table 2 shows model results for Full-Scale WISC-III IQ. Compared to children with blood lead levels of 1-2 μg/dL, children with levels of 3-4μg/dL and 5-10 μg/dL had scores that were 0.12 (p=0.94) and 6.0 (p=0.01) points lower, respectively. Other covariates included in this multivariable model, adult IQ, child age, SES, and race, were statistically significant (p<0.05) except for birthweight (p=0.14).
Table 2.
Dose-response model of WISC III full-scale IQ and blood lead (1-2, 3-4, and 5-10 μg/dL), adjusted for adult IQ, age, socioeconomic status, race and birthweight.
| Variables | Coefficient (SE) | p-value |
|---|---|---|
| Lead Level | ||
| 3-4 μg/dL vs.1-2 μg/dL | -0.12 (1.62) | 0.941 |
| 5-10 μg/dL vs. 1-2 μg/dL | -6.04 (2.39) | 0.012 |
| Adult IQ | 0.13 (0.06) | 0.018 |
| Age | -1.20 (0.44) | 0.006 |
| Socioeconomic status | 0.36 (0.11) | 0.002 |
| Race | ||
| Black vs. White | -5.14 (1.80) | 0.005 |
| Hispanic vs. White | -3.12 (3.90) | 0.424 |
| Other vs. White | -1.98 (2.46) | 0.422 |
| Birth weight | 0.002 (0.001) | 0.142 |
N=408. p-values <0.05 in bold.
Table 3 shows the association between blood level and each of the neurocognitive outcomes, in models adjusted for caregiver IQ, child age, socioeconomic status, race, and birthweight. Children with blood lead levels of 5-10 μg/dL had deficits in all components of the WIAT. Compared to children with levels of 1-2 μg/dL, they had mean scores that were 8.7 and 7.9 points lower on the Reading (p=0.001) and Mathematics (p=0.001) Composite scores. Their mean scores were 7.7 points lower on Basic Reading (p=0.003), 8.5 points on Reading Comprehension (p=0.001), 5.9 points on Mathematics Reasoning (p=0.005), 7.4 points on Numerical Operations (p=0.002), and 7.9 points on Listening Comprehension (p=0.001).
Table 3.
Dose-response models of neuropsychological test scores and blood lead (1-2, 3-4, and 5-10 μg/dL), adjusted for adult IQ, age, socioeconomic status, race, and birth weight with reference lead level 1-2 μg/dL.
| Neuropsychological Test | Lead level 3-4μg/dL Coefficient (SE)* | p-value | Lead level 5-10 μg/dL Coefficient (SE)* | p-value |
|---|---|---|---|---|
| Wechsler Intelligence Scale for Children - 3rd Edition | ||||
| Full-Scale IQ | -0.12 (1.62) | 0.941 | -6.04 (2.39) | 0.012 |
| Verbal IQ | -0.86 (1.66) | 0.605 | -5.95 (2.45) | 0.016 |
| Performance IQ | 0.05 (1.78) | 0.978 | -5.37 (2.63) | 0.042 |
| Subtest scores | ||||
| Information | -0.01 (0.37) | 0.981 | -1.16 (0.55) | 0.035 |
| Similarities | 0.16 (0.42) | 0.706 | -1.19 (0.62) | 0.054 |
| Arithmetic | 0.35 (0.37) | 0.346 | -0.63 (0.55) | 0.258 |
| Vocabulary | -0.55 (0.38) | 0.150 | -1.43 (0.56) | 0.011 |
| Comprehension | -0.19 (0.35) | 0.593 | -0.89 (0.52) | 0.088 |
| Digit Span | -0.72 (0.33) | 0.031 | -0.24 (0.50) | 0.623 |
| Picture Completion | -0.34 (0.37) | 0.355 | -1.17 (0.54) | 0.031 |
| Coding | 0.03 (0.42) | 0.937 | -0.37 (0.62) | 0.558 |
| Picture Arrangement | -0.20 (0.49) | 0.686 | -0.79 (0.73) | 0.279 |
| Block Design | 0.08 (0.38) | 0.839 | -1.23 (0.57) | 0.031 |
| Object Assembly | 0.30 (0.40) | 0.455 | -0.55 (0.60) | 0.366 |
| Symbol Search | -0.19 (0.41) | 0.651 | -1.68 (0.60) | 0.006 |
| Mazes | -0.14 (0.43) | 0.741 | -0.61 (0.63) | 0.332 |
| Wechsler Individual Achievement Test | ||||
| Composites | ||||
| Reading | -1.01 (1.74) | 0.564 | -8.74 (2.57) | 0.001 |
| Mathematics | 1.54 (1.61) | 0.340 | -7.92 (2.38) | 0.001 |
| Scales | ||||
| Basic Reading | -0.88 (1.73) | 0.612 | -7.73 (2.55) | 0.003 |
| Reading Comprehension | -0.83 (1.65) | 0.615 | -8.52 (2.44) | 0.001 |
| Mathematics Reasoning | 0.96 (1.41) | 0.497 | -5.88 (2.09) | 0.005 |
| Numeric Operations | 1.53 (1.59) | 0.336 | -7.36 (2.35) | 0.002 |
| Listening Comprehension | -0.77 (1.66) | 0.640 | -7.90 (2.44) | 0.001 |
| Wide Range Assessment of Visual Motor Ability | ||||
| Visual-Motor Composite | -0.74 (1.66) | 0.654 | -5.37 (2.45) | 0.029 |
| Subtest Scores | ||||
| Drawing | 0.08 (1.63) | 0.962 | -0.99 (2.40) | 0.680 |
| Matching | 0.55 (1.69) | 0.744 | -7.98 (2.49) | 0.002 |
| Pegboard | -2.20 (2.00) | 0.272 | -2.44 (2.95) | 0.408 |
| Wide Range Assessment of Memory and Learning | ||||
| Indices | ||||
| General Memory Index | -0.69 (1.90) | 0.718 | -6.67 (2.79) | 0.017 |
| Visual Memory Index | 0.01 (1.86) | 0.995 | -6.47 (2.75) | 0.019 |
| Verbal Memory Index | -2.92 (1.81) | 0.108 | -5.74 (2.67) | 0.032 |
| Learning Index | 1.05 (1.97) | 0.596 | -3.41 (2.90) | 0.239 |
| Scales | ||||
| Picture Memory | 0.48 (0.38) | 0.209 | -0.37 (0.56) | 0.511 |
| Design Memory | 0.23 (0.36) | 0.530 | -0.29 (0.54) | 0.587 |
| Verbal Learning | -0.20 (0.41) | 0.616 | -1.06 (0.60) | 0.076 |
| Story Memory | -0.21 (0.42) | 0.624 | -0.92 (0.62) | 0.137 |
| Finger Windows | -0.79 (0.40) | 0.051 | -1.98 (0.60) | 0.001 |
| Sound Symbol | 0.07 (0.36) | 0.855 | -0.23 (0.53) | 0.661 |
| Sentence Memory | -0.56 (0.34) | 0.103 | -1.17 (0.50) | 0.021 |
| Visual Learning | 0.55 (0.39) | 0.159 | -0.07 (0.57) | 0.901 |
| Number/Letter Memory | -0.38 (0.32) | 0.234 | -0.61 (0.48) | 0.201 |
| Wisconsin Card Sorting Test | ||||
| Perseveration errors | -1.65 (1.84) | 0.370 | -9.19 (2.74) | 0.001 |
| Number of categories achieved | -0.29 (0.19) | 0.129 | -1.06 (0.29) | 0.0003 |
| Trials to the first category | -3.76 (2.27) | 0.098 | 4.93 (3.37) | 0.144 |
| Reaction Time | ||||
| Mean reaction time on successful trials | 0.02 (0.02) | 0.169 | 0.05 (0.03) | 0.066 |
| Verbal Cancellation | ||||
| Ordered errors | -1.33 (1.05) | 0.208 | 1.62 (1.54) | 0.293 |
| Unordered errors | -0.96 (1.08) | 0.373 | 2.02 (1.58) | 0.202 |
| Stoop Test | ||||
| Color-Word Interference score | -0.33 (0.80) | 0.675 | 0.75 (1.22) | 0.541 |
| Trial-Making Test | ||||
| Time to complete Part A | 2.13 (2.39) | 0.372 | 5.54 (3.62) | 0.127 |
| Time to complete Part B | 3.84 (7.14) | 0.591 | 6.09 (10.85) | 0.575 |
| Finger Tapping | ||||
| Mean of 5 trials with dominant hand | 1.81 (0.89) | 0.042 | 1.34 (1.33) | 0.312 |
| Mean of 5 trials with non-dominant hand | 1.53 (0.73) | 0.036 | -0.37 (1.08) | 0.733 |
Sample sizes for all variables ranged between N=381-389, except perseveration errors and number of categories achieved (WCST) and time to complete part B (Trial Making Test) that ranged between N=373-380, and the color-word inference score N=312.
p-values <0.01 in bold. A conservative approach was chosen because of multiple testing (of correlated test scores).
Child lead levels of 5-10 μg/dL were related to between 5-6 points lower scores on the Full-Scale WISC-III (β=-6.0, p=0.012), as well as on the Verbal and Performance IQ domains (β=-6.0, p=0.016; β=-5.3, p=0.04, respectively).With regard to WISC-III subtests, compared to children with levels of 1-2 μg/dL, children with levels of 5-10 μg/dL performed on average between 1.2 and 1.7 points lower on Information (p=0.035), Similarities (p=0.054), Vocabulary (p=0.011), Picture Completion (p=0.031), and Symbol Search (p=0.006). Blood lead levels of 5-10 μg/dL were associated with a 5.4 point deficit on the Visual-Motor Composite (p=0.029) and an 8.0 point deficit on the Matching subtest of the WRAVMA (p=0.002). Impairments on the WRAML were evident on all memory subtests, with 6.7 (p=0.017), 6.5 (p=0.019), and 5.7 (p=0.032) mean point decreases on General Memory, Visual Memory, and Verbal Memory indices respectively, in children with blood lead levels 5-10 μg/dL. Children having blood lead between 5-10 μg/dL was associated with an average 2.0 lower score (p=0.001) on the WRAML Finger Windows scale, compared to children in the 1-2 μg/dL range. On the WCST, children with blood lead levels of 5-10 μg/dL scored lower than children with levels of 1-2 μg/dL, making more perseveration errors (mean difference 9.2, p=0.001) and achieved fewer categories (mean difference 1.1, p=0.0003). The remaining neurocognitive tests and/or subscales were not significantly related to lead exposure in our sample. The sensitivity analyses yielded similar results.
After adjustment for all covariates and children’s Full-Scale IQ, performance on most scales of the Wechsler Individual Achievement Test (WIAT) remained lower among children with lead levels of 5-10 μg/dL (Table 4). However, the magnitude of these associations was smaller but reached statistical significance at the p<0.05 level on all domains except for Mathematics Reasoning. Compared to children with blood lead levels of 1-2 μg/dL, those with 5-10 μg/dL scored 5.2 points lower on the reading composite section of the WIAT (p=0.017) and 4.2 points lower on the mathematics composite section (p=0.047). Blood lead levels of 5-10 μg/dL were associated with deficits of similar magnitude on Basic Reading, Reading Comprehension, Numerical Operations, and Listening Comprehension (β=-4.4, p=0.047; β=-5.2, p=0.013; β=-4.3, p=0.034; -4.3, p=0.033, respectively).
Table 4.
Dose-response models of the Wechsler Individual Achievement Test scores and blood lead (1-2, 3-4, and 5-10 μg/dL), adjusted for, kid’s full scale IQ, adult IQ, age, socioeconomic status, race and birth weight, with reference lead level 1-2 μg/dL, N=408
| Lead level 3-4μg/dL Coefficient (SE)* | p-value | Lead level 5-10μg/dL Coefficient (SE)* | p-value | |
|---|---|---|---|---|
| Wechsler Individual Achievement Test | ||||
| Composites | ||||
| Reading | -0.93 (1.46) | 0.523 | -5.20 (2.17) | 0.017 |
| Mathematics | 1.62 (1.23) | 0.189 | -4.02 (1.83) | 0.028 |
| Scales | ||||
| Basic Reading | -0.81 (1.49) | 0.586 | -4.42 (2.21) | 0.047 |
| Reading Comprehension | -0.75 (1.39) | 0.590 | -5.16 (2.07) | 0.013 |
| Mathematics Reasoning | 1.03 (1.06) | 0.333 | -2.40 (1.58) | 0.130 |
| Numeric Operations | 1.59 (1.37) | 0.245 | -4.32 (2.03) | 0.034 |
| Listening Comprehension | -0.70 (1.34) | 0.601 | -4.28 (2.00) | 0.033 |
DISCUSSION
Among participants in the NECAT, children with blood lead levels of 5-10 μg/dL had significantly lower scores on IQ, achievement, attention, and working memory than did children in the referent group, who had levels of 1-2 μg/dL. Children with a blood lead level of 3-4 μg/dL differed from the referent group on only a few of the many test scores analyzed. Our overall findings are thus consistent with those of previous studies showing deficits in neuropsychological status among children exposed to low levels of lead (Environmental Protection Agency, 2006). An important aspect of our findings is that, at the time of neuropsychological assessment, all of the children included in the analyses had a blood lead level below 10 μg/dL, the current screening guideline of the U.S. Centers for Disease Control (CDC, 1991). Therefore, like those of several recent studies, our findings suggest that it is inappropriate to regard 10 μg/dL as a “lowest observed adverse effect” level (Chiodo et al., 2004; Kordas et al., 2006; Lanphear et al., 2000; Lanphear et al., 2005; Tellez-Rojo et al., 2006).
In some studies, a supralinear dose-effect relationship has been observed, with the inverse slope being steeper at blood lead levels <10 μg/dL than at levels >10 μg/dL (Kordas et al., 2006; Lanphear et al., 2005; Tellez-Rojo et al., 2006). Our study does not provide information on this point insofar as children with blood lead levels >10 μg/dL were excluded from the analyses. We did find, however, that the performance deficits were greater among children with a blood lead level of 5-10 μg/dL than among children with a level of 3-4 μg/dL. Although the neurobiological basis of possible supralinearity has not been identified such a form has been observed not only in epidemiological studies of children but in animal models as well. For example, biphasic functions have also been observed in rats with regard to lead exposure and long-term potentiation (Gilbert et al., 1999) and the release of GABA and glutamate in the hippocampus (Lasley and Gilbert, 2002).
Higher blood lead levels were associated not just with lower scores on tests of neuropsychological domains, but also with lower scores on tests of academic achievement. On all subscales of the WIAT, the mean scores of children with a blood lead level of 5-10 μg/dL were lower, by approximately one-half of a standard deviation, than the mean scores of children with levels of 1-2 μg/dL. Inverse associations between blood lead level and reading and arithmetic scores have been previously reported (Fergusson et al., 1988; Fergusson et al., 1993; Fergusson et al., 1997; Lanphear et al., 2000; Needleman et al., 1990). We showed, moreover, that these academic deficits remained significant even after adjustment for children’s Full-Scale IQ scores. This implies that the children’s academic achievement was significantly lower than would be expected based on their intelligence (i.e., an aptitude-achievement discrepancy). This is frequently used as a criterion for identifying children with a “learning disability,” and for allocating school resources such as remedial assistance.
Because of the detailed neuropsychological test battery administered at the baseline visits of the NECAT, we were able to evaluate the children’s performance within specific domains that might mediate the inverse associations between lead level and apical test scores such as IQ. It appeared to be within the domain of executive functioning that the children with lead levels of 5-10 μg/dL showed their most consistent deficits, achieving significantly fewer categories and making more perseverative errors on the WCST. These findings suggest that working memory, cognitive flexibility, and ability to formulate, test, and adapt hypotheses might contribute to impaired scores on apical tests. Similar observations have been made in other studies of lead-exposed children (Bellinger and Dietrich, 1994; Evans et al., 1994; Stiles and Bellinger, 1993) and in studies using animal models. Monkeys treated with lead from birth or later (at 300 or 4000 days) had more perseverative errors on discrimination (Rice, 1992) or delayed alteration tasks 6 to 9 years later (Rice and Gilbert, 1990). In one study using an alternation task, some monkeys perseverated for as long as an hour over the longest intervals between task acquisition and performance (Rice and Karpinski, 1988).
The information we had regarding children’s lead exposure history was limited to a single measurement made at the time of enrollment in the NECAT. Therefore, we do not know what the children’s levels of lead were in earlier years or whether they had changed over time. Although the relatively short half-time of lead in blood, and the resulting risk of exposure misclassification, is often cited as a limitation of cross-sectional studies such as ours, recent studies have shown that concurrent blood lead level is often the exposure biomarker that is most strongly associated with children’s neuropsychological outcomes at school age (Lanphear et al., 2005).
We did not assess the quantity and quality of emotional and cognitive stimulation using an instrument such as the Home Observation for Measurement of the Environment Inventory. However, life stress (from the Parenting Stress Index) was not associated with child IQ in bivariate analyses. Also, we adjusted for both socioeconomic status and caregiver IQ, both of which are moderately correlated with child stimulation and parenting skills. The analyses pertain to baseline assessments conducted as part of the Children’s Amalgam Trial, before any amalgam restorations had been placed. Thus, none of the children had any exposure to dental restorations, providing an assessment of the effects of lead unconfounded by exposure to elemental mercury.
In summary, we found that blood lead levels of 5-10 μg/dL in school-age children are associated with deficits in intelligence, visual-spatial skills, executive function, and IQ-adjusted academic achievement. These findings thus contribute to the accumulating evidence that we have yet to identify a threshold for lead-induced cognitive dysfunction in children, and that 10 μg/dL is a level without biological significance.
Acknowledgments
We would like to thank senior neuropsychological technicians Mandy Pelotte, Jaime Malley, Jody Lewis, Karen Meares and Deborah Bendaor; project director Susan Alperin; data manager Joan Landon; analytical chemist Elsa Cernichiari; and clinical coordinators Katherine Gregory and Valarie Smith.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- The Children’s Amalgam Trial: design and methods. Control Clin Trials. 2003;24:795–814. doi: 10.1016/s0197-2456(03)00105-3. [DOI] [PubMed] [Google Scholar]
- Centers of Disease Control. Blood lead levels--United States, 1999-2002. MMWR Morb Mortal Wkly Rep. 2005;54:513–6. [PubMed] [Google Scholar]
- Bellinger D, Dietrich KN. Low-level lead exposure and cognitive function in children. Pediatr Ann. 1994;23:600–5. doi: 10.3928/0090-4481-19941101-08. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Trachtenberg F, Barregard L, Tavares M, Cernichiari E, Daniel D, McKinlay S. Neuropsychological and renal effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006;295:1775–83. doi: 10.1001/jama.295.15.1775. [DOI] [PubMed] [Google Scholar]
- Brody DJ, Pirkle JL, Kramer RA, Flegal KM, Matte TD, Gunter EW, Paschal DC. Blood lead levels in the US population. Phase 1 of the Third National Health and Nutrition Examination Survey (NHANES III, 1988 to 1991) JAMA. 1994;272:277–83. doi: 10.1001/jama.272.4.277. [DOI] [PubMed] [Google Scholar]
- Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348:1517–26. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo LM, Jacobson SW, Jacobson JL. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol Teratol. 2004;26:359–71. doi: 10.1016/j.ntt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency. [Accessed July 2007];Clean Air Scientific Advisory Committee (CASAC) Lead Review Panel. 2006 http://www.epa.gov/sab/panels/casac_lead_review_panel.htm.
- Evans HL, Daniel SA, Marmor M. Reversal learning tasks may provide rapid determination of cognitive deficits in lead-exposed children. Neurotoxicol Teratol. 1994;16:471–77. doi: 10.1016/0892-0362(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Ferber D. Toxicology. Overhaul of CDC panel revives lead safety debate. Science. 2002;298:732. doi: 10.1126/science.298.5594.732. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Fergusson JE, Horwood LJ, Kinzett NG. A longitudinal study of dentine lead levels, intelligence, school performance and behaviour. Part I. Dentine lead levels and exposure to environmental risk factors. J Child Psychol Psychiatry. 1988;29:781–92. doi: 10.1111/j.1469-7610.1988.tb00753.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. Early dentine lead levels and subsequent cognitive and behavioural development. J Child Psychol Psychiatry. 1993;34:215–27. doi: 10.1111/j.1469-7610.1993.tb00980.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. Early dentine lead levels and educational outcomes at 18 years. J Child Psychol Psychiatry. 1997;38:471–78. doi: 10.1111/j.1469-7610.1997.tb01532.x. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Mack CM, Lasley SM. Chronic developmental lead exposure and hippocampal long-term potentiation: biphasic dose-response relationship. Neurotoxicology. 1999;20:71–82. [PubMed] [Google Scholar]
- Gilbert SG, Weiss B. A rationale for lowering the blood lead action level from 10 to 2 microg/dL. Neurotoxicology. 2006;27:693–701. doi: 10.1016/j.neuro.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LW. Manual for scoring socioeconomic status for research on health behavior. Public Health Rep. 1970;85:815–27. [PMC free article] [PubMed] [Google Scholar]
- Juberg DR, Kleiman CF, Kwon SC. Position paper of the American Council on Science and Health: lead and human health. Ecotoxicol Environ Saf. 1997;38:162–80. doi: 10.1006/eesa.1997.1591. [DOI] [PubMed] [Google Scholar]
- Koller K, Brown T, Spurgeon A, Levy L. Recent developments in low-level lead exposure and intellectual impairment in children. Environ Health Perspect. 2004;112:987–94. doi: 10.1289/ehp.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordas K, Canfield RL, Lopez P, Rosado JL, Vargas GG, Cebrian ME, Rico JA, Ronquillo D, Stoltzfus RJ. Deficits in cognitive function and achievement in Mexican first-graders with low blood lead concentrations. Environ Res. 2006;100:371–86. doi: 10.1016/j.envres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead concentrations <10 microg/dL in US children and adolescents. Public Health Rep. 2000;115:521–29. doi: 10.1093/phr/115.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–99. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasley SM, Gilbert ME. Rat hippocampal glutamate and GABA release exhibit biphasic effects as a function of chronic lead exposure level. Toxicol Sci. 2002;66:139–47. doi: 10.1093/toxsci/66.1.139. [DOI] [PubMed] [Google Scholar]
- Needleman HL, Schell A, Bellinger D, Leviton A, Allred EN. The long-term effects of exposure to low doses of lead in childhood. An 11-year follow-up report. N Engl J Med. 1990;322:83–88. doi: 10.1056/NEJM199001113220203. [DOI] [PubMed] [Google Scholar]
- Rice DC. Behavioral effects of lead in monkeys tested during infancy and adulthood. Neurotoxicol Teratol. 1992;14:235–45. doi: 10.1016/0892-0362(92)90002-r. [DOI] [PubMed] [Google Scholar]
- Rice DC, Gilbert SG. Lack of sensitive period for lead-induced behavioral impairment on a spatial delayed alternation task in monkeys. Toxicol Appl Pharmacol. 1990;103:364–73. doi: 10.1016/0041-008x(90)90236-n. [DOI] [PubMed] [Google Scholar]
- Rice DC, Karpinski KF. Lifetime low-level lead exposure produces deficits in delayed alternation in adult monkeys. Neurotoxicol Teratol. 1988;10:207–14. doi: 10.1016/0892-0362(88)90019-0. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Low-level lead exposure and children’s IQ: a meta-analysis and search for a threshold. Environ Res. 1994;65:42–55. doi: 10.1006/enrs.1994.1020. [DOI] [PubMed] [Google Scholar]
- Stiles KM, Bellinger DC. Neuropsychological correlates of low-level lead exposure in school-age children: a prospective study. Neurotoxicol Teratol. 1993;15:27–35. doi: 10.1016/0892-0362(93)90042-m. [DOI] [PubMed] [Google Scholar]
- Tellez-Rojo MM, Bellinger DC, Arroyo-Quiroz C, Lamadrid-Figueroa H, Mercado-Garcia A, Schnaas-Arrieta L, Wright RO, Hernandez-Avila M, Hu H. Longitudinal associations between blood lead concentrations lower than 10 microg/dL and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics. 2006;118:e323–30. doi: 10.1542/peds.2005-3123. [DOI] [PubMed] [Google Scholar]
- Wakefield J. The lead effect? Environ Health Perspect. 2002;110:A574–80. doi: 10.1289/ehp.110-a574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle DT, Lanphear BP. Human health risks from low-level environmental exposures: no apparent safety thresholds. PLoS Med. 2005;2:e350. doi: 10.1371/journal.pmed.0020350. [DOI] [PMC free article] [PubMed] [Google Scholar]


