Abstract
Angiotensin II (Ang II) has powerful sodium-retaining, growth-promoting and pro-inflammatory properties in addition to its physiological role in maintaining body salt and fluid balance and blood pressure homeostasis. Increased circulating and local tissue Ang II is one of the most important factors contributing to the development of sodium and fluid retention, hypertension and target organ damage. The importance of Ang II in the pathogenesis of hypertension and target organ injury is best demonstrated by the effectiveness of angiotensin-converting enzyme (ACE) inhibitors and AT1-receptor antagonists in treating hypertension and progressive renal disease including diabetic nephropathy. The detrimental effects of Ang II are mediated primarily by the AT1-receptor, while the AT2-receptor may oppose the AT1-receptor. The classical view of the AT1-receptor-mediated effects of Ang II is that the agonist binds its receptors at the cell surface, and following receptor phosphorylation, activates downstream signal transduction pathways and intracellular responses. However, evidence is emerging that binding of Ang II to its cell surface AT1-receptors also activates endocytotic (or internalisation) processes that promote trafficking of both the effector and the receptor into intracellular compartments. Whether internalised Ang II has important intracrine and signalling actions is not well understood. The purpose of this article is to review recent advances in Ang II research with focus on the mechanisms underlying high levels of intracellular Ang II in proximal tubule cells and the contribution of receptor-mediated endocytosis of extracellular Ang II. Further attention is devoted to the question whether intracellular and/or internalised Ang II plays a physiological role by activating cytoplasmic or nuclear receptors in proximal tubule cells. This information may aid future development of drugs to prevent and treat Ang II-induced target organ injury in cardiovascular and renal diseases by blocking intracellular and/or nuclear actions of Ang II.
Keywords: angiotensin II, AT1-receptor signalling, endosomes, kidney, proximal tubule cells, receptor endocytosis
Introduction
Renin was discovered by Robert Tigerstedt in kidney extract more than a century ago, but interest in the renin-angiotensin-aldosterone system (RAAS) remains stronger than ever.1 Our knowledge of the RAAS has dramatically evolved from Tigerstedt’s early discovery of renin as a “pressure-elevating substance from the kidney” to today’s widespread recognition of the RAAS as a dual endocrine and local tissue paracrine and autocrine system.2–6 For the first half of a century, the effector of the system, angiotensin II (Ang II), was known mainly as a humoral factor that is formed by the action of kidney-derived rate-limiting enzyme renin. Renin cleaves the liver-synthesised substrate angiotensinogen to form Ang I, which is converted to Ang II by the lung endothelium-derived angiotensin-converting enzyme (ACE). Ang II raises systemic blood pressure (BP) by causing vasoconstriction and promotes sodium and fluid retention by stimulating aldosterone synthesis and release from the adrenals. Over the last two decades, the RAAS is no longer considered as a circulating system alone and its roles have been expanded and redefined. For example, prorenin, renin and its substrate angiotensinogen have been shown to be synthesised and taken up in tissues other than the kidney and liver.7–9 ACE is widely expressed in tissues beyond the pulmonary endothelium so that Ang II may be produced in any tissues where renin, angiotensinogen and ACE are co-existent. ACE is also no longer the only enzyme to convert Ang I to Ang II because chymase has been shown to produce Ang II in human tissues.10 Finally, novel biological actions have been uncovered for other components of the system, such as Ang (1–7)11 and Ang IV.12,13 It is now well accepted that Ang II and its biologically active metabolites can be produced at local tissues and act both systemically as circulating peptides and locally as paracrine and/or autocrine factors.
There is now increasing evidence that Ang II may also function as an intracrine peptide, which may be synthesised and exert biological effects within the cells.14–19 The potential role of intracellular Ang II could be traced back to an early study in which radiolabelled Ang II was found in the nuclei of vascular smooth muscle and cardiac cells following systemic administration.20 However, Relater defined the concept of intracrine Ang II as the effector peptide that is either synthesised within a cell via actions of renin, angiotensinogen and ACE or internalised from extracellular Ang II.14,21 Evidence supporting a functional role of intracellular (or intracrine) Ang II has been reported in various target tissues or cells. For instance, Re and associates have demonstrated in the liver that intracellular Ang II induced important actions in hepatocytes.22–24 De Mello and colleagues have consistently shown that intracellular dialysis of renin, angiotensinogen or Ang II into hamster cardiomyocytes significantly altered cell-to-cell communications, supporting an important role for intracellular Ang II in regulating cardiac function.25–27 Haller et al. showed that microinjection of Ang II directly into rat vascular smooth muscle cells increased intracellular calcium, which could be blocked by intracellular AT1-receptor blockers.28,29 Sigmund and associates recently provided evidence that an intracellular form of renin plays a functional role in the brain.19 Moreover, Danser’s and Mullins’ groups have recently reported that prorenin uptake or internalisation induced intracellular signalling or responses that were either dependent or independent of Ang II.30,31 The presence and the potential role(s) of intracrine or intracellular Ang II in tissues other than the kidney have been reviewed elsewhere5,7,14,17,18 and further detailed discussion of the topic in those tissues is beyond the scope of this article.
The current review instead focuses on the intracrine renin-Ang II system in the kidney with emphasis on proximal tubule cells, because little is known about the presence and potential role(s) of intracellular Ang II in this tissue. Recent evidence suggests that increased synthesis and/or uptake of circulating and locally produced extracellular Ang II by proximal tubule cells may contribute to sodium retention and promote growth and proliferation, leading to the development of hypertension-induced renal injury.32–36 Although Ang II exerts powerful and diverse effects on proximal tubular function and cell growth by activating cell surface type 1 (AT1) receptors,37–41 Ang II-mediated activation of these receptors also induces endocytosis (internalisation) of the Ang II-AT1-receptor complex, which may initiate intracellular effects.42–44 Whether internalised or intracellular Ang II plays a physiological and/or pathological role in proximal tubule cells remains to be further studied.45,46 Since proximal tubule cells reabsorb more than 65–70% of glomerular filtered sodium load, and increased sodium reabsorption in this nephron segment by extracellular and intracellular Ang II will promote sodium and fluid retention and consequently the development of hypertension. Acting as a powerful intracellular cytokine and growth factor, intracellular Ang II may also play an important role in the pathogenesis of Ang II-induced hypertensive renal injury, especially tubulo-interstitial fibrosis.35,47,48 By studying intracellular trafficking and intracrine actions of internalised Ang II, novel mechanisms by which Ang II activates intracellular receptors to elicit biological responses may be uncovered. This information may aid future development of drugs to prevent and treat Ang II-induced target organ damage in cardiovascular and renal diseases by blocking intracellular and/or nuclear actions of Ang II.
Overview of proximal tubule renin-angiotensin system
It is well recognised that proximal tubule function is regulated by both circulating and locally formed Ang II.3,4,49 In the kidney, all major components of the RAS, including angiotensinogen, renin, ACE and Ang II receptors, have been demonstrated in proximal tubule cells (figure 1).50–56 Expression of renin, angiotensinogen and ACE ensures local generation of Ang II independent of the circulating RAS, whereas expression of Ang II receptors is essential for Ang II to induce biological actions. There are at least two major classes of Ang II receptors expressed in proximal tubule cells, AT1 and AT2, where high-affinity AT1-receptors occur in both the brush border and basolateral membranes.52,55,57–60 AT1-receptors are G protein-coupled receptors belonging to the superfamily of seven transmembrane-spanning proteins.40,61–63 Two subtypes of the AT1-receptor, designated AT1a and AT1b, have been identified in rodents, but the former is the predominant isoform in the kidney, equivalent to the human AT1-receptor.61 The primary actions of Ang II in proximal tubule cells are to stimulate sodium and bicarbonate reabsorption physiologically38,39,64,65, and to promote sodium retention and induce cellular growth and differentiation in diseased states.35,66–69 Most of the known proximal tubular actions of Ang II are mediated by the AT1-receptor, which is coupled to a G-protein-regulating mechanism that activates multiple signalling pathways, including phospholipase C, D, and A2 signalling, mitogen-activated protein kinase (MAPK), and tyrosine kinase.37,52,63,70,71 Activation of AT1-receptors leads to phosphoinositide hydrolysis, mobilisation of intracellular calcium, and inhibition of adenylate cyclase.38,45,46,57,72,73 By contrast, only low levels of AT2-receptors are expressed in proximal tubules and the role of this receptor is much less clearly defined than that of the AT1-receptor.57,60,74–76
Figure 1.
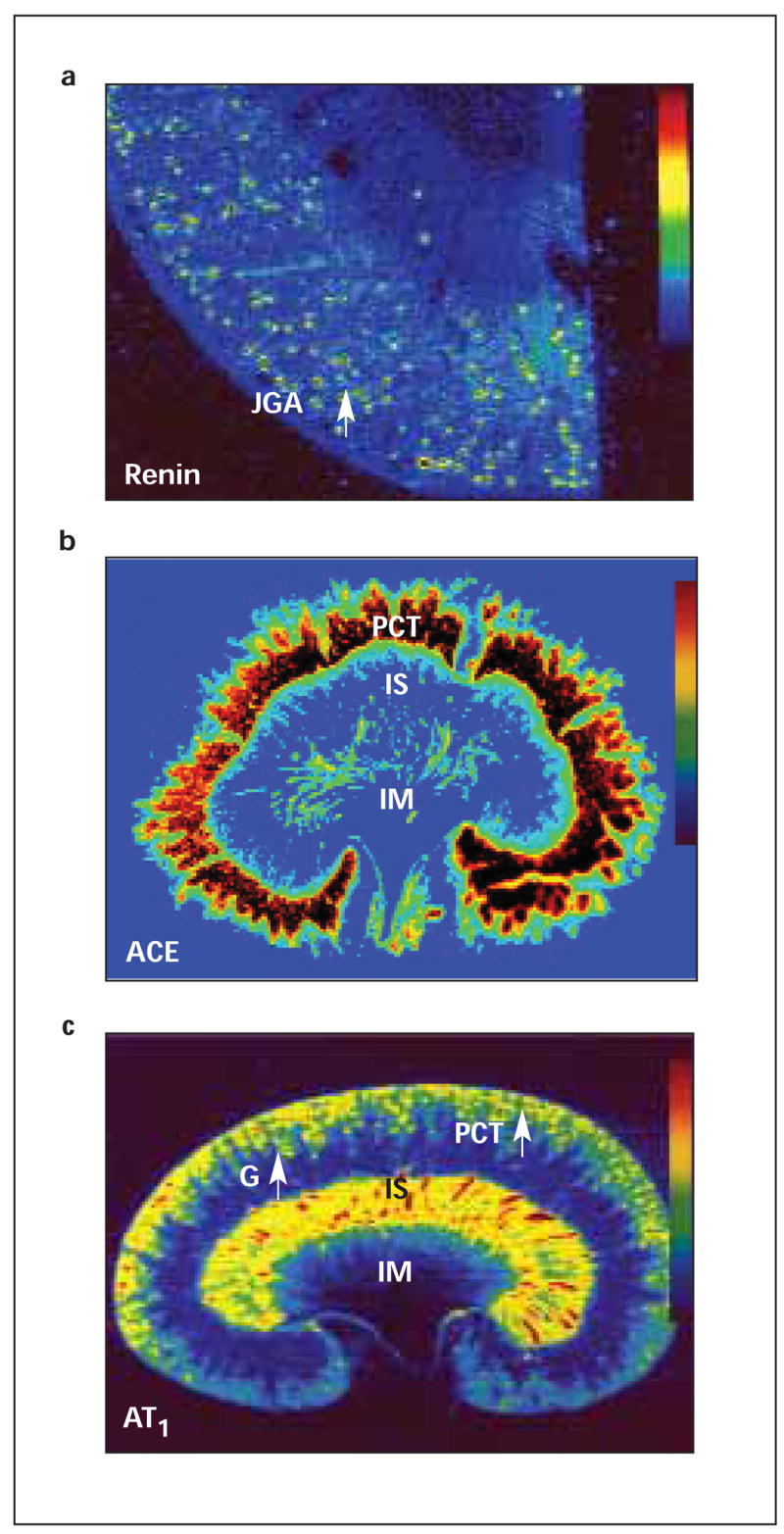
Mapping of active renin; a: angiotensin-converting enzyme (ACE); b: and Ang II AT1-receptors; c: in the kidney, as visualised by quantitative in vitro autoradiography. Active renin in the renal cortex was labelled with [125I]-H77, a renin inhibitor; ACE by [125 I]-351A, a lisinopril derivative; and AT1-receptors by [125I]-[Sar1, IIe8] Ang II. Active renin was localised in the vascular pole of glomerulus corresponding to the juxtaglomerular apparatus (JGA), ACE primarily in proximal tubules (PCT), and AT1-receptors in the glomerulus (G), proximal tubules and the inner stripe of the outer medulla (IS). IM = inner medulla. Modified from References 40,52,58,60,102
The classic view of Ang II-mediated actions is that Ang II binds its receptors at the plasma membrane and phosphorylation of the receptor activates downstream signalling and induces intracellular responses.37,38,52,57,63,70,71 However, increasing evidence suggests that binding of Ang II to its membrane AT1-receptors also activates endocytotic (or internalisation) processes that promote trafficking of both the effector and the receptor into intracellular compartments, where interaction of Ang II with its receptors may induce intracellular signalling with consequent biological effects.42–44 Whether internalised or intracellular Ang II has important intracrine actions and signalling is not completely understood. For instance, although the concept of intracellular Ang II was introduced many decades ago14,20,77,78 a careful MEDLINE search yielded only several dozens of citation on intracellular Ang II and its receptors in all tissues. Thus, it is important to understand the regulatory mechanisms of AT1-receptor-mediated Ang II endocytosis and its contribution to intracellular Ang II levels, intracellular trafficking pathways, and the potential role of internalised Ang II in proximal tubule cells and other tissues.
AT1-receptor-mediated accumulation of extracellular Ang II in proximal tubule cells
Angiotensin II levels in the kidney are often greater than can be explained by levels of circulating Ang II, but the precise levels and localisation of intrarenal Ang II are not fully understood.32,33,36,50,53,79,80 Nanomolar concentrations of Ang II have been reported in the glomerular filtrate,81 proximal tubular fluid79,80,82,83 and cortical interstitial fluid84,85 Conversely, it has recently been suggested that most intrarenal Ang II is cell-associated, though the location of cell-associated Ang II has not been identified to our knowledge.34,86 The biological significance of high levels of intrarenal Ang II is not known.
There is considerable evidence that the kidney takes up circulating or extracellular Ang II, and this process may contribute significantly to overall levels of intrarenal Ang II.32–34,36,86–88 Navar and associates were among the first to demonstrate that the kidney accumulated circulating Ang II when rats were infused with the exogenous peptide,32,33,89 and their findings were later confirmed by many others.34,36,88,90 Uptake of circulating or extracellular Ang II by the kidney appears to involve AT1-receptor-mediated internalisation, because AT1-receptor antagonists effectively prevent Ang II accumulation in this tissue (figure 2).34,36,87–90 While not internalised itself, the AT2-receptor may play a regulatory role in AT1-receptor-mediated internalisation of Ang II, since the AT2-receptor has been shown to antagonise most, if not all, of the known AT1-receptor mediated actions of Ang II.43,61,74,76,91 However, we do not know the cellular localisation of Ang II uptake in the kidney because most previous studies were only concerned with the whole kidney tissue. There is indirect evidence suggesting proximal tubule cells as potential sites of intrarenal Ang II accumulation.92 For example, we recently demonstrated increased intracellular uptake of Ang II in renal endosomes of Ang II-infused rats isolated primarily from renal cortical tubules, and this uptake was prevented by the ARB candesartan (figure 2).36 Ang II has also been shown to stimulate AT1a-receptor internalisation in AT1a-receptor transfected epithelial cell lines, such as opossum kidney (OK) epithelial cells and human embryonic kidney 293 cells (HEK293)42,43,91,93,94 but these cells normally do not express the components of the RAS including the AT1-receptors and thus their physiological relevance remains uncertain. The contribution of receptor-mediated endocytosis to intracellular levels of Ang II has been determined only recently in proximal tubule cells in vitro. Using cultured proximal tubule cells which express the RAS and respond to Ang II stimulation,95,96 we showed that intracellular Ang II levels were increased more than two-fold when they were incubated with exogenous Ang II, and this response was inhibited by the ARB losartan and the cytoskeleton microtubule inhibitor colchicine or the tyrosine phosphatase inhibitor phenylarsine oxide (PAO).46 These results support the concept that proximal tubule cells take up extracellular Ang II via AT1-receptor-mediated endocytosis and the process requires interactions with cytoskeleton microtubules and tyrosine phosphatases.
Figure 2.
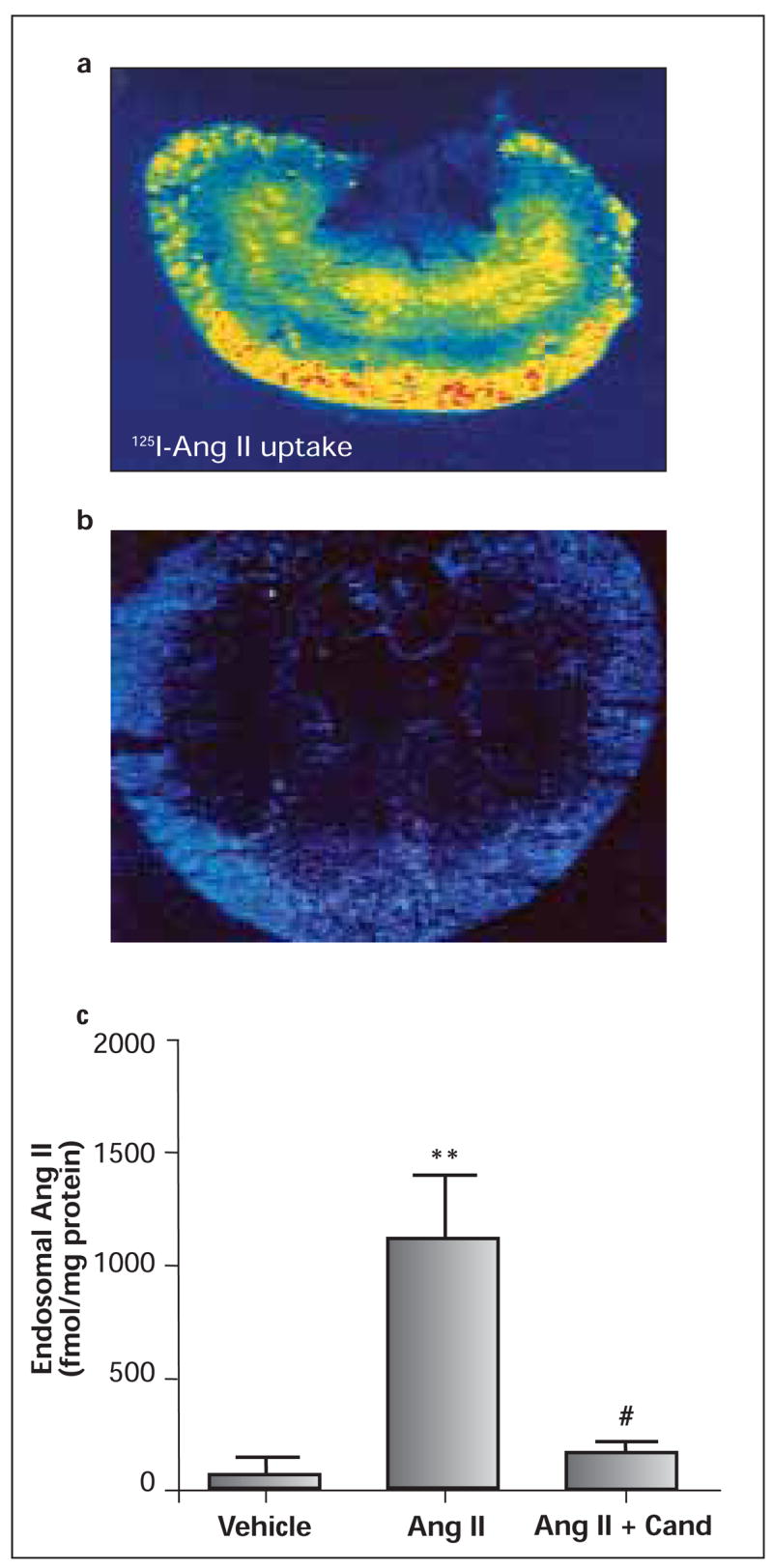
Quantitative in vitro autoradiographs showing AT1-receptor-mediated uptake of circulating [125I]-Ang II in the rat kidney. a: a control rat kidney; b: the kidney of a rat pretreated with losartan to block AT1-receptors before [125I]-Ang II was infused; c: renal cortical endosomal Ang II levels in the rats receiving vehicle, Ang II infusion for 2 weeks, or receiving concurrent administration of Ang II and candesartan. ** = p<0.01 vs. vehicle; # = p<0.05 vs. Ang II. Modified from References 36,40,58,102.
Pathways of intracellular trafficking of internalised Ang II
The specific intracellular compartment(s) where internalised Ang II is trafficked in proximal tubule cells remain to be determined. Likewise, we do not know whether Ang II is translocated to the nucleus following internalisation in these cells, where it may activate nuclear receptors to elicit a nuclear effect. Identification of the intracellular compartment(s) associated with Ang II internalisation would be important in understanding the role(s) of internalised Ang II in these cells. Recent studies suggest that endosomes may be intracellular compartments for internalised Ang II following its binding to cell membrane-bound AT1-receptors.36,86,91,92 In HEK 293 transfected with AT1a-receptors, the Ang II-AT1a-receptor complex was internalised and transported to the endosomes; however, levels of internalised Ang II were not determined.91 Although Ang II and AT1a-receptors have been reported in endosomes of the rat renal cortex, it was not determined whether they are synthesised intracellularly or accumulated through endocytosis.92 In the pig kidney, acute infusion of 125I-Ang II was associated with high levels of relative radioactivity in intracellular fractions, but the precise location of this radioactivity remains unidentified.34,86,88 None of these studies identified and/or measured intracellular Ang II levels during chronic Ang II infusion in vivo or in cultured proximal tubule cells in vitro. Using a different approach, we have recently isolated and purified intermicrovillar clefts (heavy endosomes) containing clathrin-coated vesicles and endosomes and measured intracellular Ang II levels in rats with Ang II-induced hypertension.36 Because we were able to co-localise AT1a receptors with a trapped endosomal marker, fluorescein dextran, the Ang II levels obtained from isolated and purified endosomes most likely represent internalised Ang II in this compartment (figure 2). Nevertheless, since complete elimination of potential contamination between different subcellular and intracellular fractions is impossible, intracellular localisation of Ang II and AT1-receptors in renal endosomes or other compartment(s) such as the nucleus, still awaits precise morphological confirmation. Although Ang II receptors have been demonstrated in the nuclei of epithelial cells or isolated from the kidney,97–100 there is no study specifically designed to localise internalised Ang II and AT1-receptors in proximal tubule cells in vitro and in vivo. It would be interesting to use state-of-the-art electron microscopic autoradiography and immunohistochemistry to localise internalised Ang II and AT1-receptors, focusing mainly in proximal tubule cells ex vivo, and use confocal and fluorescence microscopy for similar studies in cultured proximal tubule cells in vitro. The information obtained from the experiments will be important for our understanding of the intracrine role(s) of internalised Ang II in the regulation of proximal tubule sodium transport and BP and in the development of Ang II-induced renal injury.
Internalised Ang II induces intracellular calcium mobilisation by stimulating intracellular AT1-receptors
The autocrine or intracrine role of internalised Ang II is not well understood to our knowledge. It is generally recognised that in order to exert cellular actions, extracellular Ang II must bind to cell surface receptors and activate intracellular second messenger systems.37,61,63,70,101,102 Membrane-bound receptors may be primarily responsible for the acute or immediate effects of Ang II following activation of signal transduction systems.42,71 However, binding of Ang II to its membrane AT1-receptors also activates endocytotic processes that promote trafficking of both the effector and the receptor into intracellular compartments in vascular smooth muscle cells (VSMCs)103,104 and AT1-receptor-expressing epithelial cells.94,105,106 We do not know whether internalised Ang II activates cytoplasmic receptors to induce intracellular effects because it is difficult to differentiate intracellular effects from those mediated by the membrane receptors or intracellularly formed peptide.
The fate of internalised AT1-receptors has been suggested as: a) recycling back to the cell surface after dissociation from internalised Ang II; b) helping dispose of excess Ang II through degradation by intracellular lysosomes; or c) mediating the intracellular actions of Ang II.42,43,91,103,106 Recent studies have shown that internalised Ang II and AT1a-receptors are not necessarily sorted to lysosomes for degradation, but may be stored within the endosomal compartments and released into the cytoplasm in response to the acidic endosomal environment.104,107,108 Upon release, internalised Ang II may mediate responses within the cells.43 Consistent with the latter pathway, recent evidence suggests that internalisation of AT1-receptors and Ang II is important for full expression of physiologically relevant actions of this agonist.94,105,106 Schelling et al. have previously shown that blockade of AT1-receptor endocytosis with PAO inhibited activation of protein kinase C and formation of inositol 1,4,5-triphosphate (IP3) and sodium flux.105,106 However, PAO is not only an inhibitor of AT1-receptor-mediated endocytosis but also a potent tyrosine phosphatase inhibitor,104–106,108 which also selectively inhibits the sustained formation of phospholipase C-mediated diacylglycerol (DG) accumulation in VSMCs.108 Thus, it is difficult to know whether PAO blocks receptor-mediated endocytosis or directly inhibits membrane AT1-receptor-mediated downstream signalling. Recent studies have demonstrated that microinjection of Ang II directly into the cytosol of VSMCs increases intracellular [Ca2+]i.28,29 Since the AT1-receptor blocker was applied extracellularly to block cell membrane-bound receptors, whereas Ang II was microinjected into the cells, these results raise the possibility that intracellular Ang II acts on cytoplasmic receptors to exert intracrine effects in VSMCs. Indeed, Ang II has been shown to bind to a high-affinity cytoplasmic Ang II binding protein in non-renal tissues, although the consequences of this interaction remain to be determined.22,78,109,110
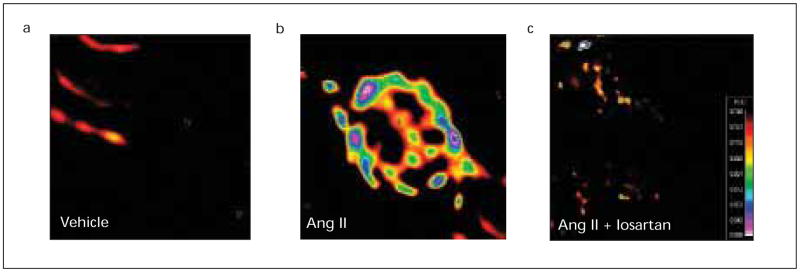
To determine whether intracellular or internalised Ang II plays any physiological role in proximal tubule cells, microinjection of Ang II directly into the cells may be one of the best approaches available, because it can differentiate the intracellular effects mediated by the microinjected Ang II from those mediated by extracellular Ang II via membrane receptors. Using this novel approach, we recently demonstrated that microinjection of Ang II into proximal tubule cells induced intracellular [Ca2+]i responses and the responses were blocked by co-microinjection with the AT1-receptor agonist losartan or by pre-treatment of the cells with thapsigargin to deplete intracellular calcium stores or with U-73122 to inhibit phospholipase C.45 Intracellular [Ca2+]i response to Ang II stimulation represents a well-described characteristic of actions of Ang II on proximal tubular transport (figure 3).37–39,45,49 Our results suggest that intracellular Ang II may play a physiological role in proximal tubule cells by stimulating cytoplasmic AT1-receptors to mobilise calcium from intracellular stores.
Figure 3.
Microinjection of Ang II directly into single proximal tubule cells increased intracellular calcium via activation of intracellular AT1-receptors. a: control; b: Ang II (1 nM); and c: co-microinjection of Ang II and losartan (10 μM). Images were taken 30 seconds after microinjection. The color bar indicates the intensity of calcium signalling with black representing background and red the highest level of signalling.
Intracellular Ang II activates nuclear transcription factor NF-κB by stimulating cytoplasmic and nuclear AT1-receptors
In addition to actively participating in physiological regulation of sodium and fluid reabsorption and BP homeostasis, Ang II has been implicated in the pathogenesis of many progressive renal diseases, including Ang II-induced hypertensive tubulo-interstitial fibrosis.35,47,48,68 Ang II is not only a powerful vasoactive peptide, but also a potent pro-inflammatory cytokine and growth factor in cardiovascular and renal tissues.111–113 There is accumulating evidence that Ang II can affect the transcription of genes related to sodium transport114,115 and cell growth and proliferation.114–117 Ang II has been shown to induce expression of the epithelial sodium channel (EnaC),115 NHE-3,115,118–120 and proto-oncogenes, growth factor genes, extracellular matrix genes and hypertrophic marker genes.47,69,111 Although not classified as gene transcription-modulating drugs, ACE inhibitors and AT1-receptor antagonists have been shown to inhibit changes in gene expression induced by Ang II.117
The growth-promoting and proliferative effects of Ang II may be partly mediated by internalised Ang II, acting on cytoplasmic and nuclear receptors.36,68,111,121 Internalised Ang II may stimulate cytoplasmic receptors to activate a variety of intracellular kinases, leading to phosphorylation of many cytoplasmic and nuclear proteins. Intracellular kinases activated by Ang II include extracellular signal-regulated protein kinase(s) (ERKs)63,70,117,122 JAK-STAT signalling117,123,124 and calcineurin phosphatase.117,125 Activation of calcineurin phosphatase by Ang II may be particularly relevant to increased intracellular [Ca2+]i by internalised Ang II, because increased intracellular [Ca2+]i is associated with increased expression of the N+/H+ exchanger and hypertrophic and proliferative responses.126,127 Internalised Ang II may also be translocated to the nucleus,20,97,99,110 where it may activate nuclear calcium28,29,45 and stimulate transcription of NHE-3 and pro-inflammatory cytokines and growth factors.20,47,69,97,111,128 Binding sites for Ang II with AT1-receptor properties have been identified in the nucleus of hepatocytes,110 CHO cells,97 HEK 293 cells99 and the kidney,98,100,109,110 but we do not know whether there are functional nuclear Ang II receptors in proximal tubule cells.
Recent studies indicate that Ang II activates nuclear transcription factor NF-κB and activator protein-1 (AP-1) in the kidney, leading to long-lasting inflammatory and growth-promoting effects.47,48,112,129,130 NF-κB is an important transcription factor in inflammatory diseases, and activation of NF-κB by Ang II stimulates transcription of many sodium transporters, cytokines and chemokines, including angiotensinogen, monocyte chemoattractant peptide-1 (MCP-1), TGFβ, and RANTES (regulated on activation normal T cell expressed and secreted).47,48,112,121 When not activated, NF-κB exists in an inactive form in the cytoplasm, binding to inhibitory IκB proteins. Stimulation of cells results in phosphorylation and degradation of IκB proteins, which releases NF-κB dimers. These dimers are translocated to the nucleus, where they activate appropriate target genes.131,132 After internalisation, Ang II has been localised to the nucleus of VSMCs.20 More recently, Chen demonstrated that Ang II induced internalisation of an AT1a-receptor-green fluorescent protein complex, which is then translocated to the nucleus in transfected CHO cells.97 It is likely that internalised Ang II activates nuclear transcription factor NF-κB by binding to cytoplasmic and nuclear AT1-receptors, thereby increasing expression of the Na+/H+ exchanger NHE-3, which promotes sodium transport in proximal tubules, and pro-inflammatory cytokines, chemokines and growth factors including angiotensinogen, MCP-1, IL-1β and TGF-β1. NF-κB activation by intracellular Ang II may be particularly relevant because activation of NF-κB by Ang II is one of the major mechanisms by which Ang II acts as an inflammatory cytokine to induce tubulo-interstitial fibrosis.47,48,112,121,129,130,133 In a recent study, we have shown that Ang II induced growth of proximal tubule cells and activated NF-κB, and these responses were blocked by inhibition of AT1-receptor-mediated endocytosis of extracellular Ang II with losartan or colchicine.134 Taken together, these studies suggest that internalised or intracellular Ang II may play an important role in renal cellular growth and fibrotic responses by activating NF-κB signalling.
Perspectives
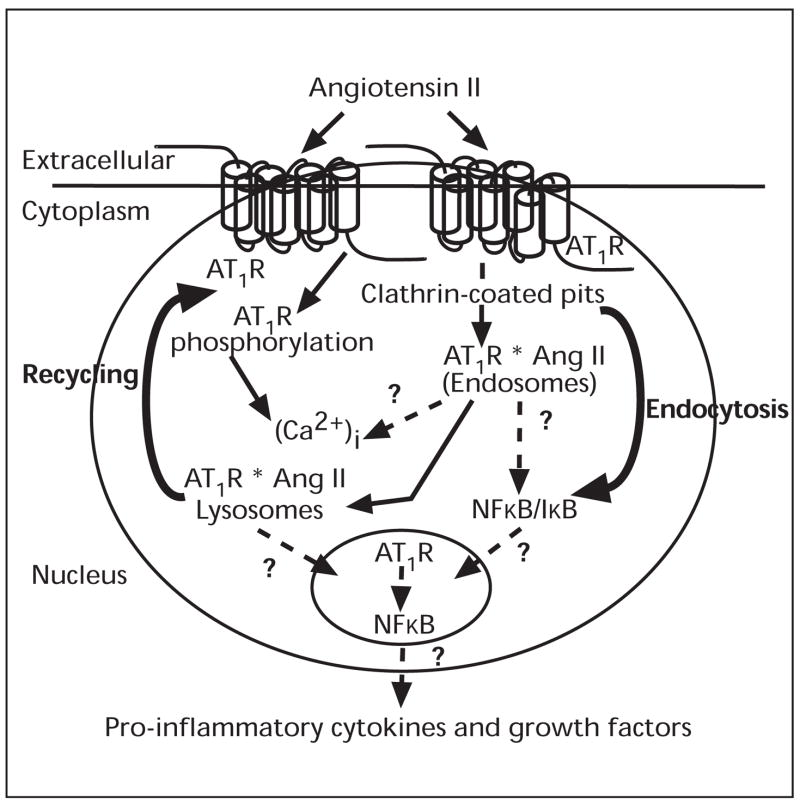
In summary, significant progress has been made during the last few years on the potential role of intracrine Ang II that is either synthesised intracellularly or internalised via AT1-receptor-mediated endocytosis in other tissues and kidney cells. Our current understanding of intracellular Ang II and its signalling in proximal tubule cells may be summarised in figure 4. Intra-proximal tubular and renal cortical interstitial fluid compartments contain high levels of extracellular Ang II under physiological conditions, which may be enhanced substantially during Ang II-induced hypertension or other renal diseases associated with activated renin-angiotensin system, such as diabetic nephropathy. The primary effects of extracellular Ang II (circulating and intrarenally formed) are still mediated by cell surface receptors, the peptide is also taken up by proximal tubule cells through AT1-receptor-mediated endocytosis. Upon internalisation, Ang II may stimulate cytoplasmic and nuclear AT1-receptors to increase intracellular [Ca2+]i and activates nuclear transcription factor NFκB, leading to increased expression of the Na+/H+ exchanger NHE-3 and pro-inflammatory cytokines and growth factors. Increased expression of the Na+/H+ exchanger NHE-3 and pro-inflammatory cytokines and growth factors by internalised Ang II may contribute to sodium retention and tubulo-interstitial injury in Ang II-dependent hypertension.
Figure 4.
A schematic diagram showing classic and alternative pathways by which extracellular Ang II induces biological actions in proximal tubule cells, namely through activation of cell surface AT1-receptors under acute physiological settings and/or intracellular AT1-receptors (cytoplasmic and nuclear) following endocytosis under pathophysiological conditions. While the classic pathway plays an essential role in the physiological regulation of proximal tubular transport, the alternative pathway may play a critical role in the development of hypertension and Ang II-induced tubulo-interstitial injury.
Further studies are required to further study the cellular mechanisms by which AT1-receptor-mediated endocytosis of extracellular Ang II is regulated, the pathways of its intracellular trafficking and signalling, and its potential role(s) in the regulation of proximal tubule function and in the development of target organ injury in hypertensive and progressive renal diseases.
Acknowledgments
The work was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases Grant 5RO1DK067299, American Heart Association Greater Midwest Affiliate Grant-in-Aid 0355551Z, National Kidney Foundation of Michigan Grant-in-Aid, and Henry Ford Hospital Institutional Grant to JLZ. The authors apologise to other investigators whose excellent work was not cited due to space restriction.
References
- 1.Tigerstedt R, Bergman PG. Kidney and circulation. Scand Arch Physiol. 1898;8:223–71. [Google Scholar]
- 2.Campbell DJ. Circulating and tissue angiotensin systems. J Clin Invest. 1987;79:1–6. doi: 10.1172/JCI112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston CI. Franz Volhard Lecture. Renin-angiotensin system: a dual tissue and hormonal system for cardiovascular control. J Hypertens Suppl. 1992;10:S13–S26. [PubMed] [Google Scholar]
- 4.Dzau VJ. Theodore Cooper Lecture: Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37:1047–52. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- 5.Paul M, Poyan MA, Kreutz R. Physiology of local rennin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 6.Lavoie JL, Sigmund CD. Minireview: overview of the rennin-angiotensin system–an endocrine and paracrine system. Endocrinology. 2003;144:2179–83. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 7.Jan Danser AH, Saris JJ. Prorenin uptake in the heart: a prerequisite for local angiotensin generation? J Mol Cell Cardiol. 2002;34:1463–72. doi: 10.1006/jmcc.2002.2078. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen G. Renin/prorenin receptors. Kidney Int. 2006;69:1503–06. doi: 10.1038/sj.ki.5000265. [DOI] [PubMed] [Google Scholar]
- 9.Campbell DJ, Valentijn AJ. Identification of vascular renin-binding proteins by chemical cross-linking: inhibition of binding of renin by renin inhibitors. J Hypertens. 1994;12:879–90. [PubMed] [Google Scholar]
- 10.Ihara M, Urata H, Kinoshita A, et al. Increased chymase-dependent angiotensin II formation in human atherosclerotic aorta. Hypertension. 1999;33:1399–405. doi: 10.1161/01.hyp.33.6.1399. [DOI] [PubMed] [Google Scholar]
- 11.Ferrario CM. Angiotensin-converting enzyme 2 and angiotensin-(1–7): an evolving story in cardiovascular regulation. Hypertension. 2006;47:515–21. doi: 10.1161/01.HYP.0000196268.08909.fb. [DOI] [PubMed] [Google Scholar]
- 12.Wright JW, Krebs LT, Stobb JW, Harding JW. The angiotensin IV system: functional implications. Front Neuroendocrinol. 1995;16:23–52. doi: 10.1006/frne.1995.1002. [DOI] [PubMed] [Google Scholar]
- 13.Albiston AL, McDowall SG, Matsacos D, et al. Evidence that the angiotensin IV (AT(4)) receptor is the enzyme insulin-regulated aminopeptidase. J Biol Chem. 2001;276:48623–26. doi: 10.1074/jbc.C100512200. [DOI] [PubMed] [Google Scholar]
- 14.Re RN. On the biological actions of intracellular angiotensin. Hypertension. 2000;35:1189–90. doi: 10.1161/01.hyp.35.6.1189. [DOI] [PubMed] [Google Scholar]
- 15.Peters J, Farrenkopf R, Clausmeyer S, et al. Functional significance of prorenin internalization in the rat heart. Circ Res. 2002;90:1135–41. doi: 10.1161/01.res.0000019242.51541.99. [DOI] [PubMed] [Google Scholar]
- 16.Cook JL, Zhang Z, Re RN. In vitro evidence for an intracellular site of angiotensin action. Circ Res. 2001;89:1138–46. doi: 10.1161/hh2401.101270. [DOI] [PubMed] [Google Scholar]
- 17.De Mello WC, Danser AH. Angiotensin II and the heart: on the intracrine renin-angiotensin system. Hypertension. 2000;35:1183–8. doi: 10.1161/01.hyp.35.6.1183. [DOI] [PubMed] [Google Scholar]
- 18.Filipeanu CM, Henning RH, Nelemans SA, de Zeeuw D. Intracellular angiotensin II: from myth to reality? JRAAS. 2001;2:219–26. doi: 10.3317/jraas.2001.035. [DOI] [PubMed] [Google Scholar]
- 19.Lavoie JL, Liu X, Bianco RA, Beltz TG, Johnson AK, Sigmund CD. Evidence supporting a functional role for intracellular renin in the brain. Hypertension. 2006;47:461–6. doi: 10.1161/01.HYP.0000203308.52919.dc. [DOI] [PubMed] [Google Scholar]
- 20.Robertson A, Khairallah P. Angiotensin II: rapid localization in nuclei of smooth and cardiac muscle. Science. 1971;172:1138–9. doi: 10.1126/science.172.3988.1138. [DOI] [PubMed] [Google Scholar]
- 21.Re RN. Intracellular renin and the nature of intracrine enzymes. Hypertension. 2003;42:117–22. doi: 10.1161/01.HYP.0000082495.93495.5B. [DOI] [PubMed] [Google Scholar]
- 22.Re RN, Vizard DL, Brown J, LeGros L, Bryan SE. Angiotensin II receptors in chromatin. J Hypertens Suppl. 1984;2:S271–S273. [PubMed] [Google Scholar]
- 23.Cook JL, Zhang Z, Re RN. Intracellular angiostensin II increases the long isoform of PDGF mRNA in rat hepatoma cells. J Mol Cell Cardiol. 2002;34:1525–37. doi: 10.1006/jmcc.2002.2106. [DOI] [PubMed] [Google Scholar]
- 24.Cook JL, Mills SJ, Naquin R, Alam J, Re RN. Nuclear accumulation of the AT1receptor in a rat vascular smooth muscle cell line: effects upon signal transduction and cellular proliferation. J Mol Cell Cardiol. 2006;40:696–707. doi: 10.1016/j.yjmcc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 25.De Mello WC. Intracellular angiotensin II regulates the inward calcium current in cardiac myocytes. Hypertension. 1998;32:976–82. doi: 10.1161/01.hyp.32.6.976. [DOI] [PubMed] [Google Scholar]
- 26.De Mello WC. Renin-angiotensin system and cell communication in the failing heart. Hypertension. 1996;27:1267–72. doi: 10.1161/01.hyp.27.6.1267. [DOI] [PubMed] [Google Scholar]
- 27.De Mello WC. Renin increments the inward calcium current in the failing heart. J Hypertens. 2006;24:1181–6. doi: 10.1097/01.hjh.0000226209.88312.db. [DOI] [PubMed] [Google Scholar]
- 28.Haller H, Lindschau C, Erdmann B, Quass P, Luft FC. Effects of intracellular angiotensin II in vascular smooth muscle cells. Circ Res. 1996;79:765–72. doi: 10.1161/01.res.79.4.765. [DOI] [PubMed] [Google Scholar]
- 29.Haller H, Lindschau C, Quass P, Luft FC. Intracellular actions of angiotensin II in vascular smooth muscle cells. J Am Soc Nephrol. 1999;10(suppl 11):S75–S83. [PubMed] [Google Scholar]
- 30.Danser AH, Deinum J. Renin, proreinin and the putative (pro)renin receptor. Hypertension. 2005;46:1069–76. doi: 10.1161/01.HYP.0000186329.92187.2e. [DOI] [PubMed] [Google Scholar]
- 31.Saris JJ, Hoen PA, Garrelds IM, et al. Prorenin induces intracellular signalling in cardiomyocytes independently of angiotensin II. Hypertension. 2006;48:564–71. doi: 10.1161/01.HYP.0000240064.19301.1b. [DOI] [PubMed] [Google Scholar]
- 32.von Thun AM, Vari RC, El Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol. 1994;266:F120–F128. doi: 10.1152/ajprenal.1994.266.1.F120. [DOI] [PubMed] [Google Scholar]
- 33.Zou LX, Hymel A, Imig JD, Navar LG. Renal accumulation of circulating angiotensin II in angiotensin II-infused rats. Hypertension. 1996;27:658–62. doi: 10.1161/01.hyp.27.3.658. [DOI] [PubMed] [Google Scholar]
- 34.van Kats JP, Schalekamp MA, Verdouw PD, Duncker DJ, Danser AH. Intrarenal angiotensin II: interstitial and cellular levels and site of production. Kidney Int. 2001;60:2311–17. doi: 10.1046/j.1523-1755.2001.00049.x. [DOI] [PubMed] [Google Scholar]
- 35.Johnson RJ, Alpers CE, Yoshimura A, et al. Renal injury from angiotensin II-mediated hypertension. Hypertension. 1992;19:464–74. doi: 10.1161/01.hyp.19.5.464. [DOI] [PubMed] [Google Scholar]
- 36.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT(1) receptor. Hypertension. 2002;39:116–21. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 37.Douglas JG, Hopfer U. Novel aspect of angiotensin receptors and signal transduction in the kidney. Annu Rev Physiol. 1994;56:649–69. doi: 10.1146/annurev.ph.56.030194.003245. [DOI] [PubMed] [Google Scholar]
- 38.Navar LG, Carmines PK, Huang WC, Mitchell KD. The tubular effects of angiotensin II. Kidney Int Suppl. 1987;20:S81–S88. [PubMed] [Google Scholar]
- 39.Harris PJ, Navar LG. Tubular transport responses to angiotensin II. Am J Physiol Renal Physiol. 1985;248:F621–F630. doi: 10.1152/ajprenal.1985.248.5.F621. [DOI] [PubMed] [Google Scholar]
- 40.Zhuo JL, Allen AM, Alcorn D, MacGregor D, Aldred GP, Mendelsohn FA. The distribution of angiotensin II receptors. In: Laragh JH, Brenner BM, editors. Hypertension: Pathology, Diagnosis & Management. 1[2nd Edition] New York: Raven Press; 1995. pp. 1739–62. [Google Scholar]
- 41.Ruster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol. 2006;17:2985–91. doi: 10.1681/ASN.2006040356. [DOI] [PubMed] [Google Scholar]
- 42.Thomas WG, Thekkumkara TJ, Baker KM. Molecular mechanisms of angiotensin II (AT1A) receptor endocytosis. Clin Exp Pharmacol Physiol Suppl. 1996;3:S74–S80. [PubMed] [Google Scholar]
- 43.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signalling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 44.Gonzalez-Gaitan M. Signal dispersal and transduction through the endocytic pathway. Nat Rev Mol Cell Biol. 2003;4:213–24. doi: 10.1038/nrm1053. [DOI] [PubMed] [Google Scholar]
- 45.Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular angiotensin II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol Renal Physiol. 2006;290:F1382–F1390. doi: 10.1152/ajprenal.00269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li XC, Carretero OA, Navar LG, Zhuo JL. AT1a receptor-mediated accumulation of extracellular angiotensin II in proximal tubule cells: role of cytoskeleton microtubules and tyrosine phosphatases. Am J Physiol Renal Physio. 2006;291:F375–F383. doi: 10.1152/ajprenal.00405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruiz-Ortega M, Ruperez M, Lorenzo O, et al. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl. 2002;82:12–22. doi: 10.1046/j.1523-1755.62.s82.4.x. [DOI] [PubMed] [Google Scholar]
- 48.Muller DN, Dechend R, Mervaala EM, et al. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000;35:193–201. doi: 10.1161/01.hyp.35.1.193. [DOI] [PubMed] [Google Scholar]
- 49.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev. 1996;76:425–536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- 50.Mendelsohn FA. Angiotensin II: evidence for its role as an intrarenal hormone. Kidney Int Suppl. 1982;12:S78–S81. [PubMed] [Google Scholar]
- 51.Tang SS, Jung F, Diamant D, et al. Temperature-sensitive SV40 immortalized rat proximal tubule cell line has functional renin-angiotensin system. Am J Physiol. 1995;268:F435–F446. doi: 10.1152/ajprenal.1995.268.3.F435. [DOI] [PubMed] [Google Scholar]
- 52.Zhuo JL, Mendelsohn FA. Intrarenal Angiotensin II Receptors. In: Robertson JIS, Nicholls MG, editors. The Renin-Angiotensin System: Biochemistry, Pathophysiology and Therapeutics. London & New York: Gower Medical Publishing; 1993. pp. 25.1–25.14. [Google Scholar]
- 53.Navar LG, Imig JD, Zou L, Wang CT. Intrarenal production of angiotensin II. Semin Nephrol. 1997;17:412–22. [PubMed] [Google Scholar]
- 54.Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT(1) receptor and ACE binding in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F19–F25. doi: 10.1152/ajprenal.00335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ingelfinger JR, Jung F, Diamant D, et al. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am J Physiol. 1999;276:F218–F227. doi: 10.1152/ajprenal.1999.276.2.F218. [DOI] [PubMed] [Google Scholar]
- 56.Tang SS, Jung F, Diamant D, et al. Temperature-sensitive SV40 immortalized rat proximal tubule cell line has functional renin-angiotensin system. Am J Physiol. 1995;268:F435–F446. doi: 10.1152/ajprenal.1995.268.3.F435. [DOI] [PubMed] [Google Scholar]
- 57.Douglas JG. Angiotensin receptor subtypes of the kidney cortex. Am J Physiol. 1987;253:F1–F7. doi: 10.1152/ajprenal.1987.253.1.F1. [DOI] [PubMed] [Google Scholar]
- 58.Zhuo J, Alcorn D, Harris PJ, Mendelsohn FA. Localization and properties of angiotensin II receptors in rat kidney. Kidney Int Suppl. 1993;42:S40–S46. [PubMed] [Google Scholar]
- 59.Dulin NO, Ernsberger P, Suciu DJ, Douglas JG. Rabbit renal epithelial angiotensin II receptors. Am J Physiol. 1994;267:F776–F782. doi: 10.1152/ajprenal.1994.267.5.F776. [DOI] [PubMed] [Google Scholar]
- 60.Zhuo J, Song K, Harris PJ, Mendelsohn FA. In vitro autoradiography reveals predominantly AT1 angiotensin II receptors in rat kidney. Ren Physiol Biochem. 1992;15:231–9. doi: 10.1159/000173458. [DOI] [PubMed] [Google Scholar]
- 61.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–72. [PubMed] [Google Scholar]
- 62.Murphy TJ, Alexander RW, Griendling KK, Runge MS, Bernstein KE. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature. 1991;351:233–6. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- 63.Mehta PK, Griendling KK. Angiotensin II cell signalling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 64.Cogan MG. Angiotensin II: a powerful controller of sodium transport in the early proximal tubule. Hypertension. 1990;15:451–8. doi: 10.1161/01.hyp.15.5.451. [DOI] [PubMed] [Google Scholar]
- 65.Zhuo J, Thomas D, Harris PJ, Skinner SL. The role of endogenous angiotensin II in the regulation of renal haemodynamics and proximal fluid reabsorption in the rat. J Physiol. 1992;453:1–13. doi: 10.1113/jphysiol.1992.sp019214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolf G, Ziyadeh FN. Renal tubular hypertrophy induced by angiotensin II. Semin Nephrol. 1997;17:448–54. [PubMed] [Google Scholar]
- 67.Luft FC. Proinflammatory effects of angiotensin II and endothelin: targets for progression of cardiovascular and renal diseases. Curr Opin Nephrol Hypertens. 2002;11:59–66. doi: 10.1097/00041552-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 68.Ruiz-Ortega M, Lorenzo O, Suzuki Y, Ruperez M, Egido J. Proinflammatory actions of angiotensins. Curr Opin Nephrol Hypertens. 2001;10:321–9. doi: 10.1097/00041552-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Wolf G, Ziyadeh FN, Zahner G, Stahl RA. Angiotensin II-stimulated expression of transforming growth factor beta in renal proximal tubular cells: attenuation after stable transfection with the c-mas oncogene. Kidney Int. 1995;48:1818–27. doi: 10.1038/ki.1995.480. [DOI] [PubMed] [Google Scholar]
- 70.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–72. [PubMed] [Google Scholar]
- 71.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–72. [PubMed] [Google Scholar]
- 72.Schelling JR, Singh H, Marzec R, Linas SL. Angiotensin II-dependent proximal tubule sodium transport is mediated by cAMP modulation of phospholipase C. Am J Physiol. 1994;267:C1239–C1245. doi: 10.1152/ajpcell.1994.267.5.C1239. [DOI] [PubMed] [Google Scholar]
- 73.Thekkumkara TJ, Cookson R, Linas SL. Angiotensin (AT1A) receptor-mediated increases in transcellular sodium transport in proximal tubule cells. Am J Physiol. 1998;274:F897–F905. doi: 10.1152/ajprenal.1998.274.5.F897. [DOI] [PubMed] [Google Scholar]
- 74.Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension. 2000;35:155–63. doi: 10.1161/01.hyp.35.1.155. [DOI] [PubMed] [Google Scholar]
- 75.Hakam AC, Hussain T. Angiotensin II AT2 receptors inhibit proximal tubular Na+-K+-ATPase activity via a NO/cGMP-dependent pathway. Am J Physiol Renal Physiol. 2006;290:F1430–F1436. doi: 10.1152/ajprenal.00218.2005. [DOI] [PubMed] [Google Scholar]
- 76.Siragy HM, Carey RM. Angiotensin type 2 receptors: potential importance in the regulation of blood pressure. Curr Opin Nephrol Hypertens. 2001;10:99–103. doi: 10.1097/00041552-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 77.De Mello WC. Is an intracellular renin-angiotensin system involved in control of cell communication in heart? J Cardiovasc Pharmacol. 1994;23:640–6. doi: 10.1097/00005344-199404000-00018. [DOI] [PubMed] [Google Scholar]
- 78.Re R, Parab M. Effect of angiotensin II on RNA synthesis by isolated nuclei. Life Sci. 1984;34:647–51. doi: 10.1016/0024-3205(84)90228-5. [DOI] [PubMed] [Google Scholar]
- 79.Navar LG, Lewis L, Hymel A, Braam B, Mitchell KD. Tubular fluid concentrations and kidney contents of angiotensins I and II in anesthetized rats. J Am Soc Nephrol. 1994;5:1153–8. doi: 10.1681/ASN.V541153. [DOI] [PubMed] [Google Scholar]
- 80.Navar LG, Harrison-Bernard LM, Wang CT, Cervenka L, Mitchell KD. Concentrations and actions of intraluminal angiotensin II. J Am Soc Nephrol. 1999;10(suppl 11):S189–S195. [PubMed] [Google Scholar]
- 81.Seikaly MG, Arant BS, Jr, Seney FD., Jr Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J Clin Invest. 1990;86:1352–7. doi: 10.1172/JCI114846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Braam B, Mitchell KD, Fox J, Navar LG. Proximal tubular secretion of angiotensin II in rats. Am J Physiol. 1993;264:F891–F898. doi: 10.1152/ajprenal.1993.264.5.F891. [DOI] [PubMed] [Google Scholar]
- 83.Mitchell KD, Jacinto SM, Mullins JJ. Proximal tubular fluid, kidney, and plasma levels of angiotensin II in hypertensive ren-2 transgenic rats. Am J Physiol. 1997;273:F246–F253. doi: 10.1152/ajprenal.1997.273.2.F246. [DOI] [PubMed] [Google Scholar]
- 84.Siragy HM, Howell NL, Ragsdale NV, Carey RM. Renal interstitial fluid angiotensin. Modulation by anesthesia, epinephrine, sodium depletion, and renin inhibition. Hypertension. 1995;25:1021–4. doi: 10.1161/01.hyp.25.5.1021. [DOI] [PubMed] [Google Scholar]
- 85.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension. 2002;39:129–34. doi: 10.1161/hy0102.100536. [DOI] [PubMed] [Google Scholar]
- 86.van Kats JP, van Meegen JR, Verdouw PD, Duncker DJ, Schalekamp MA, Danser AH. Subcellular localization of angiotensin II in kidney and adrenal. J Hypertens. 2001;19:583–9. doi: 10.1097/00004872-200103001-00010. [DOI] [PubMed] [Google Scholar]
- 87.Zou LX, Imig JD, Hymel A, Navar LG. Renal uptake of circulating angiotensin II in Val5-angiotensin II infused rats is mediated by AT1 receptor. Am J Hypertens. 1998;11:570–8. doi: 10.1016/s0895-7061(97)00410-x. [DOI] [PubMed] [Google Scholar]
- 88.van Kats JP, de Lannoy LM, Jan Danser AH, van Meegen JR, Verdouw PD, Schalekamp MA. Angiotensin II type 1 (AT1) receptor-mediated accumulation of angiotensin II in tissues and its intracellular half-life in vivo. Hypertension. 1997;30:42–9. doi: 10.1161/01.hyp.30.1.42. [DOI] [PubMed] [Google Scholar]
- 89.Zou LX, Imig JD, von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal angiotensin II augmentation in angiotensin II-infused rats. Hypertension. 1996;28:669–77. doi: 10.1161/01.hyp.28.4.669. [DOI] [PubMed] [Google Scholar]
- 90.Ingert C, Grima M, Coquard C, Barthelmebs M, Imbs JL. Contribution of angiotensin II internalization to intrarenal angiotensin II levels in rats. Am J Physiol Renal Physiol. 2002;283:F1003–F1010. doi: 10.1152/ajprenal.00322.2001. [DOI] [PubMed] [Google Scholar]
- 91.Hein L, Meinel L, Pratt RE, Dzau VJ, Kobilka BK. Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol Endocrinol. 1997;11:1266–77. doi: 10.1210/mend.11.9.9975. [DOI] [PubMed] [Google Scholar]
- 92.Imig JD, Navar GL, Zou LX, et al. Renal endosomes contain angiotensin peptides, converting enzyme, and AT(1A) receptors. Am J Physiol. 1999;277:F303–F311. doi: 10.1152/ajprenal.1999.277.2.F303. [DOI] [PubMed] [Google Scholar]
- 93.Linas SL. Role of receptor mediated endocytosis in proximal tubule epithelial function. Kidney Int Suppl. 1997;61:S18–S21. [PubMed] [Google Scholar]
- 94.Thekkumkara T, Linas SL. Role of internalization in AT(1A) receptor function in proximal tubule epithelium. Am J Physiol Renal Physiol. 2002;282:F623–F629. doi: 10.1152/ajprenal.00118.2001. [DOI] [PubMed] [Google Scholar]
- 95.Romero MF, Hopfer U, Madhun ZT, Zhuo W, Douglas JG. Angiotensin actions in the rabbit proximal tubule. Ren Physiol Biochem. 2003;14:199–207. doi: 10.1159/000173405. [DOI] [PubMed] [Google Scholar]
- 96.Romero MF, Douglas JG, Eckert RL, Hopfer U, Jacobberger JW. Development and characterization of rabbit proximal tubular epithelial cell lines. Kidney Int. 1992;42:1130–44. doi: 10.1038/ki.1992.397. [DOI] [PubMed] [Google Scholar]
- 97.Chen R, Mukhin YV, Garnovskaya MN, et al. A functional angiotensin II receptor-GFP fusion protein: evidence for agonist-dependent nuclear translocation. Am J Physiol Renal Physiol. 2000;279:F440–F448. doi: 10.1152/ajprenal.2000.279.3.F440. [DOI] [PubMed] [Google Scholar]
- 98.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2. Lewis rat. Am J Physiol Renal Physiol. 2006;290:F1497–F1506. doi: 10.1152/ajprenal.00317.2005. [DOI] [PubMed] [Google Scholar]
- 99.Morinelli TA, Raymond JR, Baldys A, et al. Identification of a putative nuclear localization sequence within the angiotensin II AT1A receptor associated with nuclear activation. Am J Physiol Cell Physiol. 2007;292:1398–408. doi: 10.1152/ajpcell.00337.2006. [DOI] [PubMed] [Google Scholar]
- 100.Licea H, Walters MR, Navar LG. Renal nuclear angiotensin II receptors in normal and hypertensive rats. Acta Physiol Hung. 2002;89:427–38. doi: 10.1556/APhysiol.89.2002.4.3. [DOI] [PubMed] [Google Scholar]
- 101.Timmermans PB, Wong PC, Chiu AT, et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45:205–51. [PubMed] [Google Scholar]
- 102.Zhuo JL, Moeller I, Jenkins T, et al. Mapping tissue angiotensin-converting enzyme and angiotensin AT1, AT2 and AT4 receptors. J Hypertens. 1997;16:2027–37. doi: 10.1097/00004872-199816121-00026. [DOI] [PubMed] [Google Scholar]
- 103.Ullian ME, Linas SL. Role of receptor cycling in the regulation of angiotensin II surface receptor number and angiotensin II uptake in rat vascular smooth muscle cells. J Clin Invest. 1989;84:840–6. doi: 10.1172/JCI114244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anderson KM, Peach MJ. Receptor binding and internalization of a unique biologically active angiotensin II-colloidal gold conjugate: morphological analysis of angiotensin II processing in isolated vascular strips. J Vasc Res. 1994;31:10–17. doi: 10.1159/000159026. [DOI] [PubMed] [Google Scholar]
- 105.Schelling JR, Linas SL. Angiotensin II-dependent proximal tubule sodium transport requires receptor-mediated endocytosis. Am J Physiol. 1994;266:C669–C675. doi: 10.1152/ajpcell.1994.266.3.C669. [DOI] [PubMed] [Google Scholar]
- 106.Schelling JR, Hanson AS, Marzec R, Linas SL. Cytoskeleton-dependent endocytosis is required for apical type 1 angiotensin II receptor-mediated phospholipase C activation in cultured rat proximal tubule cells. J Clin Invest. 1992;90:2472–80. doi: 10.1172/JCI116139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anborgh PH, Seachrist JL, Dale LB, Ferguson SS. Receptor/beta-arrestin complex formation and the differential trafficking and resensitization of beta2-adrenergic and angiotensin II type 1A receptors. Mol Endocrinol. 2000;14:2040–53. doi: 10.1210/mend.14.12.0565. [DOI] [PubMed] [Google Scholar]
- 108.Griendling KK, Delafontaine P, Rittenhouse SE, Gimbrone MA, Jr, Alexander RW. Correlation of receptor sequestration with sustained diacylglycerol accumulation in angiotensin II-stimulated cultured vascular smooth muscle cells. J Biol Chem. 1987;262:14555–62. [PubMed] [Google Scholar]
- 109.Sen I, Bull HG, Soffer RL. Isolation of an angiotensin II-binding protein from liver. Proc Natl Acad Sci USA. 1984;81:1679–83. doi: 10.1073/pnas.81.6.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang SS, Rogg H, Schumacher R, Dzau VJ. Characterization of nucler angiotensin II binding sites in rat liver and comparison with plasma membrane receptors. Endocrinology. 1992;131:374–80. doi: 10.1210/endo.131.1.1612017. [DOI] [PubMed] [Google Scholar]
- 111.Zhuo JL. Intracrine renin and angiotensin II: a novel role in cardiovascular and renal cellular regulation. J Hypertens. 2006;24:1017–20. doi: 10.1097/01.hjh.0000226188.90815.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wolf G, Wenzel U, Burns KD, Harris RC, Stahl RA, Thaiss F. Angiotensin II activates nuclear transcription factor-kappaB through AT1 and AT2 receptors. Kidney Int. 2002;61:1986–95. doi: 10.1046/j.1523-1755.2002.00365.x. [DOI] [PubMed] [Google Scholar]
- 113.Suzuki Y, Ruiz-Ortega M, Egido J. Angiotensin II: a double-edged sword in inflammation. J Nephrol. 2000;13(suppl 3):S101–S110. [PubMed] [Google Scholar]
- 114.Kwon TH, Nielsen J, Kim YH, Knepper MA, Frokiaer J, Nielsen S. Regulation of sodium transporters in the thick ascending limb of rat kidney: response to angiotensin II. Am J Physiol Renal Physiol. 2003;285:F152–F165. doi: 10.1152/ajprenal.00307.2002. [DOI] [PubMed] [Google Scholar]
- 115.Brooks HL, Allred AJ, Beutler KT, Coffman TM, Knepper MA. Targeted proteomic profiling of renal Na(+) transporter and channel abundances in angiotensin II type 1a receptor knockout mice. Hypertension. 2002;39:470–3. doi: 10.1161/hy02t2.102959. [DOI] [PubMed] [Google Scholar]
- 116.Beutler KT, Masilamani S, Turban S, et al. Long-Term Regulation of ENaC Expression in Kidney by Angiotensin II. Hypertension. 2003;41:1143–50. doi: 10.1161/01.HYP.0000066129.12106.E2. [DOI] [PubMed] [Google Scholar]
- 117.Kurtz TW, Gardner DG. Transcription-modulating drugs: a new frontier in the treatment of essential hypertension. Hypertension. 1998;32:380–6. doi: 10.1161/01.hyp.32.3.380. [DOI] [PubMed] [Google Scholar]
- 118.du CD, Chalumeau C, Defontaine N, et al. Angiotensin II stimulates NHE3 activity by exocytic insertion of the transporter: role of PI 3-kinase. Kidney Int. 2003;64:939–49. doi: 10.1046/j.1523-1755.2003.00189.x. [DOI] [PubMed] [Google Scholar]
- 119.Moe OW. Acute regulation of proximal tubule apical membrane Na? H exchanger NHE-3: role of phosphorylation, protein trafficking, and regulatory factors. J Am Soc Nephrol. 1999;10:2412–25. doi: 10.1681/ASN.V10112412. [DOI] [PubMed] [Google Scholar]
- 120.Turban S, Beutler KT, Morris RG, Masilamani S, Fenton RA, Knepper MA, Packer RK. Long-term regulation of proximal tubule acid-base transporter abundance by angiotensin II. Kidney Int. 2006;70:660–8. doi: 10.1038/sj.ki.5001571. [DOI] [PubMed] [Google Scholar]
- 121.Zhuo JL. Monocyte chemoattractant protein-1: a key mediator of angiotensin II-induced target organ damage in hypertensive heart disease? J Hypertens. 2004;22:451–4. doi: 10.1097/01.hjh.0000098211.37783.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Duff JL, Berk BC, Corson MA. Angiotensin II stimulates the pp44 and pp42 mitogen-activated protein kinases in cultured rat aortic smooth muscle cells. Biochem Biophys Res Commun. 1992;188:257–64. doi: 10.1016/0006-291x(92)92378-b. [DOI] [PubMed] [Google Scholar]
- 123.Marrero MB, Schieffer B, Paxton WG, et al. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature. 1995;375:247–50. doi: 10.1038/375247a0. [DOI] [PubMed] [Google Scholar]
- 124.Schmitz U, Ishida T, Ishida M, et al. Angiotensin II stimulates p21-activated kinase in vascular smooth muscle cells: role in activation of JNK. Circ Res. 1998;82:1272–8. doi: 10.1161/01.res.82.12.1272. [DOI] [PubMed] [Google Scholar]
- 125.Molkentin JD, Lu JR, Antos CL, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–28. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lucchesi PA, Bell JM, Willis LS, Byron KL, Corson MA, Berk BC. Ca(2+)-dependent mitogen-activated protein kinase activation in spontaneously hypertensive rat vascular smooth muscle defines a hypertensive signal transduction phenotype. Circ Res. 1996;78:962–70. doi: 10.1161/01.res.78.6.962. [DOI] [PubMed] [Google Scholar]
- 127.Mitsuka M, Nagae M, Berk BC. Na(+)-H+ exchange inhibitors decrease neointimal formation after rat carotid injury. Effects on smooth muscle cell migration and proliferation. Circ Res. 1993;73:269–75. doi: 10.1161/01.res.73.2.269. [DOI] [PubMed] [Google Scholar]
- 128.Jimenez E, Vinson GP, Montiel M. Angiotensin II (AII)-binding sites in nuclei from rat liver: partial characterization of the mechanisms of Ang II accumulation in nuclei. J Endocrinol. 1994;143:449–53. doi: 10.1677/joe.0.1430449. [DOI] [PubMed] [Google Scholar]
- 129.Muller DN, Heissmeyer V, Dechend R, et al. Aspirin inhibits NF-kappaB and protects from angiotensin II-induced organ damage. FASEB J. 2001;15:1822–4. doi: 10.1096/fj.00-0843fje. [DOI] [PubMed] [Google Scholar]
- 130.Mezzano SA, Ruiz-Ortega M, Egido J. Angiotensin II and renal fibrosis. Hypertension. 2001;38:635–8. doi: 10.1161/hy09t1.094234. [DOI] [PubMed] [Google Scholar]
- 131.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signalling module: temporal control and selective gene activation. Science. 2002;298:1241–5. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 132.Stancovski I, Baltimore D. NF-kappaB activation: the I kappaB kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 133.Ruiz-Ortega M, Lorenzo O, Ruperez M, Blanco J, Egido J. Systemic infusion of angiotensin II into normal rats activates nuclear factor-kappaB and AP-1 in the kidney: role of AT(1) and AT(2) receptors. Am J Pathol. 2001;158:1743–56. doi: 10.1016/s0002-9440(10)64130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhuo JL, Carretero OA, Li XC. Effects of AT1a receptor-mediated endocytosis of extracellular angiotensin II on activation of nuclear factor-KB in proximal tubule cells. Ann NY Acad Sci. 2006;1091:336–45. doi: 10.1196/annals.1378.078. [DOI] [PMC free article] [PubMed] [Google Scholar]