Abstract
Long-term angiotensin II (ANG II) administration is associated with increased ANG II accumulation in the kidney, but intrarenal compartment(s) involved in this response remains to be determined. We tested the hypothesis that 1) extracellular ANG II is taken up by proximal tubule cells (PTCs) through AT1 receptor-mediated endocytosis, 2) this process is regulated by cytoskeleton microtubule- and tyrosine phosphatase-dependent mechanisms, and 3) AT1 receptor-mediated endocytosis of ANG II has a functional relevance by modulating intracellular cAMP signaling. In cultured PTCs, [125I]Tyr-labeled ANG II and fluorescein labeled-ANG II were internalized in a time-dependent manner and colocalized with the endosome marker Alexa Fluor 594-transferrin. Endocytosis of extracellular ANG II was inhibited by the AT1 receptor blocker losartan (16.5 ± 4.6%, P < 0.01 vs. ANG II, 78.3 ± 6.2%) and by the tyrosine phosphatase inhibitor phenylarsine oxide (PAO; 30.0 ± 3.5%, P < 0.05 vs. ANG II). Intracellular ANG II levels were increased by ~58% (basal, 229.8 ± 11.4 vs. ANG II, 361.3 ± 11.8 pg ANG II/mg protein, P < 0.01), and the responses were blocked by losartan (P < 0.01), the cytoskeleton microtubule inhibitor colchicine (P < 0.05), and PAO (P < 0.01), whereas depletion of clathrin-coated pits with hyperosmotic sucrose had no effect (356.1 ± 25.5 pg ANG II/mg protein, not significant). ANG II accumulation was associated with significant inhibition of both basal (control, 15.5 ± 2.8 vs. ANG II, 9.1 ± 2.4 pmol/mg protein, P < 0.05) and forskolin-stimulated cAMP signaling (forskolin, 68.7 ± 8.6 vs. forskolin + ANG II, 42.8 ± 13.8 pmol/mg protein, P < 0.01). These effects were blocked by losartan and PAO. We conclude that extracellular ANG II is internalized in PTCs through AT1 receptor-mediated endocytosis and that internalized ANG II may play a functional role in proximal tubule cells by inhibiting intracellular cAMP signaling.
Keywords: kidney, receptor-mediated endocytosis
Proximal tubule cells (PTCs) are the major target of a dual vasoactive peptide angiotensin II (ANG II), acting as both a circulating endocrine hormone and a local tissue paracrine and autocrine peptide in the kidney (14, 20, 22, 27, 30, 41). ANG II, administered through the tubular lumen or via peritubular capillary perfusion, activates cell surface AT1 receptors located in apical and basolateral membranes to regulate sodium and bicarbonate reabsorption under physiological conditions (14, 22, 41). Stimulating sodium and fluid reabsorption by ANG II in proximal tubules plays a major contributory role in sodium retention during the development of ANG II-dependent hypertension (14, 18, 24). Although cell surface AT1 receptor-mediated effects of ANG II in proximal tubules have been studied extensively, there is increasing evidence that PTCs may take up extracellular ANG II (both circulating and locally produced) via AT1 receptor-mediated endocytosis, which may be important for regulation of proximal tubular transport (11, 29, 30, 34, 35, 42). Nanomolar concentrations of ANG II have been reported in the glomerular filtrate (31), proximal tubular fluid (5, 20, 23, 31), and cortical interstitial fluid (25, 32). High levels of ANG II present in interstitial and intratubular fluid compartments combined with expression of abundant AT1 receptors in both apical and basolateral membranes provide PTCs with an ideal environment to promote cellular uptake through receptor-mediated endocytosis. For instance, increased whole kidney accumulation of circulating ANG II via AT1 receptor-mediated endocytosis has been consistently demonstrated in the contralateral (nonclipped) kidney of 2-kidney, 1-clip rats, a high-endogenous ANG II model of hypertension (13), along with kidneys of Ren-2 transgenic (20, 40) and ANG II-infused rats (38, 42, 44). However, the whole kidney approach does not allow identification of specific compartment(s) that may be responsible for intrarenal accumulation of ANG II in vivo.
Our group (42) recently demonstrated that increased intra-renal uptake of ANG II occurred primarily in renal cortical endosomes of ANG II-infused rats and was prevented by the AT1 receptor blocker candesartan. However, pharmacological blockers cannot distinguish between AT1 receptor subtypes, because there is ~ 95% genomic homology between AT1A and AT1B receptors (7). Most of AT1 receptor-mediated agonist endocytosis involves AT1A receptors, whereas the role of AT1B receptors remains unclear (7). To understand the role of AT1 receptor-mediated endocytosis in renal epithelial cells, opossum kidney (OK) epithelial cells and human embryonic kidney 293 cells (HEK-293) were transfected with AT1A receptors (16, 34), but these cells do not express major components of the renin-angiotensin system (RAS; including endogenous AT1A receptors), and therefore their physiological relevance remains uncertain. Recent evidence suggests that AT1 receptor-mediated endocytosis of extracellular ANG II is important not just for trafficking ANG II to the lysosomes for degradation and recycling of the receptors back to the membranes but also for full expression of the biological actions of ANG II in various cells (16, 29, 30, 34). For example, endocytosis of the ANG II-AT1 receptor complex is accompanied by increased phospholipase C- or phospholipase A2-mediated sodium flux and decreased cAMP production in renal epithelial cells (4, 29, 30, 34). These studies suggest that AT1A receptor-mediated endocytosis plays an important role in regulating PTC transport.
In the present study, we hypothesized that 1) extracellular ANG II is taken up by PTCs through AT1 receptor-mediated endocytosis; 2) receptor-mediated ANG II endocytosis contributes to increased intracellular accumulation of ANG II in PTCs; 3) blockade of receptor-mediated endocytosis by inhibitors of cell membrane cytoskeleton microtubules or tyrosine phosphatases prevents accumulation of ANG II in PTCs; and 4) AT1 receptor-mediated ANG II endocytosis plays a physiological role by regulating intracellular cAMP signaling. Using cultured rabbit PTCs derived from the S1 segment of proximal convoluted tubules, which express major components of the RAS (including AT1 and AT2 receptors), we demonstrated that AT1 receptor-mediated endocytosis of extracellular ANG II contributes to intracellular accumulation of ANG II in PTCs in vitro and plays an important role in the regulation of proximal tubule transport by modulating intracellular cAMP signaling.
MATERIALS AND METHODS
Materials
Cultured PTCs were obtained from American Type Culture Collection (vEPT; ATCC). These cells were initially derived from the S1 segment of rabbit kidney proximal convoluted tubules and have been shown to express electrolyte transporters as well as major components of the RAS, including angiotensinogen, renin, angiotensin-converting enzyme (ACE), and ANG II receptors (26, 27). Dulbecco’s modified Eagle’s medium, nutrient mixture, Ham’s F-12 (DMEM/F-12), trypsin, heat-inactivated fetal bovine serum (FBS), and the antibiotics penicillin and streptomycin were purchased from ATCC. Human Val5-ANG II, the radioligand [125I]Tyr-ANG II, and ANG II enzyme immunoassay kits were obtained from Biochem/Peninsula Laboratories. cAMP enzyme immunoassay kits were purchased from R&D Systems. The AT1 receptor antagonist losartan was a gift from Merck Pharmaceuticals, and the AT2 receptor antagonist PD-123319 was donated by Pfizer. AT1 receptor small-inteference RNA (siRNA) and rabbit polyclonal AT1 receptor antibody targeting the NH2-terminal extracellular domain of the human AT1 receptor (N-10), scrambled siRNA, and transfection reagents were purchased from Santa Cruz Biotechnology. Western blot supplies were obtained from Amersham. Colchicine and phenylarsine oxide (PAO) were obtained from Calbiochem.
Cell culture
Unless specified otherwise, PTCs (passages 8–12) were subcultured in six-well plates in complete DMEM/F-12 growth medium supplemented with 50 nM hydrocortisone, 5% heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (26, 27, 43). After reaching 80% confluence, they were starved in serum-free medium for 24 h before the experiment (26, 27, 43).
Expression of AT1 and AT2 receptors
To determine the proportion of AT1 or AT2 receptors in PTCs, we used [125I]Tyr-ANG II binding assays as described previously (15, 39, 40). Briefly, the cells were incubated with [125I]Tyr-ANG II (~100 pmol) for 60 min at 37°C. Total ANG II receptor binding was calculated as the binding in the absence of unlabeled ANG II or its receptor subtype-selective antagonist in the incubation. Nonspecific binding was determined as the binding in the presence of 10 μM unlabeled ANG II. AT1 receptor binding was determined in the presence of 10 μM unlabeled AT2 receptor blocker PD-123319, whereas AT2 receptor binding was calculated as the binding in the presence of 10 μM unlabeled AT1 blocker losartan. To determine ANG II receptor binding affinity constant (Kd) and maximum binding capacity (Bmax), we produced saturation binding curves and Scatchard plot by incubating PTCs with increasing concentrations of [125I]Tyr-ANG II (0–10 nM) alone or with 100 pmol of [125I]Tyr-ANG II in the presence of increasing concentrations of unlabeled ANG II or losartan (0–10 μM). Kd and Bmax were calculated using GraphPad Prism 4.0.
To confirm that rabbit PTCs express AT1 receptor protein, we divided subconfluent (60%) cells into three groups (n = 6). The first group was treated with serum-free medium as a control. The second group was transfected with an AT1 receptor-specific 20- to 25-nucleotide siRNA (AT1R siRNA; Santa Cruz) (37). The third group was transfected with a negative, non-AT1 receptor-targeting, scrambled siRNA (Santa Cruz). After transfection, cells were washed twice with ice-cold phosphate-uffered saline (PBS) and lysed with a modified RIPA buffer (50 mM Tris · HCl, 1% NP-40, 0.25% Nadeoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 μg/ml each of aprotinin, leupeptin, and pepstatin, 1 mM Na3VO4, and 1 mM NaF, pH 7.4). Proteins were extracted, electrophoretically separated on 8–16% Tris-glycine gels, and transferred to Millipore Immobilon-P membranes. The membranes were incubated for 3 h at room temperature with a rabbit polyclonal antibody against the human AT1 receptor (N-10, 1:200; Santa Cruz) or a rabbit anti-AT1A receptor polyclonal antibody against the cytosolic domain of the AT1A receptor (CLSTKMSTLSYRPSDNM; 1:200) as described (11, 17, 42, 43). Western blot signals were detected using enhanced chemiluminescence (Amersham) and analyzed using a microcomputer imaging device with a digital camera (MCID, Imaging Research, Ontario, Canada).
AT1 receptor-mediated endocytosis of extracellular ANG II
To determine whether AT1 receptors are internalized by PTCs when exposed to extracellular ANG II, the cells were incubated with 100 pmol [125I]Tyr-ANG II for 2, 5, 10, 15, or 30 min at 37°C alone or in the presence of the AT1 receptor blocker losartan (10 μM) or the specific tyrosine phosphatase inhibitor PAO (1 μM), both known to inhibit AT1A receptor endocytosis (9, 12, 30). At each time point, incubations were stopped by washing the cells twice with ice-cold PBS to remove free radioligands from the medium. Acid-sensitive (noninternalized) and -insensitive radioactivity (internalized) were separated by washing the cells twice with 5 mM ice-cold acetic acid buffer in 150 mM NaCl, pH 2.5. Radioactivity was counted and the percentage of internalized or noninternalized receptors analyzed (2, 12, 34).
Effects of AT1 and AT2 receptor blockade on intracellular accumulation of ANG II
To determine the role(s) of AT1 receptor-mediated ANG II endocytosis, PTCs were treated with vehicle (serum-free medium), ANG II (Val5-ANG II; 1 nM), ANG II plus losartan (10 μM), or ANG II plus PD-123319 (10 μM) for 60 min at 37°C. After treatment, the medium was removed and the cells washed twice with ice-cold PBS and then twice with ice-cold acid buffer (5 mM acetic acid, 150 mM NaCl, pH 2.5) to remove any cell membrane-bound ANG II (2, 12, 16, 34). ANG II was extracted from PTCs in a buffer containing 20 mM Tris · HCl, 10 mM EDTA, 5 mM EGTA, 5 mM mercaptoethanol, 50 g/ml PMSF, 1 μg/ml aprotinin, and 1 μg/ml pepstatin A and measured using a sensitive and specific ANG II enzyme immunoassay kit (Biochem/Peninsula).
Effects of inhibitors of clathrin-coated pits, cytoskeleton microtubules, and tyrosine phosphatase on intracellular ANG II accumulation
To determine the role(s) of clathrin-coated pits, cytoskeleton microtubules, or tyrosine phosphatases in AT1 receptor-mediated ANG II endocytosis in PTCs, the cells were treated with serum-free medium alone, ANG II (1 nM), ANG II plus 400 mM sucrose, which depletes clathrin-coated pits (2, 9, 10), ANG II plus the cytoskeleton microtubule inhibitor colchicine (1 μM) (3, 8, 29), or ANG II plus the tyrosine phosphatase inhibitor PAO (1 μM) to block AT1 receptor-mediated endocytosis (9, 12, 30). PAO is an established tyrosine phosphatase inhibitor that has been widely used for studying G protein-coupled receptor (GPCR) endocytosis (9, 12, 30, 33, 36). After treatment, the cells were washed and ANG II was extracted as described above.
Effects of AT1 receptor-mediated endocytosis of extracellular ANG II on intracellular cAMP production
Cyclic AMP is one of the most important signaling molecules involved in the regulation of sodium and fluid transport by ANG II in proximal tubules (14, 22, 27, 34). ANG II is thought to activate mainly basolateral AT1 receptors, which are coupled to adenylyl cyclase via Gi proteins, to inhibit formation of cAMP; however, it was recently reported that after endocytosis, ANG II may directly activate Gi protein-coupled basolateral AT1 receptors (34). To determine whether AT1 receptor-mediated ANG II endocytosis can affect intracellular cAMP signaling, we followed three experimental protocols. First, subconfluent PTCs in six-well plates were treated for 15, 30, or 60 min with serum-free medium only or ANG II (1 nM) alone to determine the time-dependent responses of cAMP production to ANG II (n = 6 each). Second, based on the time-dependent responses of cAMP to ANG II (peaked at 30 min), PTCs were pretreated with the AT1 receptor blocker losartan (10 μM) or the tyrosine phosphatase inhibitor PAO (1 μM) before exposure to ANG II for 30 min (n = 6 each). Cells treated with losartan or PAO alone also were used as controls. Third, PTCs were treated for 30 min with 1) the adenylyl cyclase activator forskolin alone (10 μM), which stimulates cAMP production, 2) forskolin plus ANG II (ANG II + forskolin), 3) ANG II plus forskolin and the AT1 receptor blocker losartan (10 μM), 4) ANG II plus forskolin and the AT2 receptor blocker PD-123319 (10 μM), or 5) ANG II plus forskolin and the endocytotic inhibitor PAO (1 μM) (n = 6 each). The phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX; 1 mM) was added to all samples to prevent degradation of cAMP (29, 33). After treatment, the culture medium was removed and the cells were washed and lysed with 0.1 N HCl. The lysates were centrifuged at 1000 g, and the supernatant was collected for cAMP measurements with the use of a sensitive cAMP ELISA kit (R&D).
Statistical analysis
Results are expressed as means ± SE. Unless otherwise specified, 6–12 samples from two separate experiments were collected for each treatment and assayed in duplicate for measurements of intracellular ANG II levels. For Western blot data, at least six samples from two separate experiments were performed, with each treatment assayed in duplicate. For binding data, two separate experiments were performed, with each time point determined in duplicate. Comparisons between two treatments were made using Student’s unpaired t-test. Comparisons between more than two treatments were made with one-way analysis of variance, followed by a Newman-Keuls test for multiple comparisons. P < 0.05 was considered significant.
RESULTS
Expression of AT1 receptors in rabbit proximal tubule cells
As shown in Fig. 1A, radioreceptor binding assays using [125I]Tyr-ANG II showed that PTCs express a single class of ANG II receptor binding. Scatchard analysis suggests that these cells bound [125I]Tyr-ANG II with an apparent Kd of 6.8 ± 2.1 nM, and the Bmax averaged 1,012 ± 28 fmol/mg protein. The AT1 receptor predominates in these cells because losartan (10 μM), a competitive AT1 receptor blocker, displaced over 90% of total ANG II receptor binding, whereas the AT2 receptor-selective blocker PD-123319 (10 μM) inhibited < 10% of binding. Next, we used two approaches to confirm that PTCs express AT1 receptors. First, with the use of a rabbit anti-AT1 receptor polyclonal antibody against the NH2-terminal extracellular domain of the human AT1 receptor (11, 17, 42), Western blot detected a single protein band of ~42 kDa (Fig. 1B). The AT1 receptor protein we detected is consistent with the AT1 receptor reportedly expressed in the rat kidney (15). In previous studies, pretreatment of samples with an AT1 receptor-selective antigen blocking peptide (Santa Cruz) before running the Western blot confirmed the specificity of the AT1 receptor protein (15, 43). Second, AT1 receptor protein expression was significantly knocked down by ~80% after transfection of PTCs with an AT1-selective siRNA, whereas a scrambled, non-AT1-selective siRNA had little effect (Fig. 1B). When the same membranes were stripped and reprobed with an anti-β-actin antibody, equal protein loading was confirmed (Fig. 1A, bottom band). Thus the PTCs we used express AT1 receptor protein corresponding to human AT1 receptors.
Fig. 1.
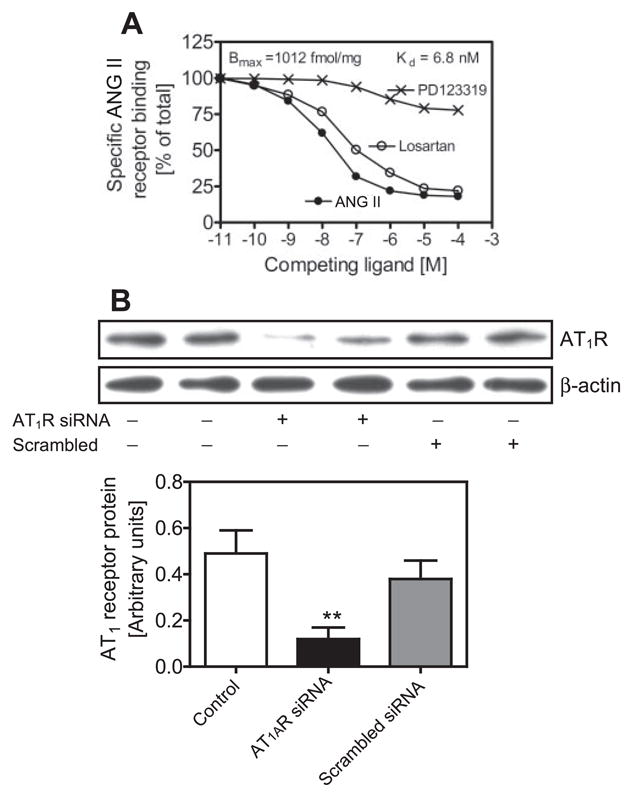
Expression of AT1 receptors (equivalent to human AT1 or rodent AT1A) in cultured rabbit proximal tubule cells (PTCs). A: [125I]Tyr-ANG II receptor binding showing binding competition by the unlabeled ANG II, AT1 receptor blocker losartan or the AT2 receptor blocker PD-123319 (10−11 to 10−4 M) and Scatchard analysis of maximum binding capacity (Bmax) and the apparent Kd. B: ANG II receptor protein expression was significantly inhibited by a specific AT1 receptor small-interference RNA (AT1R siRNA), whereas a scrambled siRNA had no effect. **P < 0.01 vs. control.
AT1 receptor-mediated endocytosis of extracellular ANG II
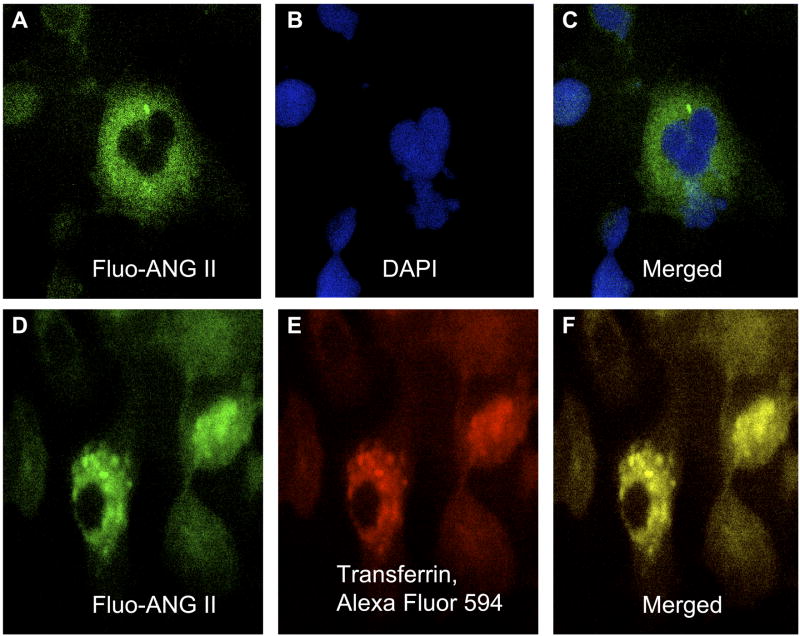
AT1A receptors have been shown to internalize after exposure to extracellular ANG II in OK or HEK-293 cells, two renal epithelial cell lines commonly used for transfection of AT1A receptors because they do not express endogenous receptors (16, 34). Using both the radioligand [125I]Tyr-ANG II and fluorescein-conjugated ANG II, we determined whether extracellular ANG II is internalized after binding to cell surface AT1 receptors in rabbit PTCs and whether losartan or PAO blocked its endocytosis. Figure 2 shows that extracellular ANG II was internalized in a time-dependent manner, with close to 80% internalized within 30 min of incubation (78.3 ± 6.2%). Time-dependent endocytosis of extracellular ANG II was almost completely inhibited by blocking of AT1 receptors with losartan (16.5 ± 4.6%, P < 0.01) or inhibiting tyrosine phosphatase with PAO (30.0 ± 3.5%, P < 0.05). Thirty minutes after incubation, fluorescence microscopy shows that fluorescein-labeled ANG II was localized to the cytoplasm of the cells (Figs. 3, A and C), where it colocalized with Alexa Fluor 594-labeled transferrin, an endosomal marker (Figs. 3, D and F). These results suggest that after endocytosis, ANG II is trafficked mainly into endosomes of PTCs.
Fig. 2.
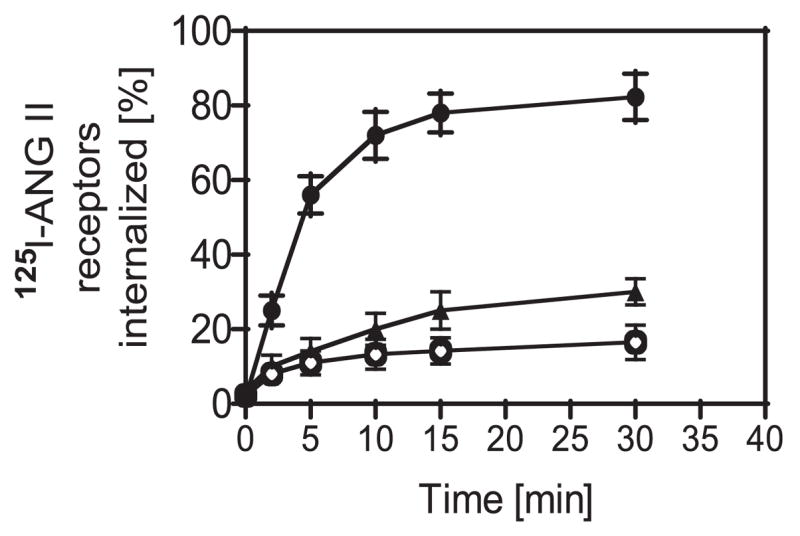
Time-dependent endocytosis of [125I]Tyr-ANG II in cultured rabbit PTCs in the absence (●) or presence of the endocytotic inhibitor losartan (10 μM; ○) or phenylarsine oxide (PAO; 1 μM; ▲). Endocytosis of [125I]Tyr-ANG II peaked at 30 min after exposure to the radioligand, which was significantly inhibited by losartan and PAO, suggesting an AT1 receptor-mediated response.
Fig. 3.
Endocytosis of fluorescein-labeled ANG II (Fluo-ANG II) in the cytoplasm of cultured rabbit PTCs, where it colocalized with Alexa Fluor 594-labeled transferrin, an endosomal marker, 30 min after the cells were exposed to Fluo-ANG II. A: cytoplasmic localization of internalized Fluo-ANG II (green). B: nucleus stained with 4,6-diamidino-2-phenylindole (DAPI; blue). C: merged image of A and B, showing the relationship between Fluo-ANG II and the nucleus. D: Fluo-ANG II (green). E: endosomes stained with Alexa Fluor 594-labeled transferrin (red). F: merged image of D and E (yellow), showing colocalization of Fluo-ANG II and transferrin. Magnification, ×40.
Effects of AT1 and AT2 receptor blockade on accumulation of ANG II
Our group (42, 44) has previously shown that extracellular ANG II is accumulated in the rat kidney via AT1 receptor-mediated endocytosis after long-term ANG II infusion. High levels of ANG II were later demonstrated in isolated renal cortical endosomes and intermicrovillar clefts of ANG II-infused rats (42). In the present study, intracellular ANG II levels in PTCs were measured using an ANG II enzyme-linked immunoassay (Biochem/Peninsula Laboratories). The assay demonstrated high levels of sensitivity and can detect up to 20 pg ANG II/ml or 2 pg ANG II per well of six-well-plates (intra-assay variation < 5%; interassay variation < 14%). There is 100% cross-reactivity with ANG II and Val5-ANG II, but only 0.5% with ANG I. Basal ANG II levels in PTCs averaged 229.8 ± 11.4 pg ANG II/mg protein (Fig. 4). Incubation of PTCs with Val5-ANG II (1 nM) for 1 h at 37°C increased intracellular ANG II by ~58% (361.3 ± 11.8 pg ANG II/mg protein, P < 0.001 vs. basal). Blockade of AT1 receptors with losartan (10 μM) effectively prevented increased ANG II (254.3 ± 8.8 pg ANG II/mg protein, P < 0.001 vs. ANG II). Losartan alone did not alter basal ANG II levels (220.5 ± 6.9 pg ANG II/mg protein, not significant vs. basal). Interestingly, coadministration of PD-123319 with ANG II also slightly reduced intracellular ANG II to the level seen with ANG II alone (287.3 ± 26 pg ANG II/mg protein, P < 0.05 vs. ANG II). These data suggest that both AT1 and AT2 receptors mediate endocytosis of extracellular ANG II in PTCs, but it is AT1 that predominated.
Fig. 4.
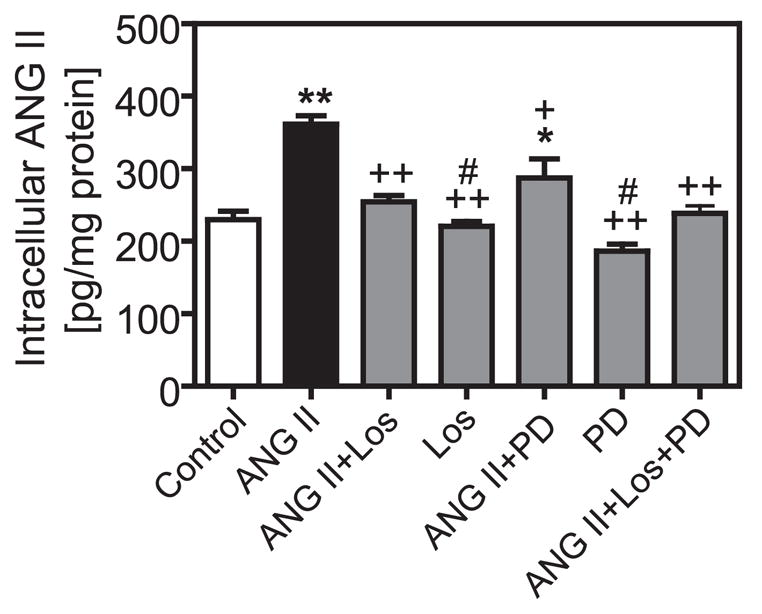
Effects of AT1 and AT2 receptors on intracellular accumulation of extracellular ANG II in PTCs. Note that both the AT1 receptor blocker losartan (10 3M) and the AT2 receptor blocker PD-123319 (10 μM) inhibited intracellular ANG II accumulation, but the effect of losartan predominated. Los, losartan; PD, PD-123319. *P < 0.05; **P < 0.01 vs. control. + P < 0.05; + + P < 0.01 vs. ANG II. #P < 0.05 vs. ANG II + Los or ANG II + PD.
Effects of inhibitors of clathrin-coated pits, cytoskeleton microtubules, and tyrosine phosphatase on ANG II accumulation
Clathrin-coated pits, cytoskeleton microtubules, and tyrosine phosphatases have been separately shown to play animportant role in AT1A receptor-mediated endocytosis in vascular smooth muscle cells (VSMCs) (2), Chinese hamster ovary (CHO) cells transfected with AT1A receptors (9), or rat PTCs (29, 30). However, it is not clear whether AT1-mediated endocytosis contributes to accumulation of extracellular ANG II in rabbit PTCs via a similar mechanism. Figure 5A shows that incubation of PTCs at 4°C with Val5-ANG II, which inhibits receptor-mediated endocytosis, prevented the AT1-mediated increase in intracellular ANG II accumulation (ANG II, 367.6 ± 18.9 pg ANG II/mg protein; ANG II plus 4°C, 213.2 ± 17.7 pg ANG II/mg protein, P < 0.001). Unexpectedly, depletion of clathrin-coated pits with 400 mM sucrose did not prevent intracellular ANG II accumulation (356.1 ± 25.5 pg ANG II/mg protein, not significant vs. ANG II). As shown in Fig. 5B, both colchicine, an inhibitor of cytoskeleton microtubule-mediated protein trafficking (8, 29), and PAO, an inhibitor of tyrosine phosphatases and receptor-mediated endocytosis (12, 29), prevented intracellular ANG II accumulation (ANG II + colchicine, 266.9 ± 28 pg ANG II/mg protein, P < 0.05 vs. ANG II; ANG II + PAO, 190.8 ± 17.9 pg ANG II/mg protein, P < 0.001 vs. ANG II). These results suggest that cytoskeleton microtubules and tyrosine phosphatases, but not clathrin-coated pits, mediate extracellular ANG II accumulation in PTCs.
Fig. 5.
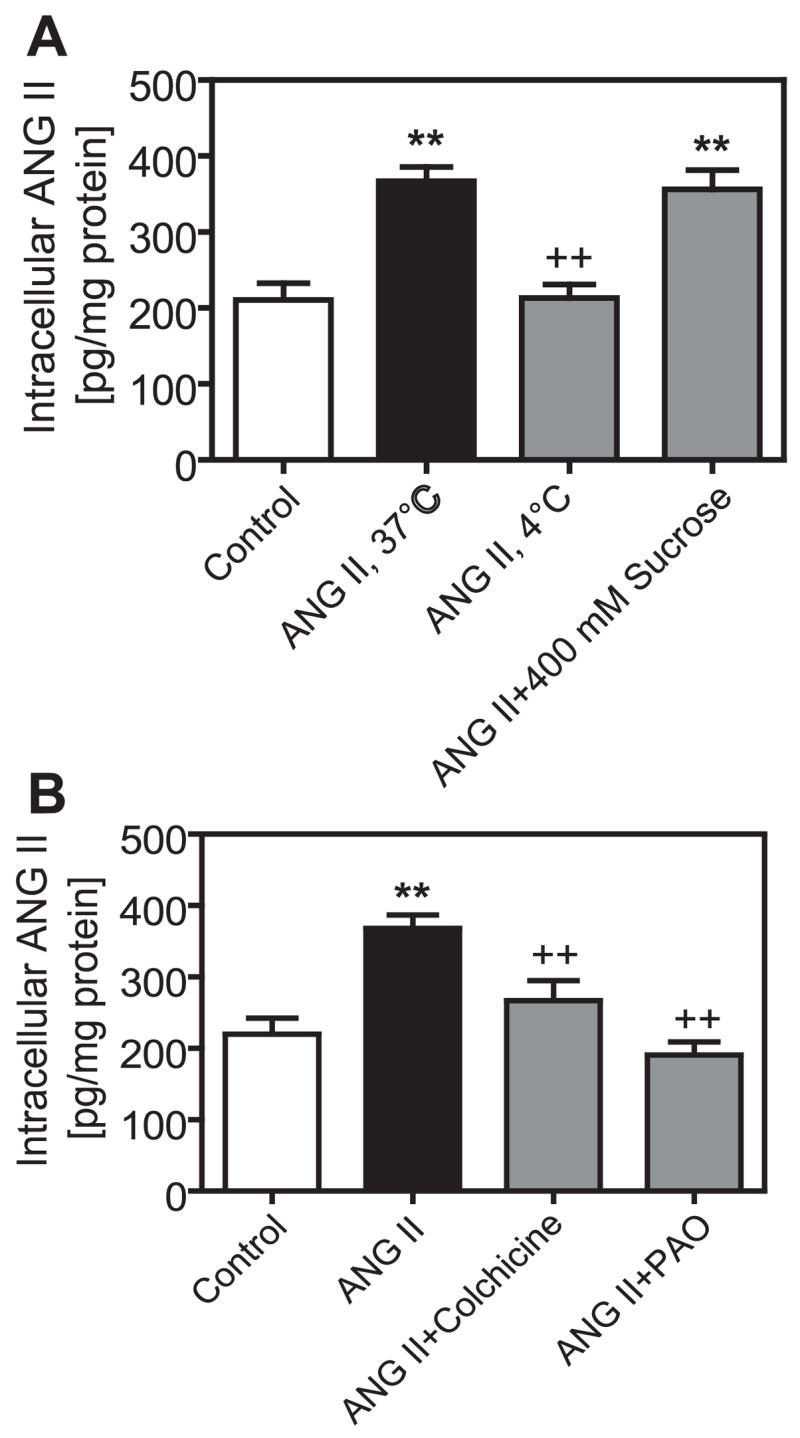
Effects of endocytotic inhibitors on AT1 receptor-mediated accumulation of ANG II in PTCs. A: effects of cold (4°C), which inhibits AT1 receptor-mediated endocytosis, or sucrose (400 mM), which depletes clathrin-coated pits in non-renal cells. B: effects of the cytoskeleton microtubule inhibitor colchicine (1 μM) or the tyrosine phosphatase inhibitor PAO (1 μM). **P < 0.01 vs. control. + + P < 0.01 vs. ANG II.
Effects of AT1 receptor-mediated endocytosis of extracellular ANG II on intracellular cAMP production
Previous studies suggested that AT1 (or AT1A) receptor-mediated endocytosis of extracellular ANG II may play an important role in full expression of biological actions of ANG II in various cells (29, 30, 34). To determine whether AT1-mediated endocytosis of extracellular ANG II has a functional relevance, we measured cAMP production in PTCs using an enzyme-linked immunoassay (R&D). AT1-mediated ANG II endocytosis was blocked by the AT1 receptor blocker losartan (10 μM) or the tyrosine phosphatase inhibitor PAO (1 μM). Figure 6A shows time-dependent inhibition of cAMP production by ANG II, which peaked at 30 min. At 30 min, ANG II inhibited basal cAMP production (basal, 15.5 ± 2.8 pmol/mg protein; ANG II, 9.1 ± 2.4 pmol/mg protein, P < 0.05). The effect of ANG II on basal cAMP production was reversed by losartan (33.3 ± 6.3 pmol/mg protein, P < 0.05) and PAO (22.5 ± 3.6 pmol/mg protein, P < 0.05) to levels significantly above control (Fig. 6B). As shown in Fig. 7, the adenylyl cyclase activator forskolin (10 μM) markedly increased intracellular cAMP levels (68.7 ± 8.6 pmol · mg−1 · protein−1, P < 0.01) (Fig. 7). ANG II significantly reduced forskolin-induced increases in cAMP (42.8 ± 13.8 pmol/mg protein, P < 0.05 vs. forskolin alone). Both losartan and PAO, which inhibit AT1-mediated endocytosis, blocked ANG II-induced inhibition of forskolin-mediated increases in intracellular cAMP production (ANG II + forskolin + losartan, 54.3 ± 14.7 pmol/mg protein, P < 0.05 vs. ANG II + forskolin; ANG II + forskolin + PAO, 36.3 ± 5.9 pmol/mg protein, P < 0.05 vs. ANG II + forskolin) (Fig. 7B). Losartan alone also slightly increased cAMP production by ~15% above control (P < 0.05), whereas PAO alone had no effect. PD-123319 had no effect on ANG II-induced inhibition of the forskolin-mediated increase in cAMP production (22.5 ± 5.6 pmol/mg protein, not significant vs. ANG II + forskolin) (Fig. 7A). These results indicate that AT1 receptor-mediated endocytosis of extracellular ANG II may affect intracellular cAMP signaling in PTCs.
Fig. 6.
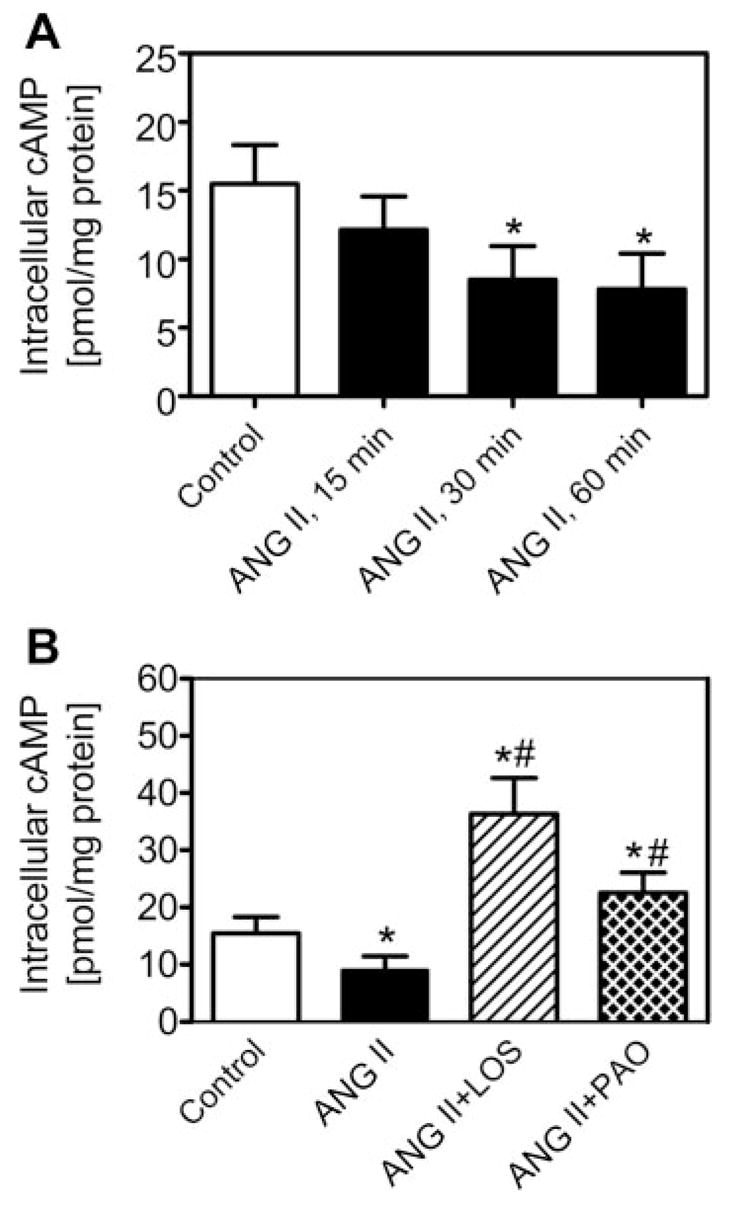
Effects of blockade of AT1-mediated ANG II endocytosis by losartan and PAO on basal intracellular cAMP responses to ANG II in PTCs. A: time-dependent inhibition of cAMP production by ANG II (1 nM), which peaked at 30 min. B: at 30 min, ANG II (1 nM) attenuated basal cAMP production and that losartan and PAO reversed ANG II-induced inhibition of basal cAMP production. *P < 0.05 vs. control. #P < 0.05 vs. ANG II.
Fig. 7.
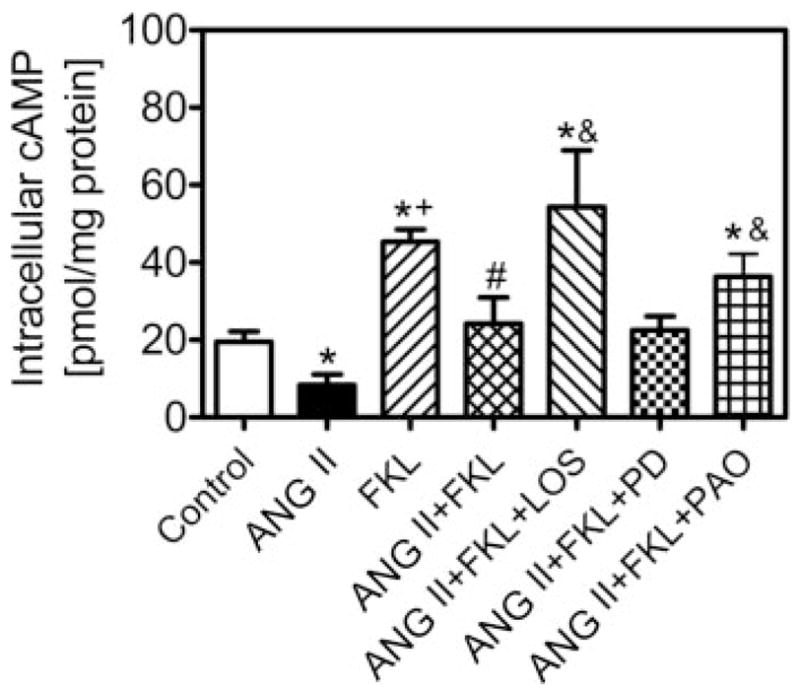
Effects of blockade of AT1-mediated ANG II endocytosis by losartan and PAO on forskolin-stimulated cAMP production in PTCs 30 min after exposure to the agonist and/or blockers. Note that forskolin stimulated cAMP production and ANG II (1 nM) significantly attenuated forskolin-stimulated cAMP production. Both losartan and PAO reversed ANG II-induced inhibition of forskolin-increased cAMP production, whereas PD-123319 did not. FKL, forskolin. *P < 0.05 vs. control. + P < 0.05 vs. ANG II. #P < 0.05 vs. FKL. &P < 0.05 vs. ANG II + FKL.
DISCUSSION
The present study produced three key findings, namely, 1) cultured PTCs derived directly from the S1 segment of rabbit proximal tubules expressed predominantly AT1 receptor protein equivalent to AT1A receptors in rodents; 2) incubating these cells with Val5-ANG II significantly increased intracellular ANG II accumulation, which was blocked by inhibition of receptor-mediated endocytosis with the AT1 receptor blocker losartan, cold (4°C), the cytoskeleton microtubule inhibitor colchicine, or the tyrosine phosphatase inhibitor PAO; and 3) AT1 receptor-mediated endocytosis of extracellular ANG II has a functional role, as indicated by the finding that blockade of AT1 receptor-mediated endocytosis with various endocytotic inhibitors prevented ANG II-induced inhibition of basal and forskolin-stimulated intracellular cAMP production. These results suggest that AT1-mediated endocytosis of extracellular ANG II in PTCs plays an important role in renal accumulation of circulating ANG II ex vivo and that after endocytosis, intracellular ANG II exerts a functional effect on ANG II receptor-activated signaling. Because colchicine, a selective inhibitor of cytoskeleton microtubules, and PAO, an inhibitor of tyrosine phosphatases, prevented AT1-mediated intracellular ANG II accumulation, our results suggest that increased endocytosis of extracellular ANG II in PTCs is cytoskeleton microtubule- and tyrosine phosphate-dependent. By contrast, hyperosmotic sucrose (400 mM), which inhibits GPCR endocytosis in VSMCs or CHO cells by depleting clathrin-coated pits (9, 28), failed to prevent intracellular ANG II accumulation, indicating that clathrin-coated pits do not play a significant role in AT1 receptor-mediated ANG II accumulation in rabbit PTCs.
The present study provides evidence that AT1-mediated ANG II endocytosis plays an important role in high intracellular accumulation of ANG II in PTCs in vitro and, by implication, that this process also may occur in PTCs ex vivo. Investigators in our group and others have previously shown that circulating ANG II accumulates in the kidney of ANG II-infused rats via an AT1-mediated mechanism(s) (36, 38, 42, 44); however, the cellular location of ANG II accumulation in the kidney as well as the mechanisms involved have not been determined. ANG II levels in the kidney are reportedly several thousand times higher than the circulating peptide, which leads to the hypothesis of compartmentalization of ANG II synthesis and/or release within the kidney (5, 23). Indeed, early studies reported nanomolar concentrations of ANG II in intrarenal fluid compartments, including the glomerular filtrate (31), proximal tubular fluid (5, 31), and renal cortical interstitial fluid (25, 32) compared with femto- to picomolar levels in the circulation. However, when van Kats et al. (35) infused 125I-labeled ANG II into pigs and measured labeled peptide levels in different cellular fractions of the kidney homogenates, they found that most 125I-ANG II is cell-associated due to AT1 receptor-mediated endocytosis. We previously measured internalized AT1A receptors and ANG II in isolated renal cortical endosomes and intermicrovillar clefts of ANG II-infused rats and found that AT1A receptor antibody binding more than doubled, whereas ANG II levels were 5–10 times higher in endosomes and more than doubled in intermicrovillar clefts compared with control (42). Because coadministration of the AT1 receptor blocker candesartan prevented accumulation of extracellular ANG II in endosomes and intermicrovillar clefts, we interpreted these findings as an AT1A-mediated response. However, it should be emphasized that all previous studies were performed in whole kidney tissue, and therefore it is not possible to determine the cellular sites responsible for increased accumulation of extracellular ANG II in the kidney after long-term ANG II infusion.
In the present study, we used cultured rabbit PTCs as a tool to determine the contribution of AT1-mediated endocytosis of extracellular ANG II to intracellular accumulation of ANG II and study the potential role(s) of clathrin-coated pits, cytoskeleton microtubules, and tyrosine phosphatases in AT1-mediated ANG II accumulation in PTCs in vitro. Use of these cells as a model offers several advantages over the whole kidney approach in vivo. These cells express all necessary components of the RAS, including ANG II receptors, and respond to ANG II stimulation by activating intracellular signaling, commonly associated with PTC function (26, 27, 43). Although proximal tubules can be isolated for measurement of ANG II, the procedures are laborious and time-consuming, and the reagents used for isolation and purification would likely alter ANG II formation and degradation. The main disadvantage with using whole kidney homogenates is perhaps that it is not possible to study the cellular mechanisms involved beyond the role of AT1 receptors. In the present study, we first demonstrated that AT1 (equivalent to human AT1 and rodent AT1A) receptor proteins are expressed in these PTCs by Western blot, using rabbit anti-AT1 receptor antibodies raised against the NH2-terminal or cytosolic domains of the receptor (11, 17, 42) and an AT1 receptor-selective siRNA. We then confirmed that incubating these cells with 125I-ANG II induced ~80% internalization of the peptide within 30 min of exposure and that this was blocked by losartan, pointing to an AT1 receptor-mediated mechanism. This phenomenon has been demonstrated previously in OK cells transfected with AT1A receptors, although basal and internalized ANG II were not measured (33). We therefore extended previous studies by measuring intracellular ANG II before and after exposure to extracellular ANG II to promote receptor-mediated endocytosis. On the basis of cell number and protein content per well and ANG II concentrations, we can estimate basal endogenous ANG II content in these cells as well as the relative contribution of AT1-mediated endocytosis of extracellular ANG II to intracellular ANG II accumulation in PTCs. There are ~ 106 cells or 360 μg protein in each well of a six-well plate when they have grown to 80% confluence. The basal concentration of ANG II is close to 200–250 pg/mg protein, which gives a calculated basal ANG II content of ~ 70–90 pg/106 cells. Intracellular ANG II content would increase by 50–70% to ~ 110–150 pg/106 cells, primarily due to AT1-mediated endocytosis.
Whether AT2 receptors mediate ANG II endocytosis and its subsequent intracellular signaling is not known (7, 9, 16, 45). Hein et al. (16) showed that unlike AT1 receptors, AT2 receptors transiently or stably expressed in HEK-293 cells do not internalize when they are stimulated by ANG II. As shown in Figs. 1 and 4, however, we found that rabbit PTCs also express low levels of endogenous AT2 receptors and that the AT2 receptor antagonist PD-123319 partially inhibited intracellular accumulation of extracellular ANG II, suggesting that endogenous AT2 receptors perhaps play a minor role in mediating ANG II endocytosis in rabbit PTCs. Indeed, AT2 receptors have been reported to mediate different biological effects in various renal cells (7).
The cellular mechanisms that regulate AT1 (or AT1A) receptor-mediated endocytosis are complex and often cell type specific. There are two recognized pathways for GPCR endocytosis, the classic clathrin-dependent and non-clathrin-dependent pathways (1, 9, 28). The most commonly cited pathways for GPCR-mediated endocytosis include clathrin-coated pits, β-arrestin and/or dynamin proteins, β-adaptin, and G protein-coupled receptor kinases (1, 9, 28). Because discussion of the role(s) of individual pathways in AT1A receptor-mediated endocytosis is beyond the scope of the present study, we focused on the potential role(s) of three important pathways in mediating intracellular ANG II accumulation in PTCs. Clathrin-coated pits or vesicles, interacting with β-arrestin and/or dynamin proteins, are widely credited with endocytosis of epidermal growth factor (9, 35) and β2-adrenergic receptors (9) and also with AT1A receptors in CHO or HEK-293 cells stably transfected with the mutant receptors (16, 33). We questioned whether clathrin-coated pits play a role in AT1-mediated ANG II accumulation in PTCs. Our group previously showed that in the ANG II-infused rat kidney, ANG II accumulated in renal cortical endosomes, where it colocalized with AT1A receptors (42); yet we could not determine whether clathrin-coated pits play any role in intracellular trafficking of ANG II/AT1 receptor complex to the endosomes in vivo. In the present study, we found that pretreating PTCs with hyperosmotic sucrose (400 mM), which is commonly used to deplete clathrin-coated pits (2, 9, 35), did not significantly prevent receptor-mediated intracellular accumulation of ANG II, suggesting that non-clathrin endocytic pathways may play an important role in PTCs. Non-clathrin endocytic pathways also can deliver molecules to classic endocytic compartments, such as endosomes, and to other intracellular compartments, such as the Golgi apparatus and endoplasmic reticulum (28). Schelling et al. (29) demonstrated that in cultured rat PTCs, blocking receptor-mediated endocytosis with the cytoskeleton microtubule inhibitor colchicine or PAO, a tyrosine phosphatase-selective inhibitor, completely eliminated apical ANG II-induced phospholipase C (PLC)-mediated intracellular inositol 1,4,5-trisphosphate (IP3) signaling and 22Na transport. In the present study, we demonstrated that colchicine and PAO completely prevented AT1-mediated intracellular ANG II accumulation in PTCs, supporting the hypothesis that AT1-mediated endocytosis of extracellular ANG II in PTCs is cytoskeleton microtubule-dependent and requires activation of tyrosine phosphatases.
How cytoskeleton microtubules or tyrosine phosphatases could modulate AT1-induced intracellular accumulation of extracellular ANG II in PTCs remains to be determined. Cytoskeleton microtubules are polarized cytoplasmic structures extending from the perinuclear region toward the periphery of the cell (3, 6, 19). Cytoskeleton microtubules, acting through the dynein activator protein dynactin, play an important role in cytoplasmic trafficking of viruses, solutes, or proteins from early endosomes to late endosomes or lysosomes and from the endoplasmic reticulum to the Golgi apparatus inside mammalian cells (3, 6, 19). PTCs are polarized epithelial cells with their apical membrane facing the tubular lumen and their basolateral membrane touching the peritubular capillaries. Solutes, amino acids, peptides, and glucose are transported into cells via receptor-mediated endocytosis or by various transporters (3, 6, 21). It is likely that colchicine prevented intra-cellular ANG II accumulation by inhibiting cytoplasmic trafficking of the peptide after endocytosis. In VSMCs, disruption of cytoskeleton microtubules with nocodazole blocked AT1 receptor trafficking into caveolae/lipid rafts (45). By contrast, PAO may block AT1A-mediated ANG II endocytosis via a different mechanism. PAO is a general inhibitor of tyrosine phosphatases that has been widely used to study AT1A receptor endocytosis (12, 30), but it is not clear which specific tyrosine phosphatase it inhibits and how it inhibits AT1A receptor endocytosis. Previous studies have shown that PAO inhibits not only AT1A receptor endocytosis (12, 30) but also other GPCR endocytosis (33, 36). Thus PAO may not act specifically at the receptor level and, instead, inhibits receptor endocytosis by targeting the endocytotic machinery such as arrestins, dynamins, or cytoskeleton microtubules. It is also likely that PAO may inhibit one of tyrosine phosphatases that play a role in GPCR endocytosis. Nevertheless, because PAO inhibits protein tyrosine phosphatases and therefore induces protein tyrosine dephosphorylation, our results suggest that tyrosine phosphatases and/or tyrosine dephosphorylation are involved in AT1A receptor-mediated intracellular accumulation of extracellular ANG II in proximal tubule cells. Further studies are required to identify which specific protein tyrosine phosphatase regulates AT1A receptor endocytosis and elucidate the cellular mechanisms involved.
Our results show that AT1-mediated endocytosis of extracellular ANG II may play a functional role in regulating proximal tubular sodium transport. In the present study, increased intracellular accumulation of extracellular ANG II via AT1 receptor-mediated endocytosis was associated with decreased basal and forskolin-stimulated intracellular cAMP production. Losartan and PAO inhibited AT1-mediated ANG II endocytosis in PTCs, and both prevented the effects of ANG II on intracellular cAMP production, indicating that internalized ANG II does indeed play a functional role in PTC function. Alternatively, because coadministration of losartan or PAO with ANG II increased cAMP production to the levels that were significantly higher than control or ANG II alone (Fig. 6B), other mechanisms unrelated to AT1-mediated ANG II endocytosis may be involved. There is evidence that receptor-mediated ANG II endocytosis is important not just for transporting the ligand to the lysosomes for destruction and recycling the receptors back to the cell surface and that receptor-mediated ANG II endocytosis may be important in regulating biological actions of ANG II in PTCs. Schelling et al. (29, 30) demonstrated that endocytosis of the ANG II-AT1 receptor complex activated PLC-IP3 signaling, increased sodium flux, and decreased cAMP signaling in cultured rat PTCs. Becker et al. (4) showed that AT1 receptor-mediated endocytosis was associated with increased phospholipase A2 activity and sodium flux in LLC-PK cells expressing rabbit AT1 receptors. Thekkumkara and Linas (34) reported that in OK cells, apical membrane AT1A receptors were internalized before they inter-act with G proteins, leading to inhibition of cAMP signaling. Accordingly, our finding that inhibition of AT1 receptor-mediated endocytosis of extracellular ANG II blocked intracellular ANG II accumulation, and therefore ANG II-induced inhibition of cAMP signaling in PTCs, is consistent with these previous observations.
In summary, we have demonstrated in cultured rabbit PTCs, which express endogenous AT1 receptors, that 1) intracellular ANG II levels increase significantly when cells are exposed to extracellular ANG II; 2) increased intracellular ANG II accumulation is inhibited by the AT1 receptor antagonist losartan, the cytoskeleton microtubule inhibitor colchicine, or the tyrosine phosphatase inhibitor PAO; 3) depletion of clathrin-coated pits with hyperosmotic sucrose has no effect on intracellular ANG II accumulation; and 4) inhibition of AT1 receptor-mediated intracellular ANG II accumulation blocks ANG II-inhibited cAMP production. These results suggest that AT1 receptor-mediated endocytosis of extracellular ANG II in PTCs contributes to increased intrarenal ANG II accumulation in vivo and also plays a functional role in the regulation of proximal tubule cell function by regulating intracellular cAMP signaling.
Acknowledgments
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant 5R01 DK-067299 (to J. L. Zhuo), American Heart Association Grant-in-Aid 0355551Z, and a National Kidney Foundation Grant-in-Aid. O. A. Carretero is supported by National Heart, Lung, and Blood Institute (NHLBI) Program Project Grant HL-28982. L. G. Navar is supported by NHLBI Grant HL-26371. Parts of this work were presented as an abstract at the Gordon Research Conference on Angiotensin in 2004 and the Federation of American Societies for Experimental Biology Summer Research Conference on Renal Microcirculatory and Tubular Dynamics in 2004.
References
- 1.Aguilar RC, Wendland B. Endocytosis of membrane receptors: two pathways are better than one. Proc Natl Acad Sci USA. 2005;102:2679–2680. doi: 10.1073/pnas.0500213102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson KM, Peach MJ. Receptor binding and internalization of a unique biologically active angiotensin II-colloidal gold conjugate: morphological analysis of angiotensin II processing in isolated vascular strips. J Vasc Res. 1994;31:10–17. doi: 10.1159/000159026. [DOI] [PubMed] [Google Scholar]
- 3.Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–159. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- 4.Becker BN, Cheng HF, Burns KD, Harris RC. Polarized rabbit type 1 angiotensin II receptors manifest differential rates of endocytosis and recycling. Am J Physiol Cell Physiol. 1995;269:C1048–C1056. doi: 10.1152/ajpcell.1995.269.4.C1048. [DOI] [PubMed] [Google Scholar]
- 5.Braam B, Mitchell KD, Fox J, Navar LG. Proximal tubular secretion of angiotensin II in rats. Am J Physiol Renal Fluid Electrolyte Physiol. 1993;264:F891–F898. doi: 10.1152/ajprenal.1993.264.5.F891. [DOI] [PubMed] [Google Scholar]
- 6.Brown D, Stow JL. Protein trafficking and polarity in kidney epithelium: from cell biology to physiology. Physiol Rev. 1996;76:245–297. doi: 10.1152/physrev.1996.76.1.245. [DOI] [PubMed] [Google Scholar]
- 7.De Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 8.Elkjaer ML, Birn H, Agre P, Christensen EI, Nielsen S. Effects of microtubule disruption on endocytosis, membrane recycling and polarized distribution of aquaporin-1 and gp330 in proximal tubule cells. Eur J Cell Biol. 1995;67:57–72. [PubMed] [Google Scholar]
- 9.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 10.Gagnon AW, Kallal L, Benovic JL. Role of clathrin-mediated endocytosis in agonist-induced down- regulation of the β2-adrenergic receptor. J Biol Chem. 1998;273:6976–6981. doi: 10.1074/jbc.273.12.6976. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Villalobos R, Klassen RB, Allen PL, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin II. Am J Physiol Renal Physiol. 2005;288:F420–F427. doi: 10.1152/ajprenal.00243.2004. [DOI] [PubMed] [Google Scholar]
- 12.Griendling KK, Delafontaine P, Rittenhouse SE, Gimbrone MA, Jr, Alexander RW. Correlation of receptor sequestration with sustained diacylglycerol accumulation in angiotensin II-stimulated cultured vascular smooth muscle cells. J Biol Chem. 1987;262:14555–14562. [PubMed] [Google Scholar]
- 13.Guan S, Fox J, Mitchell KD, Navar LG. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one-clip hypertensive rats. Hypertension. 1992;20:763–767. doi: 10.1161/01.hyp.20.6.763. [DOI] [PubMed] [Google Scholar]
- 14.Harris PJ, Navar LG. Tubular transport responses to angiotensin II. Am J Physiol Renal Fluid Electrolyte Physiol. 1985;248:F621–F630. doi: 10.1152/ajprenal.1985.248.5.F621. [DOI] [PubMed] [Google Scholar]
- 15.Harrison-Bernard LM, Zhuo JL, Kobori H, Ohishi M, Navar LG. Intrarenal AT1 receptor and ACE binding in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F19–F25. doi: 10.1152/ajprenal.00335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hein L, Meinel L, Pratt RE, Dzau VJ, Kobilka BK. Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol Endocrinol. 1997;11:1266–1277. doi: 10.1210/mend.11.9.9975. [DOI] [PubMed] [Google Scholar]
- 17.Imig JD, Navar GL, Zou LX, O’Reilly KC, Allen PL, Kaysen JH, Hammond TG, Navar LG. Renal endosomes contain angiotensin peptides, converting enzyme, and AT1A receptors. Am J Physiol Renal Physiol. 1999;277:F303–F311. doi: 10.1152/ajprenal.1999.277.2.F303. [DOI] [PubMed] [Google Scholar]
- 18.Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM. Renal injury from angiotensin II-mediated hypertension. Hypertension. 1992;19:464–474. doi: 10.1161/01.hyp.19.5.464. [DOI] [PubMed] [Google Scholar]
- 19.Kelly RB. Microtubules, membrane traffic, and cell organization. Cell. 1990;61:5–7. doi: 10.1016/0092-8674(90)90206-t. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell KD, Jacinto SM, Mullins JJ. Proximal tubular fluid, kidney, and plasma levels of angiotensin II in hypertensive ren-2 transgenic rats. Am J Physiol Renal Physiol. 1997;273:F246–F253. doi: 10.1152/ajprenal.1997.273.2.F246. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 22.Navar LG, Carmines PK, Huang WC, Mitchell KD. The tubular effects of angiotensin II. Kidney Int Suppl. 1987;20:S81–S88. [PubMed] [Google Scholar]
- 23.Navar LG, Lewis L, Hymel A, Braam B, Mitchell KD. Tubular fluid concentrations and kidney contents of angiotensins I and II in anesthetized rats. J Am Soc Nephrol. 1994;5:1153–1158. doi: 10.1681/ASN.V541153. [DOI] [PubMed] [Google Scholar]
- 24.Navar LG, Zou L, Von Thun A, Tarng WC, Imig JD, Mitchell KD. Unraveling the mystery of Goldblatt hypertension. News Physiol Sci. 1998;13:170–176. doi: 10.1152/physiologyonline.1998.13.4.170. [DOI] [PubMed] [Google Scholar]
- 25.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension. 2002;39:129–134. doi: 10.1161/hy0102.100536. [DOI] [PubMed] [Google Scholar]
- 26.Romero MF, Douglas JG, Eckert RL, Hopfer U, Jacobberger JW. Development and characterization of rabbit proximal tubular epithelial cell lines. Kidney Int. 1992;42:1130–1144. doi: 10.1038/ki.1992.397. [DOI] [PubMed] [Google Scholar]
- 27.Romero MF, Hopfer U, Madhun ZT, Zhuo W, Douglas JG. Angiotensin actions in the rabbit proximal tubule. Renal Physiol Biochem. 2003;14:199–207. doi: 10.1159/000173405. [DOI] [PubMed] [Google Scholar]
- 28.Roy CL, Wrana JL. Clathrin-and non-clathrin-mediated endocytic regulation of cell signaling. Nature. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 29.Schelling JR, Hanson AS, Marzec R, Linas SL. Cytoskeleton-dependent endocytosis is required for apical type 1 angiotensin II receptor-mediated phospholipase C activation in cultured rat proximal tubule cells. J Clin Invest. 1992;90:2472–2480. doi: 10.1172/JCI116139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schelling JR, Linas SL. Angiotensin II-dependent proximal tubule sodium transport requires receptor-mediated endocytosis. Am J Physiol Cell Physiol. 1994;266:C669–C675. doi: 10.1152/ajpcell.1994.266.3.C669. [DOI] [PubMed] [Google Scholar]
- 31.Seikaly MG, Arant BS, Jr, Seney FD., Jr Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J Clin Invest. 1990;86:1352–1357. doi: 10.1172/JCI114846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siragy HM, Howell NL, Ragsdale NV, Carey RM. Renal interstitial fluid angiotensin. Modulation by anesthesia, epinephrine, sodium depletion, and renin inhibition. Hypertension. 1995;25:1021–1024. doi: 10.1161/01.hyp.25.5.1021. [DOI] [PubMed] [Google Scholar]
- 33.Sorensen SD, Linseman DA, McEwen EL, Heacock AM, Fisher SK. A role for a wortmannin-sensitive phosphatidylinositol-4-kinase in the endocytosis of muscarinic cholinergic receptors. Mol Pharmacol. 1998;53:827–836. [PubMed] [Google Scholar]
- 34.Thekkumkara T, Linas SL. Role of internalization in AT1A receptor function in proximal tubule epithelium. Am J Physiol Renal Physiol. 2002;282:F623–F629. doi: 10.1152/ajprenal.00118.2001. [DOI] [PubMed] [Google Scholar]
- 35.Van Kats JP, Schalekamp MA, Verdouw PD, Duncker DJ, Danser AH. Intrarenal angiotensin II: interstitial and cellular levels and site of production. Kidney Int. 2001;60:2311–2317. doi: 10.1046/j.1523-1755.2001.00049.x. [DOI] [PubMed] [Google Scholar]
- 36.Veyrat-Durebex C, Pomerleau L, Langlois D, Gaudreau P. Internalization and trafficking of the human and rat growth hormone-releasing hormone receptor. J Cell Physiol. 2005;203:335–344. doi: 10.1002/jcp.20233. [DOI] [PubMed] [Google Scholar]
- 37.Vazquez J, Correa de Adjounian MF, Sumners C, Gonzalez A, Diez-Freire C, Raizada MK. Selective silencing of angiotensin receptor subtype 1a (AT1aR) by RNA interference. Hypertension. 2005;45:115–119. doi: 10.1161/01.HYP.0000150161.78556.c6. [DOI] [PubMed] [Google Scholar]
- 38.Von Thun AM, Vari RC, El Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol Renal Fluid Electrolyte Physiol. 1994;266:F120–F128. doi: 10.1152/ajprenal.1994.266.1.F120. [DOI] [PubMed] [Google Scholar]
- 39.Zhuo JL, Alcorn D, Allen AM, Mendelsohn FA. High resolution localization of angiotensin II receptors in rat renal medulla. Kidney Int. 1992;42:1372–1380. doi: 10.1038/ki.1992.429. [DOI] [PubMed] [Google Scholar]
- 40.Zhuo JL, Ohishi M, Mendelsohn FA. Roles of AT1 and AT2 receptors in the hypertensive Ren-2 gene transgenic rat kidney. Hypertension. 1999;33:347–353. doi: 10.1161/01.hyp.33.1.347. [DOI] [PubMed] [Google Scholar]
- 41.Zhuo JL, Thomas D, Harris PJ, Skinner SL. The role of endogenous angiotensin II in the regulation of renal haemodynamics and proximal fluid reabsorption in the rat. J Physiol. 1992;453:1–13. doi: 10.1113/jphysiol.1992.sp019214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT1 receptor. Hypertension. 2002;39:116–121. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 43.Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular angiotensin II induces cytoplasmic Ca2+ mobilization by stimulating intracellular AT1 receptors in single proximal tubule cells. Am J Physiol Renal Physiol. 2006;290:F1382–F1390. doi: 10.1152/ajprenal.00269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou LX, Imig JD, Hymel A, Navar LG. Renal uptake of circulating angiotensin II in Val5-angiotensin II infused rats is mediated by AT1 receptor. Am J Hypertens. 1998;11:570–578. doi: 10.1016/s0895-7061(97)00410-x. [DOI] [PubMed] [Google Scholar]
- 45.Zuo L, Ushio-Fukai M, Hilenski LL, Alexander RW. Microtubules regulate angiotensin II type 1 receptor and Rac1 localization in caveolae/lipid rafts. Arterioscler Thromb Vasc Biol. 2004;24:1223–1228. doi: 10.1161/01.ATV.0000132400.25045.2a. [DOI] [PubMed] [Google Scholar]