Summary
Perturbed DNA replication either activates a cell cycle checkpoint, which halts DNA replication, or decreases the rate of DNA synthesis without activating a checkpoint. Here we report that at low doses, replication inhibitors did not activate a cell cycle checkpoint, but they did activate a process that required functional Bloom’s syndrome-associated (BLM) helicase, Mus81 nuclease and ATR kinase to induce transient double stranded DNA breaks. The induction of transient DNA breaks was accompanied by dissociation of proliferating cell nuclear antigen (PCNA) and DNA polymerase α from replication forks. In cells with functional BLM, Mus81 and ATR, the transient breaks were promptly repaired and DNA continued to replicate at a slow pace in the presence of replication inhibitors. In cells that lacked BLM, Mus81, or ATR, transient breaks did not form, DNA replication did not resume, and exposure to low doses of replication inhibitors was toxic. These observations suggest that BLM helicase, ATR kinase, and Mus81 nuclease are required to convert perturbed replication forks to DNA breaks when cells encounter conditions that decelerate DNA replication, thereby leading to the rapid repair of those breaks and resumption of DNA replication without incurring DNA damage and without activating a cell cycle checkpoint.
Keywords: BLM, Mus81, ATR, double-strand breaks, replication fork blockage, aphidicolin
Introduction
Cells are constantly exposed to exogenous radiation and chemicals as well as to endogenous metabolic products that perturb DNA replication. Perturbed replication may lead to mutations or DNA breaks, which cause genomic instability and activate the S-phase checkpoint. This S-phase checkpoint, which is regulated by the ATR and Chk1 kinases, prevents further initiation of DNA replication as long as DNA damage persists 1. The mechanism by which perturbed replication leads to lesions that are recognized as DNA damage is unclear. It is thought that DNA polymerase collisions or replication fork collapses generate double strand DNA breaks (DSBs) that are recognized as a form of DNA damage, but the precise mechanism by which this occurs is poorly understood.
The protein implicated in Bloom’s syndrome, BLM, is a member of the RecQ family of DNA helicases. Bloom’s syndrome is associated with growth retardation, immunodeficiency, premature aging, and cancer predisposition 2; 3; 4. Cells deficient in BLM exhibit an elevated frequency of sister chromatid exchange 4, implicating BLM in suppression homologous recombination. In addition to its role in homologous recombination, BLM plays one or more roles during DNA replication. BLM, which can be found in PML bodies 5, localizes to sites of unperturbed and perturbed DNA replication (i.e., replication foci) 6; 7 and interacts with the single stranded DNA (ssDNA) binding protein RPA 8. BLM physically and functionally interacts and colocalizes with Mus81 endonuclease 9, an enzyme that is involved in the repair of UV damage 10 and intrastrand crosslinks 11. Cells deficient in BLM display are sensitive to replication inhibition by hydroxyurea (HU) and camptothecin (CPT) 12; 13 and exhibit an endogeneous level of double stranded DNA breaks even in the absence of drug treatment 14. BLM plays a role in facilitating re-start of stalled replication forks following inhibition of DNA replication 15; 16. It was suggested 15; 16 that BLM might process stalled replication forks to generate a DNA structure that serves as a substrate for the DNA repair machinery and activates checkpoint signals. Consistent with this suggestion, BLM deficient cells display a discrete replication profile characterized by a global reduced fork velocity and shorter inter-origin distance 14, suggesting that those hard-to-resolve structure might also occur at low frequency in the absence of replication inhibiting drugs. However, the nature of the lesions generated by BLM and the mechanism by which those lesions are recognized by the DNA repair and cell cycle checkpoint pathways have not yet been elucidated.
Aphidicolin (APH) is a mycotoxin isolated from Cephalosporium aphidicola that specifically inhibits the activity of DNA polymerase α in eukaryotic cells, but has little effect on RNA, protein, and nucleotide biosynthesis 17; 18; 19. APH forms a ternary complex with pol α and DNA 20 that only interferes with the elongation step of DNA replication. Thus, APH inhibits progression of S-phase cells, but does not affect cells in G2, M, or G1. High levels of APH inhibit DNA replication and induce an S-phase checkpoint mediated by active Chk1 1. Low doses of APH, which perturb DNA replication but are below the threshold for checkpoint activation, are not toxic. Because cells rapidly resume replication after APH removal, APH can be used to synchronize cells in early S phase 17; 18; 19.
This study examines the activities and functions of BLM and Mus81 in cells undergoing perturbed replication in the presence of replication inhibitors. The results show that brief exposure to low, non-toxic doses of APH induced transient DNA breaks in BLM-proficient but not in BLM-deficient cells. BLM-proficient, but not BLM-deficient cells also exhibited dissociation of DNA polymerase α and proliferating cells nuclear antigen (PCNA) from replication forks. The dissociation of replication proteins from replication forks and the induction of transient DNA breaks occurred without activating a cell cycle checkpoint. Mus81 endonuclease and a functional ATR kinase were also required for induction of APH-induced DNA breaks and unraveling of replication forks. Following the repair of APH-induced breaks, BLM-proficient, Mus81-proficient cells re-established replication forks and resumed DNA synthesis at a slow pace in the presence of APH. BLM-deficient or Mus81-deficient cells, which did not induce transient breaks, exhibited an irreversible replication arrest that eventually leads to stable DNA breaks, activation of the S-phase checkpoint and homologous recombination. These observations suggest that BLM and Mus81 are both required to induce transient DNA breaks in response to stalled replication forks. Those transient breaks, which are formed in an ATR-dependent manner, are likely to serve as intermediates in the pathway that leads to recovery from stalled replication and resumption of replication at a slow pace.
Results
Formation of transient APH-induced DSBs and γ-H2AX foci requires BLM
The role of BLM in the response to APH-induced replication stress was examined in BLM-deficient fibroblasts (PSNG13) or in BLM-deficient fibroblasts complemented with BLM cDNA (PSNF5; BLM-complemented) 21. A DNA comet assay was carried out under neutral conditions to measure double strand DNA breaks (DSBs) in these cells (Figure 1). The comet assay showed that DSBs were detected within 10 minutes after treating BLM-complemented cells with APH, but DSBs were not detected when BLM-deficient cells were treated in the same manner (Compare PSNF5 and PSNF13, Figure 1A, middle panel). Quantification of the comet assay data (50 nuclei per sample) confirmed that exposure to 1 or 10 μg/ml APH altered distribution of comet tail length in BLM-complemented but not in BLM-deficient cells (Figure 1B).
Figure 1.
DSBs formed after APH treatment evaluated by neutral comet assay. (A) Neutral comet assays were performed using cells exposed to H202 (500 μM) for 15 minutes or APH (1 μg/mL) for 10 minutes as indicated. (B) Average tail length was quantified as described in Methods. BLM-complemented PSNF5 or BLM-deficient PSNG13 cells were used, as indicated. For each data point, 50 nuclei were scored, using data from 2 independent experiments.
The kinetics of DSB formation was examined in APH-treated BLM-deficient and BLM-complemented cells by immunostaining APH-treated cells with antibodies to phosphorylated H2AX (γ-H2AX), a marker for DNA breaks 22; 23 (Figure 2). The number of cells exhibiting above-threshold levels of γ-H2AX staining was recorded using Pathway analysis. When cells are exposed to agents that are known to induce DSBs, γ-H2AX appears rapidly and associates with nascent DSBs, forming discrete foci 22; 23. Immunostaining with antibodies that detect γ-H2AX and PCNA, a marker of S-phase, showed that BLM-complemented cells that exhibited PCNA staining induced transient γ-H2AX foci within 10 minutes of treatment with low doses of APH (PSNF5, Figure 2). γ-H2AX foci dissociated within 60 minutes despite the continued presence of low doses of APH (0.5 and 1 μg/ml - Figure 2A, B). In BLM-complemented PCNA-positive cells treated with 10 μg/ml APH, γ-H2AX foci appeared with similar kinetics, but remained stable beyond 60 minutes. PCNA-negative cells did not form γ-H2AX foci (data not shown), indicating that APH-induced DSBs only formed during S-phase.
Figure 2.
BLM-dependent γ–H2AX foci. Cells were treated with APH and then immunostained with PCNA and γ–H2AX. (A) Cells were treated with 1 μg/mL APH for the indicated amount of time and stained for PCNA (green) and γ–H2AX (red) as described in Methods. Inserts show images at higher magnification. (B) and (C). The number of γ–H2AX-positive PCNA-positive PSNF5 (closed circles) and PSNG13 cells (open circles) per 100 cells was counted. APH treatment was at the concentration and for the amount of time indicated. (D) The number of γ–H2AX-positive PCNA-positive GM0037 and GM1492 cells per 100 cells was counted. Cells were treated with 1 μg/mL APH for the indicated amount of time. Experiments were repeated 3 times with independent samples. Error bars represent standard deviations.
BLM-deficient PCNA-positive cells did not form transient γ-H2AX foci after exposure to 0.5, 1.0 or 10 μg/ml APH (Figure 2, PSNF13); however, a small number of persistent γ-H2AX foci appeared gradually in BLM-deficient cells during exposure to APH for 60 min (Figure 2, B–D). Cell cycle analyses indicated that the fraction of cells in S-phase was similar in BLM- proficient and BLM-deficient cell cultures (Supplemental Figure 1). Therefore, the different response of BLM-complemented and BLM-deficient cells to APH did not result from different kinetics of cell cycle progression in these cells.
We have also investigated the effects of APH on primary normal human fibroblasts (GM00037) and primary Bloom’s Syndrome (BS) human fibroblasts (GM01492) (Figure 2D). These results confirm that BLM is required for formation of transient APH-induced DSBs. Consistent with this idea, the slow accumulation of persistent DSBs in APH-treated BLM-deficient cells was associated with significantly lower survival than BLM-proficient cells (Supplemental Figure 2A).
Since it was reported that H2AX can be phosphorylated and recruited to DNA damage sites that do not include double strand breaks 24; 25, it was important to evaluate the breaks we have detected indeed represented double stranded and not other lesions, such as single stranded DNA breaks. To insure that the comet assay we have performed above (Figure 1) was specific for DSBs, cells were treated with hydrogen peroxide to generate ssDNA breaks. Cells were then analyzed by neutral comet assays under neutral conditions, that detect only double stranded DNA breaks, or under alkaline conditions, in which strands unwind and the broken DNA can be detected by comet26. When a neutral comet assay was performed, the distribution of DNA tail length was not changed by exposure to hydrogen peroxide (500 μM for 15 minutes; Figure 1A, right panels); however, when an alkaline comet assay was performed, the distribution of DNA tail length was changed by exposure to hydrogen peroxide (data not shown). These data confirm that exposure to APH induces DSBs in BLM-proficient cells.
Formation of APH-induced γ-H2AX foci requires Mus81
Previous studies showed that BLM recruits Mus81 nuclease to stalled replication forks and that BLM enhances the nuclease activity of Mus81 9. Thus, it seemed possible that Mus81 nuclease might play a role in generating APH-induced BLM-dependent DSBs. Therefore, the kinetics of γ– H2AX foci formation was examined in APH-treated Mus81-proficient HCT116 cells as well as in Mus81-deficient cells (HCT116 Mus81−/−) and HCT116 Mus81−/− cells complemented with Mus81 cDNA (Figure 3). Since the parental HCT116 cells exhibited a higher threshold for APH sensitivity, a lower concentration of APH (0.5 mg/ml) was used in experiments with those cells and their derivatives. In Mus81-proficient and Mus81-complemented cells, transient γ-H2AX foci appeared 10 minutes after exposure to APH and disappeared about 1 hour after the beginning of the exposure, although APH was continuously present in the culture medium (Figures 3A, B). In contrast, transient γ-H2AX foci were not detected in APH-treated Mus81-deficient cells; those cells slowly accumulated stable DNA breaks. Similarly, Mus81-proficient cells treated with HU exhibited faster kinetics of accumulation of DNA breaks than Mus81-deficient cells (Figure 3C). Consistent with the notion that Mus81 contributes to the processing of stalled replication forks by induction of rapidly-repaired DNA breaks, exposure of Mus81 proficient cells to 0.5 μg/ml APH did not activate an S-phase replication checkpoint that phosphorylates the Chk1 kinase; Mus81 deficient cells, which did not exhibit transient breaks but exhibited slow accumulation of persistent DNA breaks, had activated the phosphorylation of Chk1 following 30 minutes exposure to APH (Figure 3D).
Figure 3.
Mus81-dependent γ–H2AX foci. Cells were treated with APH and then immunostained with PCNA and γ–H2AX. (A) HCT116 Mus81−/−, or HCT116 Mus81−/− complemented cells were treated with 0.5 μg/ml APH for the indicated amount of time and immunostained for PCNA (green) and γ–H2AX (red), as described in Methods. Inserts show images at higher magnification. (B) and (C). The number of γ–H2AX–positive PCNA-positive nuclei per 100 cells was counted. Parental unmodified HCT116 (circles), HCT116 Mus81−/− cells (triangles), and HCT116 Mus81−/− +Mus81 cells (squares) were treated with APH (B) or HU (C). Each experiment was repeated 3 times with independent samples. Error bars represent standard deviations. (D) Western blot analysis of Phosphorylated Chk1 expression in HCT116 derived cell lines. When treated with 0.5 μg/ml of APH for 30 minutes, only Mus 81-defiecient cells exhibited staining for p-Chk-1. PCNA was used as a loading control (lower panel).
The above observations demonstrate that Mus81 is required for formation of APH-induced DSBs and APH-induced γ-H2AX foci. Consistent with this, Mus81 deficient cells were hypersensitive to APH (Supplemental Figure 2B). These observations suggest that Mus81 contributes to formation of APH-induced BLM-dependent transient DSBs and that this activity is essential for the recovery from transient inhibition of DNA replication.
Role of ATR in the BLM–dependent response to APH-induced replication stress
BLM is phosphorylated on threonines 99 and 122 by the ATM and ATR kinases 13; 15. The effect of APH on BLM phosphorylation was examined using antibodies to phospho-BLM-T99 13, considered to be the active form of BLM. Cells were concomitantly stained for PCNA as a marker for S-phase nuclei. Phosphorylation of BLM, which was absent in untreated cells, was induced in PCNA-positive cells within 10 minutes of exposure to APH (Figure 4A). Cells that did not stain positive for PCNA did not exhibit phosphorylation of BLM (data not shown), suggesting that APH-induced BLM phosphorylation was restricted to the S-phase of the cell cycle. Although phospo-BLM was restricted to PCNA-positive cells, PCNA foci did not co-localize with phospho-BLM. By contrast, phospho-BLM and γ-H2AX co-localized and appeared with similar kinetics (supplemental Figure 3), suggesting that phospho-BLM accumulates at or near APH-induced DSBs.
Figure 4.
Phosphorylation of BLM and formation of DSBs after treatment with low levels of APH. (A) and (B). Cells were treated with 1 μg/mL of APH for 10 minutes and immunostained for PCNA (red) and p-BLM (green). Inserts show images at higher magnification. (C) ATR is required for formation of DSBs. The extent of DSB formation was measured by comet assay in cells that contain an active ATR kinase and in cells in which the ATR kinase was inactivated (ATR K/D). Comet assays were performed as described in the legend to Figure 1.
BLM phosphorylation was also examined in cells expressing a conditional, doxycycline-inducible dominant-negative kinase-dead form of ATM and Rad3-Related kinase (ATRkd). The results showed that APH induced BLM phosphorylation in ATR WT cells but not in ATRkd cells (i.e., pretreated with 2 μg/ml doxycycline for 2 days to induce ATRkd; Figure 4B). By contrast, cells deficient in the PI3 kinase ATM exhibited phosphorylation of BLM with similar kinetics as WT cells (supplemental Figure 4). These observations suggest that ATR, but not ATM, is required for APH-induced phosphorylation of BLM. To determine if ATR was required for the formation of BLM-induced DNA breaks, we performed comet assays on ATR WT and ATRkd cells. As shown in Figure 4C, comet tails did not form in APH-treated ATRkd cells, suggesting that ATR was essential for the formation of APH-induced BLM- and Mus81-dependent transient DSBs.
Effect of APH on elongation of DNA replication in BLM-deficient and BLM-proficient cells
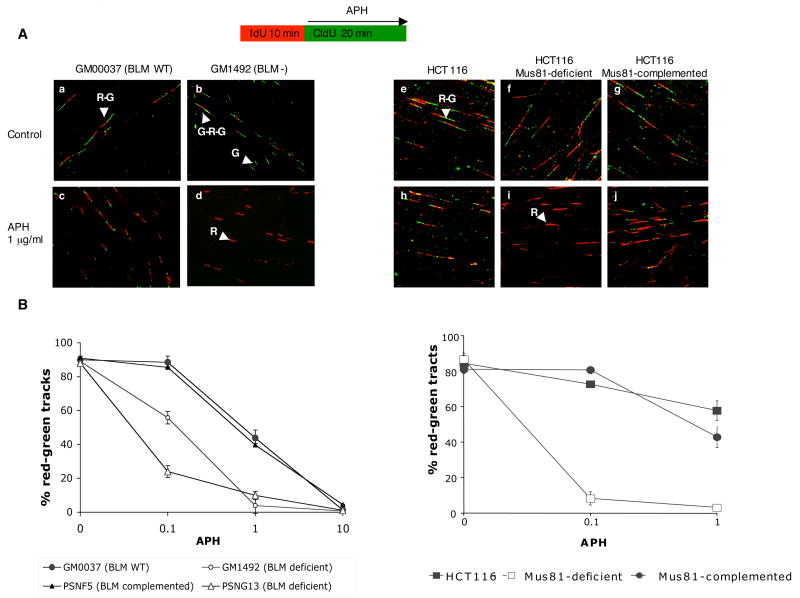
To determine whether the formation and resolution of DSBs after APH treatment play a role in the resolution of stalled replication forks and the resumption of DNA synthesis, we examined replication fork progression in APH-treated BLM-deficient and BLM-complemented cells using a DNA fiber assay 27. Cells were pulse labeled with 5-Iodo-2′-deoxyuridine (IdU), treated with APH (detected by Cy3; red signal) and labeled with 5-chloro-2′-deoxyuridine (CldU) (detected by Alexa 488; green signal). Figure 5A shows that several labeling patterns were observed in these cells: initiation of DNA replication during the second labeling period (in the presence of APH) generated green tracks (G); initiation during the first labeling period generated green-red-green tracks (G-R-G, red signal flanked by two green signals); and initiation before IdU labeling generated unidirectional red-green tracks (R-G). In addition, a few red (R) and rare R-G-R tracks were detected; these are likely due to termination events. The length of DNA fibers can be quantified, providing an alternative to the use of 2D gels, often used in yeast to estimate the extent of initiation and elongation of DNA replication.
Figure 5.
Stalled replication forks in cells treated with APH. Replication fork progression was assessed in PSNF5, PSNG13, GM00037, and GM01492 cells. Cell labeling protocol is shown schematically above Panel (A). Cells were labeled with IdU for 10 min. IdU was then washed out and the cells were exposed to APH and CldU for 20 min. IdU was immunodetected by Cy-3–labeled antibodies (red color). CldU was detected by Alexa488-labeled antibodies (green color). (A) Representative images of labeled cells are shown. Red-green tracks (R-G), red-only tracks (R), green-only tracks (G), and green-red-green tracks (G-R-G) are indicated by arrowheads. (B) Abundance of R-G (elongation), G (initiation) and R (termination/stalling) in GM00037, GM01492, PSNF5, and PSNG13 cells.(C) Abundance of R-G (elongation), G (initiation) and R (termination/stalling) in HCT116, Mus81 deficient and Mus81 complemented HCT116. Experiments were repeated at least 3 times with independent samples. Standard deviations are in parenthesis. . Experiments were repeated at least 3 times with independent samples.
The frequency of these different patterns was estimated in BLM-deficient and BLM-complemented cells treated with or without APH. Although BLM deficient cells exhibit a slightly reduced rate of DNA synthesis 14, those differences were not apparent under the short labeling conditions we have used and the length distributions of replicating DNA tracks were similar in BS and WT cells. However, significant differences were observed after exposure to APH (Figure 5; Supplemental Table 1). In BLM-complemented or BLM-proficient cells (PSNF5 and GM00037), the replicating DNA tracks (green CldU signals in R-G tracks) were significantly shorter in cells treated with APH (Figure 5A, compare panels a and c); but the frequency of initiation (green-only fibers) was similar in APH-treated and untreated cells (Supplemental Table 1). These results show that replication fork progression was suppressed, but continued at a slow rate, in BLM-proficient cells treated with =1 μg/ml APH (Figure 5B, left panel). Treatment with higher doses of APH (10 μg/ml) resulted in stalling of replication forks and reduced initiation rates in both cell lines.
In BLM-deficient cells (PSNG13 and GM01492), most replication forks were stalled (i.e., R-only tracks) after treatment with =0.1 μg/ml APH (Figure 5A, compare panels b and d; Figure 5B, left panel; Supplemental Table I) and replication was completely inhibited in cells exposed to higher doses (1–10 μg/ml APH). Similarly, elongation of replication forks was completely inhibited by APH in Mus81-deficient cells (Figure 5A, compare panels f and I; Supplemental Table I), while slow elongation of replication continued in APH-treated Mus81-proficient cells (Figure 5A, compare panel h to panels i and j; Figure 5B, right panel). These data confirm that BLM and Mus81 play a role in the response to APH-induced replication stress, allowing slow replication in cells exposed to low levels of APH.
APH-induced disassembly of replication forks requires BLM
We also investigated the levels and spatial distribution of chromatin-bound PCNA, RPA and DNA polymerases α and ε in APH-treated BLM-deficient and BLM-complemented cells. In BLM-proficient cells, the level of total PCNA in the nucleus was not affected by APH, but levels of chromatin-bound PCNA were lower in APH-treated than in untreated cells. In contrast, the levels of chromatin-bound PCNA were unaffected in APH-treated BLM deficient cells (Figure 6A). Consistent with this, we observed lower levels of PCNA in RPA foci after treatment of BLM-proficient cells by APH (Figure 6B). We have also observed that DNA polymerase α-primase leaves chromatin after a short exposure to APH in BLM proficient, but not deficient cells (Figure 6C). By contrast, Replication Protein A (RPA), which binds single stranded DNA, continues to exhibit a focal pattern after treatment with low levels of APH. RPA co-localized with PCNA in untreated cells, but not in cells that were treated with APH for 10 minutes. RPA foci co-localized with γ-H2AX foci in cells exposed to APH for 10 minutes (Supplemental Figure 5), suggesting that DSBs were formed in stalled replication factories.
Figure 6.
PCNA levels and distribution in response to inhibition of DNA replication. (A) Abundance of PCNA in soluble and insoluble fraction in BLM-deficient and BLM-complemented cells treated with APH. LaminB is for loading control. Cells were treated with 1 μg/ml APH for 1 h and then immunoblotted with PCNA and LaminB as described in Methods. (B) Immunostaining for PCNA (green) and RPA (red); (C) Immunostaining for PCNA (red) and pol α (green). (D) Immunostaining for PCNA (green) and pol ε (red). Cells were treated with 1 μg/ml of APH as indicated. Inserts show images at higher magnification.
Although PCNA and DNA polymerase α-primase dissociated from RPA foci in BLM-proficient cells, the distribution of PCNA and DNA polymerase α-primase was not affected in BLM deficient cells (Figure 6B, Supplemental Figure 6). The distribution of Pol ε, which facilitates leading strand synthesis, was not altered by exposure of BLM- deficient or BLM-complemented cells to APH (Figure 6D). PCNA co-localized with pol ε in untreated but not in APH-treated cells, consistent with the depletion of PCNA from chromatin in APH-treated BLM-complemented cells, observed above. These data suggest that BLM facilitates disassembly of replication forks in cells with APH-induced replication stress. RPA and Pol ε remain at replication forks and colocalize with DNA breaks, whereas PCNA and DNA polymerase α-primase leave replication forks.
Discussion
This study shows that low doses of APH induce DSBs in replicating cells, and that formation of these DSBs requires BLM and Mus81. APH-induced BLM-dependent DSBs are transient and appear to be rapidly repaired by non-homologous end joining (NHEJ) 28, whereas BLM-independent DSBs, which form infrequently with delayed kinetics in APH-treated BLM-deficient cells, are persistent and accumulate irreversibly. In cells that contain functional BLM, Mus81 and ATR, DNA replication proceeds slowly in the presence of low doses of APH following the repair of the transient surge of DSBs. By contrast, in cells deficient in BLM, ATR or Mus81, replication elongation is completely inhibited and cells cannot progress in S-phase. The inability to replicate slowly in the presence of APH is illustrated by the hypersensitivity of those cells to APH. These data suggest that BLM, Mus81 and ATR are required to induce transient DSBs after short exposure to APH. Since cells that are deficient in each of the above components could not induce transient DSBs, it is plausible to assume that all three proteins act in the same pathway, although the biochemical details of the interaction remain to be elucidated.
The studies presented here suggest that transient DSBs are formed in an active manner and that their rapid repair prevents the activation of the S-phase checkpoint and the accumulation of slower, stable DSBs. Our previous study 28 suggests that those transient DSBs are repaired via the NHEJ pathway, and that the repair process allows stalled replication forks to resume replication at a slow pace. The assumption that the NHEJ pathway is required to repair transient APH-induced breaks is based on the observation that APH-induced breaks persist in cells deficient in enzymes involved in the NHEJ pathway, such as DNAPK and XRCC4 ligase. We have also shown that cells that cannot form or process the transient breaks accumulate stable DNA breaks activate the S-phase checkpoint and recruit components of the homologous recombination pathway to resolve the permanent breaks. Together with the data presented here, our observations suggest that the S-phase checkpoint and homologous recombination are involved in the response to perturbed replication if DNA replication cannot resume, for example, in cells that exhibit inefficient repair (absence or inhibition of DNA-PK or XRCC1), inefficient formation of transient breaks (in cells that do not contain active forms of either BLM, Mus81 or ATR) or when cells are exposed to high doses of replication inhibitors. It is therefore hypothesized that the BLM and Mus81 depended transient breaks reported here are repaired by the non-homologous end-joining pathway to allow DNA replication to resume. The formation of irreversible DNA breaks in the absence of BLM was also reported in Xenopus cell extracts, in which BLM prevents the accumulation of DNA breaks during DNA replication 29. A model summarizing the proposed cellular response to APH-induced replication stress is presented in Figure 7.
Figure 7.
Schematic representation of the proposed events triggered by exposure to replication inhibitors such as APH. Low doses of inhibitors cause temporary inhibition of replication forks that is resolved by conversion of those forks to transient DNA breaks by the action of BLM helicase and Mus81 nuclease. This activity requires phosphorylation of BLM by ATR. The transient breaks are rapidly repaired by the non-homologous end-joining pathway (NHEJ); repair lead to recovery of replication at a slow rate even in the presence of the drug. In the absence of components of the NHEJ pathway such as DNA-PK or XRCC4, the breaks persist and lead to the activation of the S-phase checkpoint. High doses of inhibitors, BLM, Mus81 or ATR deficiencies may also lead to persistent breaks and checkpoint activation.
The suggestion that BLM/Mus81 trigger rather than resolve breaks following exposure to replication inhibitors is in line with experiments in mammalian cells proposing a role for BLM helicase in resolving replication lesions 2; 15; 30 and with data in yeast suggesting that Sgs1 represses homologous recombination after stalling of replication forks 31; 32; 33. Mus81 converts intrastrand DNA crosslinks to DSBs 34 providing a precedent for such a role for Mus81 nuclease. BLM-dependent disassembly of replication forks, indicated by the removal of PCNA and pol α from replication foci, might play a role in the conversion of stalled replication forks to DNA breaks. Disassembly of replication forks was observed during the processing of other replication-dependent lesions, such as lesions induced by etoposide 35 and MMS-induced replication arrest in Xenopus egg extracts, in which replication arrest was accompanied by dissociation of PCNA, but not RPA or polymerase ε36.
The observations reported here suggest that ATR-dependent phosphorylation of BLM plays a role in the formation of transient APH-induced DNA breaks, consistent with the notion that phosphorylation of BLM by ATR plays a role in the resolution of APH-induced replication stress 13; 15; 16. By contrast, cells treated with the topoisomerase inhibitor CPT exhibit ATM-mediated phosphorylation of BLM, which is required for rapid phosphorylation of γ-H2AX 13; 15 13; 37. The involvement of different kinases in the response to those different drugs might reflect two different pathways for the phosphorylation of BLM. When a DNA replication fork encounters a topoisomerase: CPT cleavage complex, the encounter directly forms DSBs that trigger phosphorylation of BLM by ATM. In contrast, APH-induced replication stress might create a precursor lesion that is subsequently converted to a DSB via a process that requires ATR, BLM - and Mus81. It is likely that the precursor lesion contains stretches of single stranded DNA (ssDNA) that are bound to RPA. In vitro, perturbation of DNA replication by APH forms ssDNA, possibly by uncoupling leading and lagging strand replication38. The resulting ssDNA is sufficient to activate ATR – mediated phosphorylation 39 by associating with RPA 40, thereby recruiting ATR via ATRIP 41. Consistent with this, RPA-ssDNA can function as a substrate for BLM helicase activity in vitro 8, possibly facilitating the resolution of stalled replication forks leading to chicken-foot structures 30. Thus, the transient BLM- and Mus81-mediated DNA breaks reported here might play an intermediate role in both the detection of replication lesions and in their resolution 6; 7; 13; 30.
Previous studies have shown that purified yeast Mus81-Eme1 resolves Holliday junctions by a nick and counternick mechanism 42 and that human Mus81 cleaves Holliday junctions into linear duplexes 43, supporting a role for Mus81 endonuclease in generating DNA lesion-induced DSBs. Although genetic defects in mus81 are not lethal in mice, cells deficient in Mus81 constitutively activate components of the S-phase checkpoint pathway, suggesting that Mus81 might regulate S-phase progression in the absence of exogenous DNA damage. However, it is probable that many types of non-lethal lesions are generated during normal DNA replication and cell growth, and that BLM and Mus81 promote genomic stability by facilitating repair of these non-lethal lesions.
Methods
Cells and culture conditions
BLM-deficient (PSNG13) and BLM-complemented (PSNF5) fibroblasts 21 were a gift from Dr. Ian Hickson (Oxford, UK) and were grown in Minimal Essential Alpha Medium (MEM) supplemented with 10% heat-inactivated fetal calf serum containing 350 μg/mL G418 (Invitrogen). GM00037 (normal human) and GM01492 (untransformed BS) were obtained from the Coriell Cell Repository (Camden, NJ) and were grown in DMEM supplemented with 10% fetal bovine serum. ATRkd cells were grown in DMEM supplemented with 10% heat-inactivated fetal calf serum and L-glutamine containing 400 μg/mL G418. HCT116, HCT116 Mus81−/−, and Mus81−/− +Mus81 cells 44 were grown in McCoy’s 5A medium (Invitrogen) supplemented with 10% heat-inactivated fetal calf serum. Viability after exposure to drugs was measured as described 28.
Drugs
APH was purchased from Wako, U.S.A., dissolved in Me2SO (1 mg/mL) and stored at −20°C.
Neutral comet assay
Neutral comet assay was performed using the CometAssay Kit (Trevigen) following the manufacturer’s protocol. Cells were treated with APH or H2O2 for the indicated times. Cells were collected and suspended in low-melting-point agarose. The agarose was applied to CometSlides™ and allowed to set at 4°C in the dark. After lysis of the agarose-embedded cells in lysis solution (2.5 M NaCl, 100 mM EDTA, pH 10, 10 mM Tris base, 1% sodium lauryl sarcosinate, 0.01% Triton X-100), the slides were electrophoresed in TBE, pH 8 (0.089 M Tris, 0.089 M boric acid, 0.003 M EDTA). The samples were then fixed in 70% ethanol and dried overnight before staining with SyBr® Green (Molecular Probes, Eugene, OR) to visualize cellular DNA. Images of nuclei were captured using a CCD camera (Roter Scientific; Cool SNAP FX) with epifluorescence microscopy (Olympus; IX70) using a 20X objective lens. For each sample, 50 cells were scored for tail length. Tail length was manually measured using IPLab software. Two independent experiments were performed for each data set.
DNA fiber analysis
DNA fiber analysis was performed as described previously 27. Cells were labeled with 20 μM IdU for 10 min and then labeled with 20 μM CldU for 20 min. Cells were trypsinized and resuspended in PBS at 1 × 106 cells/mL. The cell suspension (2.5 μl) was mixed with 7.5 μl lysis buffer (0.5% SDS in 200 mM Tris-HCl, pH 7.4, 50 mM EDTA) on an uncoated glass slide (Daigger). After 8 min, DNA spreads were fixed in 3:1 methanol:acetic acid for 5 min and stored in 70% ethanol at 4°C. Double immunostaining of CldU and IdU was performed according to Dimitrova and Gilbert 45. The slides were incubated in 100% methanol at room temperature for 5 min and rehydrated with PBS. DNA was denatured with 2.5 N HCl at 37°C for 30 min, then washed and incubated with primary antibodies. The anti-CldU (Accurate Chemical and Scientific Corporation) and anti-IdU (Becton Dickinson) antibodies were diluted in PBS with 0.5% bovine serum albumin (BSA). Cells were incubated with the antibodies for 1 h at 37°C. The slides were then washed 3 times with 0.1% Triton X-100 in PBS and incubated for 1 h at 37°C with secondary antibody conjugated with Alexa 488 (Molecular Probes for rat immunoglobin G) and Cy-3 (Jackson Immuno Research Laboratories, Inc. for mouse immunoglobin). The slides were washed 3 times with 0.1% Triton X-100 in PBS and counterstained for DNA with 4 μg/mL 4′-6-diamino-2-phenylindole in aqueous mounting medium (Biomeda Corp.). Images of DNA fibers were captured by epifluorescence microscopy using 100X objective lens.
FACS analysis
Cells were labeled with 20 μM BrdU and 0.25 μM fluorodeoxyuridine (FdU, Fluka), washed with PBS, and fixed in 70% ethanol overnight. DNA was denatured with 1 M HCl-0.1% Triton X-100 on ice for 10 min followed by boiling for 10 min. Cells were incubated with fluorescein isothiocyanate–conjugated anti-BrdU antibody (Becton Dickinson) for 1 h, and DNA was stained with propidium iodide in the presence of RNase. BrdU-positive cells were detected and quantified by FACScan (Becton Dickinson).
Immunofluorescence
Cells were grown on 18mmX18mm cover slips. . After treatment with APH, cells were washed with PBS, treated with a hypotonic lysis solution (10 mM Tris-HCl pH 7.4, 2.5 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, and 0.5% Nonidet P-40) for 8 min on ice. Cells were fixed in 4% paraformaldehyde in PBS for 10 min, washed in PBS, made permeable in 100% methanol at −20°C for 15 min, and then washed and blocked with PBS containing 1% BSA and 0.1% Triton X-100 for 30 min. Cells were incubated with anti-PCNA (Santa Cruz), anti-γ–H2AX (Upstate), anti-T99p-BLM 13 and anti-phospho-Chk1-Serine-317 (Cell Signaling) antibodies. Antibodies were diluted in PBS with 0.5% BSA for 1 h at 37°C. Slides were then washed 3 times with 0.1% Triton X-100 in PBS and incubated for 1 h at 37°C with secondary antibody conjugated with Alexa 488 (Molecular Probes) or Cy-3 (Jackson Immuno Research Laboratories). Slides were washed 3 times with 0.1% Triton X-100 in PBS and counterstained for DNA with 4′-6-diamino-2-phenylindol. Images were captured by confocal microscopy (Nikon; PCM 2000) using 100X objective lens. For quantitative analyses, slides were imaged on a Pathway HT™ automated fluorescence imaging workstation (Atto Bioscience, Rockville, MD) equipped with a Hamamatsu ORCA-ER CCD camera. An air Olympus 40x/340UAPO 0.9 na (0.21 × 0.15 mm field of view) objective was used to capture images in the blue (DAPI -360/10 nm excitation; 435 nm LP emission); green (Alexa 488 - 488/10 nm; 540/30 nm), and red (Cy3 - 548/20 nm; 570 nm LP) channels. Quantitative analyses of the intensity of each dye in each cell were performed IPlab for Pathway. To measure the intensity and distribution of PCNA, slides were imaged on a Pathway HT™ automated fluorescence imaging workstation (Atto Bioscience, Rockville, MD) equipped with a Hamamatsu ORCA-ER CCD camera. An air Olympus 40x/340UAPO 0.9 na (0.21 × 0.15 mm field of view) objective was used to capture images in the blue (DAPI -360/10 nm excitation; 435 nm LP emission); green (Alexa 488 - 488/10 nm; 540/30 nm. The intensity of each dye in each cell was measured using the IPlab software.
Western Blot Analysis
Western blot analysis was carried out as previously described 28 with minor variations. Membrane was blocked with 5% (w/v) non-fat milk for 1 h and incubated with rabbit anti-phopho-Chk1 (Ser317) (Cell Signaling) overnight in cold room. Secondary incubation with peroxidase-conjugated anti-rabbit IgG antibody (Santa Cruz) was performed for 1 hour and detection was achieved with Lumi-LightPLUS western blotting substrate (Roche).
Supplementary Material
Acknowledgments
We thank William M. Bonner and Kurt W. Kohn for their critical reading of the manuscript and for helpful suggestions. We are grateful to Chii-Mei Lin, Lixin Wang, Haiqing Fu, Chiara Conti, and Asako Nakamura for helpful suggestions. This study was supported by the Intramural Research Program of the NIH, Center for Cancer Research, National Cancer Institute and by fellowships from the Uehara Memorial Foundation and the Japan Society for the Promotion of Science.
Abbreviations
- APH
Aphidicolin
- ATM
ataxia telangiectasia mutated
- ATR
ATM- and Rad3-related
- ATRkd
ATR kinase dead
- BLM
Bloom
- BS
Bloom’s syndrome
- BSA
bovine serum albumin
- BrdU
bromodeoxyuridine
- CldU
5-chloro-2′-deoxyuridine
- DMEM
Dulbecco’s modified Eagle’s medium
- DNA-PK
DNA-dependent protein kinase
- DNA-PKcs
catalytic subunit of DNA-PK
- DSBs
double-strand breaks
- FACS
fluorescence-activated cell sorting
- FdU
fluorodeoxyuridine
- HU
hydroxyurea
- IdU
5-Iodo-2′-deoxyuridine
- MEM
Minimum Essential Alpha Medium
- NHEJ
nonhomologous end-joining
- PBS
phosphate-buffered saline
- pol α
polymerase lpha
- PCNA
proliferating cell nuclear antigen
- RPA
replication protein A
- SSDNA
single-strand DNA
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feijoo C, Hall-Jackson C, Wu R, Jenkins D, Leitch J, Gilbert DM, Smythe C. Activation of mammalian Chk1 during DNA replication arrest: a role for Chk1 in the intra-S phase checkpoint monitoring replication origin firing. J Cell Biol. 2001;154:913–23. doi: 10.1083/jcb.200104099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hickson ID. RecQ helicases: caretakers of the genome. Nat Rev Cancer. 2003;3:169–78. doi: 10.1038/nrc1012. [DOI] [PubMed] [Google Scholar]
- 3.Bachrati CZ, Hickson ID. RecQ helicases: suppressors of tumorigenesis and premature aging. Biochem J. 2003;374:577–606. doi: 10.1042/BJ20030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaganti RS, Schonberg S, German J. A manyfold increase in sister chromatid exchanges in Bloom’s syndrome lymphocytes. Proc Natl Acad Sci U S A. 1974;71:4508–12. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bischof O, Kim SH, Irving J, Beresten S, Ellis NA, Campisi J. Regulation and localization of the Bloom syndrome protein in response to DNA damage. J Cell Biol. 2001;153:367–80. doi: 10.1083/jcb.153.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rassool FV, North PS, Mufti GJ, Hickson ID. Constitutive DNA damage is linked to DNA replication abnormalities in Bloom’s syndrome cells. Oncogene. 2003;22:8749–57. doi: 10.1038/sj.onc.1206970. [DOI] [PubMed] [Google Scholar]
- 7.Sengupta S, Robles AI, Linke SP, Sinogeeva NI, Zhang R, Pedeux R, Ward IM, Celeste A, Nussenzweig A, Chen J, Halazonetis TD, Harris CC. Functional interaction between BLM helicase and 53BP1 in a Chk1-mediated pathway during S-phase arrest. J Cell Biol. 2004;166:801–13. doi: 10.1083/jcb.200405128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brosh RM, Jr, Li JL, Kenny MK, Karow JK, Cooper MP, Kureekattil RP, Hickson ID, Bohr VA. Replication protein A physically interacts with the Bloom’s syndrome protein and stimulates its helicase activity. J Biol Chem. 2000;275:23500–8. doi: 10.1074/jbc.M001557200. [DOI] [PubMed] [Google Scholar]
- 9.Zhang R, Sengupta S, Yang Q, Linke SP, Yanaihara N, Bradsher J, Blais V, McGowan CH, Harris CC. BLM helicase facilitates Mus81 endonuclease activity in human cells. Cancer Res. 2005;65:2526–31. doi: 10.1158/0008-5472.CAN-04-2421. [DOI] [PubMed] [Google Scholar]
- 10.Gao H, Chen XB, McGowan CH. Mus81 endonuclease localizes to nucleoli and to regions of DNA damage in human S-phase cells. Mol Biol Cell. 2003;14:4826–34. doi: 10.1091/mbc.E03-05-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wyman C, Kanaar R. DNA Double-Strand Break Repair: All’s Well That Ends Well. Annu Rev Genet. 2006 doi: 10.1146/annurev.genet.40.110405.090451. [DOI] [PubMed] [Google Scholar]
- 12.Franchitto A, Pichierri P. Bloom’s syndrome protein is required for correct relocalization of RAD50/MRE11/NBS1 complex after replication fork arrest. J Cell Biol. 2002;157:19–30. doi: 10.1083/jcb.200110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao VA, Fan AM, Meng L, Doe CF, North PS, Hickson ID, Pommier Y. Phosphorylation of BLM, dissociation from topoisomerase IIIalpha, and colocalization with gamma-H2AX after topoisomerase I-induced replication damage. Mol Cell Biol. 2005;25:8925–37. doi: 10.1128/MCB.25.20.8925-8937.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao VA, Conti C, Guirouilh-Barbat J, Nakamura A, Miao ZH, Davies SL, Sacca B, Hickson ID, Bensimon A, Pommier Y. Endogenous {gamma}-H2AX-ATM-Chk2 checkpoint activation in Bloom’s syndrome helicase deficient cells is related to DNA replication arrested forks. Mol Cancer Res. 2007;5:713–24. doi: 10.1158/1541-7786.MCR-07-0028. [DOI] [PubMed] [Google Scholar]
- 15.Davies SL, North PS, Dart A, Lakin ND, Hickson ID. Phosphorylation of the Bloom’s syndrome helicase and its role in recovery from S-phase arrest. Mol Cell Biol. 2004;24:1279–91. doi: 10.1128/MCB.24.3.1279-1291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies SL, North PS, Hickson ID. Role for BLM in replication-fork restart and suppression of origin firing after replicative stress. Nat Struct Mol Biol. 2007;14:677–9. doi: 10.1038/nsmb1267. [DOI] [PubMed] [Google Scholar]
- 17.Ikegami S, Taguchi T, Ohashi M, Oguro M, Nagano H, Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978;275:458–60. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- 18.Pedrali-Noy G, Spadari S, Miller-Faures A, Miller AO, Kruppa J, Koch G. Synchronization of HeLa cell cultures by inhibition of DNA polymerase alpha with aphidicolin. Nucleic Acids Res. 1980;8:377–87. doi: 10.1093/nar/8.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedrali-Noy G, Belvedere M, Crepaldi T, Focher F, Spadari S. Inhibition of DNA replication and growth of several human and murine neoplastic cells by aphidicolin without detectable effect upon synthesis of immunoglobulins and HLA antigens. Cancer Res. 1982;42:3810–3. [PubMed] [Google Scholar]
- 20.Sheaff R, Ilsley D, Kuchta R. Mechanism of DNA polymerase alpha inhibition by aphidicolin. Biochemistry. 1991;30:8590–7. doi: 10.1021/bi00099a014. [DOI] [PubMed] [Google Scholar]
- 21.Gaymes TJ, North PS, Brady N, Hickson ID, Mufti GJ, Rassool FV. Increased error-prone non homologous DNA end-joining--a proposed mechanism of chromosomal instability in Bloom’s syndrome. Oncogene. 2002;21:2525–33. doi: 10.1038/sj.onc.1205331. [DOI] [PubMed] [Google Scholar]
- 22.Pilch DR, Sedelnikova OA, Redon C, Celeste A, Nussenzweig A, Bonner WM. Characteristics of gamma-H2AX foci at DNA double-strand breaks sites. Biochem Cell Biol. 2003;81:123–9. doi: 10.1139/o03-042. [DOI] [PubMed] [Google Scholar]
- 23.Sedelnikova OA, Pilch DR, Redon C, Bonner WM. Histone H2AX in DNA damage and repair. Cancer Biol Ther. 2003;2:233–5. doi: 10.4161/cbt.2.3.373. [DOI] [PubMed] [Google Scholar]
- 24.Marti TM, Hefner E, Feeney L, Natale V, Cleaver JE. H2AX phosphorylation within the G1 phase after UV irradiation depends on nucleotide excision repair and not DNA double-strand breaks. Proc Natl Acad Sci U S A. 2006;103:9891–6. doi: 10.1073/pnas.0603779103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet. 2003;33:497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- 26.Singh NP, Stephens RE, Schneider EL. Modifications of alkaline microgel electrophoresis for sensitive detection of DNA damage. Int J Radiat Biol. 1994;66:23–28. doi: 10.1080/09553009414550911. [DOI] [PubMed] [Google Scholar]
- 27.Merrick CJ, Jackson D, Diffley JF. Visualization of altered replication dynamics after DNA damage in human cells. J Biol Chem. 2004;279:20067–75. doi: 10.1074/jbc.M400022200. [DOI] [PubMed] [Google Scholar]
- 28.Shimura T, Martin MM, Torres MJ, Gu C, Pluth JM, DiBernardi MA, McDonald JS, Aladjem MI. DNA-PK is involved in repairing a transient surge of DNA breaks induced by deceleration of DNA replication. J Mol Biol. 2007;367:665–80. doi: 10.1016/j.jmb.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Kim SM, Lee J, Dunphy WG. Absence of BLM leads to accumulation of chromosomal DNA breaks during both unperturbed and disrupted S phases. J Cell Biol. 2004;165:801–12. doi: 10.1083/jcb.200402095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ralf C, Hickson ID, Wu L. The Bloom’s syndrome helicase can promote the regression of a model replication fork. J Biol Chem. 2006;281:22839–46. doi: 10.1074/jbc.M604268200. [DOI] [PubMed] [Google Scholar]
- 31.Cobb JA, Bjergbaek L, Shimada K, Frei C, Gasser SM. DNA polymerase stabilization at stalled replication forks requires Mec1 and the RecQ helicase Sgs1. Embo J. 2003;22:4325–36. doi: 10.1093/emboj/cdg391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjergbaek L, Cobb JA, Tsai-Pflugfelder M, Gasser SM. Mechanistically distinct roles for Sgs1p in checkpoint activation and replication fork maintenance. Embo J. 2005;24:405–17. doi: 10.1038/sj.emboj.7600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khakhar RR, Cobb JA, Bjergbaek L, Hickson ID, Gasser SM. RecQ helicases: multiple roles in genome maintenance. Trends Cell Biol. 2003;13:493–501. doi: 10.1016/s0962-8924(03)00171-5. [DOI] [PubMed] [Google Scholar]
- 34.Hanada K, Budzowska M, Modesti M, Maas A, Wyman C, Essers J, Kanaar R. The structure-specific endonuclease Mus81-Eme1 promotes conversion of interstrand DNA crosslinks into double-strands breaks. Embo J. 2006 doi: 10.1038/sj.emboj.7601344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi R, Lidonnici MR, Soza S, Biamonti G, Montecucco A. The dispersal of replication proteins after Etoposide treatment requires the cooperation of Nbs1 with the ataxia telangiectasia Rad3-related/Chk1 pathway. Cancer Res. 2006;66:1675–83. doi: 10.1158/0008-5472.CAN-05-2741. [DOI] [PubMed] [Google Scholar]
- 36.Stokes MP, Michael WM. DNA damage-induced replication arrest in Xenopus egg extracts. J Cell Biol. 2003;163:245–55. doi: 10.1083/jcb.200306006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho CC, Siu WY, Lau A, Chan WM, Arooz T, Poon RY. Stalled replication induces p53 accumulation through distinct mechanisms from DNA damage checkpoint pathways. Cancer Res. 2006;66:2233–41. doi: 10.1158/0008-5472.CAN-05-1790. [DOI] [PubMed] [Google Scholar]
- 38.Pacek M, Walter JC. A requirement for MCM7 and Cdc45 in chromosome unwinding during eukaryotic DNA replication. Embo J. 2004;23:3667–76. doi: 10.1038/sj.emboj.7600369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacDougall CA, Byun TS, Van C, Yee MC, Cimprich KA. The structural determinants of checkpoint activation. Genes Dev. 2007;21:898–903. doi: 10.1101/gad.1522607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–8. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 41.Ball HL, Myers JS, Cortez D. ATRIP binding to replication protein A-single-stranded DNA promotes ATR-ATRIP localization but is dispensable for Chk1 phosphorylation. Mol Biol Cell. 2005;16:2372–81. doi: 10.1091/mbc.E04-11-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaillard PH, Noguchi E, Shanahan P, Russell P. The endogenous Mus81-Eme1 complex resolves Holliday junctions by a nick and counternick mechanism. Mol Cell. 2003;12:747–59. doi: 10.1016/s1097-2765(03)00342-3. [DOI] [PubMed] [Google Scholar]
- 43.Chen XB, Melchionna R, Denis CM, Gaillard PH, Blasina A, Van de Weyer I, Boddy MN, Russell P, Vialard J, McGowan CH. Human Mus81-associated endonuclease cleaves Holliday junctions in vitro. Mol Cell. 2001;8:1117–27. doi: 10.1016/s1097-2765(01)00375-6. [DOI] [PubMed] [Google Scholar]
- 44.Hiyama T, Katsura M, Yoshihara T, Ishida M, Kinomura A, Tonda T, Asahara T, Miyagawa K. Haploinsufficiency of the Mus81-Eme1 endonuclease activates the intra-S-phase and G2/M checkpoints and promotes rereplication in human cells. Nucleic Acids Res. 2006;34:880–92. doi: 10.1093/nar/gkj495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dimitrova DS, Gilbert DM. The spatial position and replication timing of chromosomal domains are both established in early G1 phase. Mol Cell. 1999;4:983–93. doi: 10.1016/s1097-2765(00)80227-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.