Abstract
In this article we describe the needed instrumentation and the methods to be followed for the observation and measurement of the birefringence of single and bundled microtubules and of their ordered arrays using a polarizing microscope. As instruments, the traditional polarizing microscope and the recently developed LC-PolScope are discussed. As methods we describe qualitative and quantitative observations, including notes on specimen preparations, that optimize the sensitivity and accuracy of measuring specimen retardance.
Keywords: microtubules, alignment, birefringence, retardance, polarization, polarizer, compensator, LC-PolScope
1. Introduction
The polarized light microscope was instrumental in the discovery of the dynamic character of microtubules, even before their molecular identity was established. As early as 1952, Shinya Inoué (1) described the reversible assembly and disassembly of fibers constituting the mitotic spindle in dividing cells that he observed with the polarizing microscope. In a number of pioneering experiments Inoué and collaborators established biophysical properties of the labile spindle fibers by observing their birefringence in living cells that were exposed to cold temperatures (2), to a partial exchange of H2O with D2O (3) and to hydrostatic pressure (4). In 1975, after the discovery of microtubules as the major structural component of the spindle, Inoué and collaborators demonstrated that the birefringence of the spindle is indeed caused by the parallel array of aligned microtubules (5); also, see review in (6).
These early observations of the dynamic properties of spindle microtubules were made possible by technical improvements to the polarizing microscope, including the introduction of microscope lenses optimized for polarized light observations and the restoration of polarization distortions using polarization rectifiers (7, 8). More recently, the use of liquid crystal devices for controlling the polarization, and of image algorithms for quickly generating quantitative birefringence maps have revitalized the use of polarized light microscopy for analyzing the architectural dynamics in living cells (9–11). The new technology, called LC-PolScope, is commercially available from Cambridge Research and Instrumentation in Woburn, Massachusetts, USA (http://www.cri-inc.com), and is used in many fields, including for the observation of microtubule structure and dynamics in dividing cells (12), for cell enucleation (13), and for assisting in-vitro fertilization procedures (14).
A single microtubule (MT) molecule is a thin but stiff cylinder that is only 25 nm wide and often many microns long. Even though its diameter is well below the resolution limit of the light microscope, a single MT molecule and its dynamics can be observed in fluorescence (15), dark field (16), differential interference contrast (DIC) (17), and polarized light microscopy (18). An excellent discussion of fluorescence and DIC methods for visualizing MT dynamics in living cells is published in a recent review by Clare Waterman-Storer (19). The observation of single MTs in living cells by phase sensitive techniques such as phase contrast, DIC, dark field, and polarized light microscopy, is often obscured by the seemingly random optical phase variations induced by organelles and other cytoskeletal components in the vicinity of the thin microtubule filaments. However, in special circumstances, such as in thin lamellipodia of epithelial cells, it is possible to visualize single MTs using DIC and possibly other phase sensitive techniques (20). In the polarizing microscope the birefringence of single and bundled microtubules can be measured quantitatively and can thus be used to determine the number of microtubules in an observed structure (12, 18). Currently, only the polarized light microscope can measure an intrinsic material property of the microtubule molecules, such as birefringence, that can be used to quantify the number of microtubules or the density of aligned MTs in the field of view. In addition, the birefringence of a dense array of microtubules, such as a spindle, reveals the prevailing alignment of the filaments, even though the individual molecules are not resolved. Thus, the polarized light microscope has the unique potential to reveal the dynamics of submicroscopic structural parameters that are usually only accessible through more intrusive and static methods such as electron microscopy.
There are several recent reviews of polarized light microscopy that are helpful in the context of this article. Inoué wrote a general introduction to polarized light microscopy in biology with many helpful hints and tricks (21); see also Appendix III in the first edition of (22). We have published review articles on observing mitotic and meiotic spindles in the polarizing microscope (23) and on the use of the LC-PolScope for live cell imaging (11). In the current article we describe the instrumentation needed and methods to be followed for improving the results when observing the birefringence associated with single microtubules and microtubule arrays inside living cells or in in-vitro preparations.
2. Instrumentation
The optical components of a traditional polarizing microscope include two linear polarizers, a compensator, and a rotation stage on which the specimen is placed. Other components, such as objective and condenser lenses, a light source, an ocular or eye piece, and a camera, are common with a standard transmitted light microscope. When used in a polarizing microscope, however, these common components might have to meet specific requirements to allow the detection of the weak birefringence of microtubule arrays. In this section I briefly discuss the critical components and define the quantities that can be measured in each resolved area of a birefringent specimen, namely the retardance and the slow axis orientation.
2.1. Polarizers
A polarizing microscope has two polarizers one of which is located in the illumination path before the condenser lens and the other in the imaging path behind the objective lens (Fig. 1). The first polarizer is used to linearly polarize the light supplied by a bright halogen or arc lamp. The second polarizer serves to analyze the polarization of the light after it passed through the lenses and the specimen. This second polarizer is therefore called the analyzer. For best results the linear polarizers should have high transmission and good extinction. Modern sheet polarizers are available with transmission coefficients for unpolarized light of higher than 40% and extinction coefficients of around 104. An ideal polarizer has a transmission of 50% and infinite extinction. The extinction is a measure of the purity of the polarization state of light, after it has passed through the polarizer. The extinction coefficient is measured by the ratio of light transmitted through two polarizers of the same type in series, with their transmission axes aligned parallel and perpendicular to each other (extinction = I∥ / I⊥). Glan-Thompson and other prism polarizers perform close to the ideal values.
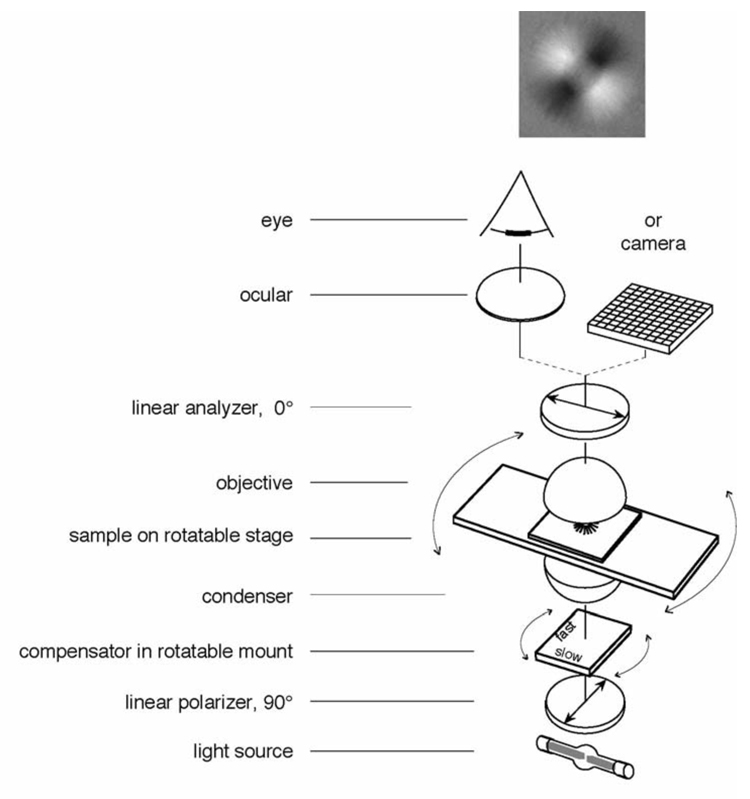
Figure 1.
Schematic of optical arrangement of the traditional polarized light microscope. The specimen image, as projected by the objective lens, is presented through an ocular to the eye and/or directly captured by a camera. The top image shows the appearance of an aster (a centrosome isolated from surf clam egg with radially arranged microtubules) between crossed polarizers and a Brace-Köhler compensator. The compensator is rotated slightly away from the extinction position in such a way that microtubule arrays that are oriented diagonally from top left to bottom right are bright because they run more parallel to the compensator slow axis. Microtubule arrays that are oriented diagonally from bottom left to top right are darker than the background because the MTs run more perpendicular to the compensator slow axis (see section 3.2).
The extinction or purity of polarization that is achieved in a polarization optical train directly affects the sensitivity for measuring the birefringence in a specimen: the higher the extinction the higher the sensitivity can be. However, the extinction of a polarization optical train is determined by the weakest elements in the sequence of optical components that are placed between the polarizer and analyzer. The weakest elements are those that introduce the strongest polarization distortions, which, in a microscope, are often the objective and condenser lenses.
2.2. Objective and condenser lenses
The purity of the polarization in a light microscope is usually limited by the polarization distortions introduced by the condenser and objective lenses, especially if they have a high numerical aperture. While a pair of sheet polarizers might have an extinction ratio of 104 or higher, if a condenser and objective lens is placed between them, the extinction usually drops to around 103 or below. Most microscope manufacturers offer lenses that are designated “Pol” to indicate low polarization distortions that can arise from a number of factors, including stress or crystalline inclusions in the lens glass and the type of antireflection coatings used on lens surfaces. Some lens types are available with “DIC” designation instead of “Pol”. “DIC” lenses do not meet the more stringent Pol requirements but pass for use in differential interference contrast microscopy and might be the only available designation for some lens types such as Plan Apochromats that feature good image correction together with reduced polarization distortions.
Whenever possible or practical, we recommend the use of oil immersion objective and condenser lenses. The immersion medium abolishes, or at least reduces, the polarization distortions typical of so-called dry lenses that have an air space between the front lens element and the slide and cover glass of the specimen preparation. The transition of a light ray between two media of substantially different refractive index (nair = 1.00, nglass = 1.52) introduces polarization distortions, especially for high NA lenses. A discussion of polarization distortions introduced by microscope lenses and possible ways of rectifying the polarization distortions can be found in (7, 24).
2.3. Compensator
In addition to the polarizer and analyzer, the polarized light microscope often includes a compensator in the optical path. While not absolutely necessary for some basic observations, especially when looking at highly birefringent objects, the compensator (a) can significantly improve the detection and visibility of weakly birefringent objects, (b) is required to determine the slow and fast axis of specimen birefringence, and (c) is an indispensable tool for the quantitative measurement of object birefringence.
There are several types of compensators, most of them are named for their original inventors. We will discuss the Brace-Köhler compensator, which is suited for the observation and measurement of weak birefringence, such as those of microtubule arrays. The Brace-Köhler compensator consists of a thin birefringent plate, often made from mica, with a retardance of a 10th to a 30th of a wavelength (λ/10 to λ/30; a definition of retardance is provided later). The birefringent plate is placed in a graduated rotatable mount and inserted either between the polarizer and condenser or between the objective lens and the analyzer. The location varies between microscope manufacturers and from one microscope type to another. In either location, the effect of the Brace-Köhler compensator on the observed image is the same and its usage does not depend on location (as long as there is not a third, strongly birefringent element between the polarizers). Further comments on the use of a Brace-Köhler compensator are found in section 3. Methods.
Recently, liquid-crystal based devices were introduced as compensators that can be controlled electronically (9). The liquid-crystal devices can be used as traditional compensators or as part of a measurement scheme that is discussed below under the heading LC-PolScope.
2.4. Retardance
The retardance of a resolved specimen area is the quantity typically measured with the compensator in a polarizing microscope. In general terms, retardance characterizes the relative phase shift between two orthogonally polarized light waves that have traversed an optically anisotropic material. When linearly polarized light travels through a birefringent material, the light is split into two orthogonally polarized waves that travel with different speeds. The difference in speed between the two waves is a consequence of the difference in the refractive index Δn of the material for the two polarization components. Retardance, also called birefringence retardation, expresses the relative phase shift between the two waves after they traversed the birefringent specimen of thickness t:
Accordingly, retardance has the dimension of a distance, typically expressed in nm or as a fraction of the wavelength of light.
2.5. Slow and fast axes
The slow and fast axes describe specific orientations in a birefringent material. For a given propagation direction, light polarized parallel to the slow axis experiences the highest refractive index and hence travels the slowest in the material. For the same propagation direction, light that is polarized perpendicular to the slow axis experiences the lowest refractive index and therefore travels the fastest in the material. The polarization direction associated with the lowest refractive index is called the fast axis. The slow and the fast axes are always perpendicular to each other. A compensator is used to determine the slow and fast axis of a birefringent specimen.
2.6. Rotation stage
The rotation stage is an indispensable accessory for a traditional polarizing microscope, because the contrast of a birefringent object depends on its orientation with respect to the microscope’s polarizer and analyzer. For qualitative observations, the systematic variation of brightness when rotating an object between crossed polars attests to the object’s optical anisotropy. For quantitative measurements, the rotation stage needs to have a graduated scale around its circular perimeter, including a vernier, so specimens can be rotated by a specific angle that usually can be measured within a tenth of a degree.
2.7. LC-PolScope
The LC-PolScope augments the traditional polarizing microscope by integrating a liquid-crystal (LC) compensator, electronic imaging, and digital image processing tools to build a birefringence imaging system (9, 10, 25). (Fig. 2). Commercial versions of the LC-PolScope are available from Cambridge Research and Instrumentation, Woburn, Massachusetts, USA (http://www.cri-inc.com). The LC-PolScope generates high-resolution maps of the retardance and slow axis distribution in the specimen. Such maps can be generated within seconds at a sensitivity that allows the detection of single microtubules.
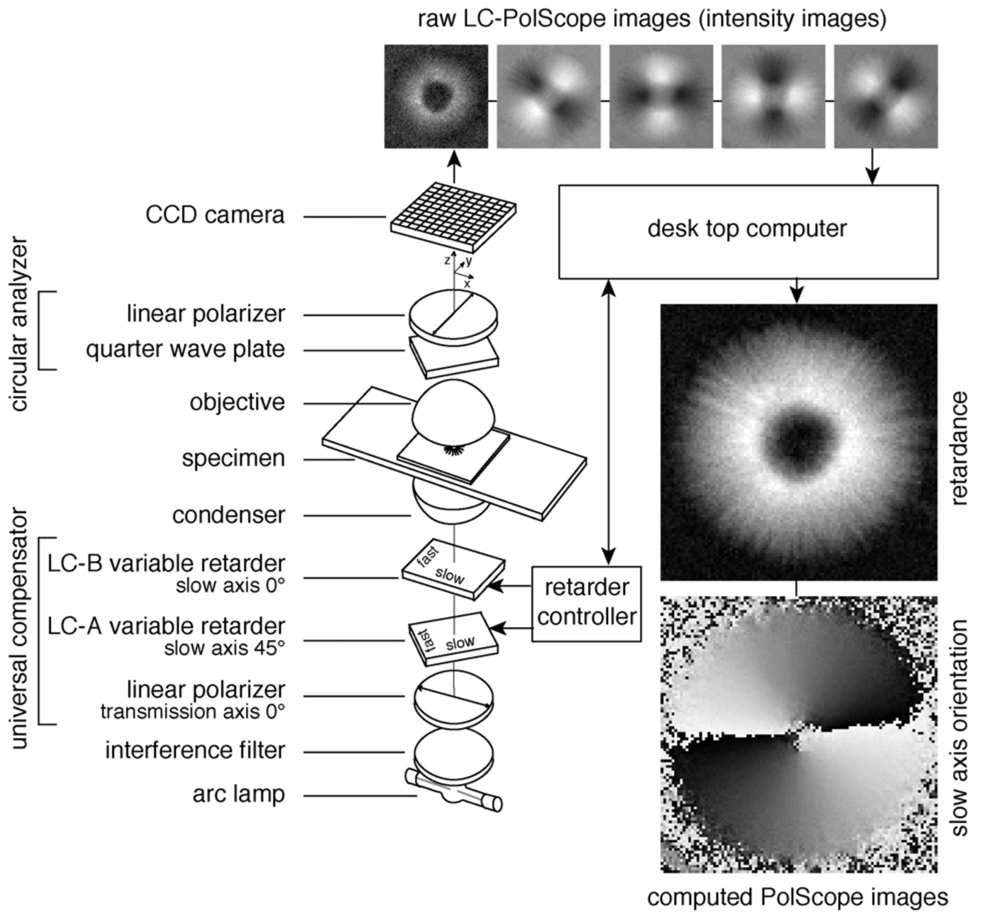
Figure 2.
Schematic of the LC-PolScope. The optical design (left) builds on the traditional polarized light microscope with the conventional compensator replaced by two variable retarders LC-A and LC-B forming the universal compensator. Images of the specimen (top row, aster isolated from surf clam egg) are captured at five predetermined retarder settings that cause the specimen to be illuminated with circularly polarized light (first, left-most image in top row) and with elliptically polarized light of different axis orientations (second to fifth images). The retardance and orientation images near the bottom right were both computed from the same five intensity images shown in the top row.
The two LC variable retarders and the polarizer are optically bonded together to form a universal compensator. The universal compensator can be used to produce, and rapidly switch between, circularly polarized light and elliptically polarized light of various axis orientations. In contrast to the traditional polarizing microscope, which uses linearly polarized light, the LC-PolScope uses near circularly polarized light that passes through the specimen. After the specimen the light passes through a polarization analyzer that blocks circularly polarized light. The universal compensator is switched to several predetermined settings producing circularly and elliptically polarized light. An electronic camera records images of the specimen for each of those settings and transfers the raw image data to a computer. In the computer the image data are combined using algorithms to calculate the specimen retardance in each resolved image point. The result of the computation is an image representing the retardance measured in each pixel independent of the orientation of the slow axis in that point. Hence, the retardance image is black where there is no birefringence in the sample and shows a specific shade of gray depending on the retardance of the sample at that point, regardless of the sample’s orientation. In addition to the retardance, the LC-PolScope also measures the orientation of the slow axis in every image point, using the same set of raw image data. The orientation is measured as an angle between the horizontal axis and the slow axis of the specimen at each image point. The orientation values can either be displayed as a separate image or be overlaid on a retardance image using color or other means of encoding orientation. Thus, computed LC-PolScope images represent material properties rather than light intensities. As a consequence, the retardance image, for example, displays specimen retardance on a linear scale, in contrast to the quadratic relationship between image intensity and specimen retardance in a traditional polarizing microscope.
3. Methods
3.1. Observation of the specimen between crossed polarizers
Usually, the two polars (short for polarizers) are in crossed position so that the analyzer blocks (absorbs) most of the light that has passed through the polarizer. Hence, the image that is projected by the objective lens is mostly dark, except for birefringent or otherwise optically anisotropic specimen parts that appear bright. When the specimen is rotated on a revolving stage, the birefringent parts change brightness, from dark to bright and back to dark four times during a full 360° rotation. The systematic variation of brightness when rotating a specimen between crossed polars is a telltale of the presence of birefringence in the specimen.
A birefringent specimen part appears darkest when its slow and fast axis is parallel to polarizer and analyzer. This specimen orientation is also called the extinction position. Rotating it by 45° away from the extinction position makes the birefringent part appear brightest. In general, not all birefringent parts in the field of view will turn dark at the same time because each part has different axis orientations. Hence, in addition to helping recognize birefringent elements, one can also determine the axis orientations by rotating the specimen between crossed polars.
3.2. Observations using a traditional compensator
In general, the birefringence of the compensator, when inserted into the optical path, causes the image background to become brighter, whereas birefringent parts, such as a mitotic spindle, become either brighter or darker than the background. Careful observations have established that a spindle appears brighter than the background when the spindle axis is more parallel to the slow axis of the compensator (26). When the spindle axis is more perpendicular to the compensator slow axis, the spindle fibers appear darker than the background. In this latter position the retardance of the spindle is said to be either fully or partially compensated. These results directly indicate that the slow axis of the spindle fibers are parallel to the fiber axis; or, in other words, the slow axis of microtubules is oriented parallel to the microtubule axis.
By following a number of steps in adjusting the mutual orientations of the polarizers, compensator and specimen, one can achieve best contrast and a quantitative analysis of the retardance of specimen birefringence. As an example, we consider spindle microtubules (MTs) that are nearly parallel to the spindle axis and that are observed using a Brace-Köhler compensator (see section 2.3) in the optical path of the microscope:
Using the rotatable mount of the compensator, rotate its slow axis parallel to polarizer or analyzer (extinction position); as a result, the background appears dark.
Using the rotation stage, rotate the spindle axis in the specimen to its extinction position; the spindle appears dark.
Now rotate the spindle axis by 45° away from the extinction position; the spindle appears brightest; during the following steps, the spindle position remains fixed at 45° to extinction.
Rotate the compensator either clockwise or counterclockwise to render the spindle either darker or brighter than the background; for observations by eye, a spindle that is darker than the background tends to be more easily recognized.
- For measuring the retardance of the spindle MTs, rotate the compensator until the spindle MTs appear darkest, i.e. the light intensity in the image region of the spindle is lowest. In this postion, the spindle retardance Rspindle is said to be compensated by the compensator retardance Rcomp:
θmin is the angle by which the compensator was rotated away from extinction to achieve minimum light intensity in the spindle region; note that the light intensity in the spindle increases on eiter side of θmin while the intensity of the surrounding background steadily increases for the same range of angles; therefore, judging the lowest spindle intensity by eye can be tricky.
It is advisable to deviate from the above protocol for generating high contrast images of small changes in orientation of the specimen’s birefringence axis. For example, microtubule arrays that reorient or fluctuate by thermal or other forces are best observed in the extinction position, with the compensator rotated slightly away from extinction. With this arrangement, small changes in microtubule orientations are translated into high-contrast intensity changes against a nearly dark background. This effect was exploited for visualizing the subtle variations of DNA ordering in cave cricket sperm heads (27).
A more complete discussion of the appearance of spindles in the polarizing microscope and the analytic interpretation of polarized light images of single, bundled and arrays of microtubules can be found in (23).
3.3. Observations using the LC-PolScope
In addition to a liquid-crystal compensator, the LC-PolScope includes an electronic camera and software for image acquisition, instrument control and data analysis. A schematic of the optical and electronic LC-PolScope components is shown in Fig. 2. The computer with software provides a user interface used for calibrating the instrument, for recording a sequence of raw images and for computing retardance and azimuth images. Observations with the LC-PolScope usually start with calibrating the instrument and recording a so-called background stack. The calibration, which is done without a birefringent specimen in the viewing field, determines the optimal settings of the liquid crystal devices. Subsequently, a background stack of raw PolScope images is recorded. The background stack is a record of the spurious birefringence induced e.g. by stress in microscope lenses and optical components other than the specimen. Then the specimen is moved into the field of view and another stack of raw PolScope images is recorded. The raw specimen stack of images contains the information of the specimen retardance superimposed on the background retardance. During the calculation of the specimen retardance, the influence of the spurious background retardance is removed, based on the separate background stack. As a result, the background corrected retardance image shows the birefringence of the specimen at high contrast on a dark background. The spurious background removal is especially important when observing and measuring the weak retardance of individual microtubules or small bundles of microtubules.
LC-PolScope images represent the retardance in every resolved image point measured simultaneously over the whole field of view. The recording and analysis process can take less than a second and can be repeated indefinitely, providing high-resolution time lapse records of dynamic events, such as the assembly and disassembly of microtubules and their ordered array (Fig. 3).
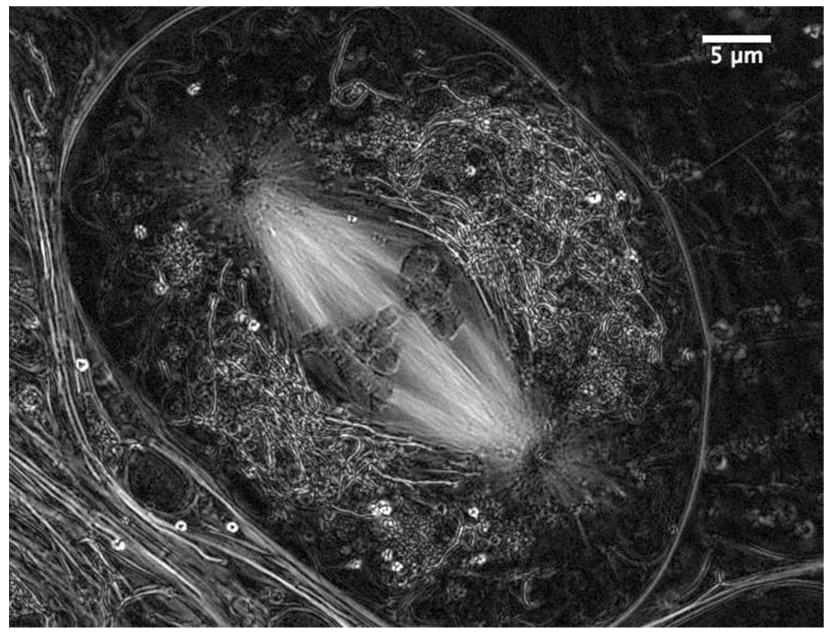
Figure 3.
Metaphase of meiosis I in a living spermatocyte from the crane fly, Nephrotoma suturalis, viewed with the LC-PolScope. The kinetochore fibers that connect the chromosomes to the spindle poles stand out with exceptional bright contrast. Each fiber is a bundle of about 60 kinetochore microtubules as inferred directly from the measured retardance (ref. 12). (Image recorded with James R. LaFountain, University at Buffalo.)
Recently, we have published a more detailed account of the use of the LC-PolScope for imaging birefringent structures in living cells (11).
3.4. Notes on specimen preparation
Don’t use plastic dishes or slides. Usually, plastic parts such as culture dishes are highly birefringent and obscure the weak birefringence of microtubule arrays. However, plastic dishes with a glass bottom, usually made of a thin cover glass, are OK.
Use oil immersion optics whenever possible (see section 2.2). When using high NA oil immersion optics, prepare the specimen in such a way that the structure of interest is located close to the coverslip. Otherwise, spherical aberration can noticeably reduce the resolution and sensitivity achieved in the image. If you need to focus deeper into an aequeous medium (more than about 20 µm), use water immersion optics to improve the resolution (28).
When using the LC-PolScope, prepare the specimen so when mounted in the microscope one can easily find a clear area without birefringent parts and move it into the viewing field when required. A clear area is needed for calibrating the LC-PolScope and for recording a stack of background images. Sometimes we find it helpful to add a tiny drop of oil or other non-toxic, immiscible liquid to the preparation. The clear drop can provide an area for calibrating the instrument and take background images in a preparation that is otherwise dense with birefringent structures.
References
- 1.Inoué S. The effect of cholchicine on the microscopic and submicroscopic structure of the mitotic spindle. Experimental Cell Research. 1952;2 Supplement:305–318. [Google Scholar]
- 2.Inoué S. Organization and function of the mitotic spindle. In: Allen RH, Kamiya N, editors. Primitive Motile Systems in Cell Biology. New York, NY: Academic Press; 1964. pp. 549–598. [Google Scholar]
- 3.Inoué S, Sato H. Cell motility by labile association of molecules: The nature of mitotic spindle fibers and their role in chromosome movement. J. Gen. Physiol. 1967;50:259–292. [PMC free article] [PubMed] [Google Scholar]
- 4.Salmon ED. Pressure-induced depolymerization of spindle microtubules. I. Changes in birefringence and spindle length. J. Cell Biol. 1975;65:603–614. doi: 10.1083/jcb.65.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato H, Ellis GW, Inoué S. Microtubular origin of mitotic spindle form birefringence Demonstration of the applicability of Wiener's equation. J. Cell Biol. 1975;67:501–517. doi: 10.1083/jcb.67.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoué S, Salmon ED. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol. Biol. Cell. 1995;6:1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoué S, Hyde WL. Studies on depolarization of light at microscope lens surfaces II. The simultaneous realization of high resolution and high sensitivity with the polarizing microscope. J. Biophys. Biochem. Cytol. 1957;3:831–838. doi: 10.1083/jcb.3.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoué S, Kubota H. Diffraction anomaly in polarizing microscopes. Nature. 1958;182:1725–1726. doi: 10.1038/1821725a0. [DOI] [PubMed] [Google Scholar]
- 9.Oldenbourg R, Mei G. New polarized light microscope with precision universal compensator. J. Microsc. 1995;180(Pt 2):140–147. doi: 10.1111/j.1365-2818.1995.tb03669.x. [DOI] [PubMed] [Google Scholar]
- 10.Shribak M, Oldenbourg R. Techniques for fast and sensitive measurements of two-dimensional birefringence distributions. Appl. Opt. 2003;42:3009–3017. doi: 10.1364/ao.42.003009. [DOI] [PubMed] [Google Scholar]
- 11.Oldenbourg R. Polarization microscopy with the LC-PolScope. In: Goldman RD, Spector DL, editors. Live Cell Imaging: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2005. pp. 205–237. [Google Scholar]
- 12.LaFountain JR, Jr, Oldenbourg R. Maloriented bivalents have metaphase positions at the spindle equator with more kinetochore microtubules to one pole than to the other. Mol. Biol. Cell. 2004;15:5346–5355. doi: 10.1091/mbc.E04-06-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Oldenbourg R, Trimarchi JR, Keefe DL. A reliable, noninvasive technique for spindle imaging and enucleation of mammalian oocytes. Nat. Biotechnol. 2000;18:223–225. doi: 10.1038/72692. [DOI] [PubMed] [Google Scholar]
- 14.Wang WH, Keefe DL. Prediction of chromosome misalignment among in vitro matured human oocytes by spindle imaging with the PolScope. Fertil. Steril. 78:1077–1081. doi: 10.1016/s0015-0282(02)04196-1. [DOI] [PubMed] [Google Scholar]
- 15.Sammak PJ, Borisy GG. Direct observation of microtubule dynamics in living cells. Nature. 1988;332:724–726. doi: 10.1038/332724a0. [DOI] [PubMed] [Google Scholar]
- 16.Horio T, Hotani H. Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature. 1986;321:605–607. doi: 10.1038/321605a0. [DOI] [PubMed] [Google Scholar]
- 17.Walker RA, O'Brien ET, Pryer NK, Soboeiro MF, Voter WA, Erickson HP, Salmon ED. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J. Cell Biol. 1988;107:1437–1448. doi: 10.1083/jcb.107.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oldenbourg R, Salmon ED, Tran PT. Birefringence of single and bundled microtubules. Biophys. J. 1998;74:645–654. doi: 10.1016/S0006-3495(98)77824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waterman-Storer CM. Microtubules and microscopes: how the development of light microscopic imaging technologies has contributed to discoveries about microtubule dynamics in living cells. Mol. Biol. Cell. 1998;9:3263–3271. doi: 10.1091/mbc.9.12.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cassimeris L, Pryer NK, Salmon ED. Real-time observations of microtubule dynamic instability in living cells. J. Cell Biol. 1988;107:2223–2231. doi: 10.1083/jcb.107.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoué S. Polarization Microscopy. In: Bonifacino JS, Dasso M, Harford JB, Lippincott-Schwartz J, Yamada KM, editors. Current Protocols in Cell Biology. Vol. 1. New York: John Wiley & Sons; 2002. pp. 4.9.1–4.9.27. [Google Scholar]
- 22.Inoué S. Video Microscopy. New York: Plenum Press; 1986. [Google Scholar]
- 23.Oldenbourg R. Polarized light microscopy of spindles. Methods Cell Biol. 1999;61:175–208. doi: 10.1016/s0091-679x(08)61981-0. [DOI] [PubMed] [Google Scholar]
- 24.Shribak M, Inoué S, Oldenbourg R. Polarization aberrations caused by differential transmission and phase shift in high NA lenses: theory, measurement and rectification. Opt. Eng. 2002;41:943–954. [Google Scholar]
- 25.Oldenbourg R. A new view on polarization microscopy. Nature. 1996;381:811–812. doi: 10.1038/381811a0. [DOI] [PubMed] [Google Scholar]
- 26.Inoué S, Dan K. Birefringence of the dividing cell. J. Morphol. 1951;89:423–456. [Google Scholar]
- 27.Inoué S, Sato H. Deoxyribonucleic acid arrangement in living sperm. In: Hayashi T, Szent-Gyorgyi AG, editors. Molecular Architecture in Cell Physiology. Englewood Cliffs, NJ: Prentice Hall; 1966. pp. 209–248. [Google Scholar]
- 28.Inoué S, Spring KR. Video Microscopy. New York: Plenum Press; 1997. [Google Scholar]