Abstract
Leishmania amazonensis infection, occurring predominantly in Central and South America, can manifest itself in several forms, including those of cutaneous and diffuse cutaneous leishmaniasis. The outcome of L. amazonensis infection depends largely on host immune responses to the parasites. While CD4+ T cell activation is a prerequisite for pathogenesis in L. amazonensis-infected mice, the roles of B cells and their antibody production are unclear. In this study, we provide evidence suggesting that B cells and antibodies are involved in disease pathogenesis. We documented a correlation between B cell activation and lesion progress in immunocompetent mice. In the absence of functional B cells and antibodies, JhD mice showed a delayed onset of disease and developed small lesions. Histological examination of these mice revealed a significant reduction in CD4+ and CD8+ T cells, but not in MAC1+ macrophages, at the infection site. In contrast to the wild-type mice that showed typical tissue necrosis, L. amazonensis-infected JhD mice showed no or minimal signs of necrotic foci. A marked reduction in CD4+ T cell proliferation and cytokine (IFN-γ and IL-10) production in infected JhD mice suggested an involvement of B cells and antibodies in the priming of parasite-specific T cells. This notion was further supported by the observations that adoptive transfer of B cells or antibodies could restore CD4+ T cell activation and migration in infected JhD mice. Moreover, antibody coating of parasites could stimulate dendritic cells to produce high levels of cytokines and increase their ability to prime naive CD4+ T cells. Since CD4+ T cells are crucial to disease pathogenesis, this study suggests that B cells and their antibody production enhanced L. amazonensis infection, partially by promoting T cell priming and cellular migration to the infection site.
Keywords: Protozoan parasites, Cutaneous leishmaniasis, B cells, Antibodies, Cellular recruitment, T cell activation
1. Introduction
Leishmaniasis, a vector-borne disease caused by parasites of the Leishmania genus, is endemic in Africa, Asia, Europe, and Central and South America. Leishmania infection can lead to various clinical manifestations ranging from benign, self-healing cutaneous lesions and/or extensive, disfiguring mucocutaneous lesions to a life-threatening visceral infection. These diverse outcomes of the disease are determined by both the genetic composition of the various parasite species and host immune responses to the infection. Studies of host defense mechanisms are, therefore, essential to our understanding of the disease pathogenesis caused by Leishmania infection. While many studies have focused on and demonstrated the integral roles of CD4+ T cells, dendritic cells (DCs) and macrophages (MΦs) in Leishmania infection, less emphasis has been placed on understanding how B cells and their antibody production may influence the outcome of infection.
Despite evidence of active B cell activation and antibody production during the progressive disease in human (Galvao-Castro et al., 1984; Hailu et al., 2001; Miles et al., 2005) and murine models (Howard et al., 1980; Palanivel et al., 1996), the biological functions of B cells and antibodies in cutaneous leishmaniasis remain a matter of debate. Several lines of evidence using Leishmania major infection as a model have suggested that B cells can enhance disease pathogenesis. These studies include those that showed disease exacerbation following an IL-7-mediated B cell expansion in susceptible BALB/c mice (Hoerauf et al., 1995) and a decrease in disease progression in B cell-deficient JhD mice (Miles et al., 2005). Moreover, the IgG immune complex was also shown to reduce leishmaniacidal activity by inducing high levels of IL-10 production by MΦs (Anderson et al., 2002; Miles et al., 2005). While the above studies strongly suggest pathogenic roles for B cells and antibodies, other studies have suggested that B cells and/or antibodies play a protective role during Leishmania infection. For example, Scott et al. (1986) and Woelbing et al. (2006) demonstrated that deletions of B cells either via anti-IgM treatment or targeted gene deletion (μMT) could increase the disease pathogenesis caused by L. major infection in otherwise resistant C3H/HeN and C57BL/6 mice. On the other hand, some reports have suggested that B cells and antibodies do not play any significant role during the course of L. major infection, as evidenced by comparable disease outcomes and T cell responses in B cell-deficient μMT mice and their wild- type counterparts on both C57BL/6 and BALB/c backgrounds. Similar observations were documented using anti-IgM-treated C3H/HeJ mice (Sacks et al., 1984; Brown and Reiner, 1999).
The observed discrepancies in the involvement of B cells may be attributed to multiple factors, including the host’s genetic background, infection routes and doses, specific growth characteristics, and differential host immunosuppressive mechanisms employed by individual parasite species and strains. While the results obtained from studies of the L. major infection model have been variable, studies of B cell functions using parasites of the Leishmania mexicana complex have provided more consistent conclusions. B cells and/or antibodies have been suggested to enhance disease pathogenesis in L. mexicana, Leishmania pifanoi and Leishmania amazonensis. For example, studies by Kima et Al. (2000) have shown that, when infected with either L. pifanoi or L. amazonensis, JhD mice that lack functional B cells and antibodies displayed a reduced lesion size, compared with that of wild-type counterparts. Focusing on an L. pifanoi infection model, Colmenares et al. (2002) subsequently demonstrated that the presence of antibodies is associated with active cellular recruitment at the infection site. While this study suggested the induction of cell infiltration as a potential mechanism by which antibodies can enhance disease pathogenesis in L. pifanoi infection, it remained to be investigated whether this mechanism can explain the disease pathogenesis caused by other species of the L. mexicana complex. Given the diversity in genetic backgrounds of these parasites and the various disease symptoms these can cause, we further investigated the mechanisms of B cells and antibodies in disease pathogenesis caused by another member of the L. mexicana complex, L. amazonensis.
Cutaneous leishmaniasis caused by L. amazonensis can lead to non-healing lesions in all tested inbred strains of mice; however, varying degrees of severity have been observed (Cupolilo et al., 2003; Qi et al., 2001; Vanloubbeeck and Jones, 2004). While the cellular mechanisms responsible for this generalized susceptibility of mice to L. amazonensis infection are unresolved, CD4+ T cells are known to play an integral role in lesion development and disease pathogenesis (Soong et al., 1997; Jones et al., 2000). Mice deficient in functional CD4+ T cells (RAG2−/− and MHC class II−/− mice) did not develop lesions of an appreciable size, even at late stages of infection (Soong et al., 1997). While it is clear that CD4+ T cells are required for disease pathogenesis, it appeared that other cellular components also contribute to disease formation, because RAG2−/− mice that were adoptively transferred with unfractionated splenocytes developed more progressive lesions than did mice that received purified CD4+ T cells alone (Soong et al., 1997). Therefore, other host immune components, such as B cells and/or antibodies, are potentially involved in disease development following L. amazonensis infection.
In this study, we assessed the pathogenic roles of B cells and antibodies during L. amazonensis infection by examining parasite-specific cellular immune responses in two immunocompetent mouse strains with different disease susceptibilities, as well as in JhD mice that lack functional B cells due to targeted deletion of the J segments of the Ig heavy chain. We found that B cells do play significant roles in disease formation, in particular in the induction of local cellular recruitment and the priming of parasite-specific CD4+ T cells. Since CD4+ T cells in L. pifanoi-infected JhD mice displayed normal proliferative responses (Colmenares et al., 2002), B cell involvement in the priming of CD4+ T cells appeared to be unique to L. amazonensis infection. A restoration of T cell activation and migratory functions following adoptive transfer of B cells or passive transfer of immune sera into JhD mice further suggests that B cells could assist in the activation of CD4+ T cells via both antibody-dependent and antibody-independent mechanisms. In vitro examination of antibody-mediated priming of CD4+ T cells also revealed that antibodies could enhance the ability of DCs to prime naive CD4+ T cells. Since the priming of parasite-specific CD4+ T cells is required for disease pathogenesis (Soong et al., 1997), B cell-mediated enhancement of CD4+ T cells could directly contribute to disease pathogenesis. Collectively, this study provides evidence of various mechanisms of B cells and their antibody production during the course of L. amazonensis infection. It also demonstrates the unique characteristics of B cell involvement during infection caused by L. amazonensis.
2. Materials and methods
2.1. Animals
Female JhD mice and wild-type BALB/c controls were purchased from Taconic (Hudson, NY). Female C3H/HeJ (C3H) mice were purchased from Jackson Laboratory (Bar Harbor, ME). All animals were maintained under specific-pathogen free conditions and used for experimentation at 6–8 weeks of age according to protocols approved by the Animal Care and Use Committee of the University of Texas Medical Branch (Galveston, TX).
2.2. Parasites
The infectivity of L. amazonensis (MHOM/BR/77/LTB00016 and RAT/BA/74/LV78 clone 12–1) was maintained by regular passage through BALB/c mice. Promastigotes were cultured at 23°C in Schneider’s Drosophila medium (Invitrogen, Carlsbad, CA), pH 7.0, supplemented with 20% FBS (Hyclone, Logan, UT). Lesion-derived amastigotes (LTB strain) were obtained from foot tissue of BALB/c mice infected with 2 x 106 promastigotes for 7–8 weeks. Amastigotes were cultured at 33°C in complete Schneider’s medium, pH 5.0, containing 20% FBS, for 2–3 days prior to use. Axenic amastigotes (LV78 strain) were maintained as previously described (Dutta et al., 2005). Briefly, the parasites were cultured in complete Grace's insect cell culture medium (Invitrogen), supplemented with 20% FBS, pH 5.0 at 33°C. These parasites remained in amastigote form under the described culture conditions. To obtain a crude parasite antigen preparation, axenic amastigotes (2 x 108 parasites/ml) were washed twice with PBS and subjected to three freeze/thaw cycles and sonication.
2.3. Infection and evaluation of lesion development
To examine disease progression, mice were inoculated with 2 x 106 stationary promastigotes or lesion-derived amastigotes of L. amazonensis (LTB strain) in the upper right hind foot. Lesion development was monitored weekly by measuring foot thickness with a digital caliper (Control Company, Friendswood, TX). At 7–8 weeks p.i., mice were sacrificed and tissue parasite burdens were determined via a limiting dilution assay, as described previously (Soong et al., 1995). For B cell adoptive transfer, JhD mice (three to four mice per group) were injected i.v. 1 day prior to infection with 5 x 107 purified CD19+ B cells derived from spleens of naïve BALB/c mice. CD19+ B cells were purified using MACS microbeads (Miltenyi, Germany), and the purity of B cells was ≥95%. For antibody passive transfer, JhD mice were injected i.p. at 1, 3 and 7 days p.i. with 200 μl of sera derived from 8–10 weeks infected BALB/c mice. Draining lymph nodes (DLNs), infected foot tissue, and sera were collected from individual mice for analysis at 3 weeks p.i.
2.4. Flow cytometric analysis
To evaluate cellular immune responses in infected animals, DLN cells were harvested from naïve and infected BALB/c, JhD or C3H mice at indicated time points. After blocking of non-specific binding sites with rat anti-mouse CD16/32 (eBioscience, San Diego, CA), cells were stained with the following specific mAbs (0.5 μg/106 cells): fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD4 (GK1.5); FITC-conjugated rat anti-mouse CD8 (53–6.7); phycoerythrin (PE)-Cy5 conjugated rat anti-mouse CD19 (6D5); PE-conjugated rat anti-mouse CD69 (H1.2F3); FITC-conjugated rat anti-mouse CD25 (PC61.5); PE-conjugated rat-anti mouse CD62L (MEL-14); PE-Cy5-conjugated rat anti-mouse CD44 (IM7); and isotype controls, including PE-conjugated rat IgG1, IgG2a and IgG2b (all from eBioscience, San Diego, CA). For detection of intracellular cytokines, DLN cells (1 x 106/ml) were re-stimulated with phorbol myristate acetate (PMA)/ionomycin in the presence of GolgiPlug™ (BD Biosciences) for 6 h. Subsequently, cells were stained for surface antigens, fixed/permeabilized with a Cytofix/Cytoperm solution (BD Biosciences), and then incubated for 20 min with PE-conjugated rat anti-mouse IL-10 (JES5-16E3, eBioscience) and FITC-conjugated rat anti-mouse IFN-γ (XMG1.2, eBioscience). For the analysis of lesion-derived CD4+ T cells, tissues of infected mice were processed as previously described (Ji et al., 2005), with some modifications. Briefly, the soft foot tissue of 3-week infected mice were harvested, cut and torn into small pieces by forceps. Tissues were then ground, transferred into a 40-μm cell strainer (BD Biosciences) and disrupted with a 5-ml syringe pestle. After red blood cells were removed by lysing buffer, cells were washed, stained for surface CD4 and CD8, and analyzed on a FACScan (BD Biosciences). The percentages of CD4+ and CD8+ T cells were analyzed with FlowJo software (TreeStar, San Carlos, CA).
2.5. Histopathology and immunohistochemistry
At 7–8 weeks p.i., foot tissue was collected and treated with 2% paraformaldehyde in 5% sucrose solution at 4°C overnight, then with 20% sucrose in PBS for 8 h before being embedded in the Opimal Cutting Temperature (O.C.T.) compound (Sakura Finetek USA, Inc, Torrance, CA). Tissue sections (5-μm thick) were stained with H&E. For immunohistochemical staining, tissue sections were fixed with ice-cold acetone for 5 min and then rehydrated in 1 x Tris-buffered saline (TBS). After non-specific binding sites were blocked with blocking solution (1% BSA, 0.01% Triton X-100, 5% dry-milk and 10% normal rabbit serum in 1 x TBS), tissue sections were incubated overnight at 4°C with mAbs specific to mouse CD4 (clone RM4-5, BD Biosciences), CD8 (clone 53–6.7, BD Biosciences), MAC-1 (clone M1/70, BD Bioscienes), Gr-1 (clone RB6-8C5, Invitrogen) or rat isotype antibodies (Serotec, Raleigh, NC). Following three washes, tissues were treated with 0.8% H2O2 in methanol for 30 min to deplete endogenous peroxidase activities. After extensive washing, a secondary antibody (mouse-adsorbed, biotinylated rabbit anti-rat IgGs, Vector Laboratories, Burlingame, CA) was applied for 1 h at room temperature. Color development was achieved using an Avidin:Biotinylated Enzyme Complex provided in a Vectastain Elite ABC kit (Vector Laboratories) and a 3,3-diaminobenzidine/metal substrate solution (Pierce, Rockford, IL). The sections were counterstained with 1% methyl green and mounted.
At least two sections per animal and eight to nine mice per group were evaluated for each primary antibody. No significant staining was observed in tissues stained with isotype antibodies (data not shown). Sections were photographed with an Olympus BX51 microscope (Olympus Corp., New Hyde Park, NY) equipped with a digital camera (Olympus DP70). Positively stained cells were scored semiquantitatively under 40 x visual fields for CD4+ and CD8+ T cells, and 10 x visual fields for MAC-1+ cells. The average scores from three randomly selected visual fields were used for subsequent statistical analysis. For CD4+ cells, the following scores were used: 1, ≤50 cells/field; 2, 51–80 cells/field; 3, 81–110 cells/field; 4, 111–140 cells/field; and 5, ≥ 141 cells/field. For CD8+ cells, the stained tissues were scored as follows: 1, ≤ 10 cells/field; 2, 11–20 cells/field; 3, 21–30 cells/field; 4, 31–40 cells/field; and 5, ≥ 41 cells/field. For MAC-1+ cells, the stained tissues were scored as follows: 1, ≤ 20 cells/field; 2, 21–40 cells/field; 3, 41–60 cells/field; 4, 61–80 cells/field; and 5, ≥ 81 cells/field.
2.6. CD4+ T cell isolation and activation
To evaluate antigen-specific recalled responses, CD4+ T cells were isolated. Because cell numbers in the DLNs of naïve and JhD mice were limited (3.0 ± 0.9 x 106 cells/DLN of naïve mice, and 6.2 ± 0.5 x 106 cells/DLN of JhD mice at 7–8 weeks of infection), we isolated CD4+ T cells from the spleens of JhD and control mice. Briefly, splenocytes were consecutively labeled with biotinylated rat anti-mouse CD4 (eBioscience) and streptavidin-conjugated Microbeads (Miltenyi). CD4-positive selection was obtained by passing cells through a MidiMACs column (Miltenyi). The purity of CD4+ cells was ≥95% routinely. Purified CD4+ T cells (2 x 105) were co-cultured with mitomycin C-treated naïve splenocytes (2 x 105) in the presence or absence of amastigote lysate (equivalent to 2 x 105 parasites per well) in U-bottomed, 96-well culture plates for 72 h. One μCi of [3H]-thymidine was added at 18–22 h before harvest and the incorporated radioactivity was determined using a direct beta counter (Matrix 9600; Packard Instrument Co. Ltd., Meriden, CT).
2.7. Cytokine measurement of DLN cells
To examine parasite-specific cytokine production in infected mice, DLN cells were harvested from infected BALB/c or JhD mice (2.5 x 106 cells/0.5 ml) restimulated with amastigote lysate (equivalent to 2.5 x 106) in 24-well plates for 72 h. Cytokine levels in culture supernatants were measured at the institutional Gastrointestinal Immunology Core of the Texas Gulf Coast Digestive Diseases Center using a mouse 23-Plex cytokine assay (Bio-Rad, Richmond, CA). Briefly, supernatants and cytokine standards were incubated with antibody-conjugated beads for 30 min with shaking. After three washes, diluted biotinylated detection antibody was added, followed by the addition of PE-conjugated streptavidin. After extensive washes, beads were suspended in a Bio-Plex assay buffer and analyzed on a Bio-Plex array reader (Bio-Rad). The tested cytokines/chemokines include IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-17, exotaxin, M-CSF, GM-CSF, IFN-γ, KC, MCP-1, MIP-1α, MIP-1β, RANTES and TNF-α.
2.8. Bone marrow-derived DC cultures
Bone marrow-derived DC (BM-DC) cultures were prepared, as previously described (Qi et al., 2001). Briefly, bone marrow cells were obtained from the femurs of naïve BALB/c mice and cultured at 2 x 106 cells per 10 ml in the presence of murine rGM-CSF (20 ng/ml) in complete Iscove's Modified Dulbecco's Medium (IMDM) media, supplemented with 10% FBS, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol and 100 U/ml penicillin. On day 3, 10 ml of fresh GM-CSF-containing medium was added, and 10 ml of culture medium was replaced with fresh GM-CSF-containing medium on day 6. Loosely attached cells, containing > 70% of CD11c+ cells, were harvested on day 8 and used for experiments.
2.9. Infection of BM-DCs and DC-T cell cocultures
BM-DCs were adjusted to 2 x 106 cells per well in 12-well plates and infected with either promastigotes or axenic amastigotes of L. amazonensis LV78 at the ratio of 8:1 parasites-to-DC. In certain groups, BM-DCs were infected with parasites that were coated with serum derived either from naïve BALB/c mice (non-immune serum, NS) or BALB/c mice infected with 2 x 106 L. amazonensis promastigotes for 10 weeks (immune serum, IS).
Following incubations at 33°C for 12 h and then 37°C for another 12 h, supernatants of infected BM-DC cultures were collected for cytokine measurement via ELISA. Infected BM-DCs were harvested and treated with mitomycin C for subsequent cocultures with purified naïve CD4+ T cells. Naïve CD4+ T cells obtained from uninfected BALB/c mice were purified using MACS microbead methods, similar to that used for the purification of CD4+ T cells obtained from infected mice (see Section 2.6). BM-DCs (2 x 104) were co-cultured with purified naïve CD4+ T cells (2 x 105) in U-bottomed, 96-well plates for 72 h, and supernatants were collected for the measurement of cytokine production via ELISA. One μCi of [3H]-thymidine was added at 18–22 h before harvest, and the incorporated radioactivity was determined using a direct beta counter (Matrix 9600; Packard Instrument Co. Ltd).
2.10. Evaluation of serum antibody titer
To assess parasite-specific antibody titers, 96-well assay plates were coated with amastigote lysates (50 μg/ml) overnight at 4°C. After blocking, plates were incubated with individual mouse serum samples (1:50 dilution) for 1 h at 37°C. Next, plates were incubated with goat anti-mouse IgGs (1:1,000; Sigma) for 1 h at 37°C and then incubated with horseradish peroxidase-conjugated rabbit anti-goat IgG (1:1,000; Pierce, Rockford, Ill.). Color was developed with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate reagents, according to the manufacturer’s instructions (BD Biosciences).
2.11. Statistical analyses
To evaluate the significance of differences between experimental groups, statistical analyses (student's t-test and one-way ANOVA) were performed using Graphpad Prism 4.0 (GraphPad Software, Inc. San Diego, CA). For one-way ANOVA analysis, the Newman-Keuls post test was used to compare the differences between treatment groups. The differences between groups were considered significant when P ≤ 0.05.
3. Results
3.1. B cell outgrowth and antibody production correlated with lesion progression
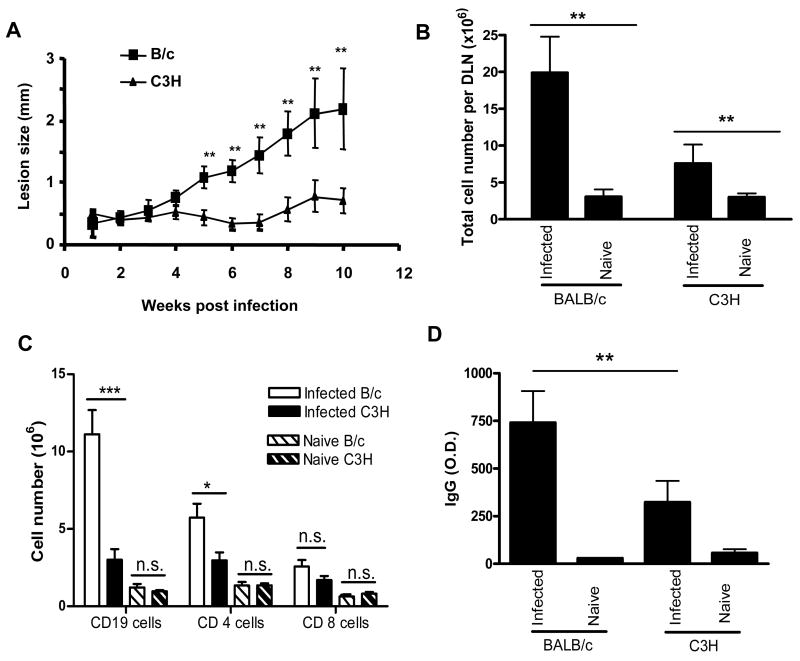
To examine the involvement of B cells and antibodies in disease pathogenesis, we first examined whether there was a correlation between disease severity and cellular distribution during L. amazonensis infection. Using highly susceptible BALB/c and relatively resistant C3H mice (Fig. 1A), we observed a correlation between lesion sizes and the number of DLN cells. Infected BALB/c mice displayed a striking enlargement of DLNs, containing 6.5-fold more cells than naïve controls (Fig. 1B), while the DLNs of infected C3H mice exhibited only a 2.5-fold increase from their naïve controls. Interestingly, the expansion of DLN cells from infected BALB/c was primarily due to the expansion of CD19+ B cells. As shown in Fig. 1C, the number of B cells in infected BALB/c increased 9.1-fold from uninfected animals, while the number of CD4+ and CD8+ T cells increased only 4-fold from the uninfected animals. This striking expansion of B cells was specific to highly susceptible BALB/c mice, as the expansion of CD19+ B cells in infected C3H mice was comparable to that of CD4+ and CD8+ T cells (3-fold increase for CD 19+ cells and 2-fold for CD4+ and CD8+ T cells). Examination of antibody titers showed that the levels of parasite-specific IgGs in infected BALB/c mice were significantly higher than those of infected C3H mice (Fig. 1D). Together, these data demonstrated a direct correlation between lesion development and B cell activation during L. amazonensis infection.
Fig. 1.
B cell outgrowth and antibody production correlated with lesion development in Leishmania amazonensis-infected mice. BALB/c and C3H mice (five per group) were left untreated or injected s.c. in the right hind foot with 2 x 106 promastigotes. (A) Lesion sizes were measured weekly with a digital caliper. (B) At 10 weeks p.i., draining lymph nodes (DLNs) were collected and the total number of cells per LN was counted using a hemacytometer. (C) Cell surface markers were stained for flow cytometry and the absolute number of each cell population in DLNs was calculated. (D) The titers of total IgGs in the sera were measured via direct ELISA. Data are presented as mean ± S.D. for each group. Similar results were observed from two independent experiments. (*P < 0.05; **P < 0.01; *** P < 0.001; n.s., not significant)
3.2. Lesion development in B cell-deficient BALB/c mice
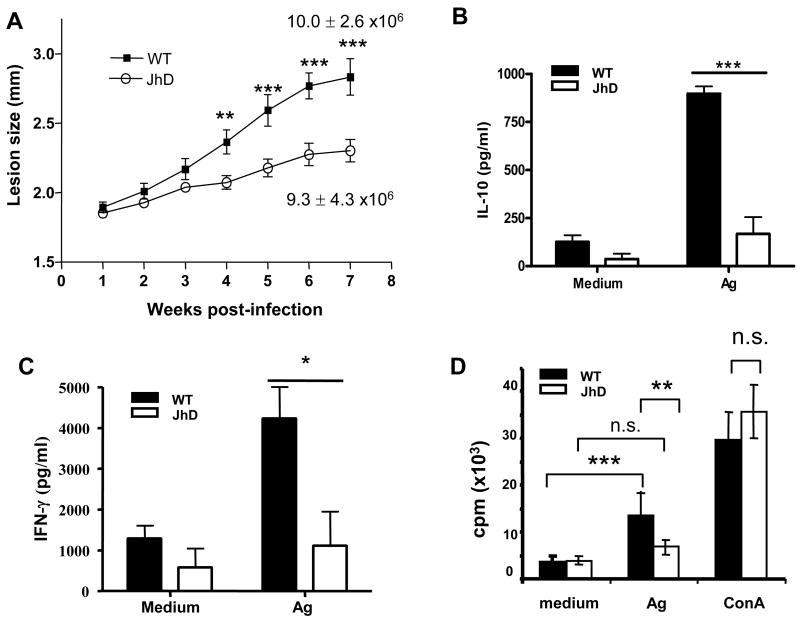
Although elevated B cell activation was found to be correlated with disease susceptibility and severity, other factors including the differences in genetic background of BALB/c and C3H mice may influence the outcome of disease (Foote and Handman, 2005). We therefore investigated the specific roles of B cells and antibodies in disease pathogenesis by examining the course of L. amazonensis infection in B cell-deficient JhD mice on the BALB/c background. Compared with wild-type controls, JhD mice infected with 2 x 106 L. amazonensis amastigotes exhibited a delayed onset of lesion development. Starting from 4 weeks p.i., lesions of JhD mice were significantly smaller than those of wild-type mice (P < 0.01, Fig. 2A). Surprisingly, these two groups of mice showed no major differences in tissue parasite burdens at 7 weeks p.i.. As expected, L. amazonensis infection induced high levels of IgG production in wild-type mice, while no measurable IgG levels were detected in infected JhD mice (data not shown). Similar results were observed when mice were infected with a lower dose (2 x 105) of amastigotes (data not shown). Together, these observations suggest that B cells play a role in enhancing disease development following L. amazonensis infection.
Fig. 2.
B cell-deficient Jh mice developed small cutaneous lesions and displayed impaired cytokine production and CD4+ T cell proliferative responses following Leishmania amazonensis infection. (A) Wild-type BALB/c and Jh mice (10 per group) were injected s.c. with 2 x 106 amastigotes. Lesion sizes were measured weekly with a digital caliper. Parasite burden in foot tissue was determined at 7 weeks p.i.. At 7-8 weeks p.i., draining lymph node (DLN) cells were collected and restimulated with parasite antigen (Ag) for 72 h. (B–C) Cytokine profiles from the supernatants were determined with a mouse 23-Bio-Plex cytokine assay. Cytokines that were significantly different between wild-type and Jh mice are shown. (D) Splenic CD4+ T cells were purified from infected wild-type and Jh mice and cocultured with mitomycin C-treated splenocytes from naïve wild-type mice (1:1 ratio) in the presence or absence of parasite Ag or 2 ng/ml of Con A for 72 h. Proliferation of CD4+ T cells was indicated by [3H] thymidine incorporation. Data are presented as mean ± S.D. for each group. Similar results were observed from two independent experiments. (* P < 0.05; *** P < 0.001; n.s., not significant)
3.3. Deficiency in antigen-specific CD4+ T cell responses in infected JhD mice
Given the importance of cytokine/chemokine responses in L. amazonensis infection, we then examined whether the absence of B cells also influences cytokine production by DLN cells. As shown in Figs. 2B and 2C, DLN cells derived from infected JhD mice showed a marked impairment of antigen-specific IL-10 and IFN-γ production when re-stimulated in vitro with parasite lysates for 72 h. Of note, CCL-2/MCP-1 levels appeared to be significantly higher in JhD mice than wild-type mice regardless of antigen re-stimulation (data not shown), suggesting that high levels of MCP-1 production may be an intrinsic phenotype of B cell-deficient mice (Rivera et al., 2005). No significant differences between the two groups of mice were found in the effects of other cytokines and chemokines tested using a BioRad mouse-23-Plex cytokine assay (data not shown).
Given the integral role of CD4+ T cells in the disease pathogenesis caused by L. amazonensis infection (Soong et al., 1997), we further examined recalled responses of antigen-specific CD4+ T cells. We isolated CD4+ T cells from the spleens of infected wild-type and JhD mice and co-cultured those with mitomycin C-treated naïve splenocytes in the absence or presence of parasite lysates or Con A. Incorporation of [3H]-labeled thymidine at 72 h post-stimulation showed that CD4+ T cells from infected JhD mice were markedly impaired in their responses to parasite antigens, but not to Con A (Fig. 2D), indicating that their proliferative impairment was antigen-specific. These data suggest involvement of B cells in T cell priming during L. amazonensis infection.
3.4. Differential histopathology in lesions of Jh and wild-type mice
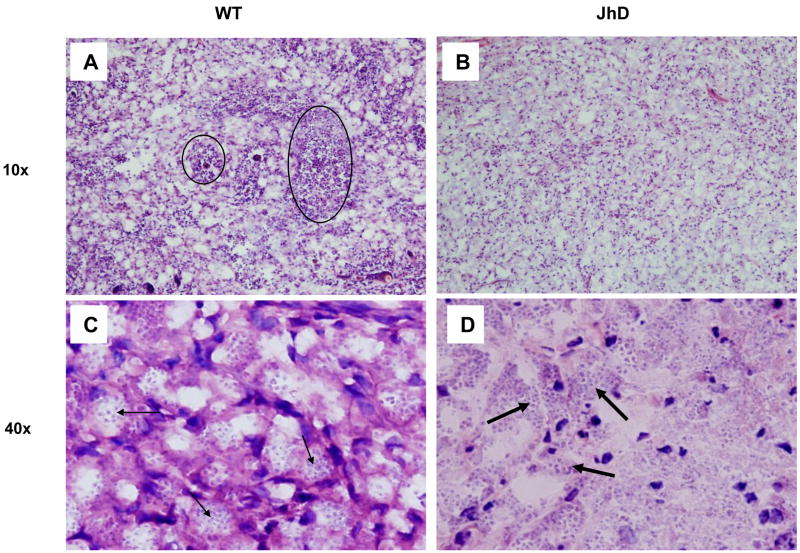
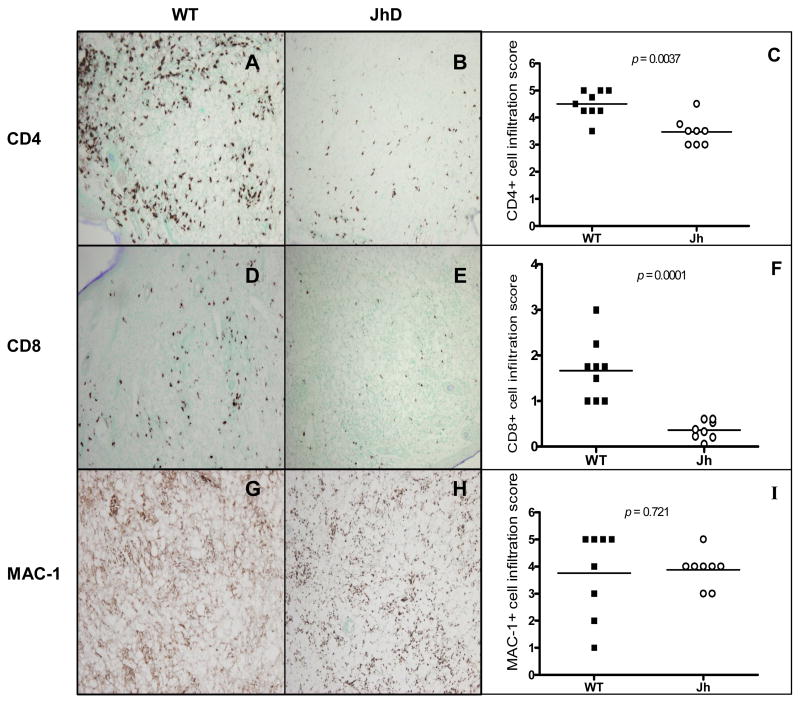
Given that JhD mice developed small lesions, but contained comparable numbers of parasites to wild-type mice, we performed histological and immunohistochemical examinations. H&E staining of foot sections obtained at 7–8 weeks p.i. revealed different cellular infiltrates in these two groups of mice. While the foot tissue of wild-type mice presented an extensive and monomorphic collection of vacuolated, heavily parasitized MΦs (Figs. 3A and 3C, small arrows), tissues of infected Jh mice showed a mixture of vacuolated MΦs and non-vacuolated cells, with some parasites appearing to be packed in the extracellular space (Fig. 3D, large arrows). In addition, lesions of wild-type mice showed moderate-to-large clusters of necrotic cells (Fig. 3A, circles), suggesting an aggressive progression of disease in immunocompetent mice; however, there were only minimal signs of tissue necrosis in lesions of JhD mice or none at all (Fig. 3B). To further define cellular immune responses at the infection sites, we performed immunohistochemical studies using mAbs specific to CD4, CD8 and MAC-1. Although significantly lower numbers of CD4+ and CD8+ T cells were detected in lesions of infected JhD mice, compared with those in the wild-type mice (P < 0.01, Figs. 4A–F), the number of MAC-1+ MΦs remained comparable in tissues of both JhD and wild-type mice (Figs. 4G–I). B220+ B cells were only detected in tissues of infected wild-type mice (data not known). Collectively, these histological data suggest a significant role for B cells in recruiting and retaining T cells at the infection site.
Fig. 3.
Differential histopathology in lesions of infected Jh and wild-type mice. Mice (eight per group) were injected s.c. in the right hind foot with 2 x 106 Leishmania amazonensis amastigotes. At 7–8 weeks p.i., infected foot tissues were collected for histological analysis. Representative H&E staining of tissues from wild-type mice (A, C) showed tissue necrosis (circles) and monomorphic collection of vacuolated and heavily parasitized macrophages (small arrows). Sections from Jh mice (B, D) showed no or minimal necrosis, as well as a mixture of vacuolated and non-vacuolated macrophages. Some parasites appeared to be packed in the extracellular space (big arrows).
Fig. 4.
Impaired recruitment of CD4+ and CD8+ T cells, but not MAC-1+ cells, in Leishmania amazonensis-infected Jh mice. Mice were grouped and infected as described in Fig. 3. At 7–8 weeks p.i., foot tissues were collected and stained with anti-CD4 (A, B), anti-CD8 (D, E) or anti-MAC-1 (G, H) and then counterstained with methyl green. Representative sections show extensive infiltrates for CD4+ cells (A) and a significant number of CD8+ cells (D) in the wild-type mice. In contrast, tissues of infected Jh mice contained limited numbers of CD4+ (B) and CD8+ cells (E). MAC-1+ macrophages appeared distributed throughout tissues of wild-type (G) and Jh mice (H), although the staining patterns appeared differently in these two groups. Tissue sections stained side-by-side with isotype controls had no/minimal background staining (data not shown). All images were taken at 10 x magnification. The total number of CD4+ (C), CD8+ T cells (D) and MAC-1+ macrophages (I) on each side were counted and scored semiquantitatively under 40 x (for CD4+ and CD8+ T cells) and 10x (for MAC-1+ cells) (see Materials and methods). The average scores from three visual fields from each group are plotted. Data represent mean ± S.D. for each group.
3.5. Recovery of T cell activation and migration after adoptive transfer B cells and antibodies to JhD mice
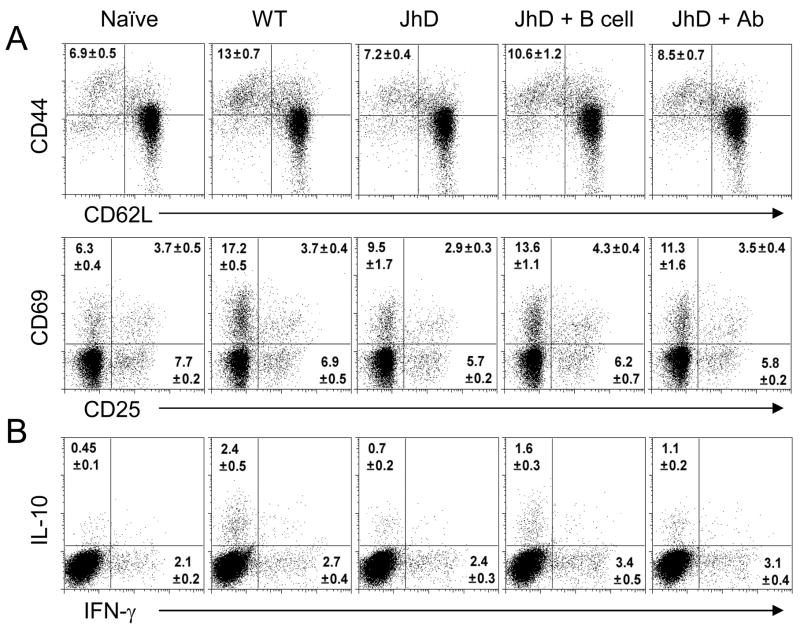
Given that JhD mice displayed a defective T cell response (Fig. 2D) and migration (Fig. 4), we further investigated whether these defects could be due to the lack of B cells or antibodies. JhD mice (three to four mice per group) were given 5 x 107 of CD19+ naive B cells 1 day prior to infection, or 200 μl of sera obtained from chronically infected BALB/c mice on days 1, 3 and 7 p.i. We observed a significant increase in total DLN cell numbers in B cell-transferred JhD mice, and an increase in serum antibody titers in antibody-treated JhD mice (Table 1), suggesting that our transfer protocols were effective. To evaluate the effect of B cell and antibody transfers on the priming of CD4+ T cells, activation markers of CD4+ T cells and percentages of lesional CD4+ T cells were measured via flow cytometry at 3 weeks p.i. We found that, in comparison to infected BALB/c mice, infected JhD mice contained a significantly reduced population of activated CD4+ T cells (CD44hiCD62LloCD69+ population) in their DLNs (Fig. 5A). However, this cell population was significantly restored in B cell-transferred JhD mice. Similar observations were made in the case of an IL-10-producing CD4+ T cell population (Fig. 5B). Interestingly, antibody treatment of JhD mice also restored the populations of activated and IL-10-producing CD4+ T cells; however, it was not as effective as those observed following B cell transfer (Figs. 5A and 5B). It appeared that antibody treatment alone was equally effective as B cell transfer in its ability to induce T cell migration to the infected tissues, as judged by the comparable percentages of CD4+ T cells recovered from antibody- and B cell-transferred JhD mice (Table 1). Altogether, these data suggest that antibody alone was sufficient for the induction of CD4+ T cell migration; however, high levels of T cell activation could only be achieved when B cells were given. At the time examined, B cell-treated JhD mice showed only minimal antibody production (antibody titer was comparable to those of naive mice, Table 1), thus these data also suggest that B cells exhibit antibody-independent mechanisms for the induction of CD4+ T cell activation and migration.
Table 1.
LN and CD4+ T cell number, lesional CD4+ T cell percentage and serum Ab titer from naïve or amastigote-infected mice.
| Naïve | WT | JhD | JhD+B cell | JhD+Ab | |
|---|---|---|---|---|---|
| LN cells (106 per mouse) | 2.25 ± 0.25 | 28.15 ± 3.11a | 3.67 ± 1.05 | 5.58 ± 0.34b | 3.35 ± 0.4 |
| CD4+ T cells (106 per LN) | 1.14 ± 0.18 | 7.57 ± 1.15a | 2.34 ± 0.77 | 3.25 ± 0.41b | 2.08 ± 0.28 |
| Lesional CD4+ T cell (%) | - | 2.3 ± 0.2a | 0.6 ± 0.1 | 1.7 ± 0.2a | 1.8 ± 0.1a |
| Ab titer (1:50) (OD450 value) | 0.185 ± 0.01 | 0.377 ± 0.05a | 0.086 ± 0.02 | 0.165 ± 0.06a | 0.426 ± 0.02a |
, P < 0.001;
, P < 0.05 when indicated group compared with the JhD group.
LN, lymph node; Ab, antibody.
Fig. 5.
Ex vivo lymph node (LN) CD4+ T cell activation and cytokine production from amastigote (Am)-infected mice. For B cell adoptive transfer, purified spleen B cells (5 × 107) were injected i.v. in to JhD mice 1 day prior to Am infection. For antibody adoptive transfer, sera (200 μl) from chronically infected BALB/c mice were adoptively transferred (i.p.) into JhD mice at 1, 3and 7 days after Am infection. (A) At 3 weeks, individual (WT) or pooled draining LNs were extracted and stained for CD4 and T cell activation markers. (B) LN cells (106/ml) were restimulated with PMA/ionomycin with GolgiPlug™ for 6 h. Intracellular staining for IFN-γ and IL-10 were performed after CD4 surface marker. Percentages of positively stained CD4+ T cells are shown as mean ± SD from two independent repeats.
3.6. Parasite-specific antibody enhances CD4+ T cell priming and IL-10 production in vitro
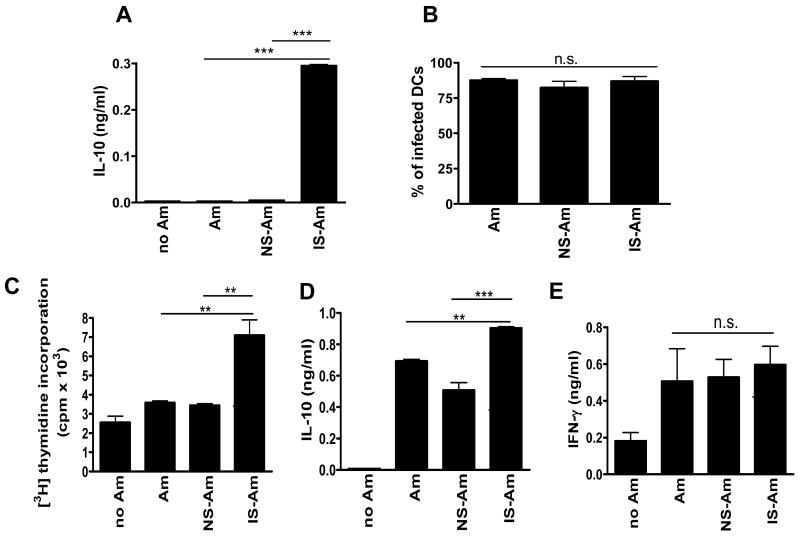
Having observed an increase in CD4+ T cell activation and IL-10 production in antibody-treated JhD mice, we further examined the potential mechanisms underlying antibody-mediated T cell activation in vitro. It has previously been shown that IgG can alter the cytokine profile of Leishmania-infected MΦs (Kane and Mosser, 2001); however, the effect of IgG on Leishmania-infected DCs is unclear. Since DCs are key antigen-presenting cells (APCs) involved in the priming and instigating of CD4+ T cell responses during L. amazonensis infection (Qi et al., 2001), the effect of antibodies on cytokine production of infected DCs was examined. Cytokine production was measured from BM-DCs infected with L. amazonensis amastigotes that were pre-exposed to the sera of naïve mice (non-immune sera-coated amastigotes, NS-Am) or of L. amazonensis-infected BALB/c mice (immune sera-coated amastigotes, IS-Am). As shown in Fig. 6A, IS-Am triggered BM-DCs to secrete levels of IL-10 significantly higher than those of DCs exposed to untreated parasites (Am) or NS-Am (approximately 68-fold increase). These changes in cytokine production were likely due to antibody-mediated cell signaling (Yang et al., 2007) rather than enhanced parasite uptake, since comparable infection rates were observed in all of the infected DC groups (Fig. 6B). To further investigate whether these IS-Am-exposed DCs could subsequently alter the phenotypes of CD4+ T cells, we performed in vitro priming assays using Leishmania-infected BM-DCs as APCs. Following infection with Am, NS-Am or IS-Am, BM-DCs were treated with mitomycin C and co-cultured with purified naive CD4+ T cells, and T cell proliferative responses were measured. As shown in Fig. 6C, we found that BM-DCs infected with IS-Am were able to induce CD4+ T cells to proliferate at an almost 2-fold higher rate than that of DCs infected with untreated Am or NS-Am. Accordingly, CD4+ T cells co-cultured with IS-Am-infected DCs also produced enhanced levels of IL-10 (Fig. 6D). Interestingly, T cell production of IFN-γ appeared to be unaffected by the presence of antibodies (Fig. 6E). Similar trends in T cell activation and cytokine production were observed when DCs were infected with IS- or NS-coated promastigotes (data not shown). Together, these in vitro data suggest that parasite-specific antibodies can greatly enhance the priming of antigen-specific CD4+ T cells and their IL-10 production via the modulation of DC functions.
Fig. 6.
Enhanced cytokine production and increased priming efficiency of CD4+ T cells by dendritic cells (DCs) infected with antibody-coated parasites. Bone marrow-derived dendritic cells (BM-DCs) from naive BALB/c were infected with Leishmania amazonensis axenic amastigotes at an 8:1 parasite-to-cell ratio. These parasites were untreated, previously coated with non-immune serum (NS-Am) or with serum obtained from infected BALB/c mice (immune serum, IS-Am). (A) Supernatants were collected at 24 h p.i. cytokine levels were measured using ELISA. (B) Infection rates of DC cultures were determined by counting 200 cells from each condition under a light microscope. Infected BM-DCs were then used for cocultures with purified naïve CD4+ T cells (2 x 105 cells/well). (C) The proliferation of CD4+ T cells was indicated by [3H] thymidine incorporation. (D–E) Culture supernatants were collected at 72 h p.i. and cytokine levels were measured using ELISA. Data are presented as mean ± S.D. for each group. Similar results were observed from three independent experiments. (** P < 0.01; *** P < 0.001; n.s., not significant)
4. Discussion
While it is generally accepted that CD4+ T cells play an integral role in determining the outcome of Leishmania infection, the functionary roles of B cells during disease development are still a matter of controversy and deserve more detailed investigation. Several studies have documented that B cells and/or antibodies play a pathogenic role in disease pathogenesis caused by parasites in the L. mexicana complex (McMahon-Pratt and Alexander, 2004). While the detailed investigation of B cell involvement in the L. pifanoi infection model has been reported (Colmenares et al., 2002), the pathogenic roles of B cells during infection caused by a closely related parasite species, L. amazonensis, are unclear. In this study, we examined in detail the functional roles of B cells during L. amazonensis infection by utilizing both in vivo and in vitro systems. Compared with the L. pifanoi infection model, we found both similarities and unique characteristics by which B cells play a pathogenic role in L. amazonensis infection. During the course of L. amazonensis infection, we observed extensive B cell expansion and high-titer antibody production associated with elevated disease severity in immunocompetent mice (Fig. 1). Similar to an L. pifanoi infection model (Kima et al., 2000), the disease severity caused by L. amazonensis infection was significantly reduced in the absence of B cells (Fig. 2). Leishmania amazonensis-infected, B cell-deficient JhD mice showed a decrease in cellular recruitment, in particular CD4+ and CD8+ T cells (Fig. 4). On the other hand, we found the role of B cells in L. amazonensis infection to be different from that in the L. pifanoi infection model in terms of their involvement in CD4+ T cell activation. CD4+ T cell responses were reported to be normal in L. pifanoi-infected JhD mice (Colmenares et al., 2002), but we found that L. amazonensis-infected JhD mice developed a weak CD4+ T cell proliferation in response to parasite antigens (Figs. 2D and 5). We further demonstrated that these defective CD4+ T cell responses were due specifically to the lack of B cells and antibodies, as proven by the recovery of T cell activation and migration after administration of B cells or immune antibodies in JhD mice (Fig. 5 and Table 1). We then further confirmed that antibodies could influence T cell priming using in vitro DC infection and T cell priming studies. We found that the presence of parasite-specific antibodies could markedly induce DC cytokine production and their APC function for the priming of naive CD4+ T cells (Fig. 6). As CD4+ T cells are required for lesion development following L. amazonensis infection (Soong et al., 1997), our data suggest that B cells and antibodies can enhance L. amazonensis infection, partially by promoting T cell priming and cellular migration to infection sites.
CD4+ T cells play an indispensable role in the disease pathogenesis associated with L. amazonensis infection. We previously reported that mice deficient in MHC II-restricted CD4+ T cells have minimal tissue parasite burdens, no measurable lesions even at 12 to 14 weeks p.i. and a dramatic impairment of MAC-1+ MΦ migration (Soong et al., 1997). Our current studies of JhD mice clearly showed a distinctive disease pattern in the absence of B cells. Although B cells are not required for lesion development, their activation can significantly promote disease progression. One of the most interesting findings in this study comes from our immunohistochemical studies showing a marked reduction in CD4+ and CD8+ T cells, but a comparable influx of MAC-1+ MΦs in lesions of JhD mice, compared with those in wild-type mice (Fig. 4). This rich supply of MAC-1+ MΦs may explain the high parasite burdens in Jh mice (Fig. 2). It is worth mentioning that, although the total number of MAC-1+ MΦs appeared unaltered, lesions of infected JhD mice appeared different from those of infected wild-type controls in several ways: limited necrotic foci and a mixed population of vacuolated and non-vacuolated cells, with some parasites packed in the extracellular space (Fig. 3). While the detailed mechanisms of how B cells can regulate cellular infiltration remain to be investigated, we documented herein that the presence of B cells and antibodies could significantly influence CD4+ T cell migration (Table 1). Our findings suggesting the influence of B cells on local tissue infiltration are in agreement with previous reports both in Leishmania and other infection models. For example, Colmenares et Al. (2002) observed reduced monocyte and lymphocyte migration at the infection sites of L. pifanoi-infected JhD mice. Moreover, no inflammatory lesion was detected in the absence of B cells following Cryptosporidium parvum infection of TCRα−/− mice, normally associated with massive leukocyte infiltration (Waters et al., 2000). While we showed that B cell treatment of JhD mice could restore CD4+ T cells at the infection site (Table 1), we also demonstrated that antibody treatment alone could exert a similar effect. This is not unexpected because antibody is known to promote inflammation by activating the complement pathways to produce the powerful chemoattractant C3a and C5a fragments (Tagami, 1992). Antibody can also induce inflammation via FcγR-signaling cascades, as evidenced by a reduced inflammation in mice lacking FcRγI or a common FcRγ-chain (Ravetch and Bolland, 2001; Barnes et al., 2002). Altogether, these studies suggest various mechanisms by which B cells are involved in promoting local inflammation.
In addition to decreased inflammatory responses in the infection sites, infected JhD mice displayed reduced cellular immune responses, as indicated by reduced cytokine production and CD4+ T cell proliferation in response to parasite antigens (Figs. 2 and 5). While it is unclear how B cells participate in the priming of cellular immune responses, we have shown herein that there may be more than one mechanism involved in this process. While parasite-specific antibody appeared to significantly enhance CD4+ T cell activation, its effect seemed to be inferior to that of intact B cells, suggesting that B cells may exhibit antibody-independent mechanisms that participate in the priming of CD4+ T cells. While the details of these mechanisms remain to be investigated, several known functions of B cells may potentially be involved. Firstly, B cells may serve as APCs, participating in the maintenance and expansion of L. amazonensis-specific Th cells. It is known that antigen taken up via an Ig receptor, or targeted to CD19 on the surface of B cells, can activate antigen-specific T cells more efficiently than would non-specific uptake (Constant, 1999; Yan et al., 2005). In agreement with this view, we found that at the late stage of infection (7 to 8 weeks p.i.), antigen-specific T cell responses were significantly reduced in the absence of B cells (Fig. 2D). Second, B cells can act in concert with DCs to induce higher levels of T cell proliferation and cytokine production in vivo (Kleindienst and Brocker, 2005). Lastly, B cells can produce several cytokines and influence various parts of host immunity. Studies have shown that activated B cells can produce many cytokines, including TNF-γ, lymphotoxin and IL-6 (Goldfeld et al., 1992; Duddy et al., 2004). In Leishmania infection, Palanivel et al. (1996) showed that B220+ B cells from L. major-infected BALB/c mice are capable of producing IL-10 when restimulated with soluble leishmanial extracts. Our preliminary data also suggested that CD19+ B cells derived from L. amazonensis-infected BALB/c mice produced IFN-γ, IL-2 and IL-3 when exposed to L. amazonensis lysates (data not shown). The relative contribution of these B cell-derived cytokines in the activation and recruitment of T cells and macrophages warrants further investigation.
As shown in Fig. 5, we demonstrated that a passive transfer of antibodies could partially restore T cell activation normally defective in L. amazonensis-infected JhD mice. We further investigated the role of parasite-specific antibody in T-cell priming in vitro. As indicated in Fig. 6, DCs infected with antibody-coated parasites produced high levels of IL-10 and were more potent in prime naive CD4+ T cells. As shown in Fig. 6, T cells co-cultured with IS-Am-infected DCs also produced increased levels of IL-10, but not IFN-γ, suggesting a role for antibodies in the induction of predominant IL-10 production. Several studies have previously suggested the role of antibodies in the induction of IL-10 during Leishmania infection. For example, JhD mice receiving anti-L. major IgG injection produced high levels of IL-10 in DLN and developed progressive lesions following L. major infection (Miles et al., 2005). It has been suggested that this enhanced susceptibility was due to an increase in IL-10 production by infected-MΦs. It was also shown that infection of hyaluronic acid-treated MΦ with IS-coated parasites can trigger MAPK/ERK1/2 activation and subsequently induce strong IL-10 production (Yang et al., 2007). While these studies explain how IgG promotes IL-10 production in Leishmania-infected MΦs at the molecular level, our studies indicate that parasite-specific IgGs can also induce predominant IL-10 production by L. amazonensis-infected DCs, as well as enhance their antigen-presenting function to prime CD4+ T cells. Our observation of the lack of enhancement of IFN-γ production from T cells co-cultured with IS-Am-infected DCs was contradictory to that of a previous study by Woelbing et al. (2006), which showed that B cell-deficient μMT mice on the C57BL/6 background infected with IS-coated parasites produced high levels of IFN-γ and thus increased resistance to L. major infection. Discrepancies in the cytokine profile obtained in that study and ours study may be due to the different genetic backgrounds of the mice or the different parasite species used.
In conclusion, data presented in this report suggest that B cells play significant roles in the disease caused by L. amazonensis, in particular in the induction of local cellular recruitment and the priming of parasite-specific CD4+ T cells. Following infection with L. amazonensis parasites, there was extensive expansion of B cells, activation of CD4+ T cells and extensive tissue necrosis in highly susceptible, immunocompetent hosts. A targeted deletion of B cells and abolished antibody production altered cell-mediated responses at the site of infection as well as CD4+ T cell responses. B cells and antibodies were shown to influence the activation of CD4+ T cells, as suggested by the recovery of CD4+ T cell activation and migration after the administration of B cells or antibodies in JhD mice. Our in vitro studies further demonstrated that parasite-specific antibodies can alter DC cytokine production and ultimately enhance the expansion of parasite-specific CD4+ T cells. This study has potential implications for the future design of treatment regimens for Leishmania infection, in general, and L. amazonensis infection, in particular, as it calls for caution in using antibody titers as an indication of protection in vaccine-based studies. It also suggests that while modulating the expansion of pathogenic CD4+ T cells should be the primary target for the design of therapeutic treatments for L. amazonensis infection, additional strategies aimed at controlling B cell-mediated recruitment and activation may provide more efficacious treatment regimens. A better understanding of the molecular basis of B cell activation and the nature of B cell-stimulating antigens would help in the design of efficient treatment for the control of immunopathogenesis associated with leishmaniasis.
Acknowledgments
This work is supported by a James W. McLaughlin Predoctoral Fellowship to N. Wanasen and an NIH grant AI043003 to L. Soong. The authors would like to thank Ms. Mardelle Susman for her editorial assistance in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson CF, Gerber JS, Mosser DM. Modulating macrophage function with IgG immune complexes. J Endotoxin Res. 2002;8:477–81. doi: 10.1179/096805102125001118. [DOI] [PubMed] [Google Scholar]
- Barnes N, Gavin AL, Tan PS, Mottram P, Koentgen F, Hogarth PM. FcgammaRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity. 2002;16:379–89. doi: 10.1016/s1074-7613(02)00287-x. [DOI] [PubMed] [Google Scholar]
- Brown DR, Reiner SL. Polarized helper-T-cell responses against Leishmania major in the absence of B cells. Infect Immun. 1999;67:266–70. doi: 10.1128/iai.67.1.266-270.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenares M, Constant SL, Kima PE, McMahon-Pratt D. Leishmania pifanoi pathogenesis: selective lack of a local cutaneous response in the absence of circulating antibody. Infect Immun. 2002;70:6597–605. doi: 10.1128/IAI.70.12.6597-6605.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant SL. B lymphocytes as antigen-presenting cells for CD4+ T cell priming in vivo. J Immunol. 1999;162:5695–703. [PubMed] [Google Scholar]
- Cupolilo SM, Souza CS, Abreu-Silva AL, Calabrese KS, Goncalves da Costa SC. Biological behavior of Leishmania (L.) amazonensis isolated from a human diffuse cutaneous leishmaniasis in inbred strains of mice. Histol Histopathol. 2003;18:1059–65. doi: 10.14670/HH-18.1059. [DOI] [PubMed] [Google Scholar]
- Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol. 2004;172:3422–7. doi: 10.4049/jimmunol.172.6.3422. [DOI] [PubMed] [Google Scholar]
- Dutta S, Ray D, Kolli BK, Chang KP. Photodynamic sensitization of Leishmania amazonensis in both extracellular and intracellular stages with aluminum phthalocyanine chloride for photolysis in vitro. Antimicrob Agents Chemother. 2005;49:4474–84. doi: 10.1128/AAC.49.11.4474-4484.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SJ, Handman E. Genetics of murine leishmaniasis. Brief Funct Genomic Proteomic. 2005;4:270–6. doi: 10.1093/bfgp/4.3.270. [DOI] [PubMed] [Google Scholar]
- Galvao-Castro B, Sa Ferreira JA, Marzochi KF, Marzochi MC, Coutinho SG, Lambert PH. Polyclonal B cell activation, circulating immune complexes and autoimmunity in human American visceral leishmaniasis. Clin Exp Immunol. 1984;56:58–66. [PMC free article] [PubMed] [Google Scholar]
- Goldfeld AE, Flemington EK, Boussiotis VA, Theodos CM, Titus RG, Strominger JL, Speck SH. Transcription of the tumor necrosis factor alpha gene is rapidly induced by anti-immunoglobulin and blocked by cyclosporin A and FK506 in human B cells. Proc Natl Acad Sci U S A. 1992;89:12198–201. doi: 10.1073/pnas.89.24.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailu A, Menon JN, Berhe N, Gedamu L, Hassard TH, Kager PA, Olobo J, Bretscher PA. Distinct immunity in patients with visceral leishmaniasis from that in subclinically infected and drug-cured people: implications for the mechanism underlying drug cure. J Infect Dis. 2001;184:112–5. doi: 10.1086/320994. [DOI] [PubMed] [Google Scholar]
- Hoerauf A, Solbach W, Rollinghoff M, Gessner A. Effect of IL-7 treatment on Leishmania major-infected BALB.Xid mice: enhanced lymphopoiesis with sustained lack of B1 cells and clinical aggravation of disease. Int Immunol. 1995;7:1879–84. [PubMed] [Google Scholar]
- Howard JG, Hale C, Liew FY. Immunological regulation of experimental cutaneous leishmaniasis. III. Nature and significance of specific suppression of cell-mediated immunity in mice highly susceptible to Leishmania tropica. J Exp Med. 1980;152:594–607. doi: 10.1084/jem.152.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Masterson J, Sun J, Soong L. CD4+CD25+ regulatory T cells restrain pathogenic responses during Leishmania amazonensis infection. J Immunol. 2005;174:7147–53. doi: 10.4049/jimmunol.174.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DE, Buxbaum LU, Scott P. IL-4-independent inhibition of IL-12 responsiveness during Leishmania amazonensis infection. J Immunol. 2000;165:364–72. doi: 10.4049/jimmunol.165.1.364. [DOI] [PubMed] [Google Scholar]
- Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol. 2001;166:1141–7. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- Kima PE, Constant SL, Hannum L, Colmenares M, Lee KS, Haberman AM, Shlomchik MJ, McMahon-Pratt D. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J Exp Med. 2000;191:1063–8. doi: 10.1084/jem.191.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst P, Brocker T. Concerted antigen presentation by dendritic cells and B cells is necessary for optimal CD4 T-cell immunity in vivo. Immunology. 2005;115:556–64. doi: 10.1111/j.1365-2567.2005.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon-Pratt D, Alexander J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol Rev. 2004;201:206–24. doi: 10.1111/j.0105-2896.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- Miles SA, Conrad SM, Alves RG, Jeronimo SM, Mosser DM. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J Exp Med. 2005;201:747–54. doi: 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanivel V, Posey C, Horauf AM, Solbach W, Piessens WF, Harn DA. B-cell outgrowth and ligand-specific production of IL-10 correlate with Th2 dominance in certain parasitic diseases. Exp Parasitol. 1996;84:168–77. doi: 10.1006/expr.1996.0102. [DOI] [PubMed] [Google Scholar]
- Qi H, Popov V, Soong L. Leishmania amazonensis-dendritic cell interactions in vitro and the priming of parasite-specific CD4(+) T cells in vivo. J Immunol. 2001;167:4534–42. doi: 10.4049/jimmunol.167.8.4534. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–90. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- Rivera J, Zaragoza O, Casadevall A. Antibody-mediated protection against Cryptococcus neoformans pulmonary infection is dependent on B cells. Infect Immun. 2005;73:1141–50. doi: 10.1128/IAI.73.2.1141-1150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks DL, Scott PA, Asofsky R, Sher FA. Cutaneous leishmaniasis in anti-IgM-treated mice: enhanced resistance due to functional depletion of a B cell-dependent T cell involved in the suppressor pathway. J Immunol. 1984;132:2072–7. [PubMed] [Google Scholar]
- Scott P, Natovitz P, Sher A. B lymphocytes are required for the generation of T cells that mediate healing of cutaneous leishmaniasis. J Immunol. 1986;137:1017–21. [PubMed] [Google Scholar]
- Soong L, Chang CH, Sun J, Longley BJ, Jr, Ruddle NH, Flavell RA, McMahon-Pratt D. Role of CD4+ T cells in pathogenesis associated with Leishmania amazonensis infection. J Immunol. 1997;158:5374–83. [PubMed] [Google Scholar]
- Soong L, Duboise SM, Kima P, McMahon-Pratt D. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect Immun. 1995;63:3559–66. doi: 10.1128/iai.63.9.3559-3566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami H. The role of complement-derived mediators in inflammatory skin diseases. Arch Dermatol Res. 1992;284(Suppl 1):S2–9. doi: 10.1007/BF00638232. [DOI] [PubMed] [Google Scholar]
- Vanloubbeeck Y, Jones DE. Protection of C3HeB/FeJ mice against Leishmania amazonensis challenge after previous Leishmania major infection. Am J Trop Med Hyg. 2004;71:407–11. [PubMed] [Google Scholar]
- Waters WR, Palmer MV, Wannemuehler MJ, Sacco RE, Harp JA. B cells are required for the induction of intestinal inflammatory lesions in TCRalpha-deficient mice persistently infected with Cryptosporidium parvum. J Parasitol. 2000;86:1073–7. doi: 10.1645/0022-3395(2000)086[1073:BCARFT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Woelbing F, Kostka SL, Moelle K, Belkaid Y, Sunderkoetter C, Verbeek S, Waisman A, Nigg AP, Knop J, Udey MC, von Stebut E. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J Exp Med. 2006;203:177–88. doi: 10.1084/jem.20052288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Wolff MJ, Unternaehrer J, Mellman I, Mamula MJ. Targeting antigen to CD19 on B cells efficiently activates T cells. Int Immunol. 2005;17:869–77. doi: 10.1093/intimm/dxh266. [DOI] [PubMed] [Google Scholar]
- Yang Z, Mosser DM, Zhang X. Activation of the MAPK, ERK, following Leishmania amazonensis infection of macrophages. J Immunol. 2007;178:1077–85. doi: 10.4049/jimmunol.178.2.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]