Abstract
We report the design and synthesis of a tetraethylene glycol- (TEG-) based bidentate ligand functionalized with dihydrolipoic acid (DHLA) and biotin (DHLA—TEG—biotin) to promote biocompatibility of luminescent quantum dots (QD's). This new ligand readily binds to CdSe—ZnS core-shell QDs via surface ligand exchange. QDs capped with a mixture of DHLA and DHLA—TEG—biotin or polyethylene glycol- (PEG-) (molecular weight average ∼600) modified DHLA (DHLA—PEG600) and DHLA—TEG—biotin are easily dispersed in aqueous buffer solutions. In particular, homogeneous buffer solutions of QDs capped with a mixture of DHLA—PEG600 and DHLA—TEG—biotin that are stable over broad pH range have been prepared. QDs coated with mixtures of DHLA/DHLA—TEG—biotin and with DHLA—PEG600/DHLA—TEG—biotin were tested in surface binding assays and the results indicate that biotin groups on the QD surface interact specifically with NeutrAvidin-functionalized microtiter well plates.
1. INTRODUCTION
Luminescent semiconductor nanocrystals, such as those made of CdSe–ZnS core-shell quantum dots (QD's), provide substantial advantages for use as stable fluorophores in biological assays and imaging. As synthesized by conventional methods using high-temperature solution reaction from organometallic precursors, highly luminescent QD's are capped with hydrophobic organic ligands primarily made of a mixture of trioctylphosphine/trioctylphosphine oxide (TOP/TOPO). Further surface modification is required to make them water-soluble and biocompatible. Methods reported to date for achieving water solubility of such materials include silica coating [1], encapsulation of the native TOP/TOPO-capped QD's within amphiphilic polymer shells [2] or lipid micelles [3], and cap exchange of the native TOP/TOPO caps with hydrophilic ligands [4–6]. The strategy based on cap exchange with bifunctional ligands is relatively simple to implement and has the potential to provide compact hydrophilic QD's, a desired property in targeted studies, including fluorescence resonance energy transfer- (FRET-) based sensing and cellular uptake [7–11].
We have previously utilized readily available thioctic acid and polyethylene glycols (PEGs) in simple esterification schemes, followed by reduction of the 1,2-dithiolane to synthesize a series of PEG-terminated dihydrolipoic acid (DHLA–PEG) capping substrates [12]. The cap exchange reaction of TOP/TOPO-capped QD's with these substrates produced water-soluble nanocrystals that are stable over extended periods of time and over a relatively broad pH range, from weakly acidic to basic conditions (pH 5∼12). Though compact and stable, those ligands lack specific functional end groups and do not allow easy implementation of simple conjugation techniques, such as avidin-biotin binding.
In this study, we further expanded those findings and report the design and synthesis of ligands functionalized with a biotin end group. The designed ligands have a central tetraethylene glycol (TEG) segment, a dithiol terminal group for anchoring on the QD surface and a lateral biotin. Appending biotin at the end of surface-attached ligands should permit the use of the ubiquitous avidin-biotin binding motif to conjugate QD's to proteins and other biomolecules via an avidin bridge. Cap exchange reactions were carried out with mixed ligands and preliminary binding assays of the biotin-coated water-soluble QD's to NeutrAvidin-functionalized substrates showed that specific capture of the QD's due to avidin-biotin interactions was achieved.
2. EXPERIMENTAL SECTION
All manipulations were carried out under dry nitrogen and air-sensitive solids were handled in an MBraun Labmaster 130 glovebox. TEG was purchased from Sigma-Aldrich (Milwaukee, Wis, USA). Triphenylphosphine, thioctic acid, 4-(N,N-dimethylamino)pyridine, N,N′-dicyclohexylcarbodiimide, and N-hydroxysuccinimide were purchased from Acros Organics (Morris Plains, NJ, USA). Sodium azide and biotin were purchased from Alfa Aesar (Ward Hill, Mass, USA). Methanesulfonyl chloride was purchased from GFS Chemicals (Powell, Ohio, USA). Sodium borohydride was purchased from Strem Chemicals (Newburyport, Mass, USA). All the other chemicals (including solvents) were purchased from Sigma-Aldrich and Acros Organics. Tetrahydrofuran (THF) was dried over CaH2 before use. Deuterated solvents employed in all NMR measurements were used as received. Chemical shifts for 1H NMR spectra are reported relative to tetramethylsilane (TMS) signal in the deuterated solvent (TMS, δ = 0.00 ppm). All J values are reported in Hertz. Column chromatography was performed on bench top, using silica gel (Bodman Industries, Aston, Pa, USA, 60 Å, 230–400 mesh).
1H NMR spectra were recorded on a Bruker SpectroSpin 400 MHz spectrometer. Electronic absorption spectra were recorded using an HP 8453 diode array spectrophotometer (Agilent technologies, Santa Clara, Calif, USA), while fluorescence spectra were collected using a Spex Fluorolog-3 spectrophotometer (Jobin Yvon Inc, Edison, NJ, USA). To account for the nonlinear (wavelength-dependent) quantum efficiency of the PMT detector, the fluorescence spectra were corrected using calibration curves accounting for the wavelength-dependence of the PMT's detection efficiency.
2.1. Ligand synthesis
The biotin-terminated ligands were synthesized stepwise using commercially available TEG. The choice of a short TEG segment to test this synthetic scheme was motivated by the well-defined chain length of the TEG molecules compared to longer PEG chains, which is expected to make separation using column chromatography easier. In the following section we detail the synthesis of each intermediate compound necessary for preparation of the final DHLA–TEG–biotin ligand. All the compounds were characterized by thin layer chromatography (TLC) and 1H NMR.
2.1.1. 1,11-Diazido-3,6,9-trioxaundecane
Diazide-functionalization of TEG (3): compound 3 was prepared following published procedures [13]. TEG (40.0 g, 206 mmol), tetrahydrofuran (THF) (350 mL), and methanesulfonyl chloride (53.0 g, 463 mmol) were mixed in a 1 L round bottom flask and cooled to 0°C. A solution of triethylamine (49.0 g, 484 mmol) in THF (50 mL) was added drop-wise over 30 minutes and the mixture was stirred at room temperature over 20 hours. The reaction was then diluted with water (200 mL) and NaHCO3 (12.5 g). Sodium azide (36.0 g, 554 mmol) was added and the biphasic reaction mixture was first heated to 65°C to distill off the THF, and then to 70°C for 14 hours. The reaction mixture was cooled to room temperature and extracted with ether (3 × 75 mL). The combined organic extracts were dried over MgSO4, filtered, and evaporated to give brownish oil. The product was purified by flash column chromatography (hexane : EtOAc 1 : 1) and the solvent evaporated to give 39 g (a yield of 78%) as a colorless oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 3.66–3.71 (m, 12H, –OCH2 CH2–), 3.40 (t, 4H, J = 5.1 Hz, –CH2N3).
2.1.2. 1-Amino-11-azido-3,6,9-trioxaundecane
Transformation to monoamine-terminated TEG (4) [13]: 1,11-Diazido-3,6,9-trioxaundecane (3) (33.0 g, 135 mmol) and 250 mL of 0.7 M H3PO4 were placed in a 1 L round bottom flask and cooled to 0°C using an ice bath while stirring. A solution of triphenylphosphine (PPh3) (29.0 g, 110 mmol) in ether (250 mL) was slowly added via cannula and the temperature of the reaction was maintained below 5°C. Once addition was complete, the reaction mixture was warmed to room temperature and stirred under nitrogen for an additional 16 hours. The biphasic solution was separated and the aqueous layer was washed with ether (3 × 100 mL). Potassium hydroxide (KOH) (30 g) was slowly added to the aqueous layer and cooled to 0°C overnight. The solution was filtered and the filtrate was basified with an additional 40 g of KOH and cooled to room temperature. The reaction mixture was extracted with CHCl3 (4 × 75 mL) and the combined organic extracts were dried over MgSO4, filtered, and the solvent was evaporated to give 18.5 g of an oil with a slight yellow color. The monoamine transformation reaction has a yield of 63%. 1H NMR (400 MHz, CDCl3): δ (ppm) 3.57 (m, 10H, –OCH2CH2–), 3.40 (t, 2H, J = 5.2 Hz, –CH2CH2NH2), 3.29 (t, 2H, J = 5.0 Hz, –CH2N3), 2.75 (t, 2H, J = 5.2 Hz, –CH2CH2NH2).
2.1.3. 5-([1,2]Dithiolan-3-yl)pentanoic acid-N-(3′,6′,9′-trioxaundecane-11′-azido)amide
Coupling of amino-terminated TEG to thioctic acid: TA-TEG-N3, compound 5: thioctic acid (11.1 g, 53.8 mmol), 1-amino-11-azido-3,6,9-trioxaundecane (4) (11.2 g, 51.3 mmol), 4-dimethylaminopyridine (1.26 g, 10.3 mmol), and CH2Cl2 (200 mL) were placed in a 500 mL round bottom flask, cooled to 0°C and stirred under N2 atmosphere. N,N′-Dicyclohexylcarbodiimide (DCC) (11.1 g, 53.8 mmol) was slowly added and reaction mixture was stirred at 0°C for 2 hours, then warmed to room temperature and stirred for an additional 16 hours. The reaction mixture was filtered over a plug of celite and rinsed with EtOAc. The filtrate was evaporated and the crude material was purified by flash column chromatography (CHCl3 : MeOH 97 : 3 as the eluent) to give 11.7 g (yield of 56%) of a yellow oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 6.15 (m, 1H, CO–NH), 3.50–3.62 (m, 11H, –OCH2CH2– and CH), 3.47 (t, 2H, J = 5.0 Hz, CO–NH–CH2CH2O–), 3.36 (t, 2H, J = 5.5 Hz, CO–NH–CH2–), 3.31 (t, 2H, J = 5.0 Hz, –CH2N3), 2.98–3.13 (m, 2H, CH2), 2.32–2.42 (m, 1H, CH), 2.10 (t, 2H, J = 7.4 Hz, –CH2–CO–NH–), 1.77–1.87 (m, 1H, CH), 1.50–1.68 (m, 4H, CH2), 1.30–1.45 (m, 2H, CH2).
2.1.4. 5-([1,2]Dithiolan-3-yl)pentanoic acid-N-(3′6′9′-trioxaundecane-11-amino)amide
Transformation to amine-terminated TA-TEG, compound 6: compound 5 (11.0 g, 27 mmol) was dissolved in THF (150 mL) in a 300 mL round bottom flask fitted with a condenser and nitrogen inlet. PPh3 (15.0 g, 81 mmol) was added and the reaction was heated to reflux for 20 hours. The reaction mixture was cooled to room temperature and diluted with 15 mL of water and stirred for an additional 20 hours. The solvent was evaporated and the crude product was purified by flash column chromatography. Unreacted PPh3 and triphenylphosphine oxide were removed with CHCl3 and the eluent was switched to CHCl3 : MeOH : Et3N (45 : 45 : 10) to collect the desired product. Removal of the solvent gave 9.0 g (yield of 88%) of gelatinous yellow oil. 1H NMR (400 MHz, methanol-d4): δ (ppm) 3.41–3.59 (m, 12H, –OCH2CH2–), 3.26 (t, 2H, J = 5.1 Hz, –CO–NH–CH2–), 3.22 (m, 1H, CH–), 2.97–3.12 (m, 2H, CH2), 2.71 (t, 2H, J = 5.3 Hz, –CH2CH2–NH2), 2.32–2.42 (m, 1H, CH), 2.12 (t, 2H, J = 7.4 Hz, –CH2–CO–NH–), 1.75–1.85 (m, 1H, CH), 1.47–1.69 (m, 4H, CH2), 1.29–1.44 (m, 2H, CH2).
2.1.5. TA-TEG-Biotin (7)
Biotinyl-N-hydroxysuccinimide was first prepared following the synthetic procedure previously reported [14–16]. Compound 6 (2.3 g, 6.0 mmol), biotinyl-N-hydroxysuccinimide (2.05 g, 6.0 mmol) were dissolved in DMF (50 mL) and stirred at room temperature under N2 atmosphere. Et3N (3.0 g, 30 mmol) was added dropwise via syringe and the reaction was stirred for 16 hours. The solvent was evaporated under reduced pressure and the yellow residue was purified by flash column chromatography (using CHCl3 : MeOH 95 : 5 as the eluent). The solvent was evaporated to give 3.1 g (a yield of 85%) of viscous yellow oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 6.79 (m, 1H, CO–NH), 6.59 (m, 1H, CO–NH), 6.49 (s, 1H, biotin CO–NH), 6.02 (s, 1H, biotin CO–NH), 4.41 (m, 1H, biotin CO–NH–CH), 4.22 (m, 1H, biotin CO–NH–CH), 3.52 (m, 9H, –OCH2CH2– and CH), 3.45 (m, 4H, CO–NH–CH2CH2O–), 3.30 (m, 4H, CO–NH–CH2), 2.96–3.10 (m, 3H, CH2 and CH–S), 2.76–2.83 (m, 1H, CH–S), 2.65 (d, 1H, J = 12.8 Hz, CH–S), 2.30–2.40 (m, 1H, CH), 2.11 (m, 4H, NH–CO–CH2), 1.75–1.85 (m, 1H, CH), 1.45–1.68 (m, 8H, CH2), 1.26–1.42 (m, 4H, CH2).
2.1.6. DHLA–TEG–biotin (8)
Compound 7 (2.0 g, 3.3 mmol) was dispersed in a mixture of ethanol (40 mL) and water (20 mL) while stirring in a 125 mL flask. NaBH4 (700 mg, 18.5 mmol) was slowly added and the solution was stirred at room temperature for 4 hours. The reaction mixture was diluted with water (200 ml) and extracted with CHCl3 (4 × 50 ml), dried over MgSO4, filtered, and the solvent was evaporated. The crude product was purified by flash column chromatography (CHCl3 : MeOH 95 : 5 as the eluent) to give 1.53 g (a yield of 77%) of colorless viscous oil. 1H NMR (400 MHz, CDCl3): δ (ppm) 6.96 (m, 1H, CO–NH), 6.69 (m, 1H, CO–NH), 6.63 (s, 1H, biotin CO–NH), 6.03 (s, 1H, biotin CO–NH), 4.37 (m, 1H, biotin CO–NH–CH), 4.18 (m, 1H, biotin CO–NH–CH), 3.50 (m, 8H, –OCH2CH2–), 3.42 (m, 4H, CO–NH–CH2CH2O–), 3.29 (m, 4H, CO–NH–CH2), 2.96–3.06 (m, 1H, CH–S), 2.70–2.84 (m, 2H, CH–S and CH–SH), 2.46–2.66 (m, 3H, CH–S and CH2–SH), 2.07 (m, 4H, NH–CO–CH2), 1.71–1.83 (m, 1H, CH), 1.35–1.67 (m, 13H, CH2), 1.28 (t, 1H, J = 8.0 Hz, CH2–SH), 1.23 (d, 1H, J = 7.6 Hz, CH–SH).
2.2. Quantum dot synthesis and cap exchange
The CdSe–ZnS core-shell QD's used were synthesized using high-temperature reaction of organometallic precursors in a mixture of TOP/TOPO and alkylamine, as described in the literature [17–20]. Cap exchange of the TOP/TOPO-capped QD's with the newly synthesized ligands to achieve water solubility (a mixture of 1 and 8, and a mixture of 2 and 8) was carried out following procedures described previously [5, 12, 21]. For cap exchange with a mixture of 2 and 8, ∼50–300 mg of TOP/TOPO-capped QD's were precipitated using EtOH and the supernatant was discarded. To the precipitate ∼0.5 mL in total of pure or mixed ligands and ∼0.5 mL of EtOH were added. The mixture was then heated to 60 ∼ 80°C while stirring for a period of 6 to 12 hours. Once homogenized, the sample was then precipitated out with mixtures of hexane, EtOH, and CHCl3 (approximate ratio is 11 : 10 : 1, this ratio may vary from batch to batch); the precipitate was dispersed in water. The homogenized solution was further purified using 3 ∼ 4 cycles of concentration/dilution with an ultrafree centrifugal filtration device (Millipore, Mw cutoff ∼50,000 Da) to remove excess ligands and other materials from the solution. Cap exchange with a mixture of 1 and 8 was done similarly though some modifications are required for purification steps. [21] DMF (∼5 mL) was added to the reaction mixture after the solution was homogenized. The QD's were precipitated out by adding excess potassium tert-butoxide. The mixture was centrifuged and the supernatant was discarded. The precipitate was dispersed in water and the homogenized solution was further purified as described above.
2.3. Gel electrophoresis
Samples were separated in agarose gels as described previously [22]. Briefly, samples were mixed with 30% glycerol, loaded into 1.5% agarose gels buffered with 1X Tris borate EDTA (TBE) in TBE running buffer (pH ∼8.3) and run at 10 volts/cm for ∼1 hour at ambient temperature. The gel-shift bands were visualized using the QD photoluminescence collected on a Kodak 440 Digital Image Station (Rochester, NY, USA) equipped with a long-pass cutoff filter; samples were illuminated with UV light (365 nm excitation).
2.4. Surface binding assay
The binding capacity of NeutrAvidin-covered 96-well microtiter flat-bottom plates from Pierce Biotechnology is 60 picomoles of biotin per well. 100 μL aliquots of QD samples (with the desired cap mixture at ∼7 pM concentration) were added to the wells and let incubate overnight at room temperature. The fluorescence intensity was measured using a Tecan Safire Dual Monochromator Multifunction Microtiter Plate Reader (Tecan, Research Triangle Park, NC, USA), then plates were washed 3 times with 10 mM Na tetraborate buffer (pH 9) supplemented with 0.02% Tween and the fluorescence signal was measured again; 300 nm excitation line was used for all the samples. The remaining fluorescence intensities were calculated as approximate binding percentages. Those values were normalized by setting the highest binding percentage to 100%.
3. RESULTS AND DISCUSSION
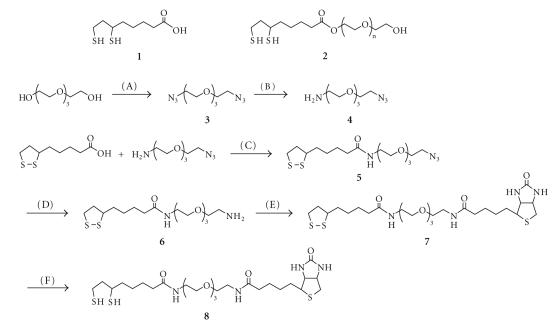
The chemical structures and synthetic schemes of a few representative ligands (namely DHLA, DHLA–PEG600, and DHLA–TEG–biotin) and reaction steps involved are summarized in Figure 1. TEG was first transformed into diazide-terminated-TEG (3) using a two-step reaction with methanesulfonyl chloride and sodium azide. Monosubstitution of one azide into an amine group was carried out in biphasic acidic solution to improve the efficiency of selective formation of the monsubstituted product (4) [13]. Thioctic acid (TA) and N3–TEG–NH2 (4) were coupled with N,N′-dicyclohexylcarbodiimide (DCC) to provide azide-terminated compound 5. The terminal azido group of 5 was reduced with PPh3 and H2O to obtain an amine-terminated TA-TEG ligand (Compound 6). Biotin-functionalized compound 7 was synthesized by coupling between 6 and Biotin N-hydroxysuccinimide ester. Finally, the terminal 1,2-dithiolane group in compound 7 was reduced with NaBH4 to obtain DHLA terminal group as a bidentate thiol anchoring unit.
Figure 1.
Chemical structures and synthetic routes of the surface ligands used in this study: (A) (i) MsCl, Et3N, THF, 0°C→20°C, 20 hours, (ii) NaN3, NaHCO3, H2O, 70°C, 14 hours; (B) PPh3, 0.7 M H3PO4, Et2O, <5°→room temperature, 16 hours; (C) DCC, DMAP, CH2Cl2, 0°C, 2 hour→room temperature, 20 hours; (D) PPh3, H2O, THF, reflux, 20 hour→room temperature, 20 hours; (E) Biotin N-hydroxysuccinimide ester, Et3N, DMF, room temperature, 16 hours; (F) NaBH4, EtOH, H2O, room temperature, 4 hours.
We verified quality of the new compounds by collecting 1H-NMR spectra throughout the various steps employed. The 1H-NMR spectra show that TEG-modified compounds have large peaks around ∼3.6 ppm, which are ascribed to CH2 groups of TEG chains. After coupling between 6 and biotin N-hydroxysuccinimide ester, new distinct multiplet peaks (2.6 ∼ 2.9, 4.22, and 4.41 ppm) are measured in the NMR spectra, which were ascribed to biotin-ring protons. In addition, following reduction of the 1,2-dithiolane group with NaBH4, new doublet and triplet peaks appeared at 1.23 and 1.28 ppm, respectively. These two peaks were assigned as thiol protons of DHLA unit (open dithiol) [12]. The observed changes in each collected spectrum indicate that each reaction step produced the desired compound.
We have previously shown that QD's capped with DHLA–PEG600 or DHLA–PEG1000 ligands can be dispersed in buffers with pH ranging between 5 ∼ 12 [12]. However, cap exchange of TOP/TOPO with DHLA–TEG–biotin alone did not produce water-soluble QD's, a property attributable to the rather short TEG segment combined with the hydrophobic nature of the biotin group [12]. To achieve water solubility using the new DHLA–TEG–biotin ligands (compound 8), TOP/TOPO-capped QD's were instead dispersed (during cap exchange) with mixtures of ligand 8 and either DHLA (1) or DHLA–PEG600 (2). Rather low to modest DHLA–TEG–biotin ratios (5–25%) were used in the mixtures for cap exchange. In addition to promoting easy transfer into buffer solutions, this approach ensures that only controlled and low density of biotin groups are available on each QD. This should also allow control over the number of biological receptors coupled to a single QD and potentially biological activity of the QD-bioconjugates. In this particular study, we used a mixture of compound 1 and compound 8 (at 7 : 1 molar ratio), and a mixture of compound 2 and compound 8 (at 7:1 molar ratio) for the cap exchange.
In the first characterization experiment of the cap exchange, we monitored changes in the electrophoretic mobility of QD's (run on a 1.5% agarose gel) as a function of the capping mixture used. The gel image in Figure 2 shows a side-by-side comparison of the mobility shift of 510 nm emitting QD cap exchanged with either a mixture of DHLA : DHLA–TEG–biotin (7 : 1) (lane C) or a mixture of DHLA–PEG600 : DHLA–TEG–biotin (7 : 1) (lane D), together with control samples made of either QD's capped with DHLA (lanes A and E) or QD's capped with DHLA–PEG600 (lane B). The data clearly show that on the one hand QD's capped with only DHLA experienced the highest mobility shift (towards the positive electrode) in this series; this result is attributed to the presence of carboxyl groups on DHLA molecules, which can be deprotonated and negatively charged in basic buffer solutions. On the other hand, DHLA–PEG600-capped QD's showed no mobility shift under applied voltage (materials did not migrate from the loading well), indicating that these QD's are essentially neutral; water solubility for this sample is mainly promoted by hydrophilicity of the long PEG chains. In comparison, the gel shift of the QD with DHLA : DHLA–TEG–Biotin (7 : 1) was slightly lower than that of the DHLA-capped QD's, whereas QD's capped with DHLA–PEG600 : DHLA–TEG–Biotin (7 : 1) mixture showed a very small shift compared with DHLA–PEG600-capped QD's. For QD's capped with DHLA : DHLA–TEG–Biotin (7 : 1) mixture, partial occupation of the nanocrystal surface by DHLA–TEG–Biotin (compared with DHLA-capped QD's) has led to reduced number of charges per QD and thus small reduction in the gel mobility shift (lane C). The nonzero mobility shift (even though extremely small) measured for QD's capped with a mixture of DHLA–PEG600 : DHLA–TEG–Biotin may be due to a slight/partial charging of biotin or amide groups on the chain under applied voltage. Overall, the present gel electrophoresis data confirm that cap exchange of TOP/TOPO-capped QD's with a ligand mixture produced nanocrystals that have both types of capping ligands; furthermore, the overall relative proportions of each ligand used during cap exchange is preserved in the final QD samples.
Figure 2.
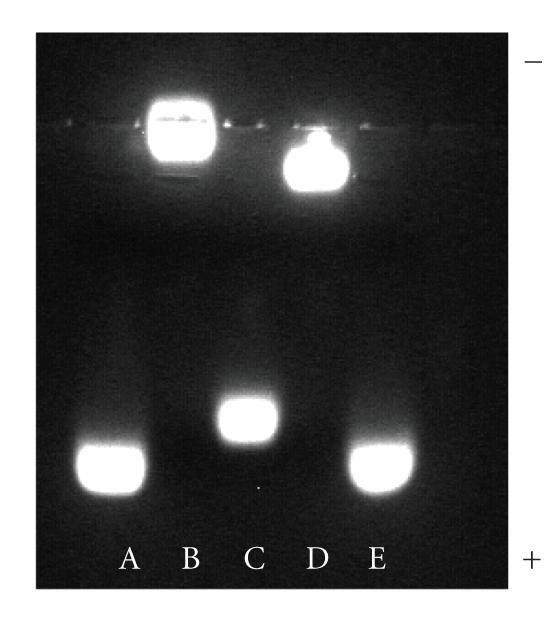
Gel shift of 510-nm emitting CdSe–ZnS QD's coated with different ligands in 1.5% agarose gel buffered with TBE buffer: (A,E) DHLA; (B) DHLA–PEG600; (C) DHLA : DHLA–TEG–Biotin (7 : 1); (D) DHLA–PEG600 : DHLA–TEG–Biotin (7 : 1).
Absorption and fluorescence spectra were measured for both the native TOP/TOPO-capped QD's in toluene and the new hydrophilic QD's capped with DHLA–TEG derivatives in H2O (data not shown). Absorption spectra measured before and after the cap exchange were essentially unchanged, though a few nm red shift of the lowest absorption maximum of the hydrophilic QD's was occasionally measured compared with that of QD's capped with TOP/TOPO ligands. The fluorescence spectra showed similar trends. These occasional small changes in the optical properties of QD's following transfer into aqueous solutions are commonly observed [1, 12, 23]. The fluorescence quantum yields of the water-dispersed QD's change from batch to batch. The quantum yields in aqueous solutions (after cap exchange and transfer) are ∼50% of the original values measured for TOP/TOPO-capped QD's in organic solutions. The overall quantum yield of the hydrophilic QD's thus varies anywhere between 10–40%.
QD's cap-exchanged with mixture containing the new DHLA–TEG–biotin ligands were also stable and aggregate-free over extended periods of time (months). Figure 3 shows solutions of QD's capped with a mixture of compound 2 and compound 8 (7 : 1) in H2O of which pH ranged from 5.5 to 9. The new water-soluble QD's were stable and well dispersed in the wide pH range including acidic conditions. This feature is quite different from what we have observed for solutions of QD's capped with either DHLA or mercaptoundecanoic acid (MUA). The DHLA- or MUA-capped QD solutions were well dispersed in aqueous basic media but showed aggregation and eventual precipitation at pH lower than 7 (data not shown), indicating that deprotonation of carboxyl groups is a crucial factor to solubilize those QD's in aqueous solutions. In contrast, appending a short PEG chain at the end of DHLA molecules extended the stability of QD capped with either 100% DHLA–PEG600 or a mixture of DHLA–PEG600 and Biotin-functionalized DHLA–TEG to acidic buffer solutions.
Figure 3.
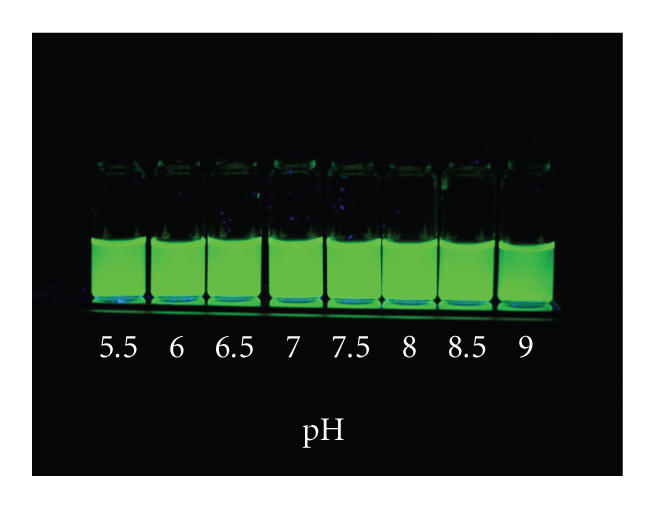
Luminescence image set of 540 nm emitting CdSe–ZnS QD with DHLA–PEG600 : DHLA–TEG–Biotin (4 : 1) at pH 5.5 ∼ 9 in phosphate buffer saline at various pH values at room temperature. Samples were excited with a handheld UV lamp at 365 nm.
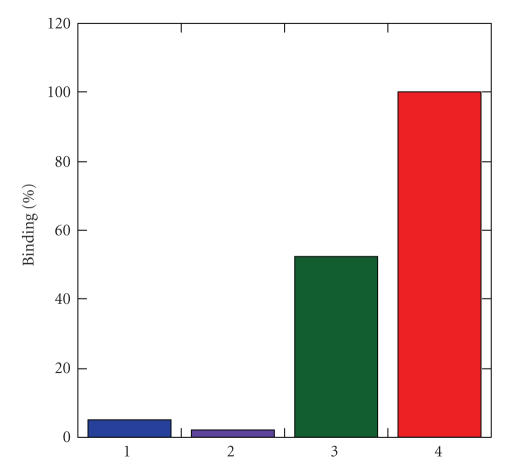
Once cap exchange with biotin-terminated DHLA–TEG and transfer into aqueous environment was successfully realized, targeted biological assays were carried out. The binding properties of QD's partially capped with biotin-terminated DHLA–TEG (8) were investigated for their ability to interact with NeutrAvidin-functionalized substrates [24]. NeutrAvidin, which is a deglycosylated form of avidin, was chosen due to its low nonspecific interactions (compared with avidin or streptavidin) while maintaining the strong binding affinity with biotin [24]. Following incubation of the NeutrAvidin-functionalized substrates with the QD samples, substrates were rinsed 3 times with borate buffer with Tween and the fluorescence signals were collected. Figure 4 shows the fluorescence intensities measured for 4 different QD solutions incubated with NeutrAvidin-coated plates: one capped with DHLA (1), one capped with DHLA–PEG600 (2), one capped with a mixture of 1 and DHLA–TEG–Biotin (8) (at 4 : 1 ratio), and one capped with a mixture of 2 and 8 (at 4 : 1 ratio). The data clearly show that only substrates incubated with QD's that were capped with a mixture of biotin-terminated DHLA–TEG produced a large signal-to-background fluorescence signal. In comparison, the substrates incubated with DHLA- or DHLA–PEG600-capped QD's produced only background contribution to the signal. This result indicates that efficient and specific interactions between the biotin groups present on the QD surface and NeutrAvidin on the substrate drive the surface capture of the nanocrystals. It is important to note that the QD's capped with DHLA–TEG–biotin alone were, however, not dispersible in buffer solutions, a result we attribute to the poor water compatibility of biotin by itself and the short TEG. We are exploring the use of longer chain PEG segments for insertion between the dithiol group and biotin. This should make dispersion in buffer solution achievable even at high biotin fractions and promote strong interactions with NeutrAvidin. Those findings will be discussed in future reports.
Figure 4.
Surface binding plate assay of QD's with different surface ligands: (1) DHLA, (2) DHLA–PEG600, (3) DHLA : DHLA–TEG–Biotin (at 4 : 1 ratio), and (4) DHLA–PEG600 : DHLA–TEG–Biotin (at 4 : 1 ratio). 540 nm emitting QD's were used.
4. CONCLUSION
We have demonstrated simple and efficient synthetic procedures to prepare new biotin-functionalized ligands based on the DHLA motif and employing short TEG segment. The present synthetic route provided high quality and stable compounds, which were further employed to make biotin-functionalized luminescent QD's, using easy-to-implement cap exchange procedure. The new biotin-appended ligand mixed with either DHLA or DHLA–PEG600 effectively cap exchanged with the native TOP/TOPO and provided QD's that are water-soluble over extended periods of time and biologically active. QD's cap-exchanged with a mixture of DHLA–PEG600 (neutral) and DHLA–TEG–biotin showed specific interactions with NeutrAvidin in surface binding assays. The present synthetic methodologies of hydrophilic surface ligands and cap-exchange reactions promise access to a variety of biological entities. Further studies of these surface-functionalized QD's for coupling with a variety of bioreceptors and biological assays are in progress.
ACKNOWLEDGMENTS
The authors acknowledge NRL, Office of Naval Research (ONR), and the Army Research Office for financial support.
References
- 1.Bruchez M, Jr, Moronne M, Gin P, Weiss S, Alivisatos AP. Semiconductor nanocrystals as fluorescent biological labels. Science. 1998;281(5385):2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 2.Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotechnology. 2004;22(8):969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 3.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298(5599):1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 4.Chan WCW, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281(5385):2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 5.Mattoussi H, Mauro JM, Goldman ER, et al. Self-assembly of CdSe-ZnS quantum dot bioconjugates using an engineered recombinant protein. Journal of the American Chemical Society. 2000;122(49):12142–12150. [Google Scholar]
- 6.Wang YA, Li JJ, Chen H, Peng X. Stabilization of inorganic nanocrystals by organic dendrons. Journal of the American Chemical Society. 2002;124(10):2293–2298. doi: 10.1021/ja016711u. [DOI] [PubMed] [Google Scholar]
- 7.Medintz IL, Clapp AR, Mattoussi H, Goldman ER, Fisher BR, Mauro JM. Self-assembled nanoscale biosensors based on quantum dot FRET donors. Nature Materials. 2003;2(9):630–638. doi: 10.1038/nmat961. [DOI] [PubMed] [Google Scholar]
- 8.Clapp AR, Medintz IL, Mauro JM, Fisher BR, Bawendi MG, Mattoussi H. Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. Journal of the American Chemical Society. 2004;126(1):301–310. doi: 10.1021/ja037088b. [DOI] [PubMed] [Google Scholar]
- 9.Clapp AR, Medintz IL, Mattoussi H. Förster resonance energy transfer investigations using quantum-dot fluorophores. ChemPhysChem. 2006;7(1):47–57. doi: 10.1002/cphc.200500217. [DOI] [PubMed] [Google Scholar]
- 10.Patolsky F, Gill R, Weizmann Y, Mokari T, Banin U, Wiliner I. Lighting-up the dynamics of telomerization and DNA replication by CdSe-ZnS quantum dots. Journal of the American Chemical Society. 2003;125(46):13918–13919. doi: 10.1021/ja035848c. [DOI] [PubMed] [Google Scholar]
- 11.Delehanty JB, Medintz IL, Pons T, Brunel FM, Dawson PE, Mattoussi H. Self-assembled quantum dot-peptide bioconjugates for selective intracellular delivery. Bioconjugate Chemistry. 2006;17(4):920–927. doi: 10.1021/bc060044i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uyeda HT, Medintz IL, Jaiswal JK, Simon SM, Mattoussi H. Synthesis of compact multidentate ligands to prepare stable hydrophilic quantum dot fluorophores. Journal of the American Chemical Society. 2005;127(11):3870–3878. doi: 10.1021/ja044031w. [DOI] [PubMed] [Google Scholar]
- 13.Schwabacher AW, Lane JW, Schiesher MW, Leigh KM, Johnson CW. Desymmetrization reactions: efficient preparation of unsymmetrically substituted linker molecules. The Journal of Organic Chemistry. 1998;63(5):1727–1729. [Google Scholar]
- 14.Um SH, Lee GS, Lee Y-J, Koo K-K, Lee C, Yoon KB. Self-assembly of avidin and D-biotin-tethering zeolite microcrystals into fibrous aggregates. Langmuir. 2002;18(11):4455–4459. [Google Scholar]
- 15.Charvet N, Reiss P, Roget A, et al. Biotinylated CdSe/ZnSe nanocrystals for specific fluorescent labeling. Journal of Materials Chemistry. 2004;14(17):2638–2642. [Google Scholar]
- 16.Tong D, Yao J, Li H, Han S. Synthesis and characterization of thermo- and pH-sensitive block copolymers bearing a biotin group at the poly(ethylene oxide) chain end. Journal of Applied Polymer Science. 2006;102(4):3552–3558. [Google Scholar]
- 17.Murray CB, Norris DJ, Bawendi MG. Synthesis and characterization of nearly monodisperse CdE (E = S, Se, Te) semiconductor nanocrystallites. Journal of the American Chemical Society. 1993;115(19):8706–8715. [Google Scholar]
- 18.Hines MA, Guyot-Sionnest P. Synthesis and characterization of strongly luminescing ZnS-capped CdSe nanocrystals. Journal of Physical Chemistry. 1996;100(2):468–471. [Google Scholar]
- 19.Dabbousi BO, Rodriguez-Viejo J, Mikulec FV, et al. (CdSe)ZnS core-shell quantum dots: synthesis and characterization of a size series of highly luminescent nanocrystallites. Journal of Physical Chemistry B. 1997;101(46):9463–9475. [Google Scholar]
- 20.Peng ZA, Peng X. Formation of high-quality CdTe, CdSe, and CdS nanocrystals using CdO as precursor. Journal of the American Chemical Society. 2001;123(1):183–184. doi: 10.1021/ja003633m. [DOI] [PubMed] [Google Scholar]
- 21.Clapp AR, Goldman ER, Mattoussi H. Capping of CdSe-ZnS quantum dots with DHLA and subsequent conjugation with proteins. Nature Protocols. 2006;1(3):1258–1266. doi: 10.1038/nprot.2006.184. [DOI] [PubMed] [Google Scholar]
- 22.Medintz IL, Berti L, Pons T, et al. A reactive peptidic linker for self-assembling hybrid quantum dot-DNA bioconjugates. Nano Letters. 2007;7(6):1741–1748. doi: 10.1021/nl070782v. [DOI] [PubMed] [Google Scholar]
- 23.Pinaud F. (fpinaud@chem.ucla.edu), King D, Moore H-P, Weiss S. Bioactivation and cell targeting of semiconductor CdSe/ZnS nanocrystals with phytochelatin-related peptides. Journal of the American Chemical Society. 2004;126(19):6115–6123. doi: 10.1021/ja031691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermanson GT. Bioconjugate Techniques. San Diego, Calif, USA: Academic Press; 1996. [Google Scholar]