Abstract
Estrogens play a physiologic role during prostate development with regard to programming stromal cells and directing early morphogenic events. However, if estrogenic exposures are abnormally high during the critical developmental period, permanent alterations in prostate branching morphogenesis and cellular differentiation will result, a process referred to as neonatal imprinting or developmental estrogenization. These perturbations are associated with an increased incidence of prostatic lesions with aging, which include hyperplasia, inflammation, and dysplasia. To understand how early estrogenic exposures can permanently alter the prostate and predispose it to neoplasia, we examined the effects of estrogens on prostatic steroid receptors and key developmental genes. Transient and permanent alterations in prostatic AR, ERα, ERβ, and RARs are observed. We propose that estrogen-induced alterations in these critical transcription factors play a fundamental role in initiating prostatic growth and differentiation defects by shifting the prostate from an androgen-dominated gland to one whose development is regulated by estrogens and retinoids. This in turn leads to specific disruptions in the expression patterns of key prostatic developmental genes that normally dictate morphogenesis and differentiation. Specifically, we find transient reductions in Nkx3.1 and permanent reductions in Hoxb-13, which lead to differentiation defects particularly within the ventral lobe. Prolonged developmental expression of Bmp-4 contributes to hypomorphic growth throughout the prostatic complex. Reduced expression of Fgf 10 and Shh and their cognate receptors in the dorsolateral lobes leads to branching defects in those specific regions in response to neonatal estrogens. We hypothesize that these molecular changes initiated early in life predispose the prostate to the neoplastic state upon aging.
Keywords: prostate, estradiol, estrogen, steroid receptors, Hoxb-13, Nkx3.1, sonic hedgehog, fibroblast growth factor-10
INTRODUCTION
Prostate gland development is under hormonal regulation, primarily mediated by androgens that dictate its growth and differentiation.1,2 In addition, the developing prostate is sensitive to other protein and steroid hormones including estrogens.2 In humans, under the continuous influence of maternal and placental estrogens during fetal development, extensive squamous metaplasia arises within the prostatic epithelium, which sloughs at birth after maternal estrogen levels fall.3 These effects are mediated through estrogen receptors α and β (ERα and ERβ), which are expressed at high levels in the developing prostate stroma and epithelium, respectively.4 The natural role, if any, for estrogens during human prostatic development is unknown; however, it is possible that estrogens normally program the prostate gland in a manner that affects their growth and function throughout life.
Studies with various rodent models support a physiologic role for estrogens in prostate development, particularly with regard to programming of prostatic stromal cells.5,6 In contrast to humans, in whom prostate development is completed in utero, the rodent prostate gland is rudimentary at birth and undergoes extensive branching morphogenesis followed by functional differentiation during the first 15 days of life.7 Thus the neonatal rodent prostate has emerged as a useful model for evaluating the role of endogenous and exogenous estrogens during prostate development. With this rodent model, it has been shown that elevated perinatal levels of endogenous estrogens (maternal or excess local production) or exogenous estrogens (diethylstilbesterol or, potentially, environmental estrogens) lead to permanent disturbances in prostate growth and predispose to precancerous lesions, a process referred to as developmental estrogenization or estrogen imprinting.8 The specific model used in our laboratory to study developmental estrogenization of the prostate gland is the Sprague–Dawley rat given injections of 25 μg estradiol on neonatal days 1, 3, and 5. While considered a “high-dose” exposure, most of this estradiol is bound to the high levels of circulating α-fetoprotein in the neonate, and free circulating estradiol levels are estimated at 1/100th of this amount. This neonatal exposure to estradiol results in a permanent reduction in prostatic growth and activational response to androgens during adulthood, an effect mediated in part through a permanent reduction in androgen receptor (AR) expression.9–12 Upon aging, prostatic hyperplasia and dysplasia are prominent in neonatally estrogenized rats, and PIN-3 (prostatic intraepithelial neoplasia) lesions are observed when these animals are given exogenous testosterone.13,14 Although all prostate lobes are permanently imprinted by elevated estrogens, there is some lobe specificity to the response. Thus branching deficiencies are notable in the dorsal and lateral lobes while the ventral prostate, although hypomorphic, shows normal branching patterns.15 Structural and functional epithelial cytodifferentiation during development is perturbed or, for some end-points, permanently blocked by neonatal estrogens as determined by markers for basal and luminal cytokeratins and secretory proteins (PBP, DLP protein, urokinase, 26 kD protease) 10,16,17 and the greatest differentiation defects are consistently observed in the ventral prostate lobe. The molecular basis for neonatal estrogenization of the prostate gland with lobe-specific responses will be the focus of this review article.
MECHANISM OF ACTION: STEROID RECEPTORS
Estrogen action in the rat prostate gland is mediated through stromal ERα18 and epithelial ERβ.19 Importantly, studies with ER knockout mice (αERKO and βERKO) showed that stromal cell ERα is the dominant ER mediating developmental estrogenization of the prostate.17 During the first 5–10 days of life, ERα is present in the mesenchymal cells of the proximal regions of the growing ducts. Following estrogenic exposure, there is a transient upregulation of this protein within periductal stromal cells along the length of the ducts, which allows for amplification of estrogenic action during this critical period.18 This, in turn, leads to a transient expression of progesterone receptor (PR) in these stromal cells that are normally negative for PR.20 In addition, prostatic retinoid receptors (RARs and RXRs) and intraprostatic retinoid levels are immediately and permanently elevated following neonatal estrogenic exposure, which allows for the amplification of retinoid signals during development and with aging.21,22 It is particularly significant that these increases in ER, PR, and RAR levels occur at the same time that AR is drastically downregulated.10,12 These changes are summarized in the schematic shown in FIGURE 1. Following a brief exposure to high levels of estrogen during the neonatal critical period, the temporal expression patterns as well as quantitative levels of several key steroid receptors (transcription factors), are drastically altered. Thus, the prostate is no longer under predominant androgen regulation, but is rather driven by estrogenic and retinoid signals through the ERs, PR, RARs, and RXRs. We propose that the net effect of these changes is that the programming and organizational signals that normally dictate and determine prostate development during discrete temporal windows are permanently and irretrievably altered.
FIGURE 1.

Schematic representation of steroid receptor expression in postnatal day 5–10 developing prostates from oil-treated control rats (A) and neonatally estrogenized rats (B). In the normal developing prostate (A), androgen receptor (AR) is the dominant steroid receptor in both epithelial and stromal cells. Under the influence of androgens, the stromal cells produce and secrete specific paracrine factors that dictate growth and differentiation of the gland. As epithelial cells differentiate, AR levels markedly increase and ERβ expression is induced. Other steroid receptors are expressed in a cell-specific manner and regulate cell-specific gene expression during critical developmental windows. Estrogen receptor α (ERα) is expressed at low levels in periductal stromal cells in the proximal region of the elongating ducts. RARβ is expressed in a subpopulation of basal epithelial cells, whereas RARα and γ are localized to periductal stromal cells along the ductal length. RXRα and RXRγ are expressed by basal cells, while RXRβ localizes to periductal stromal cells. Following a brief exposure to high levels of estrogens during the neonatal critical period, the prostatic steroid receptor profile is drastically altered (B). AR is absent in epithelial cells and is present at very low levels in stromal cells, thus dampening the androgen signaling pathway in the developing prostate. ERα is upregulated and expressed at high levels in periductal stromal cells along the length of the ducts, and progesterone receptor (PR) is induced in those same cells under the influence of estrogen. The number of cells expressing RARα and RARβ is markedly increased. Thus, estrogen exposure has switched the developing prostate from an androgen-dominated tissue to one that is regulated by estrogen, progesterone, and retinoids.
DEVELOPMENTAL GENES
Continuous branching morphogenesis of glandular structures is dictated by time-specific and region-specific expression of master regulatory genes.23 Although common morphogenetic paradigms exist for all branched structures studied to date, the critical difference is that spatial and temporal combinations of these genes give rise to unique structures. While the “prostatic code” is not well defined at the present time, recent activity in this field has led to an early map.17,24–31,42 We are currently interested in determining whether neonatal exposure to estrogens can alter prostatic development through changes in the expression of these key developmental genes.
HOMEOBOX GENES: Nkx 3.1 AND Hoxb 13
Several specific homeobox genes have been identified within the developing prostate tissue and are thought to account for prostate determination and to play a role in morphogenesis and cellular differentiation. These include members of the Hox gene family,32 and the NK gene family.31 Importantly, a growing body of evidence supports a role for estrogens, progesterone, and retinoids in regulating homeobox genes in a variety of structures including the lungs and uterus.33–35 Furthermore, fetal exposure to diethylstilbestrol (DES) resulted in strong downregulation of uterine Hoxa-10 expression.33 Thus we explored the effects of neonatal estradiol exposure on prostatic Nkx3.1 and Hoxb 13 genes to determine whether these genes may be downstream of estrogen action in the prostate.
Nkx3.1 is a member of the NK family of homeobox transcription factors whose expression in the murine male reproductive tract is restricted to UGS-derived prostate and bulbourethral gland epithelium.25,36 Importantly, this gene is expressed in the fetal mouse UGS epithelium at the sites of bud formation, suggesting a role for Nkx3.1 in prostate determination.24,25 Continued expression of this gene is believed to be important for epithelial cell differentiation. Using qRT-PCR, we measured the ontogeny of Nkx3.1 expression in the rat ventral prostate and found peak expression at postnatal day 10 (FIG. 2A) when the epithelial cells undergo cytodifferentiation.10 Although continued expression in the adult prostate is believed to be important in maintaining differentiation, the relative levels are small compared to the expression on postnatal days 5–15. To determine the effects of early estrogen exposure on Nkx3.1 expression, we performed in situ hybridization17 and real-time RT-PCR42 on developing and adult prostates of control and estrogenized rats. Neonatal estrogens immediately and strongly suppressed Nkx3.1 expression in the developing prostate (FIG. 2B). However, this downregulation of Nkx3.1 was transient, and similar expression levels were observed between controls and estrogenized prostates by day 30 and thereafter.17 Nonetheless, it is important to stress that the developmental peak in Nkx3.1 expression was abolished by neonatal estrogens. We propose that a transient disruption in Nkx3.1 expression at a critical time when epithelial cells normally differentiate into basal and luminal layers10 may contribute to the perturbed epithelial differentiation seen throughout the prostate lobes of the estrogen-exposed animals.
FIGURE 2.
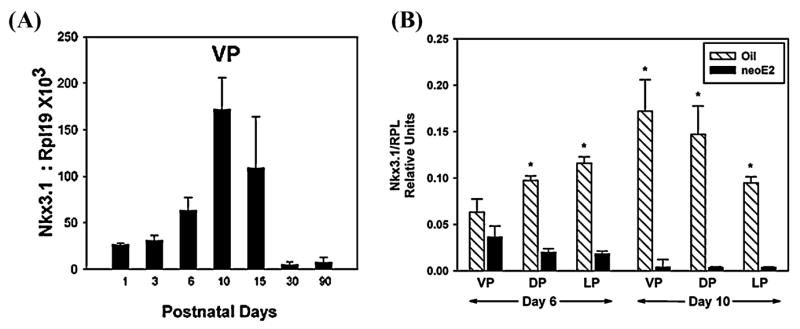
Real-time qRT-PCR of Nkx3.1expression in the developing rat prostate lobes. Samples were amplified in duplex using a SYBR-green assay and data were normalized to the ribosomal protein, RPL19 mRNA co-amplified for each tissue. A: Ventral lobe (VP) expression levels of days 1, 3, 6, 10, 15, 30, and 90 of life show a peak of expression at postnatal day 10 with low steady-state expression observed in adulthood. B: Postnatal days 6 and 10 expression levels in the VP, dorsal (DP), and lateral (LP) prostate lobes of rats exposed to oil (hatched bars) or 25 μg estradiol benzoate on days 1, 3, and 5 of life (solid bars). N = 4–10 assays/group. *P < 0.01 compared to estradiol-treated rats at same time point. (from Pu et al. Reprinted by permission.)
Prostate specification has been shown to be regulated in part through the most posterior genes of the Hox cluster, the Hox13 genes.32 Of the three Hox13 genes expressed in the prostate (Hoxa-13, Hoxb-13, and Hoxd-13), Hoxb-13 is unique in that it is expressed solely by epithelial cells and its increased expression postnatally correlates with epithelial differentiation.17,30,37 In the rat prostate, we quantitated Hoxb-13 mRNA by qRT-PCR and in situ hybridization throughout development and into adulthood and found an increasing anterior-to-posterior expression gradient with low levels in the dorsal lobe, moderate levels in the lateral lobe, and high expression throughout the epithelium of the ventral prostate lobes.15 This suggests an increasingly important role for Hoxb-13 in the rat ventral lobe, which is similar to findings reported for the mouse prostate, where expression was exclusively confined to the ventral prostate and targeted deletion of Hoxb-13 resulted in ventral lobe-specific defects. When we transfected Hoxb-13 into cultured rat prostate basal cells, they differentiated into a luminal phenotype suggesting a specific role for Hoxb-13 in epithelial cell differentiation.38 Following neonatal exposure to estrogen, Hoxb-13 expression was immediately and permanently suppressed in all prostate lobes with the most significant reduction (80%) observed in the ventral prostate gland.17 Since this loss directly correlated with the loss of epithelial cell differentiation and secretory gene expression, we propose that estrogen-initiated loss of prostatic Hoxb-13 may play a critical role in mediating the differentiation defects observed in the developmentally estrogenized prostate gland. Furthermore, since Hoxb13 was most severely reduced in the ventral lobe, where it plays a more prominent role as compared to the more anterior prostate lobes (unpublished data), its suppression may lead to the marked differentiation defects preferentially seen in the ventral prostate.
SECRETED SIGNALING MOLECULES
In addition to developmental regulation by homeobox genes, branching morphogenesis occurs as a complex interplay between epithelial and mesenchymal cells. While many secreted epithelial–mesenchymal signals have been characterized, a small number of signaling molecules have been found to be critical during embryogenesis.23 In particular, combinations of Hedgehogs, Wnts, Bmps, and Fgfs to a large extent control soft tissue development. These positive and negative regulatory molecules are spatially and temporally regulated and communicate signals between cells via their cognate receptors. We have recently examined the ontogeny and localization of Bmp-4, sonic hedgehog (Shh), and Fgf -10 in the normal developing rat prostate lobes and those exposed neonatally to estradiol to determine whether alterations in their signaling pathways are involved in mediating specific aspects of the estrogenized phenotype.
Bmp-4 as a Negative Regulator of Prostate Ductal Outgrowth
Bone morphogenetic proteins (Bmps) are members of the Tgfβ superfamily and, in general, act as inhibitors of proliferation during development.39 Bmps initiate cell signaling by activating Type I and Type II transmembrane receptors with intracellular pathways involving Smads 1, 3, and 5. In the mouse prostate, Bmp4 mRNA is localized to the mesenchyme and levels decline postnatally.46 While targeted disruption of Bmp4 is embryonically lethal, heterozygotes possessed an increased number of branching tips in the murine ventral prostate implicating Bmp4 as a negative regulator of prostate growth.46 We localized and quantitated Bmp4 mRNA expression in the prostate lobes of the developing rat (FIG. 3A) and found that it was initially intense and broad throughout the prostatic mesenchyme (seen in dorsal and lateral lobes at day 1) and subsequently condensed to cells adjacent to the elongating ducts as morphogenesis progressed (seen in the more developed ventral lobe at day 1). Expression levels of Bmp4 mRNA rapidly declined in control prostate lobes within 6 days following birth (FIG. 3B, hatched bars). These findings support the concept that broad Bmp4 expression in the mesenchyme suppresses ductal outgrowth during early developmental stages and that rapid loss postnatally is permissive for ductal outgrowth and branching morphogenesis. Following neonatal estrogen exposure, Bmp4 expression did not decline, but rather remained at relatively high perinatal levels until after day 15 (FIG. 3B, solid bars), which may contribute to prolonged growth suppression in the prostate lobes. To directly test whether elevated Bmp4 contributes to growth inhibition following estrogen exposure, we cultured newborn ventral lobes (VPs) as previously described in 10−8 M testosterone (T), T + 1.0 μg/mL noggin (a specific Bmp4 antagonist), T + 20 μM estradiol (E) or T + E + noggin. Rudimentary prostates at day 0 grew to fully branched lobes by day 8 in the presence of T and this was further enhanced by the addition of noggin (FIG. 4). Culture in T + E retarded distal prostate outgrowth (P < 0.05) and addition of noggin partially reversed (by ~40%) this estrogen-induced growth inhibition (FIG. 3C). Thus we propose that the continued postnatal expression of Bmp4 in estrogen-exposed rats prolongs the growth-inhibitory actions of this secreted factor, which contributes to the hypomorphic prostate gland phenotype.
FIGURE 3.
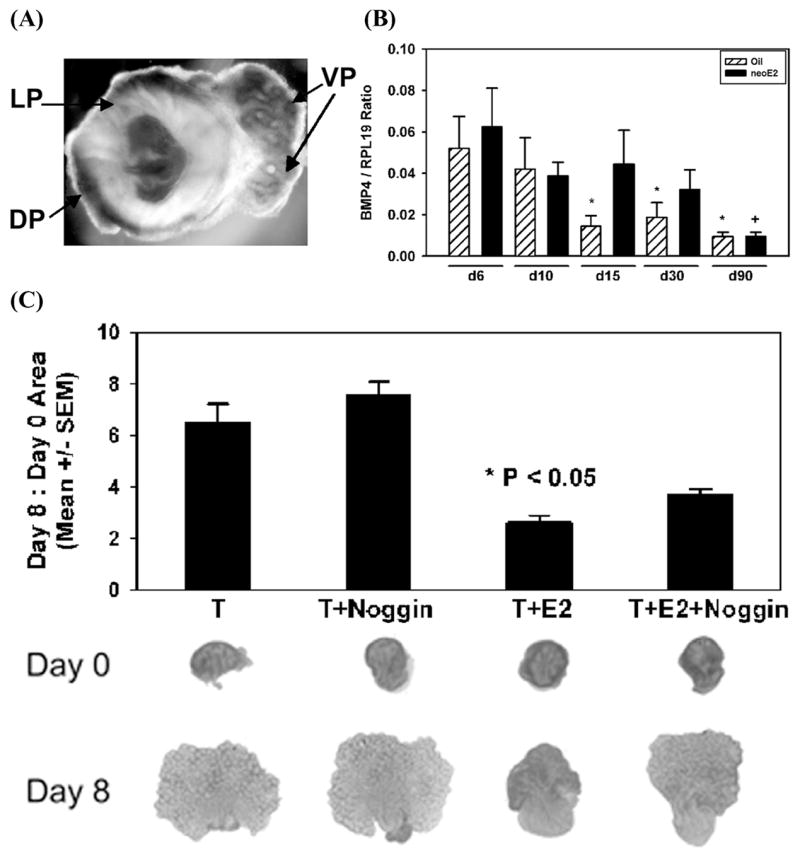
Expression and role of Bmp4 in the developing rat prostate. A: Whole-mount in situ hybridization of Bmp4 mRNA expression in a day 1 prostate complex shows broad mesenchymal Bmp4 expression in dorsal (DP) and lateral (LP) lobes at the early budding stage and signal condensation in periductal mesenchyme as ducts elongate and branch in the more advanced ventral (VP) prostate. B: VP Bmp4 mRNA levels over time as measured by real time qRT-PCR. Expression declined in control rats (hatched bar) as morphogenesis proceeds with significant loss by day 15 (*P < 0.05 vs. day 6 oil-treated tissue). Following neonatal estradiol exposure (solid bars), Bmp4 expression remained at high perinatal levels until after day 30 (+ P < 0.01 vs. day 6 estrogen-treated tissue). Bars represent mean ± SEM of 4–6 samples. C: VP organ culture for 8 days in the presence of 10−8 M testosterone (T), T + noggin (a Bmp4 anatgonist), T plus 20 μg estradiol (T + E) and T + E + noggin. Contralateral lobes were used for the T-only group and the T + E groups. A bar graph shows the area on day 8 normalized to day 0 (bars represent mean ± SEM for 6 experiments), while representative photos from each group at start of culture (day 0) and after 8 days are shown below. *P < 0.05 vs. T + E + noggin.
FIGURE 4.
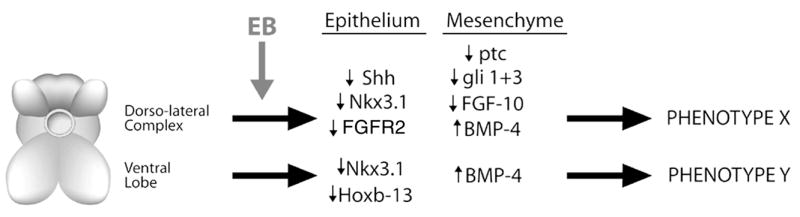
A schematic representation of the lobe-specific, estrogen-induced alterations in critical morphoregulatory genes in the rodent prostate. Brief neonatal exposure to high-dose estradiol results in alterations in expression, of key developmental genes in a lobe-specific manner that produces lobe-specific phenotypes. In the dorsoalateral lobes, estrogens suppress Shh signaling and Fgf 10 signaling, transiently reduce Nkx3.1 expression, and increase Bmp-4 levels. This results in phenotype X, which consists of branching deficiencies, hypomorphic growth, and mild epithelial differentiation defects. In the ventral lobe, estrogens permanently suppress Hoxb-13, transiently suppress Nkx3.1, and increase Bmp-4 expression postnatally. This leads to phenotype Y, which consists of severe differentiation defects and hypomorphic growth. In total, both common and unique phenotypes are proposed to result from differential regulation of key morphoregulatory genes by early estrogenic exposures.
Sonic Hedgehog (Shh) and Fibroblast Growth Factor 10 (Fgf 10) as Regulators of Branching Morphogenesis
Shh is a secreted glycoprotein produced by epithelial cells at mesenchymal interfaces in developing tissues, where it plays a critical role in growth and branching morphogenesis.40 Importantly, Shh has been shown to regulate many other developmental genes including Hox genes as well as Nkx3.1. The murine UGS and prostate epithelial cells produce Shh as early as fetal day 17 and the levels decline with development.28 Secreted Shh protein activates target cells through a membrane receptor patched (ptc) localized in mesenchymal cells. Following a cascade of molecular signals, this ultimately results in increased levels of gli transcription factors.41 We recently characterized the Shh expression patterns in the developing rat prostate lobes and localized Shh to the distal tip epithelial cells of outgrowing ducts while ptc and glis were expressed by periductal mesenchymal cells in the distal aspects of the gland.47 Between days 1–15 of postnatal life, a marked decrease was observed in Shh expression with the greatest decrease occurring between days 1–6. Through cross talk with other secreted morphogens including Bmp4 and Fgf 10, a specific role in ductal outgrowth was determined. Importantly, neonatal exposure to estradiol resulted in a significant reduction in Shh, ptc, gli1, and gli3 expression in the dorsal and lateral lobes by neonatal day 1, which persisted through development. Interestingly, ventral prostate expression of Shh, ptc, and glis was unaffected by the estrogenic exposure. This differential effect on Shh expression by estrogens may play a role in the previously noted lobe-specific estradiol response where branching was abolished in the dorsolateral region, whereas ventral branching was minimally affected by neonatal estrogen exposure.
Developmental studies have also shown an essential role for Fg f 10 in stimulating prostatic budding, ductal outgrowth, and branching morphogenesis.31,43 Fgf 10 is secreted by prostate mesenchymal cells and interacts with a unique splice variant of the Fgf transmembrane receptor family, the Fgf R2iiib, which is expressed on prostatic epithelial cells,44 thus establishing a specific paracrine communication. We recently determined that Fgf 10 and Fgf R2iiib localize to the distal periductal mesenchymal cells and distal tip epithelial cells, respectively (distal signaling center), of elongating and branching ducts in the separate rat prostate lobes where they regulate the expression of multiple morphoregulatory genes including increased Shh, ptc, Bmp7, Hoxb13, and Nkx3.1 and decreased Bmp4 levels.45 Ventral and lateral lobe organ cultures and mesenchyme-free ductal cultures were used to demonstrate a direct role for Fg f 10/Fg f R2iiib in ductal elongation, branching, epithelial proliferation, and differentiation. On the basis of these findings, we propose that localized expression and feedback loops between several morphoregulatory factors in the developing prostate contribute to tightly regulated branching morphogenesis.45
Similar to Shh-ptc-gli, neonatal estrogen exposure downregulated Fgf 10 and Fg f R2iiib expression in the dorsolateral prostate specifically, whereas ventral lobe expression of these genes was unaffected.43 Furthermore, lateral prostate organ culture studies demonstrated that growth and branching inhibition as well as Fgf 10/Fg f R2iiib suppression are mediated directly at the prostatic level. Finally, exogenous Fgf 10 added to lateral lobe cultures in the presence of T + E fully rescued the growth and branching deficits due to estrogen exposure.45 Together with the Shh-ptc-gli experiments, our findings demonstrate that reductions in Fg f 10 signaling are a proximate cause of Shh-ptc-gli down-regulation, which together results in branching inhibition of the dorsolateral prostate following neonatal estrogen exposure.
CONCLUSIONS
In summary, we have shown that early exposure to high levels of estrogens initiates permanent structural and functional alterations in the prostate gland which last throughout life. We propose that this effect is initiated through upregulated levels of stromal ERα, which in turn results in altered steroid receptor expression throughout the developing gland. Rather than being an androgen-dominated process, prostatic development becomes regulated by alternate steroids including estrogens and retinoids. This, in turn, leads to disruptions in the coordinated expression of several critical developmental genes including the homeobox genes Hoxb13 and Nkx3.1 as well as secreted ligands Shh, Fg f -10, and Bmp4 (schematized in Fig. 4). Since a precise temporal expression pattern of these and other molecules is normally required for appropriate growth and differentiation of the prostatic epithelium and stroma, the estrogen-initiated disruption in this pattern would lead to permanent growth as well as branching and differentiation defects of the prostate gland. The ultimate consequences of this developmental estrogenization are a prostate predisposed to hyperplasia and dysplasia in adulthood and sensitized to more severe lesions, including cancer, as the animals age.
Acknowledgments
We gratefully acknowledge Oliver Putz for the graphic representation of our data. This work has been supported by NIH Grant DK 40890.
References
- 1.Lasnitzki I, Mizuno T. Antagonistic effects of cyproterone acetate and oestradiol on the development of the fetal rat prostate gland induced by androgens in organ culture. Prostate. 1980;1:147–156. doi: 10.1002/pros.2990010202. [DOI] [PubMed] [Google Scholar]
- 2.Price D. Normal development of the prostate and seminal vesicles of the rat with a study of experimental postnatal modifications. Am J Anat. 1936;60:79–127. [Google Scholar]
- 3.Zondek LH, Zondek T. Congenital malformations of the male accessory sex glands in the fetus and neonate. In: Spring-Mills E, Hafez ESE, editors. Male Accessory Sex Glands. Elsevier–North Holland; New York: 1980. pp. 17–37. [Google Scholar]
- 4.Adams JY, Leav I, Lau KM, et al. Expression of estrogen receptor beta in the fetal, neonatal, and prepubertal human prostate. Prostate. 2002;52:69–81. doi: 10.1002/pros.10103. [DOI] [PubMed] [Google Scholar]
- 5.Jarred RA, McPherson SJ, Bianco JJ, et al. Prostate phenotypes in estrogen-modulated transgenic mice. Trends Endocrinol Metab. 2002;13:163–168. doi: 10.1016/s1043-2760(02)00575-1. [DOI] [PubMed] [Google Scholar]
- 6.Tilley W, Horsfall D, MckCant E, Marshall V. Specific binding of oestradiol to guinea-pig prostate cytosol and nuclear fractions. J Steroid Biochem. 1985;22:705–711. doi: 10.1016/0022-4731(85)90275-4. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi N, Sugimura Y, Kawamura J, et al. Morphological and functional heterogeneity in the rat prostatic gland. Biol Reprod. 1991;45:308–321. doi: 10.1095/biolreprod45.2.308. [DOI] [PubMed] [Google Scholar]
- 8.Santti R, Newbold RR, Makela S, et al. Developmental estrogenization and prostatic neoplasia. Prostate. 1994;24:67–78. doi: 10.1002/pros.2990240204. [DOI] [PubMed] [Google Scholar]
- 9.Prins GS. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology. 1992;130:3703–3714. doi: 10.1210/endo.130.6.1597166. [DOI] [PubMed] [Google Scholar]
- 10.Prins GS, Birch L. The developmental pattern of androgen receptor expression in rat prostate lobes is altered after neonatal exposure to estrogen. Endocrinology. 1995;136:1303–1314. doi: 10.1210/endo.136.3.7867585. [DOI] [PubMed] [Google Scholar]
- 11.Prins GS, Woodham C, Lepinske M, Birch L. Effects of neonatal estrogen exposure on prostatic secretory genes and their correlation with androgen receptor expression in the separate prostate lobes of the adult rat. Endocrinology. 1993;132:2387–2398. doi: 10.1210/endo.132.6.8504743. [DOI] [PubMed] [Google Scholar]
- 12.Woodham C, Birch L, Prins GS. Neonatal estrogens down regulate prostatic androgen receptor levels through a proteosome-mediated protein degradation pathway. Endocrinology. 2003;144:4841–4850. doi: 10.1210/en.2003-0035. [DOI] [PubMed] [Google Scholar]
- 13.Prins GS. Developmental estrogenization of the prostate gland. In: Naz RK, editor. Prostate: Basic and Clinical Aspects. CRC Press; Boca Raton, FL: 1977. pp. 247–265. [Google Scholar]
- 14.Putz O, Prins GS. Prostate gland development and estrogenic imprinting. In: Burnstein KL, editor. Steroid Hormones and Cell Cycle Regulation. Kluwer; Boston, MA: 2002. pp. 73–89. [Google Scholar]
- 15.Pu Y, Huang L, Prins GS. Neonatal estrogen exposure alters epithelial differentiation in rat prostate through down regulation of Hoxb-13. J Androl. 2004;25:330–337. doi: 10.1002/j.1939-4640.2004.tb02796.x. [DOI] [PubMed] [Google Scholar]
- 16.Chang WY, Wilson MJ, Birch L, Prins GS. Neonatal estrogen stimulates proliferation of periductal fibroblasts and alters the extracellular matrix composition in the rat prostate. Endocrinology. 1999;140:405–415. doi: 10.1210/endo.140.1.6401. [DOI] [PubMed] [Google Scholar]
- 17.Prins GS, Birch L, Habermann H, et al. Influence of neonatal estrogens on rat prostate development. Reprod Fertil Dev. 2001;13:241–252. doi: 10.1071/rd00107. [DOI] [PubMed] [Google Scholar]
- 18.Prins GS, Birch L. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology. 1997;138:1801–1809. doi: 10.1210/endo.138.5.5106. [DOI] [PubMed] [Google Scholar]
- 19.Prins GS, Marmer M, Woodham C, et al. Estrogen receptor-β messenger ribonucleic acid ontogeny in the prostate of normal and neonatally estrogenized rats. Endocrinology. 1998;139:874–883. doi: 10.1210/endo.139.3.5827. [DOI] [PubMed] [Google Scholar]
- 20.Sabharwal V, Putz O, Prins GS. Neonatal estrogen exposure induces progesterone receptor expression in the developing prostate gland. Presented at the 95th Annual Meeting of the American Urologic Association; Atlanta, GA. 2003. p. 97. [Google Scholar]
- 21.Prins GS, Chang WY, Wang Y, van Breemen RB. Retinoic acid receptors and retinoids are up-regulated in the developing and adult rat prostate by neonatal estrogen exposure. Endocrinology. 2002;143:3628–3640. doi: 10.1210/en.2002-220184. [DOI] [PubMed] [Google Scholar]
- 22.Pu Y, Deng L, Davies PJP, Prins GS. Retinoic acid metabolizing enzymes, binding proteins, and RXRs are differentially expressed in the developing and adult rat prostate lobes and are altered by neonatal estrogens in a lobe-specific manner. Presented at the 85th Annual Meeting of the Endocrine Society; Philadelphia, PA. 2003. p. 530. [Google Scholar]
- 23.Hogan BLM. Morphogenesis. Cell. 1999;96:225–233. doi: 10.1016/s0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 24.Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, et al. Roles for Nkx3.1 in prostate development and cancer. Gen Devel. 1999;13:966–977. doi: 10.1101/gad.13.8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bieberich CJ, Fujita K, He WW, Jay G. Prostate-specific and androgen-dependent expression of a novel homeobox gene. J Biol Chem. 1996;271:31779–31782. doi: 10.1074/jbc.271.50.31779. [DOI] [PubMed] [Google Scholar]
- 26.Kopachik W, Hayward SW, Cunha GR. Expression of hepatocyte nuclear factor-3a in rat prostate, seminal vesicle, and bladder. Dev Dyn. 1998;211:131–140. doi: 10.1002/(SICI)1097-0177(199802)211:2<131::AID-AJA2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 27.Lamm ML, Catbagan WS, Laciak RJ, et al. Sonic hedgehog activates mesenchymal Gli1 expression during prostate ductal bud formation. Dev Biol. 2002;249:349–366. doi: 10.1006/dbio.2002.0774. [DOI] [PubMed] [Google Scholar]
- 28.Podlasek CA, Barnett DH, Clemens JQ, et al. Prostate development requires sonic hedgehog expressed by the urogenital sinus epithelium. Dev Biol. 1999;209:28–39. doi: 10.1006/dbio.1999.9229. [DOI] [PubMed] [Google Scholar]
- 29.Podlasek CA, Clemens JQ, Bushman W. Hoxa-13 gene mutation results in abnormal seminal vesicle and prostate development. J Urol. 1999;161:1655–1661. [PubMed] [Google Scholar]
- 30.Sreenath T, Orosz A, Fujita K, Bieberich CJ. Androgen-independent expression of hoxb-13 in the mouse prostate. Prostate. 1999;41:203–207. doi: 10.1002/(sici)1097-0045(19991101)41:3<203::aid-pros8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 31.Thomson AA, Cunha GR. Prostatic growth and development are regulated by FGF10. Development. 1999;126:3693–3701. doi: 10.1242/dev.126.16.3693. [DOI] [PubMed] [Google Scholar]
- 32.Warot X, Fromental-Ramain C, Fraulob V, et al. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997;124:4781–4791. doi: 10.1242/dev.124.23.4781. [DOI] [PubMed] [Google Scholar]
- 33.Ma L, Benson GV, Lim H, et al. Abdominal B(AbdB) hoxa genes: regulation in adult uterus by estrogen and progesterone and repression in mullerian duct by the synthetic estrogen diethylstilbestrol (DES) Dev Biol. 1998;197:141–154. doi: 10.1006/dbio.1998.8907. [DOI] [PubMed] [Google Scholar]
- 34.Marshall H, Morrison A, Studer M, et al. Retinoids and hox genes. FASEB. 1996;9:969–978. [PubMed] [Google Scholar]
- 35.Wood HB, Ward SJ, Morriss-Kay GM. Effects of all-trans-retinoic acid on skeletal pattern, 5’HoxD gene expression, and RARb2/b4 promoter activity in embryonic mouse limbs. Dev Gen. 1996;18:74–84. doi: 10.1002/(SICI)1520-6408(1996)19:1<74::AID-DVG8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 36.Schiavolino PJ, Abrams EW, Yang L, et al. Tissue-specific expression of murine Nkx3.1 in the male urogenital system. Dev Dyn. 1997;209:127–138. doi: 10.1002/(SICI)1097-0177(199705)209:1<127::AID-AJA12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 37.Economides KD, Capecchi MR. Hoxb13 is required for normal differentiation and secretory function of the ventral prostate. Development. 2003;130:2061–2069. doi: 10.1242/dev.00432. [DOI] [PubMed] [Google Scholar]
- 38.Huang L, Pu Y, Birch L, Prins GS. Posterior Hox gene expression and differential androgen regulation in the developing and adult rat prostate lobes. Endocrinology. 2006 doi: 10.1210/en.2006-1250. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogan BLM. Bone morphogenetic proteins in development. Curr Opin Gen & Dev. 1996;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- 40.Bellusci S, Furuta Y, Rush M, et al. Involvement of sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development. 1997;124:53–63. doi: 10.1242/dev.124.1.53. [DOI] [PubMed] [Google Scholar]
- 41.Walterhouse D, Yoon J, Iannaccone P. Developmental pathways: sonic hedgehog-patched-GLI. Environ Health Persp. 1999;107:167–171. doi: 10.1289/ehp.99107167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang L, Pu Y, Alam S, et al. Estrogenic regulation of signaling pathways and homeobox genes during rat prostate development. J Androl. 2004;25:330–337. doi: 10.1002/j.1939-4640.2004.tb02796.x. [DOI] [PubMed] [Google Scholar]
- 43.Donjacour AA, Thomson AA, Cunha G. FGF-10 plays an essential role in the growth of the fetal prostate. Dev Biol. 2003;261:39–54. doi: 10.1016/s0012-1606(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 44.Finch P, Cunha G, Rubin J, et al. Pattern of keratinocyte growth factor and keratinocyte growth factor receptor expression during mouse fetal development suggests a role in mediating morphogenetic mesenchymal-epithelial interactions. Dev Dynam. 1995;203:223–240. doi: 10.1002/aja.1002030210. [DOI] [PubMed] [Google Scholar]
- 45.Huang L, Pu Y, Alam S, et al. The role of Fgf10 signaling in branching morphogenesis and gene expression in the rat prostate gland: lobe-specific suppression by neonatal estrogens. Dev Biol. 2005;278:396–414. doi: 10.1016/j.ydbio.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 46.Podlasek J, Clemens JQ, Lee J, Bushman W. Bone morphogenetic protein-4 is a negative regulator of prostate ductal branching. J Urol. 1999;161:125. [Google Scholar]
- 47.Pu Y, Huang L, Prins GS. Sonic hedgehog-patched-gli signaling in the developing rat 44. Prostate gland: lobe-specific suppression by neonatal estrogens reduces ductal growth and branching. Dev Biol. 2004;273:257–275. doi: 10.1016/j.ydbio.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


