Abstract
Early developmental perturbations have been linked to adult-onset prostate pathology, including excessive exposure to estrogenic compounds; however, the molecular basis for this imprinting event is not known. An important and controversial health concern is whether low-dose exposures to hormonally active environmental estrogens, such as bisphenol A, can promote human diseases, including prostate cancer. Here, we show that transient developmental exposure of rats to low, environmentally relevant doses of bisphenol A or estradiol increases prostate gland susceptibility to adult-onset precancerous lesions and hormonal carcinogenesis. We found permanent alterations in the DNA methylation patterns of multiple cell signaling genes, suggesting an epigenetic basis for estrogen imprinting. For phosphodiesterase type 4 variant 4 (PDE4D4), an enzyme responsible for cyclic AMP breakdown, a specific methylation cluster was identified in the 5′-flanking CpG island that was gradually hypermethylated with aging in normal prostates, resulting in loss of gene expression. Early and prolonged hypomethylation at this site following neonatal estradiol or bisphenol A exposure resulted in continued, elevated PDE4D4 expression. Cell line studies confirmed that site-specific methylation is involved in transcriptional silencing of the PDE4D4 gene and showed hypomethylation of this gene in prostate cancer cells. Importantly, the PDE4D4 alterations in the estrogen-exposed prostates were distinguishable before histopathologic changes of the gland, making PDE4D4 a candidate molecular marker for prostate cancer risk assessment as a result of endocrine disruptors. In total, these findings indicate that low-dose exposures to ubiquitous environmental estrogens affect the prostate epigenome during development and, in so doing, promote prostate disease with aging.
Introduction
There are increasing human health and wildlife concerns about low-dose estrogenic exposures because hormonally active xenoestrogens are ubiquitous in the environment (1). Bisphenol A, initially synthesized as a synthetic estrogen (2), is widely used as a cross-linking chemical in the manufacture of polycarbonate plastics and epoxy resins. Although bisphenol A binds to classic estrogen receptors with reduced affinity relative to 17β-estradiol (3), it possesses equivalent activational capacity of the nonclassic membrane estrogen receptors (4). Importantly, bisphenol A leaches from food and beverage containers as well as dental sealants and is found in the serum of humans with higher concentrations in placental and fetal tissues (5). Thus, there is potential for this compound as a toxicant for developing human tissues, particularly the sensitive reproductive end organs (6, 7).
Prostate gland development, which occurs during fetal life in humans and the perinatal period in rodents, is exquisitely sensitive to estrogen imprinting. The in utero estrogen environment of African-American mothers has been suggested to affect the elevated prostate cancer risk of their offspring because they have higher estradiol levels during pregnancy when compared with their Caucasian counterparts (8, 9). In rodent models, brief perinatal exposure to pharmacologic doses of natural or synthetic estrogens permanently alters prostate growth and differentiation (10–12) and results in precancerous lesions and tumors with aging (13). However, although perinatal exposures to environmentally relevant doses of bisphenol A or estradiol have been shown to augment prostatic size (14), they have not, as yet, been shown to induce pathologic prostatic lesions. Thus, it remains unclear whether low-dose exposures to estradiol or environmental estrogens can influence prostate cancer risk.
Because early exposure to low-dose estrogen augments estrogen responsiveness of adult female reproductive organs (15, 16), we asked whether analogous circumstances exist in the prostate. This is particularly relevant because relative estradiol levels increase in the aging male, partly due to increased body fat content and aromatase activity, at a time when prostate cancer incidence increases (17). Furthermore, estrogens have been associated with increased prostate cancer risk in men (18), whereas, in the Noble rat model, prolonged adult exposure to conjoint estradiol and testosterone drives prostatic carcinogenesis (19). In this context, we established a carcinogenesis model that involved neonatal exposure to high- or low-dose estradiol or low-dose bisphenol A followed by adult exposure to elevated but nonpharmacologic testosterone plus estradiol in the Sprague-Dawley rat, a strain less sensitive to hormone-induced prostate carcinogenesis. Our goal was to determine if neonatal low-dose exposures to estradiol or bisphenol A might increase cancer susceptibility as a result of adult exposure to elevated estradiol. We herein present the first evidence that indeed low-dose as well as high-dose estrogenic exposures predispose to neoplastic prostatic lesions in the aging male.
We next sought to determine the molecular underpinnings by which developmental estrogenic exposures can imprint or transform the prostate long after the initial hormone exposure. One distinct possibility is through permanent epigenetic modifications of the genome by DNA methylation at CpG-rich regions (CpG islands), which can silence (hypermethylation) or activate (hypomethylation) gene transcription (20). Once established in somatic cells, CpG methylation patterns within the genome are stable and heritable through subsequent cell divisions, except during early embryonic development and tumorigenesis. Importantly, alterations in DNA methylation have been shown to contribute to both cancer initiation and promotion (20, 21). Furthermore, previous studies have revealed an association between aberrant CpG methylation of specific genes in the reproductive tract and neonatal exposures to phytoestrogens, diethylstilbestrol, and the environmental toxicants vinclozolin and methoxychlor (22–25). To determine whether neonatal estrogenic exposures imprint the prostate gland via this epigenetic modification, we did methylation-sensitive restriction fingerprinting (MSRF) followed by specific methylation analysis to identify and characterize candidate genes as methylation targets in prostate glands exposed to our two-hit model. We herein present evidence for altered methylation patterns of several candidate genes and characterize phosphodiesterase type 4 variant 4 (PDE4D4), the enzyme involved in cyclic AMP (cAMP) degradation, as an estrogen-imprinted gene directly associated with preneoplastic prostatic lesions.
Materials and Methods
Animal housing and treatments
All animal treatments were approved by the Animal Use Committee at the University of Illinois (Chicago, IL). Pregnant Sprague-Dawley rats (Zivic-Miller Laboratories, Pittsburgh, PA) were shipped on gestation day 12 and immediately transferred to strict housing conditions. Rooms were maintained at 21°C with 50% relative humidity and a 14-hour-light/10-hour-dark schedule. To avoid bisphenol A leaching from polycarbonate plastic, all rats were housed in new polysulfone solid-bottom cages with steel covers, and water was supplied from glass bottles. Animals were fed ad libitum a special soy-free, phytoestrogen-reduced diet (Zeigler Reduced Rodent Diet 2, Zeigler Brothers, Inc., Gardners, PA) with 12 ppm phytoestrogens as determined by high-pressure liquid chromatography. To avoid variability, a single feed lot was purchased for the entire study, packaged in 25-pound bags, autoclaved, and stored at −30°C to minimize growth of microbes that might contribute estrogenic by-products. Pregnant dams were monitored, and the day of birth was designated postnatal day 0. The pups were sexed by anogenital distance, and each litter was culled to 10 pups by removing or adding female pups as necessary.
The hormonal treatment regime, consisting of newborn rats briefly exposed to estrogens followed by prolonged adult exposure to elevated estradiol with appropriate controls, resulted in a total of eight animal groups. Newborn pups were assigned to one of four neonatal treatment groups with 20 to 30 pups per group: (a) controls given tocopherol-stripped corn oil vehicle alone (ICN Biomedicals, Inc., Aurora, OH), (b) high-dose 17β-estradiol 3-benzoate (EB) at 25 µg/pup (or 2,500 µg EB/kg body weight), (c) low-dose EB at 0.001 µg EB/pup (or 0.1 µg EB/kg body weight), or (c) bisphenol A at 0.1 µg/pup (or 10 µg/kg body weight). All steroids were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). The high-dose EB was chosen based on our published data of an estrogenized phenotype with adult-onset prostatic intraepithelial neoplasia (PIN; ref. 13). The low-dose EB was chosen because this dose delayed puberty and permanently affected other reproductive structures in our dose-response study (26). An environmentally relevant dose of bisphenol A was chosen based on a predicted exposure range from leached bisphenol A in the environment (27). To avoid litter effects, male pups within each litter were randomly assigned to a treatment and toe clipped for permanent identification. Treatments were given on postnatal days 1, 3, and 5 by s.c. injections in the nape of the neck. The pups were weaned at postnatal day 21, and siblings were housed three per cage until postnatal day 90 and individually thereafter. At postnatal day 90, half of the rats from each treatment group were given implants of Silastic capsules (inside diameter 1.5 mm, outer diameter 2.0 mm; Dow Corning, Midland, MI) packed with estradiol (one 1-cm tube) and testosterone (two 2-cm tubes) for 16 weeks (replaced after 8 weeks), whereas the remaining half were given empty tubes. The testosterone capsules were necessary to maintain physiologic levels of testosterone because estradiol treatment alone results in hypothalamic-testicular feedback inhibition of endogenous testosterone secretion with resultant prostatic involution. These testosterone plus estradiol capsule lengths result in ~75 pg/mL serum estradiol and 3 ng/mL serum testosterone (28) and produce PIN in the dorsolateral prostates at 100% incidence in Noble rats (29) but only 33% incidence in Sprague-Dawley rats (30). At 28 weeks of age, the animals were sacrificed by decapitation, and prostate glands were quickly removed and microdissected into ventral, lateral, and dorsal lobes. Half of each lobe was snap frozen and stored in liquid nitrogen for subsequent methylation analysis, whereas the contra-lateral lobe was fixed in 10% buffered formalin overnight and stored in 70% ethanol for histopathologic diagnosis. In addition to the above animals sacrificed on day 200, rats from the four neonatal treatment groups were sacrificed, and prostates were removed on postnatal days 10 and 90 (n = 5–7 per group) for DNA methylation analysis.
Histopathology
Fixed prostatic tissues were processed, paraffin embedded, and sectioned along the longitudinal axis at three levels of the tissue block (10 sections per lobe). The sections were coded to prevent reader bias and stained with H&E. Each lobe was scored in a blinded fashion for epithelial and stromal hyperplasia, inflammation, and the presence of PIN and other notable pathology (adenoma, metaplasia, basement membrane breakdown, microinvasion, etc). PIN lesions were characterized by the presence of nuclear atypia (enlarged and elongated nuclei, hyperchromasia, and prominent nucleoli) with or without aberrant cellular piling and ductal formation (31). PIN lesions were graded on a scale of 0 to 3 (0, no atypia; 1, low-grade PIN; 2, focal high-grade PIN; and 3, extensive high-grade PIN). For PIN lesions, the incidence and the mean PIN score per treatment group were determined. Incidence was analyzed by χ2, and PIN scores were analyzed by ANOVA after square root transformation of the data followed by Fisher’s exact test with significance accepted at P < 0.05.
Immunohistochemistry and in situ apoptosis labeling
Proliferation was measured by immunohistochemistry using a polyclonal Ki-67 primary antibody (1:2,500; Novocastra, Newcastle upon Tyne, United Kingdom). For apoptosis assessment, terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) staining was used with ApopTag peroxidase in situ apoptosis detection kit (Chemicon International, Temecula, CA). To calculate the proliferation and apoptotic indices, multiple representative areas of each lobe were captured with a color digital AxioCam camera on an Axioskop microscope (Carl Zeiss, Inc., Thornwood, NY). Positive and negative Ki-67-stained or TUNEL-labeled epithelial cells were counted using Zeiss Image version 3.0 (Carl Zeiss), with an average of 1,000 cells counted per slide. Data were analyzed by ANOVA and post hoc Bonferroni tests, and P < 0.05 was considered significant.
Methylation-sensitive restriction fingerprinting
MSRF was done as described (32), with minor modifications. In brief, 1 µg genomic DNA extracted from tissues with the DNeasy Tissue kit (Qiagen, Valencia, CA) was digested with MseI alone or double digested with BstUI and MseI (New England Biolabs, Beverly, MA). Digested DNA was amplified by PCR using 2 µCi [α-32P] dCTP (3,000 Ci/mmol; NEN, Boston, MA) with various combinations of paired arbitrary primers chosen from the following: Bs7, 5′-GAGGTGCGCG; Bs11, 5′-GAGAGGCGCG; Bs17, 5′-GGGGACGCGA; PCG1, 5′-AAGGAAGACG; and PCG4, 5′-TCCTTCCTCG. PCR products were separated on 6% nondenaturing polyacrylamide gels, which were dried and exposed to Kodak MS film (Kodak, New Haven, CT) to visualize the labeled bands. Candidate bands displaying the appropriate differential methylation status among “control” and “comparative” samples were cut, reamplified, and cloned directly into pCR2.1 vector (Invitrogen, Carlsbad, CA) for sequencing. The sequence obtained was aligned with database from Genbank and RefSeq using BLAST, expressed sequence tag homology (National Center for Biotechnology Information), and BLAT search (University of California Genome Research, Santa Cruz, CA).
5′-Rapid amplification of cDNA ends
The first-strand cDNA of PDE4D4 was amplified using a reverse specific primer (5′-AAAGACGAGGGCCAGGACAT-3′) and the GeneRacer 5′ Primer (Invitrogen). Nested PCR was done, and products were subcloned into pCR4-TOPO vector (Invitrogen). At least 10 clones were chosen and sequenced.
Bisulfite genomic sequencing
Genomic DNA (200 ng) from rat prostate tissue samples or rat cell lines was modified with sodium bisulfite using CpGenome DNA Modification kit (Chemicon International) and used in nested PCR for bisulfite sequencing. Primers for amplifying the PDE4D4 gene promoter/exon 1 region in completely converted DNA were designed with Primer3 and MethPrimer (33). First PCR was done using forward (5′-AGTGGTTTTGGAGAAGTTAGAGTTTA-3′) and reverse (5′-CCAAAACATCCTAAATTTCTTCAAA-3′) primers. Nested PCR was done with forward (5′-TTATTGTTGTGAAGAGTAGATTTTGTG-3′) and reverse (5′-ATCCTAAATTTCTTCAAACCTAACC-3′) primers. Both PCRs were done at 94°C for 9 minutes, 40 cycles of denaturing (94°C for 30 seconds), annealing (56°C for 1 minute), and extension (72°C for 1 minute) followed by a 12-minute final extension. The PCR product was gel purified and cloned into pCR2.1 vector. Six clones were picked from each sample for sequencing (Macrogen, Rockville, MD), and at least three sets of samples from each group were used. The DNA methylation data from sequencing were analyzed by BiQ Analyzer (34).
Methylation-specific PCR
PCR was done on bisulfite-treated DNA samples (40 ng) using primers specific for methylated (5′-GGTACGAGTAGTATTATTAGTATTCGTTTC-3′ and 5′-CACGACAATACAAATAACGCTCCGT-3′) or unmethylated (5′-GGTATGAGTAGTATTATTAGTATTTGTTTT-3′ and 5’-CACAACAATACAAATAACACTCCAT-3′) DNA. Forty PCR cycles were done with the following conditions: denature at 94°C for 30 seconds, anneal at 58°C for 1 minute, and extension at 72°C for 1 minute followed by 12-minute final extension. PCR products were separated on 2% agarose gel and visualized with ethidium bromide.
Real-time reverse transcription-PCR
Total RNA was isolated and reverse transcribed, and PDE4D4 expression was quantitated by a fluorogenic method with 2× SYBR Green Master Mix using an iCycler iQ Real-time PCR Detection System (Bio-Rad Laboratories, Hercules, CA) as described previously (35). Primers specific for PDE4D4 (AF031373) were designed in the exon/exon spanning region and were as follows: PDE4D4, 5′-ACGAGCAGCACCACC AGTA-3′ (forward) and 5′-CTTGAGGCGTAGCGACCAC-3′ (reverse). PDE4D4 mRNA levels were normalized to RPL19 and the postnatal day 10 oil-treated control value was arbitrarily assigned an abundance value of 1. All data groups were analyzed by ANOVA followed by post hoc Bonferroni tests.
Prostate cell cultures and demethylation assay
Normal prostate epithelial NbE-1 cells and tumorigenic AIT cells were established from the Noble rat and immortalized as described previously (36). Cell cultures were treated with 0.5 or 1 µmol/L 5-aza-2′-deoxycytidine (5-Aza-dC; Sigma-Aldrich) for 8 days. Drugs were replenished every 4 days, and equivalent concentrations of DMSO were added in replicate control samples. At the end of the treatment, DNA and RNA were extracted from the cells and subjected to bisulfite genomic sequencing to determine the methylation status of the 5′-flanking region of the PDE4D4 gene and real-time reverse transcription-PCR (RT-PCR) to quantitate PDE4D4 gene expression.
Results
Low-dose estradiol and bisphenol A increase susceptibility to prostate neoplastic lesions
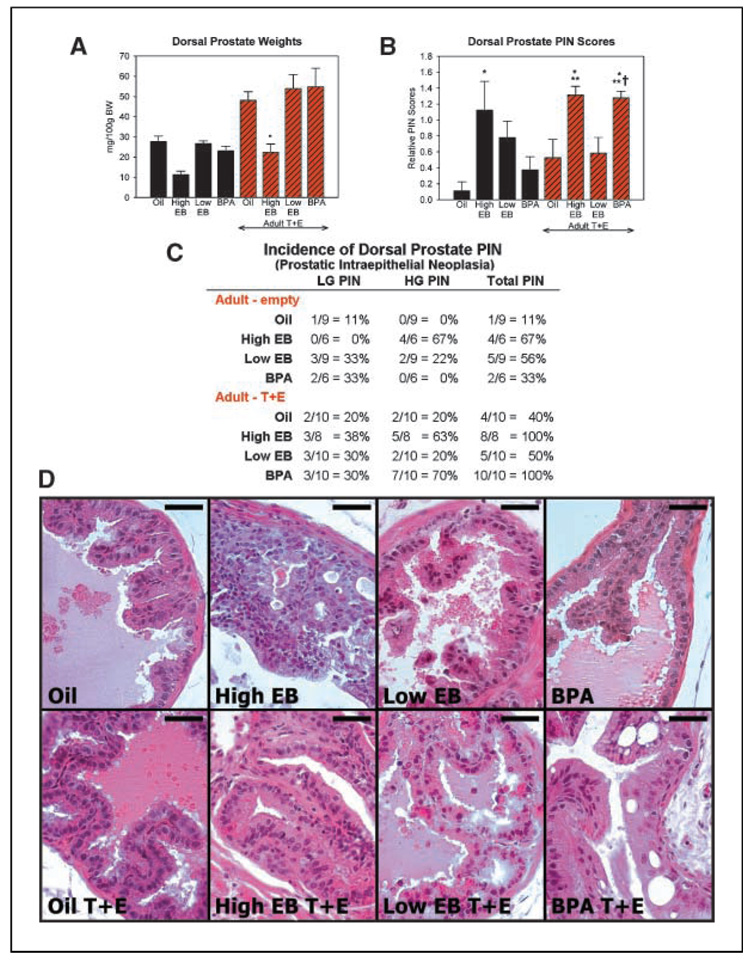
Responses to neonatal and adult hormone treatments were similar among the separate prostate lobes with only minor lobe-specific differences noted, and representative data are presented from the dorsal prostate (see Supplementary Fig. S1 for ventral and lateral data). Similar to our previous studies with rats (26), neonatal exposure to high-dose estrogen decreased adult prostate weights, whereas developmental exposure to low-dose estradiol or bisphenol A did not affect prostate size (Fig. 1A). Adult testosterone plus estradiol increased prostate weights but did not change the response pattern to neonatal exposures (Fig. 1A). Prostate histopathology was assessed in a blinded manner for hyperplasia, chronic inflammation, and PIN, the precursor lesion of prostate cancer. PIN scores, based on grade and frequency, and PIN incidence showed marked differences across treatment groups (Fig. 1B–D). The oil-control group had a low PIN incidence (11%) and score (0.11) and normal prostate histology. Neonatal exposure to high-dose EB alone resulted in a 66% incidence of high-grade PIN and a markedly increased PIN score (1.12; P < 0.05). Areas of severe nuclear atypia, adenoma, and cellular piling were typically observed (Fig. 1D). Importantly, exposure to low-dose estradiol alone also increased the PIN incidence to 56% with mixed low-grade and high-grade PIN and elevated the PIN score (0.8). Focal areas of mild nuclear atypia were frequently observed in low-dose estradiol prostates. In contrast, neonatal low-dose bisphenol A alone did not induce PIN lesions in the aged prostates. Stromal and epithelial hyperplasia as well as inflammatory cell infiltration were observed in the high-dose EB prostate but not in the low-dose EB or the bisphenol A–exposed animals (data not shown).
Figure 1.
Effects of neonatal estrogens on adult prostate. Representative data for the dorsal prostate at 6 months. A, dorsal prostate weights at day 200. *, P < 0.05 versus oil/testosterone + estradiol (T + E). B, columns, mean PIN scores; bars, SE. *, P < 0.05 versus oil alone; **, P < 0.05 versus oil/testosterone + estradiol; †, P < 0.01 versus bisphenol A (BPA) alone. C, incidence of PIN lesions across treatment groups at day 200. LGPIN, low-grade PIN; HGPIN, high-grade PIN. D, representative H&E sections from dorsal prostates of the eight treatment groups. Bar, 50 µm.
As expected (19), prolonged adult testosterone plus estradiol exposure increased PIN incidence (40%) and score (0.52) in oil-control prostates (Fig. 1B and C), and this was further increased (incidence, 100% and score, 1.3; P < 0.05) by initial early exposure to high-dose EB. Neonatal low-dose EB before adult hormones did not augment PIN lesions further than that seen with adult testosterone plus estradiol or neonatal low-EB exposures alone. In contrast, neonatal exposure to low-dose bisphenol A significantly increased the PIN incidence (100%, mostly high-grade PIN) and score (1.3; P < 0.01) following adult exposure to elevated testosterone plus estradiol. Further, the neoplastic severity produced by bisphenol A was equivalent to high-dose EB exposures. Histologically, severe atypia was common with nuclear elongation and irregular size, cellular piling, and adenoma formation (Fig. 1D).
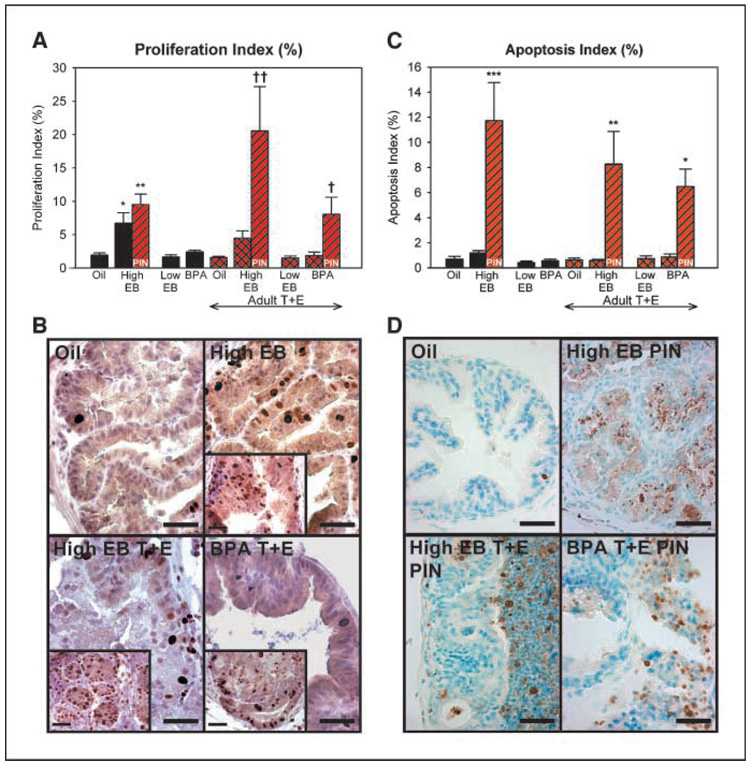
The prostatic tissues were assessed for alterations in epithelial cell proliferation and apoptosis, which are normally low in the adult prostate gland. Low rates of proliferation and apoptosis were consistently observed in all areas of the treated prostates, except for those exposed neonatally to high-dose EB or bisphenol A (Fig. 2A–B). Neonatal high EB treatment alone or with adult hormones increased basal proliferation rates throughout the tissue, with a higher rate observed in high-grade PIN regions (Fig. 2B, inset). Bisphenol A exposure followed by adult hormones also significantly increased proliferation in regions with high-grade PIN (Fig. 2A and B, inset). Similarly, low basal rates of apoptosis were detected throughout the prostate tissues, except for regions of high-grade PIN in the animals exposed neonatally to high-dose EB or bisphenol A with adult hormones (Fig. 2C–D). This provides support for the hypothesis that developmental estrogenic exposures initiate or activate precancerous pathways, resulting in an imbalance in cell proliferation and apoptosis that may contribute to prostatic pathology with aging. Taken together, the present experimental paradigm suggests that early low-dose estrogen exposures predispose the prostate to PIN with aging and that environmentally relevant doses of bisphenol A during development increase prostatic susceptibility to carcinogenesis following additional adult insults.
Figure 2.
Proliferation and apoptosis rates following estrogenic exposures. A, proliferation index for dorsal prostate epithelial cells as determined by Ki-67 immunostaining. Black and red-hatched columns, counts in histologically normal regions; red-stripped columns, areas of high-grade PIN. Basal proliferation was elevated in high-dose EB prostates. Proliferation rates were further elevated in high-grade PIN lesions of high-dose EB and bisphenol A/testosterone + estradiol prostates. *, P < 0.05 versus oil and low-dose EB; **, P < 0.01 versus oil and high-dose EB/testosterone + estradiol/PIN region; †, P < 0.05 versus bisphenol A/testosterone + estradiol/normal region; † †, P < 0.001 versus normal regions of all testosterone + estradiol treatment groups. B, representative regions of histologically normal dorsal prostates immunostained for Ki-67. Inset, regions of high-grade PIN within each group. C, apoptotic index for dorsal prostate epithelial cells as determined by TUNEL. Areas of high-grade PIN in high-dose EB and bisphenol A/testosterone + estradiol tissues showed increased apoptosis. *, P < 0.05 versus bisphenol A/testosterone + estradiol; **, P < 0.01 versus normal regions of all testosterone + estradiol treatment groups; ***, P < 0.001 versus normal regions in all treatments. D, representative TUNEL-labeled dorsal prostates. Normal region for oil and high-grade PIN region for all others. Bar, 50 µm.
Neonatal estrogens epigenetically modify the prostate through alterations in DNA methylation
We sought to determine whether permanent alterations in prostate growth and carcinogenic susceptibility long after early estrogenic exposures could be mediated through epigenetic alterations. To examine genome-wide methylation changes, MSRF was done using DNA from the neonatally exposed tissues removed on days 10, 90 (before adult hormone treatment), and 200 (schematized in Supplementary Fig. S2). Differential methylation changes were sought between control versus neonatally treated tissues across time. We were additionally interested in identifying candidates with early onset methylation changes that can potentially be used as markers for risk assessment. More than 50 candidate bands were chosen for cloning and sequencing, and 28 unique DNA candidate clones were identified (Table 1). Of the identified candidates, 16 showed no homology with known rat genes, 6 were identified once, and 2 (CAR-XI and SLC12A2) were identified multiple times with similar methylation patterns observed each time. Importantly, these eight candidate genes were homologous (>95%) to known genes involved in signal transduction pathways: Na-K-Cl cotransporter (SLC12A2), mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway (GPCR14 and PDGFRα), phosphokinase C pathway (PLCβ3), cAMP pathways (PDE4D4 and HPCAL1), and neural or cardiac development (CARXI and CARK).
Table 1.
Differentially methylated candidate genes identified with MSRF
| Clone name | Primer 1 | Primer 2 | Hypermethylation | Chromosomal band | Gene homology | Location | Related pathways |
|---|---|---|---|---|---|---|---|
| 2p717 | 7 | 17 | Low estradiol, high estradiol, bisphenol A (10, 90, 200) | 1q22 | CAR-XI | 5′-End | Neural cell development |
| 3p717 | 7 | 17 | Low estradiol (200) | 1q22 | CAR-XI | 5′-End | Neural cell development |
| 1P11G1 | 11 | G01 | Low estradiol, bisphenol A (200) | 1q43 | PLC β 3 | Exon19 | PKC and phospholipase signaling |
| 3p11G1 | 11 | G01 | Low estradiol, bisphenol A (200) | 7q12 | NA | ||
| 4p11G1 | 11 | G01 | Low estradiol, bisphenol A(90,200) | 18q12.1 | SLC12A2 | Exon17 | Na-K-2Cl cotransport |
| 5p11G1 | 11 | G01 | Low estradiol, bisphenol A (200) | 18q12.1 | SLC12A2 | Exon17 | Na-K-2Cl cotransport |
| 6p11G1 | 11 | G01 | Low estradiol, bisphenol A (200) | 18q12.1 | SLC12A2 | Exon17 | Na-K-2Cl cotransport |
| 8p11G1 | 11 | G01 | Control (10, 90) | 6q32 | NA | ||
| 9p11G1 | 11 | G01 | Low estradiol, bisphenol A (200) | 6q16 | HPCAL1 | Intron1 | cAMP signaling |
| 10p11G1 | 11 | G01 | Low estradiol, bisphenol A (200) | 18p12 | NA | ||
| 11p11G1 | 11 | G01 | Control (10, 90) | 2q14 | NA | ||
| 12p11G1 | 11 | G01 | Low estradiol (10, 90, 200) | 6q32 | NA | ||
| 3p11G4 | 11 | G04 | Low estradiol, high estradiol, bisphenol A (90,200) | 7q34 | NA | ||
| 5p11G4 | 11 | G04 | Low estradiol, high estradiol, bisphenol A (90, 200) | 6q24 | NA | ||
| 6p11G4 | 11 | G04 | Low estradiol, high estradiol, bisphenol A (90,200) | 18q12.1 | SLC12A2 | Exon17 | Na-K-2Cl cotransport |
| 7p11G4 | 11 | G04 | Low estradiol, high estradiol, bisphenol A (90, 200) | 8q22 | NA | ||
| 8p11G4 | 11 | G04 | Low estradiol, high estradiol, bisphenol A (90, 200) | 2q45 | CARK | 5′-End | Ca2+ dependent signaling |
| 9p11G4 | 11 | G04 | Low estradiol (90, 200) | 4q31 | NA | ||
| 10p11G4 | 11 | G04 | Low estradiol, bisphenol A (90, 200) | 4q31 | NA | ||
| 11p11G4 | 11 | G04 | Low estradiol, bisphenol A (90, 200) | 4q31 | NA | ||
| 14p11G4 | 11 | G04 | Low estradiol (90, 200) | 4q31 | NA | ||
| 15p11G4 | 11 | G04 | Low estradiol (90, 200) | 19q12 | NA | ||
| 17p11G4 | 11 | G04 | Low estradiol (90, 200) | 8q32 | GPCR14 | 5′-End | G-protein coupled receptor signaling |
| 18p11G4 | 11 | G04 | Control (10, 90, 200) | 2q14 | PDE4D4 | 5′-End | cAMP signaling |
| 2p1117 | 11 | 17 | Low estradiol, high estradiol, bisphenol A (200 testosterone + estradiol) | 20q13 | NA | ||
| 3p1117 | 11 | 17 | bisphenol A (10) | 7q11 | NA | ||
| 4p1117 | 11 | 17 | Low estradiol, high estradiol, bisphenol A (10) | 17p12 | NA | ||
| 5p1117 | 11 | 17 | Control (10) | 114p11 | PDGFR α | Intron4 | MAPK, ERK signaling |
NOTE: Fragments were identified based on SWISS-PROT, TrEMBL, mRNA, and RefSeq search. Shown for each candidates are MSRF primers used, the hypermethylation pattern observed in the control, high and low estradiol, and bisphenol A samples (days of adult testosterone + estradiol treatment), the chromosomal band to which the fragment localized, the gene homology, and the location of the methylated fragment on the gene and the known related pathways.
Abbreviation: NA, not available.
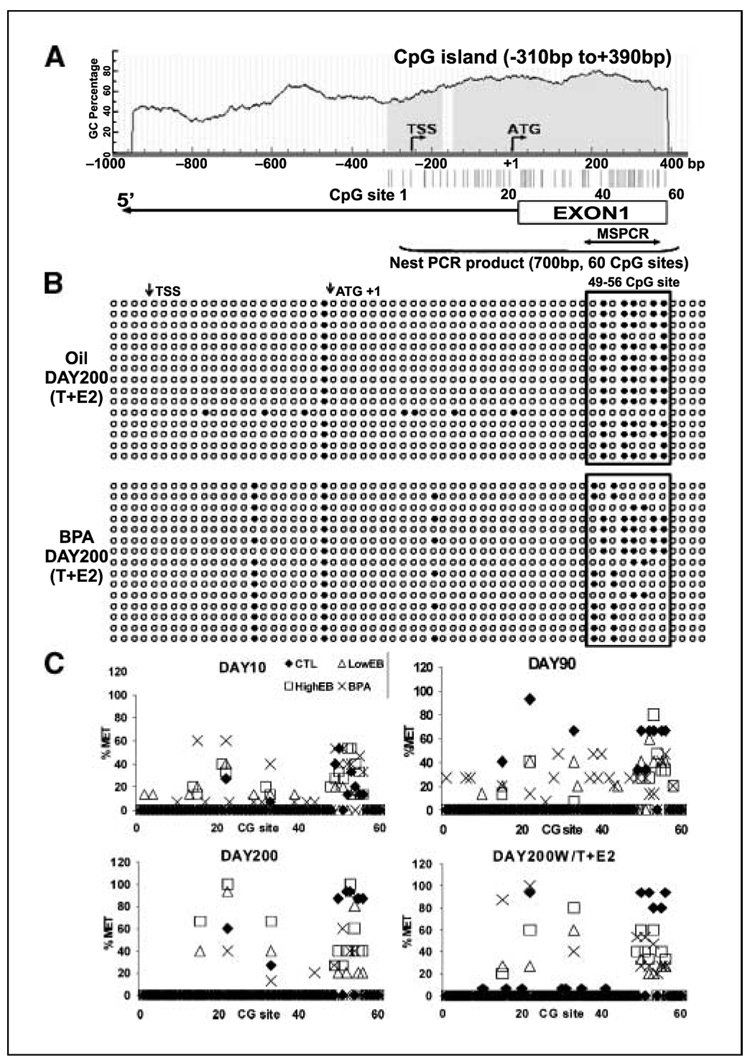
PDE4D4, which breaks down intracellular cAMP, was chosen for further characterization because the differentially methylated candidate clone corresponded to the 5′-region of the gene, and the methylation differences between control and estrogen-exposed tissues were observed as early as postnatal day 10. The 5′-flanking/promoter region of PDE4D4 was first identified by 5′-rapid amplification of cDNA ends and a 700-bp CpG island with 60 CpG sites was found to encompass its transcription and translation start sites (Fig. 3A). Importantly, multiple transcription factor response elements, including cAMP response element, estrogen response element half-site, and Sp1, were computationally identified in this CpG island (Supplementary Fig. S3).
Figure 3.
Bisulfite genomic sequencing of prostatic PDE4D4 gene methylation. A, schematic of CpG content (%) in the 5′-flanking region of the rat PDE4D4 gene identifies a CpG island (blue) between −310 to +390 bp. Vertical lines, individual CpG sites and the translation start site (ATG+1) and transcription start site (TSS). Blanket, 700-bp nested PCR-amplified region used for bisulfite sequencing. B, bisulfite genomic sequencing data from 4 to 6 clones each of three individual DNA samples taken from day 200 oil/testosterone + estradiol and bisphenol A/testosterone + estradiol dorsal prostates. Methylation status of specific CpG sites. ○, unmethylated; ●, methylated. Boxed region, potential CpG sites epigenetically altered by bisphenol A. C, percentage methylation at each CpG site within the 5′-CpG island of PDE4D4 in the separate treatment groups at days 10, 90, and 200 with or without adult testosterone + estradiol. The percentage methylation at each site was averaged from three individual sample sets. ◆, oil control; □, high-dose estradiol; △, low-dose estradiol; ×, bisphenol A.
Methylation site mapping of this CpG island was done by bisulfite genomic sequencing in prostates from all treatment groups at postnatal days 10, 90, and 200. Figure 3B shows an example of methylation mapping at the 60 CpG sites in day 200 oil (Fig. 3B, top) and bisphenol A–treated prostates (Fig. 3B, bottom), whereas Fig. 3C shows the percentage methylation at the 60 CpG sites in all dorsal prostate tissues calculated over time for the different treatment groups. Although most CpG sites were unmethylated, a methylated cluster was noted between CpG sites 49 to 56 (Fig. 3B, boxed region), and methylation frequency at these sites progressively increased in the oil-control prostates as the animals aged, reaching 100% methylation by day 200 (Fig. 3C, solid diamonds). In contrast, the CpGs 49 to 56 remained relatively hypomethylated in aging prostates exposed neonatally to high- or low-dose estradiol or low-dose bisphenol A (Fig. 3B–C).
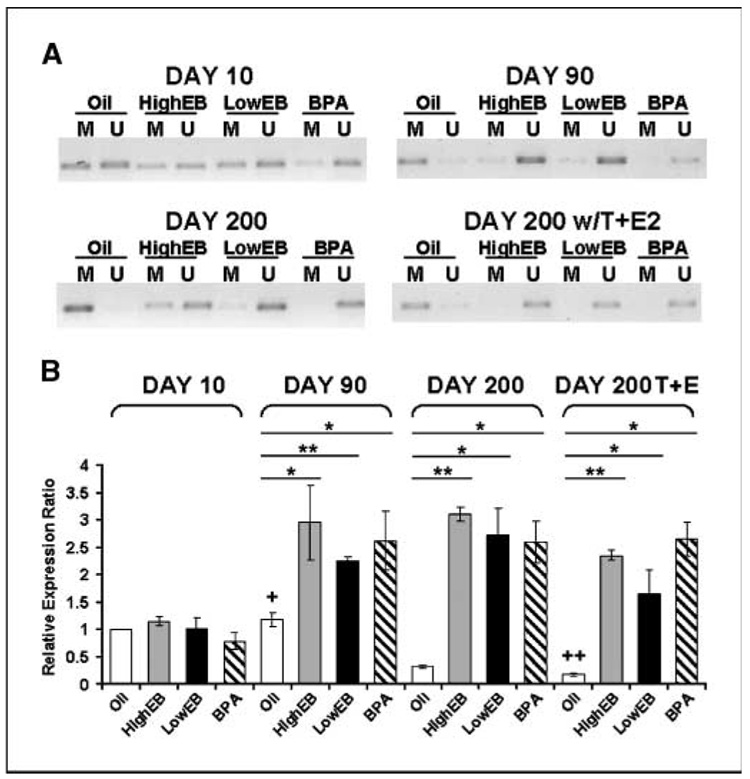
Direct association of DNA methylation at CpGs 49 to 56 and its resultant effect on PDE4D4 gene expression were shown by using methylation-specific PCR and real-time RT-PCR. There were no differences in DNA methylation or gene expression between treatment groups at day 10 (Fig. 4A–B). However, as the animals aged, CpGs 49 to 56 became entirely methylated in oil-control prostates, whereas neonatal high EB-treated, low EB-treated, and bisphenol A–treated prostates possessed completely unmethylated sequences (Fig. 4A). Importantly, these differential methylation patterns were inversely correlated to PDE4D4 gene expression (Fig. 4B). PDE4D4 message levels in prostates exposed neonatally to estradiol or bisphenol A were markedly higher at day 90 than control tissues and remained elevated with aging. We thus conclude that the prostatic PDE4D4 gene is normally silenced with aging through promoter hypermethylation but remains expressed in neonatally estrogenized prostates by virtue of hypomethylation at CpGs 49 to 56. Notably, this phenomenon was observed in all neonatal high- and low-dose EB and low-dose bisphenol A groups before the “second hit” of hormones and before adult-onset PIN lesions. Thus PDE4D4 may have potential as a marker for prostate cancer risk assessment.
Figure 4.
Comparison of PDE4D4 CpG methylation and mRNA transcript levels. A, methylation-specific PCR analysis: dorsal prostate genomic DNA was bisulfite treated followed by methylation-specific PCR using methylated specific (M) or unmethylated-specific (U) primer sets. The amplified region is indicated in Fig. 3. The PCR products are representative data from three individual sets of samples. B, PDE4D4 mRNA transcript levels as determined by real-time RT-PCR. Relative expression of day 10 oil samples was set to 1. Columns, mean; bars, SD. All EB/bisphenol A groups at days 90, 200, and 200/testosterone + estradiol were significantly different (P < 0.05) from respective groups at day 10. *, P < 0.05; **, P < 0.01 versus oil controls at the same time interval; †, P < 0.05 versus day 200 oil controls; † †, P < 0.05 versus day 10 oil controls.
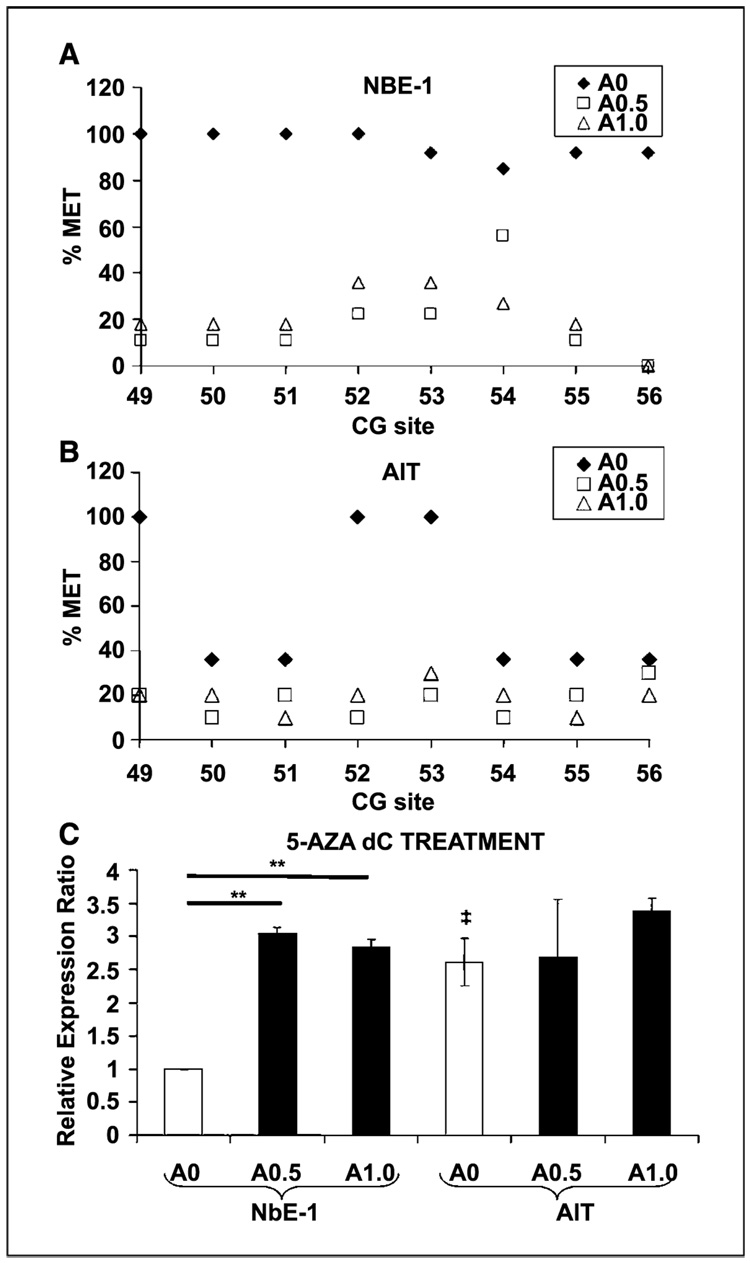
We further showed that PDE4D4 transcription is dependent on the methylation status of its promoter region, specifically at CpGs 49 to 56, by using rat prostate NbE-1 cells, an immortalized normal epithelial cell line, and AIT, a dorsal prostate tumor-derived cell line. We found low levels of PDE4D4 expression and a methylated 49 to 56 CpG cluster in NbE-1 cells but high levels of gene expression in AIT cells and unmethylated cluster at CpGs 49 to 56 (Fig. 5A–C). These in vitro findings mirror the in vivo data of control versus estrogenized prostate tissues. Treatment of NbE-1 cells with 5-Aza-dC induced loss of methylation at CpGs 49 to 56 and increased PDE4D4 gene expression (P < 0.01; Fig. 5A and C). Similar treatment of AIT cells completely demethylated these previously hypomethylated CpG sites; however, it did not further increase the already high levels of PDE4D4 expression (Fig. 5B–C). Taken together, these data provide direct evidence that hypermethylation of the PDE4D4 promoter at CpGs 49 to 56 is involved in PDE4D4 transcriptional silencing and that deregulation of methylation at this locus occurs in prostate cancer cells.
Figure 5.
Alterations in PDE4D4 CpG methylation and gene expression in NbE-1 and AIT cell lines by a demethylating agent. A, percentage CpG methylation for PDE4D4 CpGs 49 to 56 in normal NbE-1 cells without (◆) or with 5-Aza-dC treatment (□, 0.5 µmol/L 5-Aza-dC; △, 1 µmol/L 5-Aza-dC). B, percentage CpG methylation for PDE4D4 CpGs 49 to 56 in tumorigenic AIT cells without (◆) or with 5-Aza-dC treatment. C, relative PDE4D4 mRNA levels in NbE-1 and AIT cells. White columns, control cells (A0); bars, SD. Black columns, cells treated with 5-Aza-dC. Expression of control NbE-1 cells was set to 1. **, P < 0.01 versus controls; ‡, P < 0.05 versus control NbE-1 cells.
Discussion
The present findings provide the first evidence of a direct link between developmental low-dose bisphenol A or estradiol exposures and carcinogenesis of the prostate gland. Specifically, the data show that exposure to low doses of estradiol or environmentally relevant doses of bisphenol A during the neonatal developmental period in rats increases susceptibility to precancerous prostatic lesions as the animals aged and sensitizes the prostate gland to adult-induced hormonal carcinogenesis. This data thus contribute to the increasing body of evidence for a link between fetal exposures to endocrine disruptors and cancer (37–39). The human male fetus is exposed to elevated levels of maternal and exogenous estrogenic compounds, including bisphenol A (5, 40), and these estrogens could sensitize the prostate, perhaps through epigenetic mechanisms. Furthermore, relative increases in estradiol levels in the aging male (17) are adequate to promote carcinogenesis in a sensitized organ. Alternatively, a neonatally sensitized organ may be more vulnerable to adult exposures to bisphenol A or other xenoestrogens that bioaccumulate in fat cells. In this regard, recent evidence has shown that low-dose bisphenol A inappropriately activates the androgen receptor and mitogenesis in prostate adenocarcinoma cells in vitro and sensitizes cells with previous mutations (41). It is particularly relevant that the developmentally estrogenized rodent has accurately modeled multiple male and female reproductive tract lesions in humans (42). Thus, the present findings may have implications for human prostatic adenocarcinoma, which occurs with a relatively high frequency in the aging population and whose etiology remains unclear.
Although the mechanism(s) by which developmental exposures to endogenous and environmental estrogens alter the carcinogenic potential of the prostate have not been fully clarified, the present findings support the hypothesis, initially proposed by McLachlan (43), that altered epigenetic memory by endocrine disruptors may play a critical role. In this study, we have provided direct evidence in support of this premise. Our data show that several genes exhibit methylation changes in response to the neonatal estrogen treatments, many of which are permanent. It is noteworthy that several of these genes encode signaling pathway proteins that are involved in cell cycle and/or apoptosis, suggesting that neonatal estrogen exposures may perturb proliferation/apoptosis equilibrium through epigenetic gene (de)regulation. It is also interesting that overlapping as well as unique methylation alterations were observed for high- and low-dose estrogen and bisphenol A. This suggests two important points. First, common prostatic genes may be epigenetically imprinted by different estrogenic compounds and doses, suggesting common pathways that predispose to prostate carcinogenesis with aging. Second, unique candidate genes specific to the neonatal estrogenic exposure and/or dose may mediate the subtle differences in phenotypes that were observed following the separate neonatal exposures.
The epigenetic regulation of gene expression by neonatal estrogen exposure was confirmed by detailed analysis of the PDE4D4 gene. The estradiol and bisphenol A–initiated alterations in PDE4D4 gene methylation occurred at a CpG island that spans the promoter/exon 1 region, a site typically involved in epigenetic regulation. Importantly, the degree of methylation at this site was inversely related to PDE4D4 gene expression in the prostate tissues. Thus, the PDE4D4 promoter undergoes gradual hypermethylation with aging in normal prostates, resulting in PDE4D4 gene repression in the adult gland. In contrast, it remains hypomethylated in animals briefly exposed to neonatal estradiol or bisphenol A, thus engendering persistent PDE4D4 overexpression throughout life. This pattern of PDE4D4 methylation and transcriptional regulation was also observed in normal and malignant prostate epithelial cells where normal NbE-1 cells with hypermethylated PDE4D4 gene had low gene expression, whereas tumorigenic AIT cells had hypomethylation at the CpG island and elevated PDE4D4 expression. Taken together, these findings suggest the potential involvement of epigenetically mediated PDE4D4 dysregulation in prostate epithelial cell transformation.
At present, it is premature to suggest that PDE4D4 dysregulation is a direct mediator of the prostatic dysgenesis as a result of early exposures to low- and high-dose estradiol or low-dose bisphenol A, particularly because the phenotypic response to the hormonal agents has specific differences widely, whereas the PDE4D4 methylation and expression alterations are quite similar. Nonetheless, PDE4 is a promising lead candidate that deserves further discussion. PDE4 is a member of a large family of intracellular PDE enzymes involved in cyclic nucleotide monophosphate breakdown, and it specifically degrades cAMP (44). There are multiple downstream signals for cAMP in the cell, including activation of protein kinase A with resultant phosphorylation of cAMP-responsive element binding protein, which regulates transcription of genes involved in cell growth and differentiation (45). PDE4D has been shown to regulate cAMP levels in hormone-targeted cells, and the PDE4D4 variant, which localizes to the cytoskeletal structures, is itself activated by hormones (46). Sustained expression of PDE4D4 by hypomethylation could thus result in decreased intracellular cAMP in specific subcellular locations, creating a potential for aberrant cell signaling and potentially neoplastic transformation. In this regard, recent studies have shown a tight association between PDE4 expression and cancer cell proliferation, including glioma cells (47), osteosarcomas (48), and chronic lymphocytic leukemia (49). Importantly, PDE4 is currently being pursued as a possible chemotherapeutic target (50).
In addition to providing insight into the molecular underpinnings of estrogen imprinting, the methylated candidate genes identified herein have potential to serve as molecular markers for risk assessment of prostate disease due to early environmental exposures. PDE4D4 shows particular promise in this regard because alterations in both gene methylation and expression were apparent before adult hormonal exposures and, importantly, before the onset of histopathologic changes in the prostate gland. This suggests that subtle alterations in gene expression may be more sensitive indicators of underlying pathology than the histologic alterations that occur when the disease is further progressed. Future studies are planned to develop a panel of methylated genes that may be used as markers for prostatic disease following early bisphenol A exposures.
In summary, we have shown that a range of estrogenic exposures during the developmental critical period, from environmentally relevant bisphenol A exposure to low-dose and pharmacologic estradiol exposures, results in an increased incidence and susceptibility to neoplastic prostatic lesions in the aging male, which may provide a fetal basis for this adult disease. Furthermore, the present findings provide evidence that developmental exposure to environmental endocrine disruptors (bisphenol A) and natural estrogens impacts the prostate epigenome during early life, which suggests an epigenetic basis for estrogen imprinting of the prostate gland. Methylation patterns and/or expression of candidate genes, such as PDE4D4, may serve as early biomarkers of prostate malignancy due to developmental exposure to endocrine disruptors or the in utero estrogenic environment.
Supplementary Material
Acknowledgments
Grant support: NIH grants ES12281 (G.S. Prins and S-M. Ho) and DK40890 (G.S. Prins) and the Department of Defense awards DAMD W81XWH-04-1-0165 (S-M. Ho) and W81XWH-06-1-0373 (W-Y. Tang).
We thank the Department of Surgery of the University of Massachusetts (Worcester, MA), David Hepps, MD, and Lynn Birch for technical contributions.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Colborn T. Environmental estrogens: health implications for humans and wildlife. Environ Health Perspect. 1995;103:135–136. doi: 10.1289/ehp.95103s7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dodds EC, Lawson W. Synthetic estrogenic agents without phenanthrene nucleus. Nature. 1936;137:996–997. [Google Scholar]
- 3.Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- 4.Quesada I, Fuentes E, Viso-Leon MC, Ripoll C, Nadal A. Low doses of the endocrine disruptor bisphenol-A and the native hormone 17β-estradiol rapidly activate the transcription factor CREB. FASEB J. 2002;16:1671–1673. doi: 10.1096/fj.02-0313fje. [DOI] [PubMed] [Google Scholar]
- 5.Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Environmental toxins: exposure to bisphenol A advances puberty. Nature. 1999;401:763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 7.Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72:1344–1351. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 8.Henderson BE, Bernstein L, Ross RK, Depue RH, Judd HL. The early in utero oestrogen and testosterone environment of blacks and whites: potential effects on male offspring. Br J Cancer. 1988;57:216–218. doi: 10.1038/bjc.1988.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell IJ, Meyskens FL., Jr African American men and hereditary/familial prostate cancer: intermediate-risk populations for chemoprevention trials. Urology. 2001;57:178–181. doi: 10.1016/s0090-4295(00)00968-7. [DOI] [PubMed] [Google Scholar]
- 10.Rajfer J, Coffey DS. Effects of neonatal steroids on male sex tissues. Invest Urol. 1979;17:3–8. [PubMed] [Google Scholar]
- 11.Prins GS. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology. 1992;130:3703–3714. doi: 10.1210/endo.130.6.1597166. [DOI] [PubMed] [Google Scholar]
- 12.Huang L, Pu Y, Alam S, Birch L, Prins GS. Estrogenic regulation of signaling pathways and homeobox genes during rat prostate development. J Androl. 2004;25:330–337. doi: 10.1002/j.1939-4640.2004.tb02796.x. [DOI] [PubMed] [Google Scholar]
- 13.Prins GS. Prostate: basic and clinical aspects. Chapter 10. In: Naz RK, editor. Developmental estrogenization of the prostate gland. Boca Raton: CRC Press; 1997. pp. 247–265. [Google Scholar]
- 14.vom Saal FS, Timms BG, Montano MM, et al. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci USA. 1997;94:2056–2061. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newbold RR, Jefferson WN, Padilla-Banks E, Haseman J. Developmental exposure to diethylstilbestrol (DES) alters uterine response to estrogens in prepubescent mice: low versus high dose effects. Reprod Toxicol. 2004;18:399–406. doi: 10.1016/j.reprotox.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Munoz-de-Toro M, Markey CM, Wadia PR, et al. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146:4138–4147. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 18.Modugno F, Weissfeld JL, Trump DL, et al. Allelic variants of aromatase and androgen and estrogen receptors: toward a multigenic model of prostate cancer risk. Clin Cancer Res. 2001;7:3092–3096. [PubMed] [Google Scholar]
- 19.Leav I, Ho S, Ofner P, Merk F, Kwan P, Damassa D. Biochemical alterations in sex hormone-induced hyperplasia and dysplasia of the dorsolateral prostates of Noble rats. J Natl Cancer Inst. 1988;80:1045–1053. doi: 10.1093/jnci/80.13.1045. [DOI] [PubMed] [Google Scholar]
- 20.Momparler R, Bovenzi V. DNA methylation and cancer. J Cell Physiol. 2000;183:145–154. doi: 10.1002/(SICI)1097-4652(200005)183:2<145::AID-JCP1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 21.Esteller M. Aberrant DNA methylation as a cancer-inducing mechanism. Annu Rev Pharmacol Toxicol. 2005;45:629–656. doi: 10.1146/annurev.pharmtox.45.120403.095832. [DOI] [PubMed] [Google Scholar]
- 22.Lyn-Cook BD, Blann E, Payne PW, Bo J, Sheehan D, Medlock K. Methylation profile and amplification of proto-oncogenes in rat pancreas induced with phytoestrogens. Proc Soc Exp Biol Med. 1995;208:116–119. doi: 10.3181/00379727-208-43842. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Washburn KA, Moore R, et al. Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactorferrin gene in mouse uterus. Cancer Res. 1997;57:4356–4359. [PubMed] [Google Scholar]
- 24.Alworth LC, Howdeshell KL, Ruhlen RL, et al. Uterine responsiveness to estradiol and DNA methylation are altered by fetal exposure to diethylstilbestrol and methoxychlor in CD-1 mice: effects of low versus high doses. Toxicol Appl Pharmacol. 2002;183:10–22. doi: 10.1006/taap.2002.9459. [DOI] [PubMed] [Google Scholar]
- 25.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disuptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putz O, Schwartz CB, Kim S, LeBlanc GA, Cooper RL, Prins GS. Neonatal low- and high-dose exposure to estradiol benzoate in the male rat. I. Effects on the prostate gland. Biol Reprod. 2001;65:1496–1505. doi: 10.1095/biolreprod65.5.1496. [DOI] [PubMed] [Google Scholar]
- 27.Nagel SC, vom Saal FS, Thayer KA, Dhar MG, Boechler M, Weshons WV. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ Health Perspect. 1997;105:70–76. doi: 10.1289/ehp.9710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee C, Prins GS, Henneberry MO, Grayhack JT. Effect of estradiol on the rat prostate in the presence and absence of testosterone and pituitary. J Androl. 1981;2:293–299. [Google Scholar]
- 29.Yu M, Cates J, Leav I, Ho S. Heterogeneity of [3H]estradiol binding sites in the rat prostate: properties and distribution of type I and type II sites. J Steroid Biochem. 1989;33:449–457. doi: 10.1016/0022-4731(89)90336-1. [DOI] [PubMed] [Google Scholar]
- 30.Bosland MC, Ford H, Horton L. Induction at high incidence of ductal prostate adenocarcinomas in NBL/Cr and Sprague-Dawley Hsd:SD rats treated with a combination of testosterone and estradiol-17β or dietlhylstilbestrol. Carcinogenesis. 1995;16:1311–1317. doi: 10.1093/carcin/16.6.1311. [DOI] [PubMed] [Google Scholar]
- 31.Shappell S, Thomas D, Roberts R, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Biology Committee. Cancer Res. 2004;64:2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 32.Huang TH, Laux DE, Hamlin BC, Tran P, Tran H, Lubahn DB. Identification of DNA methylation markers for human breast carcinomas using the methylation-sensitive restriction fingerprinting technique. Cancer Res. 1997;57:1030–1034. [PubMed] [Google Scholar]
- 33.Li LC, Dahiva R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- 34.Bock C, Reither S, Mikeska T, Paulsen M, Walter J, Lengauer T. BiQ Analyzer: visualization and quality control for DNA methylation data from bisulphite sequencing. Bioinformatics. 2005;21:4067–4068. doi: 10.1093/bioinformatics/bti652. [DOI] [PubMed] [Google Scholar]
- 35.Lau KM, LaSprina M, long J, Ho SM. Expression of estrogen receptor (ER)-α and ER-β in normal and malignant prostatic epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res. 2000;60:702–706. [PubMed] [Google Scholar]
- 36.Chang SM, Chung LW. Interaction between prostatic fibroblast and epithelial cells in culture: role of androgen. Endocrinology. 1989;125:2719–2727. doi: 10.1210/endo-125-5-2719. [DOI] [PubMed] [Google Scholar]
- 37.Newbold RR, Bullock BC, McLachlan JA. Uterine adenocarcinoma in mice following developmental treatment with estrogens: a model for hormonal carcinogenesis. Cancer Res. 1990;50:7677–7681. [PubMed] [Google Scholar]
- 38.Shibata A, Minn AY. Perinatal sex hormones and risk of breast and prostate cancers in adulthood. Epidemiol Rev. 2000;22:239–248. doi: 10.1093/oxfordjournals.epirev.a018036. [DOI] [PubMed] [Google Scholar]
- 39.Birnbaum LS, Fenton SE. Cancer and developmental exposure to endocrine disruptors. Environ Health Perspect. 2003;111:389–394. doi: 10.1289/ehp.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baik I, Devito WJ, Ballen K, et al. Association of fetal hormone levels with stem cell potential: evidence for early life roots of human cancer. Cancer Res. 2005;65:358–363. [PubMed] [Google Scholar]
- 41.Wetherill YB, Petre CE, Monk KR, Puga A, Knudsen KE. The xenoestrogen bisphenol A induces inappropriate androgen receptor activation and mitogenesis in prostatic adenocarcinoma cells. Mol Cancer Ther. 2002;1:515–524. [PubMed] [Google Scholar]
- 42.Greco T, Duello T, Gorski J. Estrogen receptors, estradiol, and diethylstilbestrol in early development: the mouse as a model for the study of estrogen receptors and estrogen sensitivity in embryonic development of male and female reproductive tracts. Endocr Rev. 1993;14:59–71. doi: 10.1210/edrv-14-1-59. [DOI] [PubMed] [Google Scholar]
- 43.McLachlan JA. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocr Rev. 2001;22:319–341. doi: 10.1210/edrv.22.3.0432. [DOI] [PubMed] [Google Scholar]
- 44.Conti M, Swimnen JV. Structure and function of the rolipram-sensitive, low-Km cyclic AMP phosphodiesterase: a family of highly related enzymes. In: Houslay MD, Beavo JA, editors. Molecular pharmacology of cell regulation: cyclic nucleotide phosphodiesterase structure, regulation, and drug action. New York: Wiley; 1990. pp. 243–266. [Google Scholar]
- 45.Fimia GM, Sassone-Corsi P. Cyclic AMP signalling. J Cell Sci. 2001;114:1971–1972. doi: 10.1242/jcs.114.11.1971. [DOI] [PubMed] [Google Scholar]
- 46.Jin S-LC, Bushnik T, Lan L, Conti M. Subcellular localization of rolipram-sensitive, cAMP-specific phosphodiesterases. J Biol Chem. 1998;273:19672–19678. doi: 10.1074/jbc.273.31.19672. [DOI] [PubMed] [Google Scholar]
- 47.Chen TC, Wadsten P, Su S, et al. The type IV phosphodiesterase inhibitor rolipram induces expression of the cell cycle inhibitiors p21(Cip1) and p27(Kip1), resulting in growth inhibition, increased differentiation, and subsequent apoptosis of malignant A-172 glioma cells. Cancer Biol Ther. 2002;1:268–276. doi: 10.4161/cbt.80. [DOI] [PubMed] [Google Scholar]
- 48.Narita M, Murata T, Shimizu K, et al. Phosphodiesterase 4 in osteoblastic osteosarcoma cells as a potential target for growth inhibition. Anticancer Drugs. 2003;14:377–381. doi: 10.1097/00001813-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Lerner A, Kim DH, Lee R. The cAMP signaling pathway as a therapeutic target in lymphoid malignancies. Leuk Lymphoma. 2000;37:39–51. doi: 10.3109/10428190009057627. [DOI] [PubMed] [Google Scholar]
- 50.Hirsh L, Dantes A, Suh B-S, et al. Phosphodiesterase inhibitors as anti-cancer drugs. Biochem Pharmacol. 2004;68:981–988. doi: 10.1016/j.bcp.2004.05.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.