Abstract
OBJECTIVE
To determine whether group prenatal care improves pregnancy outcomes, psychosocial function, and patient satisfaction and to examine potential cost differences.
METHODS
A multisite randomized controlled trial was conducted at two university-affiliated hospital prenatal clinics. Pregnant women aged 14−25 years (n=1,047) were randomly assigned to either standard or group care. Women with medical conditions requiring individualized care were excluded from randomization. Group participants received care in a group setting with women having the same expected delivery month. Timing and content of visits followed obstetric guidelines from week 18 through delivery. Each 2-hour prenatal care session included physical assessment, education and skills building, and support through facilitated group discussion. Structured interviews were conducted at study entry, during the third trimester, and postpartum.
RESULTS
Mean age of participants was 20.4 years; 80% were African American. Using intent-to-treat analyses, women assigned to group care were significantly less likely to have preterm births compared with those in standard care: 9.8% compared with 13.8%, with no differences in age, parity, education, or income between study conditions. This is equivalent to a risk reduction of 33% (odds ratio 0.67, 95% confidence interval 0.44−0.99, P=.045), or 40 per 1,000 births. Effects were strengthened for African-American women: 10.0% compared with 15.8% (odds ratio 0.59, 95% confidence interval 0.38−0.92, P=.02). Women in group sessions were less likely to have suboptimal prenatal care (P<.01), had significantly better prenatal knowledge (P<.001), felt more ready for labor and delivery (P<.001), and had greater satisfaction with care (P<.001). Breastfeeding initiation was higher in group care: 66.5% compared with 54.6%, P<.001. There were no differences in birth weight nor in costs associated with prenatal care or delivery.
CONCLUSION
Group prenatal care resulted in equal or improved perinatal outcomes at no added cost.
CLINICAL TRIAL REGISTRATION
LEVEL OF EVIDENCE
I
Preterm birth rates have increased globally over the past quarter century. Although assisted reproductive technology and the increase in multifetal gestations account for some of the increase, the etiology for most preterm delivery remains elusive. To date, pharmacological, clinical, and psychosocial interventions have had limited success in preventing preterm birth.1 Racial disparities persist, with a twofold higher rate of preterm birth and low birth weight among African-American women. Preterm birth has numerous adverse consequences, including neonatal and infant deaths, childhood neurologic disability, prolonged hospitalization, increased cost, and potential lifelong adverse developmental and medical consequences.1–3
There have been prior randomized controlled trials on augmented prenatal care to reduce preterm birth.4–10 Hobel et al4 reported a 19% reduction in preterm birth among high-risk patients in county clinics randomized to an enhanced program that included education and increased visits. Klerman et al5 reported significantly increased patient satisfaction and knowledge. Although rates of preterm delivery, cesarean delivery, and length of stay in the neonatal intensive care unit decreased, there was no statistically significant difference. Results of other randomized controlled trials of augmented care are equivocal,6–10 except among certain subgroups: primiparous mothers7 and high-risk African-American women.8,10 Lu et al11 suggest that preterm birth prevention will require a reconceptualization of prenatal care as part of a broader strategic approach.
Group prenatal care (CenteringPregnancy, Cheshire, CT) has been implemented in over 100 clinical practices in the United States and abroad since 1995.12–13 It provides an integrated approach to prenatal care in a group setting, incorporating family members, peer support, and education (Table 1). In prior studies of group prenatal care among minority teens14 and women,13 investigators documented lower rates of preterm birth and low birth weight. However, these studies were limited by lack of randomization and potential self-selection bias.
Table 1.
Traditional Prenatal Care Compared With Group Prenatal Care
| Traditional | Group Care | |
|---|---|---|
| Delivery of care | 1. Accepted model of prenatal care using one-to-one examination room visits. | 1. Prenatal care provided within the group space (community or conference room). |
| 2. Care is provided by a credentialed prenatal provider. | 2. Care is provided through a partnership of a credentialed provider and pregnant woman. | |
| 3. Variable continuity of provider throughout pregnancy. | 3. Continuity of care from a single provider. | |
| Content of care | 4. Physical assessment completed inside an examination room by a provider. | 4. Patient participation in physical assessment (eg, blood pressure, weight) and documentation. Fundal height and heart rate monitoring occur in group space. If required, health concerns that require private consultation and cervical examinations are conducted in ancillary visits in a private examination room. |
| 5. Education is provider-dependent and may be random based on time available for education and/or response to patient-initiated queries. | 5. Education runs throughout the 10 sessions with trained providers and structured materials. Self-assessment sheets at sessions provide continuous feedback. | |
| 6. Few opportunities for women to interact socially with other pregnant women. | 6. Opportunities for community building are present throughout prenatal and postpartum period. | |
| 7. Care is focused on medical outcomes and recommended testing. | 7. Care is focused on health outcomes and personal empowerment. Testing, such as blood draw, can be done in group setting. | |
| Patient access to or involvement in care | 8. Prenatal care records are maintained by the provider and not shared with the patient unless requested. | 8. Women contribute data to their own record by performing their weight and blood pressure as well as documentation. They are encouraged to keep copies of their progress for their personal records. Transparency of the medical chart should contribute to increased safety. |
| 9. Provider schedule determines patient appointment dates and times. | 9. Schedule of group visits is available at first session, which occurs at approximately 16 weeks. | |
| 10. Patient services are often fragmented (eg, smoking cessation and nutrition counseling, WIC, labor preparation). | 10. Group provides “one-stop shopping” with all services available within the group, providing services more efficiently. | |
| 11. Limited opportunity for women to have contact with other women after delivery. | 11. Community building throughout pregnancy often leads to ongoing support postpartum | |
| Time spent by providers and patients | 12. Variable waiting time. | 12. All care, education, and support take place within the 2-hour time period. No waiting room. |
| 13. May be difficult to adapt care to accommodate cultural issues. | 13. Group can provide a setting that is supportive of cultural and language differences. | |
| 14. Providers may find the provision of prenatal care to be repetitive and often lack sufficient time to go into more detail regarding specific patient questions or concerns. | 14. Groups minimize repetition and permit sufficient time for more in-depth discussion. | |
| 15. Average visit time is limited by provider schedule. | 15. Total provider/patient time throughout pregnancy is approximately 20 hours. | |
| Administration and scheduling | 16. Efficiency marked by scheduling of patients at 10- to 15-minute intervals. | 16. Within a 2-hour period, 8−10 women can receive total care in a conference or community room. This allows examination rooms to be used for other purposes. |
| Provider, resident, student education | 17. Student education is limited by examination room space and time constraints. | 17. Students and preceptors work together within the group, incorporating student education and direct supervision. |
WIC, Special Supplemental Nutrition Program for Women, Infants, and Children.
The quality and context of prenatal services differs between the United States and other developed and developing nations. The description of prenatal care in this table reflects typical traditional individual care in a public health care setting and may not be inclusive of the quality of services in other settings. However, it is noteworthy that nearly all prenatal care is provided in this group space with the same health care provider. Moreover, there is substantially more time shared between patient and provider (20 hours across the pregnancy in the group setting), and there is typically no need for separate visits for labor preparation or laboratory testing. More information is available at www.centeringpregnancy.com.
The primary objective of this study was to conduct a multisite randomized controlled trial to evaluate whether group prenatal care would result in decreases in human immunodeficiency virus (HIV) risk behavior and sexually transmitted diseases. This is a secondary analysis to determine whether group prenatal care leads to better reproductive health outcomes, such as reductions in the numbers of preterm births and low birth weight infants, as well as improved psychosocial outcomes and patient satisfaction, and also to examine potential differences in health care costs.
MATERIALS AND METHODS
Young women (aged 14−25 years, n=1,047) entering prenatal care at two publicly funded clinics were randomly assigned to standard individual care or group care (Fig. 1). The differences in the quantity and quality of prenatal care are substantial between individual care and group care as described in Table 1. Individual prenatal care across the pregnancy occurs over the course of approximately 2 hours. Group prenatal care across the pregnancy occurs over the course of approximately 20 hours.
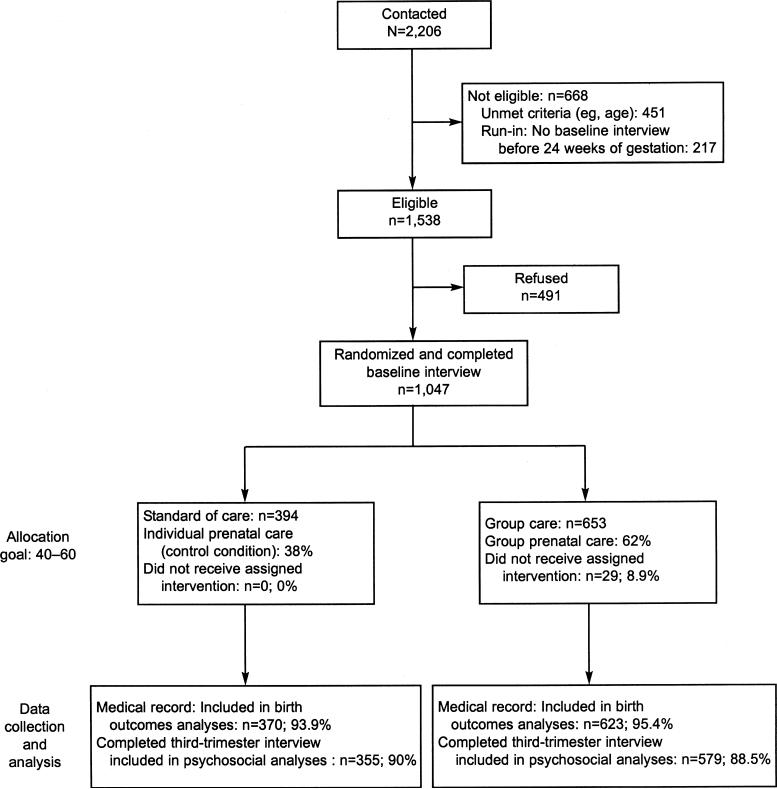
Fig. 1.
CONSORT study description. All outcomes were measured using medical records or at the trimester 3 interview, with the exception of breastfeeding initiation, which was measured at the interview conducted 6 months postpartum (n=783). There was no differential dropout between group and individual care (P=.95).
Participants were randomly assigned by using a blocked randomized controlled design, stratified based on site and expected month of delivery. Allocation was concealed from participant and research staff until eligibility screening was completed and study condition was assigned. These tasks were completed by trained research team members who were independent of prenatal care. A computer-generated randomization sequence, password protected to recruitment staff and participants, was used to assign participants. Although it was not possible to have treatment blinded (common practice in clinical interventions), all measurement and data collection were conducted in blinded fashion independently of the care setting. Moreover, medical record abstracters were independent of clinical care.
Participants were recruited from large obstetrics clinics in two university-affiliated hospitals. Procedures were approved by Human Investigation Committees at both sites (No. 11972, Yale University, New Haven, CT, and No. 197−2001, Emory University, Atlanta, GA). African-American women with limited financial resources are overrepresented, reflecting clinic use patterns. There were no deviations from the study procedures as originally planned, with the exception of expanded access by age at study entry from 14−19 years to 14−25 years; this expanded access was implemented before randomization.
Between September 2001 and December 2004, women attending their first or second prenatal care visit were referred by a provider or approached directly by research staff. Inclusion criteria were as follows: less than 24 weeks of gestation, age 25 years or less, no medical problems requiring individualized care as “high-risk pregnancy” (eg, diabetes, HIV), English or Spanish language, and willingness to be randomized. Potential participants were screened; if eligible, research staff explained the study in detail and obtained informed consent. Baseline interviews occurred at an average gestational age of 18 weeks (standard deviation [SD] 3.3). Each patient underwent second-trimester ultrasonography for confirmation of dating and anatomy. Estimated date of confinement was established by an attending obstetrician who was independent of the study, and this was confirmed by ultrasonography. Participants were followed prospectively through 1 year postpartum. All participants were paid $20 for each interview.
Of the 1,538 eligible women, 1,047 (68%) enrolled. Compared with those who declined enrollment, participants were more likely to be African American, older, and at a later gestational age at initial screening (all P<.01). Recruitment was nearly equivalent between the two study sites: Atlanta (n=546, 52%) and New Haven (n=503, 48%). Intervention effects were not statistically different on primary outcomes by study site; therefore, analyses were combined across sites.
Even with randomization, baseline differences can emerge by chance. To evaluate this, we conducted χ2 and t tests comparing the study conditions on demographic, medical history, and major study variables assessed at the baseline interview (Table 2). Despite randomization, three differences by study condition were documented. By chance, individuals assigned to group prenatal care were more likely to be African American, less likely to have a history of preterm birth, and more likely to have high levels of prenatal distress. Therefore, all subsequent analyses controlled for these variables.
Table 2.
Baseline Differences by Study Condition
| Group Prenatal Care (n=623) | Individual Prenatal Care (n=370) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Race | |||
| African American | 81.3 | 73.8 | .014 |
| Latina | 11.1 | 17.2 | |
| White or other | 7.5 | 9.0 | |
| Age (y, mean±SD) | 20.3±2.6 | 20.6±2.7 | .07 |
| Last year of education (mean±SD) | 11.4±1.5 | 11.3±1.6 | .51 |
| Median household income from census (US $, mean±SD) | 34,415±15,291 | 33,198±13,774 | .22 |
| Clinical characteristics | |||
| Nulliparous | 61.8 | 61.4 | .30 |
| History of preterm birth | 4.0 | 7.1 | .04 |
| Prior miscarriage or stillbirth | 18.7 | 17.9 | .75 |
| Prepregnancy BMI (kg/m2, mean±SD) | 27.0±7.1 | 26.7±7.4 | .54 |
| Gestational age at study entry (wk, mean±SD) | 18.0±3.4 | 18.4±3.3 | .11 |
| History of sexually transmitted infection | 52.5 | 50.0 | .43 |
| Smoking prior to pregnancy | 35.8 | 33.8 | .55 |
| Smoking since pregnancy | 20.9 | 20.0 | .74 |
| Drinking prior to pregnancy | 39.7 | 38.9 | .80 |
| Drinking during pregnancy | 8.8 | 7.9 | .62 |
| General life stress (mean±SD) | 17.8±6.9 | 17.7±6.9 | .70 |
| Prenatal distress (mean±SD) | 15.2±7.1 | 13.7±7.3 | <.001 |
SD, standard deviation; BMI, body mass index.
Data are expressed as percentages except where otherwise indicated.
Groups of eight women (on average) are formed based on estimated delivery month and led by a trained practitioner (eg, midwife, obstetrician). The model provides integrative prenatal care by combining three primary components: assessment, education and skills building, and support. All prenatal care occurs within the group setting, except for the initial assessment at entry to care, health concerns involving need for privacy, and cervical assessments late in pregnancy. After the first visit, participants in the study were randomly assigned to continue care individually or in the group setting. When group participants arrive, they engage in self-care activities of weight and blood pressure assessment and update their medical records. Individual prenatal assessments (eg, fundal height, fetal heart rate) are completed by the practitioner during the first 25−30 minutes within the group space. The majority of time is spent with women and clinicians engaging in discussion, education, and skills building to address explicit learning objectives in prenatal care, child birth preparation, and postpartum and parenting roles. Handouts and self-assessment sheets facilitate group discussions and stimulate self-care and evaluation. The full curriculum consists of 10 structured sessions (120 minutes each) conducted from 16 through 40 weeks of gestation. Table 1 provides a comparative assessment of traditional care and group care.
Structured interviews by audio computer-assisted self-interviewing (audio-CASI) were conducted upon study entry: before session 1 among group participants, and before 24 weeks of gestation in individual care. Audio-CASI allows respondents to simultaneously listen with headphones and see questions on a computer laptop, facilitating completion for participants with lower reading skills. Audio-CASI has been previously validated among pregnant women.15 Trained study staff was present to facilitate the self-interview process by answering any questions and assisting with any technical issues. Medical records were reviewed for 993 participants (95%) by trained medical abstractors who were independent of care and blinded to study assignment.
All encounters to one of the facilities (Yale New Haven Hospital) are recorded electronically in a computerized database used for billing purposes, which identifies sites of care, inpatient compared with outpatient status, International Classification of Diseases, 9th Revision (ICD-9) codes, and cost of care. Therefore, cost data were available at this site (n=503). Cost data included charges, revenue, and actual costs, but only actual costs were used because they are not dependent on reimbursement rates.
Primary outcomes included gestational age at delivery, dichotomized as term or preterm (less than 37 weeks), and infant birth weight, dichotomized as normal or low birth weight (less than 2,500 g).16 All patients underwent second-trimester ultrasound examination for confirmation of dating and anatomy. Estimated date of confinement was established by a consulting obstetrician who was independent of the study, and the date was confirmed by ultrasonography. Decisions on inpatient management and delivery were made by attending physicians and midwives, who were independent of the site of outpatient care, on a pre-established rotating call schedule.
Adequacy of prenatal care was measured by using standard scoring on the Kotelchuck Index.17 Apgar scores at 5 minutes were taken from hospital labor logs. Breastfeeding initiation was based on participant self-report at the first postpartum interview.
All psychosocial outcomes were measured during the third trimester of pregnancy (average gestational age 35 weeks, SD 3.1). Psychosocial outcomes included five domains. Pregnancy knowledge was measured by using a tool developed by the research team to assess prenatal and infant care knowledge; this was not validated. Prenatal distress was measured with the established Pregnancy Distress Questionnaire.18 Readiness for labor and delivery and readiness for infant care scales queried preparedness for delivery and infant care. Satisfaction with prenatal care was measured by using an adaptation of the Patient Participation and Satisfaction Questionnaire.19
Initial analyses were based on intention-to-treat models, with randomized study condition as the primary independent variable: individual compared with group prenatal care. General linear model and logistic regression analyses for basic group differences on birth outcomes, psychosocial factors, and patient satisfaction were conducted. Given differences despite randomization, race, preterm distress, and history of preterm birth were statistically controlled. Additionally, analyses controlled for relevant clinical risks for adverse perinatal outcomes (ie, smoking, history of preterm birth, history of miscarriage or stillborn birth).
Beyond the primary intention-to-treat analyses, several additional analytic approaches were used. Cox proportional hazards analysis was conducted to provide more detailed assessment of time to preterm delivery. Post hoc analysis was conducted to determine if group care had differential outcome for African Americans, who represented 80% of participants. Finally, we evaluated whether there was a potential “dose-response” intervention effect for the primary outcome variables of gestational age and birth weight.
Because the study was originally powered statistically to detect differences in incident sexually transmitted infection, secondary power analyses were conducted for the purposes of these analyses, based on preterm birth as the outcome. With a targeted sample size of 1,040 (n=416 in control and n=624 in the intervention group), we calculated 80% power to detect a 33% reduction in preterm birth (P<.05). This calculation was based on national U.S. base rates for preterm birth, weighted by racial and ethnic distribution in this sample, equivalent to a weighted preterm birth rate of 16.4% (U.S. National Center for Health Statistics, 2006).
RESULTS
The average age was 20.4 years (SD 2.6), with 49% aged 14−19 years. Eighty percent were African American. Thirty-eight percent had completed high school (or graduate equivalency degree), 36% were still in high school, and 26% had dropped out. Only 31% were currently employed; the remainder received economic support from a partner or family member (47%) or from public assistance (22%). There were no significant differences in age, parity, education, or median income between study conditions (Table 2). There were no systematic differences between those who were retained and those who were lost to medical record review nor any differential loss to follow-up between those randomly assigned to group care and those assigned to individual care. In addition, among those who were lost to follow-up, there were no differences between group and nongroup participants on demographic and main study variables.
To examine birth outcomes, only singleton infants were evaluated. Excluded from analyses were eight sets of twins and three infants not viable using clinical standards, ie, gestational age 20 weeks or less or birth weight 350 g or less. Women assigned to group care were significantly less likely to have preterm births than those in individual care: 9.8% compared with 13.8% (61 of 623 compared with 51 of 370, respectively; Table 3 and Fig. 2). This is equivalent to a risk reduction of 33% (odds ratio [OR] 0.67, 95% confidence interval [CI] 0.44−0.99, P=.045), or 40 per 1,000 births. Excluding those with prior pre-term birth (n=48), results remain significantly different favoring group care (P=.05).
Table 3.
Pregnancy and Psychosocial Outcomes, by Study Condition
| Group Prenatal Care (n=623) | Individual Prenatal Care (n=370) | Statistic | P | OR (95% CI) | |
|---|---|---|---|---|---|
| Birth outcomes and prenatal care | |||||
| Preterm birth | 9.8 | 13.8 | χ2=4.01 | .045 | 0.67 (0.44−0.98) |
| Gestational age (wk, mean±SD) | 39.1±2.8 | 38.9±2.5 | F=0.70 | .40 | |
| Low birth weight (less than 2,500 g) | 11.3 | 10.7 | χ2=0.03 | .90 | 0.98 (0.64−1.50) |
| Birth weight (g, mean±SD) | 3,160.6±626.3 | 3,111.8±636.8 | F=1.40 | .24 | |
| Small for gestational age | 14.3 | 15.1 | χ2=0.67 | .42 | 0.86 (0.59−1.24) |
| Fetal demise | 1.3 | 2.2 | χ2=1.34 | .25 | 0.55 (0.20−1.50) |
| Less than adequate PNC (based on Kotelchuck Index) | 26.6 | 33.0 | χ2=6.49 | .01 | 0.68 (0.50−0.91) |
| Neonatal outcomes | |||||
| Apgar, 5 minutes [mean±SD (median)] | 8.8±1.1 (9) | 8.8±1.0 (9) | F=0.60 | .44 | |
| Admitted to NICU | 8.5 | 7.8 | χ2=0.07 | .80 | 1.06 (0.66−1.72) |
| Breastfeeding initiation* | 66.5 | 54.6 | χ2=12.5 | .001 | 1.73 (1.28−2.35) |
| Psychosocial outcomes (mean±SD) | |||||
| Prenatal knowledge | 41.1±7.3 | 38.5±6.8 | F=27.08 | <.001 | |
| Prenatal distress | 12.43±7.0 | 12.93±7.1 | F=1.96 | .16 | |
| Readiness for labor and delivery | 76.2±30.6 | 68.6±33.2 | F=12.77 | <.001 | |
| Readiness for infant care | 90.0±21.9 | 86.9±26.0 | F=3.68 | .056 | |
| Satisfaction with prenatal care | 113.3±13.3 | 108.4±14.4 | F=27.16 | <.001 |
OR, odds ratio; CI, confidence interval; SD, standard deviation; PNC, prenatal care; NICU, neonatal intensive care unit.
Data are expressed as percentages except where otherwise indicated.
All analyses controlled for factors that were different by study condition (P<.10) despite randomization (race, age, prenatal distress, history of preterm birth) and clinical risk factors strongly associated with birth outcomes (smoking, prior miscarriage, or stillbirth). Analyses for continuous variables were conducted with analysis of covariance, and analyses for dichotomous variables were conducted with logistic regression with covariates.
At 6-month postpartum interview (n=783).
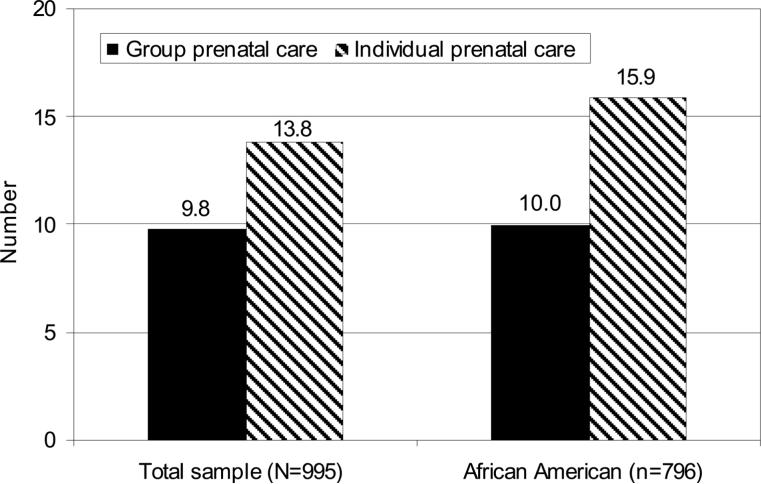
Fig. 2.
Preterm delivery for total sample and African Americans only. All analyses were controlled for factors that were different by study condition (P<.10), despite randomization (race, age, prenatal distress, history of preterm birth) and clinical risk factors strongly associated with birth outcomes (smoking, prior miscarriage or stillbirth). Total sample: odds ratio (OR) 0.67, 95% confidence interval (CI) 0.44−0.99, P=.045; African American only: OR 0.59, 95% CI 0.38−0.92, P=.02.
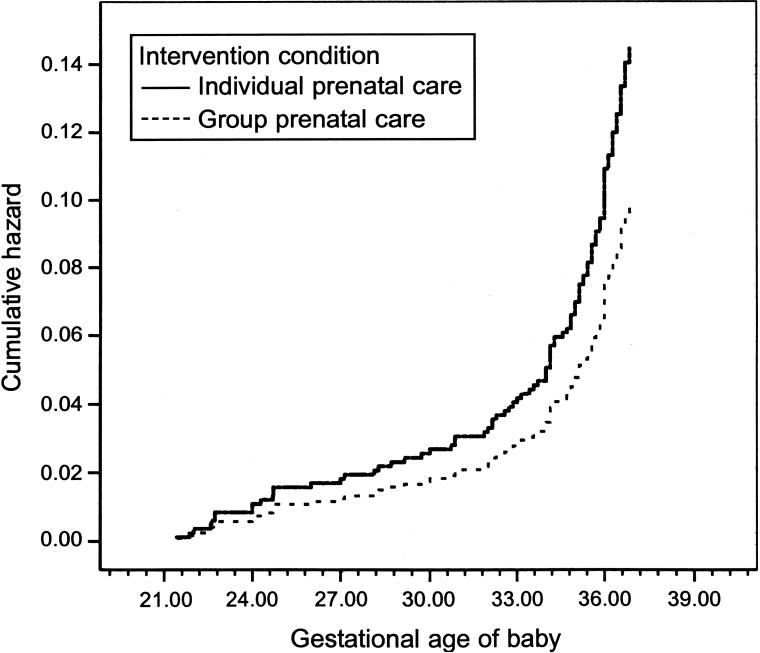
To explore the nature of the difference on pre-term birth, Cox proportional hazards was conducted to model weeks of gestational age until preterm birth (censored outcome). Results indicate that group prenatal care significantly influenced the preterm hazard function after adjustment for race, age, prenatal distress, history of preterm birth, smoking, and prior miscarriage or stillbirth (χ2=3.79, P=.048). By 26 weeks of gestation, women in individual care were more likely to deliver preterm, continuing until maximum differentiation between individual and group care at 35−37 weeks of gestation (Fig. 3).
Fig. 3.
Hazard function for preterm birth. χ2=3.79, P=.048.
Post hoc analysis was conducted to determine if group care had differential outcome for African Americans, who represented 80% of participants. When African Americans were examined alone, the impact of group care on reduced risk for preterm birth was strengthened: 10.0% compared with 15.8% (χ2=5.22, P=.02; OR 0.59, 95% CI 0.38−0.92) (Fig. 2).
Using intention-to-treat analyses, we found no significant differences in gestational age (measured in weeks), birth weight, percentage of low birth weight infants, or percentage of small for gestational age infants (less than 10th percentile by gestational age) (Table 2). Therefore, we evaluated whether there was a potential “dose-response” intervention effect. The number of visits was significantly associated with both gestational age (r=0.31, P<.001) and birth weight (r=0.28, P<.001). This effect remained significant, although attenuated, when attendance was adjusted to include eligible visits (ie, date of health care entry to birth or demise, even if preterm) (r=0.14, P=.003 for gestational age; r=0.13, P=.003 for birth weight). To illustrate, we trichotomized the number of eligible visits attended (less than 33%, 33−66%, 67−100%). Mean gestational age and birth weight for each category increased sequentially from 37.9 to 39.0 to 39.2 weeks and from 2,874.3 to 3,103.2 to 3,181.6 g, respectively.
Using intent-to-treat analyses, adequacy of prenatal care indicates that group patients were significantly less likely to have inadequate care: 26.6% compared with 33% (P = .01). There was no difference in Apgar score at 5 minutes nor in the percentage of infants admitted to the neonatal intensive care unit. Rates of breastfeeding initiation were significantly improved for women in group care compared with those in individual care: 66.5% compared with 54.6% (P<.001). There were no adverse effects.
Women in group care had significantly better psychosocial outcomes compared with those in individual care. They had more prenatal care knowledge and felt more prepared for labor and delivery (both P<.001). They also had significantly higher satisfaction with prenatal care (P<.001) (Table 3).
Basic billing data from hospital records was available at one site only (Yale-New Haven Hospital, n=503). Results indicated no significant difference in raw costs (in U.S. dollars) of prenatal care (M=$4,149 compared with $4,091, P=.69) or delivery care costs (M=$3,433 compared with $3,417, P=.94). These analyses controlled for variables as in all other prior analyses (race, prenatal distress, history of preterm birth, smoking, history of miscarriage or stillborn birth).
DISCUSSION
Investigators and clinicians have called for changes in the health care delivery system to address intransigent problems like preterm birth.11,20 Based on the results of this randomized controlled trial, it appears that group prenatal care may be one potential approach toward meeting this aim. Davidoff et al21 specifically identify a need for further investigation of optimal obstetric and neonatal management for late preterm infants. These late preterm births account for three fourths of all preterm births in the United States and Europe and, therefore, are important from a public health perspective of cumulative adverse consequences and costs. We documented a 33% reduction in the odds of preterm birth, with time to preterm birth delayed for those randomized to group prenatal care. This delay began at 26 weeks of gestation, and the largest differences were documented in the late preterm period. In the United States, these late pre-term births (34−36 6/7 weeks) represent the fastest-growing segment and the largest proportion (74%) of singleton preterm births.21 Despite their relatively large size and apparent functional maturity, compared with term infants, late preterm infants are at increased risk for neonatal morbidity (eg, respiratory distress, jaundice) and mortality, along with consequent excess hospital costs.22–24 In a study quantifying the costs of prematurity by gestational age, Gilbert et al24 document that the total costs for each gestational age group from 25 to 36 weeks were roughly the same, concluding that opportunities to prevent pre-term delivery and decrease costs are potentially available at all preterm gestational ages.
Young women assigned to group prenatal care had other clinical and psychosocial advantages compared with those receiving individual care. Birth weight was not significantly different using intent-to-treat analyses, although a dose-response effect was observed: the greater the exposure to the intervention (ie, more group visits), the longer the gestation and higher the birth weight, even after adjusting for important clinical factors and preterm birth. This reflects our current clinical understanding of only partial concordance between gestational age and birth weight overall, with only two thirds of low birth weight babies also being premature.1 Being born too early and being born too small have distinct multifactorial causes and risk factors.20 Unfortunately, risk factor screening has no demonstrated effect on reducing adverse perinatal outcomes, and few interventions have successfully reduced preterm birth or low birth weight.25,26 Group prenatal care is more multifaceted than many other clinical and psychosocial interventions that seek to augment care with more visits or more information using didactic approaches, which may be one reason for these relatively favorable outcomes.
This study is limited in several ways. First, favorable results of the intervention were not uniform. The intervention resulted in some documented benefits as well as some nonsignificant differences with intent-to-treat analyses. Nonetheless, there were no apparent adverse effects, and costs were neutral. Given rising rates of preterm birth with few effective interventions documented, group prenatal care may provide an alternative model of prenatal care. Second, the sample represents a relatively restricted group of young, ethnic minority women of low socioeconomic status who attend urban hospital clinics for prenatal care. This is a group at high risk of adverse perinatal outcomes and, therefore, may be most in need of substantive clinical interventions to reduce risk. Replication with diverse patient populations and within diverse clinical settings is essential to ensure reliability, generalizability, and clinical effectiveness. Rigorous clinical assessment through larger multicenter trials is warranted.
Future research will evaluate the biologic, behavioral, and social mechanisms by which group care may have its effects. For example, one potential biologic mechanism for our salutary effect on preterm delivery is stress reduction, altering the maternal and fetal hypothalamic pituitary axes, which can precipitate preterm delivery by way of endocrine changes.27 A clinical and social benefit is that group prenatal care provides substantially more contact with providers; medical and ancillary support services are integrated to respond to the complex needs of pregnant women.28 Mechanisms should be identified by which groups may facilitate development of community norms to enhance healthy behaviors in general and to reduce perinatal risk specifically. Finally, future research on group prenatal care will include a full cost-effectiveness analysis and evaluation of service use for mother and baby from pregnancy through first year of life.
In the United States alone, which ranks at the bottom among developed nations for infant mortality, preterm birth accounts for 35% of all U.S. health care spending for infants, with direct charges of $15.5 billion in 2002.20 Even modest risk reduction may have beneficial effects on lifetime costs and risks if changes occur when the likelihood of adverse outcomes is high.29 Any intervention that shows promise to reduce preterm birth warrants further clinical and empirical attention.
Acknowledgments
Funded by National Institute of Mental Health grant R01 MH/HD61175 to Jeannette R. Ickovics, PhD.
Footnotes
Financial Disclosure Dr. Westdahl receives approximately $3,000 per year from the Centering Pregnancy and Parenting Association Inc (Cheshire, CT) for training facilitation. Ms. Rising is the executive director of the nonprofit entity, the Centering Pregnancy and Parenting Association Inc, which promotes the Centering Pregnancy model of care nationally and internationally. The other authors have no potential conflicts to disclose.
REFERENCES
- 1.Tucker J, McGuire W. Epidemiology of preterm birth. BMJ. 2004;329:675–8. doi: 10.1136/bmj.329.7467.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofman PL, Regan F, Jackson WE, Jefferies C, Knight DB, Robinson EM, et al. Premature birth and later insulin resistance. N Engl J Med. 2004;351:2179–86. doi: 10.1056/NEJMoa042275. [DOI] [PubMed] [Google Scholar]
- 3.Foulder-Hughes LA, Cooke RW. Motor, cognitive, and behavioural disorders in children born very preterm. Dev Med Child Neurol. 2003;45:97–103. [PubMed] [Google Scholar]
- 4.Hobel CJ, Ross MG, Bemis RL, Bragonier JR, Nessim S, Sandhu M, et al. The West Los Angeles Preterm Birth Prevention Project. I. Program impact on high-risk women. Am J Obstet Gynecol. 1994;170:54–62. doi: 10.1016/s0002-9378(94)70384-1. [DOI] [PubMed] [Google Scholar]
- 5.Klerman LV, Ramey SL, Goldenberg RL, Marbury S, Hou J, Cliver SP. A randomized trial of augmented prenatal care for multiple-risk, Medicaid-eligible African American women. Am J Pub Health. 2001;91:105–11. doi: 10.2105/ajph.91.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitzman H, Olds DL, Henderson CR, Hanks C, Cole R, Tatelbaum R, et al. Effect of prenatal and infancy home visitation by nurses on pregnancy outcomes, childhood injuries, and repeated childbearing: a randomized controlled trial. JAMA. 1997;278:644–52. [PubMed] [Google Scholar]
- 7.McLaughlin FJ, Altemeier WA, Christensen MJ, Sherrod KB, Dietrich MS, Stern DT. Randomized trial of comprehensive prenatal care for low-income women: effect on infant birth weight. Pediatrics. 1992;89:128–32. [PubMed] [Google Scholar]
- 8.Heins HC, Jr, Nance NW, McCarthy BJ, Efird CM. A randomized trial of nurse-midwifery prenatal care to reduce low birth weight. Obstet Gynecol. 1990;75:341–5. [PubMed] [Google Scholar]
- 9.Belizan JM, Barros F, Langer A, Farnot U, Victora C, Villar J. Impact of health education during pregnancy on behavior and utilization of health resources. Latin American Network for Perinatal and Reproductive Research. Am J Obstet Gynecol. 1995;173:894–9. doi: 10.1016/0002-9378(95)90362-3. [DOI] [PubMed] [Google Scholar]
- 10.Moore ML, Meis PJ, Ernest JM, Wells HB, Zaccaro DJ, Terrell T. A randomized trial of nurse intervention to reduce preterm and low birth weight births. Obstet Gynecol. 1998;91:656–61. doi: 10.1016/s0029-7844(98)00012-x. [DOI] [PubMed] [Google Scholar]
- 11.Lu MC, Tache V, Alexander GR, Kotelchuck M, Halfon N. Preventing low birth weight: is prenatal care the answer? J Matern Fetal Neonatal Med. 2003;13:362–80. doi: 10.1080/jmf.13.6.362.380. [DOI] [PubMed] [Google Scholar]
- 12.Rising SS. CenteringPregnancy: an interdisciplinary model of empowerment. J Nurse Midwifery. 1998;43:46–54. doi: 10.1016/s0091-2182(97)00117-1. [DOI] [PubMed] [Google Scholar]
- 13.Ickovics JR, Kershaw TS, Westdahl C, Rising SS, Klima C, Reynolds H, et al. Group prenatal care and preterm birth weight: results from a matched cohort study at public clinics. Obstet Gynecol. 2003;102:1051–7. doi: 10.1016/s0029-7844(03)00765-8. [DOI] [PubMed] [Google Scholar]
- 14.Grady MA, Bloom KC. Pregnancy outcomes of adolescents enrolled in a CenteringPregnancy program. J Midwifery Womens Health. 2004;49:412–20. doi: 10.1016/j.jmwh.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 15.C'De Baca J, Lapham SC, Skipper BJ, Watkins ML. Use of computer interview data to test associations between risk factors and pregnancy outcomes. Comput Biomed Res. 1997;30:232–43. doi: 10.1006/cbmr.1997.1449. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham FG, Hauth JC, Leveno KJ, Gilstrap L, Bloom SL, Wenstrom KD. Williams obstetrics. 22nd ed. McGraw Hill; New York (NY): 2005. [Google Scholar]
- 17.Kotelchuck M. The Adequacy of Prenatal Care Utilization Index: its U.S. distribution and association with low birth-weight. Am J Public Health. 1994;84:1486–9. doi: 10.2105/ajph.84.9.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobel M. The Revised Pregnancy Distress Questionnaire (NUPDQ) State University of New York at Stony Brook; Stony Brook (NY): 1996. [Google Scholar]
- 19.Littlefield VM, Adams BN. Patient participation in alternative perinatal care: impact on satisfaction and health locus of control. Res Nurs Health. 1987;10:139–48. doi: 10.1002/nur.4770100305. [DOI] [PubMed] [Google Scholar]
- 20.Institute of Medicine . Preterm birth: causes, consequences, prevention. National Academy Press; Washington (DC): 2006. [Google Scholar]
- 21.Davidoff MJ, Dias T, Damus K, Russell R, Bettegowda VR, Dolan S, et al. Changes in the gestational age distribution among U.S. singleton births: impact on rates of late preterm birth, 1992 to 2002. Semin Perinatol. 2006;30:8–15. doi: 10.1053/j.semperi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Wang ML, Dorer DJ, Fleming MP, Catlin EA. Clinical outcomes of near-term infants. Pediatrics. 2004;114:372–6. doi: 10.1542/peds.114.2.372. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro-Mendoza CK, Tomashek KM, Kotelchuck M, Barfield W, Weiss J, Evans S. Risk factors for neonatal morbidity and mortality among “healthy,” late preterm newborns. Semin Perinatol. 2006;30:54–60. doi: 10.1053/j.semperi.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert WM, Nesbitt TS, Danielsen B. The cost of prematurity: quantification by gestational age and birth weight. Obstet Gynecol. 2003;102:488–92. doi: 10.1016/s0029-7844(03)00617-3. [DOI] [PubMed] [Google Scholar]
- 25.Green NS, Damus K, Simpson JL, Iams J, Reece EA, Hobel CJ, et al. Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol. 2005;193:626–35. doi: 10.1016/j.ajog.2005.02.106. [DOI] [PubMed] [Google Scholar]
- 26.Sperling MA. Prematurity: a window of opportunity? N Engl J Med. 2004;351:2229–31. doi: 10.1056/NEJMe048274. [DOI] [PubMed] [Google Scholar]
- 27.Hobel CJ. Stress and preterm birth. Clin Obstet Gynecol. 2004;47:856–80. doi: 10.1097/01.grf.0000142512.38733.8c. [DOI] [PubMed] [Google Scholar]
- 28.Halbreich U. The association between pregnancy processes, preterm delivery, low birth weight, and postpartum depressions: the need for interdisciplinary integration. Am J Obstet Gynecol. 2005;193:1312–22. doi: 10.1016/j.ajog.2005.02.103. [DOI] [PubMed] [Google Scholar]
- 29.Rose G. The strategy of preventive medicine. Oxford University Press; New York (NY): 1992. [Google Scholar]