Abstract
Hyperproliferative epidermal disorders range from benign hyperplasias such as psoriasis to basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), the two most common cancers in the US. While they all arise from the epidermis, these diseases differ dramatically in biological behavior and their underlying gene expression patterns have not been compared. We thus examined mRNA transcript levels in these disorders to identify and further characterize differentially expressed genes. Transcript expression patterns distinguish these disorders and identify EGR1, among other genes, whose epidermal expression is decreased in BCC and SCC but is elevated in psoriasis. Egr-1 inhibits growth of benign and malignant epidermal cells in vitro and appears to suppress both Cdc25A expression and Cdk2 dephosphorylation. These data indicate that gene expression profiling can differentiate epidermal hyperproliferative diseases and suggest that Egr-1 may play a role in preventing uncontrolled epidermal growth.
Keywords: Cancer, Epidermis, Psoriasis, Gene expression, EGR1
Introduction
Cutaneous basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) are the two most common cancers in the US. (Miller 1994). Among epidermal malignancies, BCC causes significant local destruction but is generally non-metastatic while SCC is biologically more aggressive and can cause significant mortality. BCC variants include superficial multicentric, nodular, sclerosing-morpheaform and pigmented types. Invasive SCC usually develops from a precancerous condition (actinic keratosis) or carcinoma in-situ, often on sun-exposed sites, and has a capacity to metastasize.
Psoriasis, on the other hand, is a benign chronic disease affecting more than 5 million Americans and is characterized by epidermal hyperplasia with inflammatory infiltrates (http://www.nlm.nih.gov/medlineplus/psoriasis.html). Psoriasis consists of psorasis vulgaris, guttate psoriasis, pustular psorasis and erythrodermic variants. The classic clinical lesions are sharply marginated papules and plaques with marked silvery-white scale typically on the elbows, knees, scalp and intertriginous areas. Histopathologically, fully developed clinical lesions show marked epidermal hyperplasia with characteristic features including regular elongation of epidermal rete ridges with characteristic bulbous enlargement of their tips, reciprocal elongation of intervening dermal papillae, thinning of the epidermis that lies immediately above the dermal papillae, and marked hyperkeratosis. A sparse superficial dermal perivascular lymphocytic inflammatory infiltrate with occasional neutrophils is typically present.
Genetic studies have identified the PTC gene as a candidate tumor suppressor gene in BCC and linked the sonic hedgehog signaling pathway to the proliferative expansion of basal epidermal cells (Bale and Yu 2001). Recent studies of SCC have elucidated roles of various oncogenes (Ras, Myc, epidermal growth factor receptor, and cyclin D1), tumor suppressor genes (TP53 and p16), and other gene dosage changes at various chromosomal loci (Mercurio 2003; O’Connor et al. 2001; Quinn et al. 1994). Searches for psoriasis susceptibility genes have led to the identification of several chromosomal loci, including those at 6p21 within the HLA complex, 17q, 1q, and 19q13 (Capon et al. 2000). Despite recent insights into the genetic basis of these epidermal hyperproliferative disorders, the underlying differences in their global gene expression profiles are not fully recognized and understood.
Gene expression profiling has proven informative in analysis of human malignancies with regard to tumor classification and identification of relevant signaling pathways and drug targets. Among epidermal malignancies, molecular profiling of transformed and metastatic murine SCC cells revealed altered expression of genes related to growth, apoptosis, and angiogenesis (Dong et al. 2001). Differential display PCR and expression arrays were applied to elucidate alterations in BCC-associated gene expression (Welss et al. 2003). Comparison between the molecular profiles of BCC and SCC identified four genes specifically modulated in SCC including RhoC and EMMPRIN genes (Marionnet et al. 2003). Reports on gene expression profiles of psoriasis mainly focused on the inflammatory aspect of the disease (Nomura et al. 2003; Oestreicher et al. 2001; Zhou et al. 2003). Systematic comparisons of these three epidermal hyperproliferative disorders, however, have not been performed.
In this study, we have examined and compared gene expression profiles of BCC, SCC and psoriasis. Our aims were to identify differentially expressed genes, especially those that distinguish benign from malignant hyperplasia, and further study the functional roles of such gene(s) in epidermal growth regulation. Here, we report that distinct expression patterns can distinguish among these epidermal hyperproliferative disorders. Differentially expressed genes include early growth response gene 1 (EGR1), which was induced in psoriatic hyperplasia but suppressed in BCC and SCC. We present evidence that Egr-1 appeared to inhibit hyperproliferation of benign and malignant epidermal cells in association with decreased Cdc25A expression and persistent inhibitory phosphorylation of Cdk2. Thus EGR1 represents a candidate growth regulatory gene contributing to differences between benign and neoplastic epidermal hyperproliferative disorders.
Material and methods
Human subjects
This study was approved by the Institutional Review Board at both Stanford University and the University of Connecticut Health Center. Biopsies were performed from both lesional and site-matched normal skin of each patient with a confirmed clinical and histopathologic diagnosis. For psoriasis, patients were selected from those not under active treatment for at least 6 months; all were of the psorasis vulgaris subtype.
Cell culture, gene transfer, and cell proliferation assays
Primary human epidermal cells were isolated from discarded surgical specimens and grown in a 1:1 mixture of SFM (GIBCO-BRL, Grand Island, NY) and Medium 154 (Cascade Biologics, Portland, OR) as described (Choate et al. 1996). The cDNA sequences corresponding to the coding regions of human Egr-1 and DBD[-]-Egr-1 (Gashler et al. 1993) were subcloned into EcoRI-NotI site of the LZRS retroviral vector (Kinsella and Nolan 1996). High titer retrovirus was prepared in human 293T packaging cells and human keratinocytes were transduced as described (Deng et al. 1998). Human keratinocytes immortalized with the E6/E7 virion of human papilloma virus (HPV) were from P. Marinkovich (Barbosa and Schlegel 1989), as are SCC-25 cells (Rheinwald and Beckett 1981). Appropriate protein expression was confirmed by Western blot analysis. The cell proliferation assays were performed by counting cell numbers daily from triplicate independent transductions initiated with identical cell numbers.
Gene expression profiling
Each biopsy specimen was divided into two parts. One was flash-frozen in liquid nitrogen prior to RNA extraction. The other was frozen in OCT at −80°C for histological confirmation. Atlas Human Cancer 1.2 Arrays were purchased from Clontech (Palo Alto, CA). RNA samples were isolated with RNeasy Kits (Qiagen, CA). 32P labeled cDNA probes were generated using gene-specific primer mix and hybridizations were performed according to manufacturer’s recommendations. Arrays were performed in triplicate for each patient and were subjected to both autoradiography and phosphorimaging for quantitation, with data values normalized to housekeeping gene controls. Cluster analysis was performed as described (Eisen et al. 1998).
Histochemistry and protein expression
For immunohistochemistry, paraffin sections were rehydrated and pretreated by boiling in 50 mM Tris-HCl (pH 9.5) for 4 min. Slides were washed in PBS, blocked with 10% normal serum, followed by overnight incubation at 4°C with 1:50 dilution of the respective antibodies. Biotin-streptavidin-horse radish peroxidase (HRP) secondary antibody detection system and DAB chromagen were employed. The expression level was arbitrarily defined as strong and moderate based on staining intensity. For immunoblotting, whole cell extracts were prepared from keratinocytes grown in vitro 12–48 h after transduction. All immunoblots were also incubated with antibodies to human β-actin control. Antibodies included those to Egr-1, FA synthase, PDGF-A, Cdc25A, Cdk6, cyclin A, p21, p53, p107, Rb, p130 and actin (Santa Cruz, CA); Cdk2, cyclin D1, cyclin E, and p27KIP1 (Pharmingen, San Diego, CA); anti-phospho Cdk2Tyr15 (Calbiochem, La Jolla, CA).
mRNA analysis
RNA samples from disease and site-matched control skin from the same patient were used both for microarray analysis as well as for northern blotting using Nytran membranes (Schleicher and Schuell, Keene, NH). Signals were scanned and normalized for loading against actin control for the same lane with all blots performed in triplicate. For in situ hybridization, tissues were fixed in 4% paraformaldehyde then 5 μm paraffin sections prepared, rehydrated and treated with 20 mg/ml proteinase K. After washing, sections were fixed again, rinsed with RNase free water and 0.1 M triethanolamine, incubated with acetic anhydride for 10 min, re-washed then dehydrated. Dried sections were hybridized with riboprobes prepared using the Riboprobe transcription system (Promega, Madison, WI). For real-time quantitative PCR (Q-PCR), Assay-on-demand probes for human EGR1, Cdc25A, p21, and GAPDH were purchased from Applied Biosystems (Norwalk, CT). One μg of total RNA was reverse-transcribed with random hexamers and Superscript II (Invitrogen, CA). Q-PCR was performed and analyzed with an ABI 7500 sequence detector using the relative quantification methods according to the manufacturer’s recommendations. GAPDH was used as an endogenous control.
Transgenic mice
The wild-type Egr-1 cDNA was subcloned via a blunt-ended BamHI site downstream of the 2,075 bp human K14 promoter and used to produce transgenic mice in C57B/16 (Byrne and Fuchs 1993). Transgene integration and expression was confirmed by PCR analysis, Q-PCR, and epidermal immunohistochmistry in F1 mice derived from six transgenic founder lines. For PMA-induced epidermal hyperproliferation, PMA (0.3 mg/ml) in ethanol was topically applied to four F1 mice and skin biopsies obtained 48 h later.
Results
Gene expression profiling of BCC, SCC and psoriasis
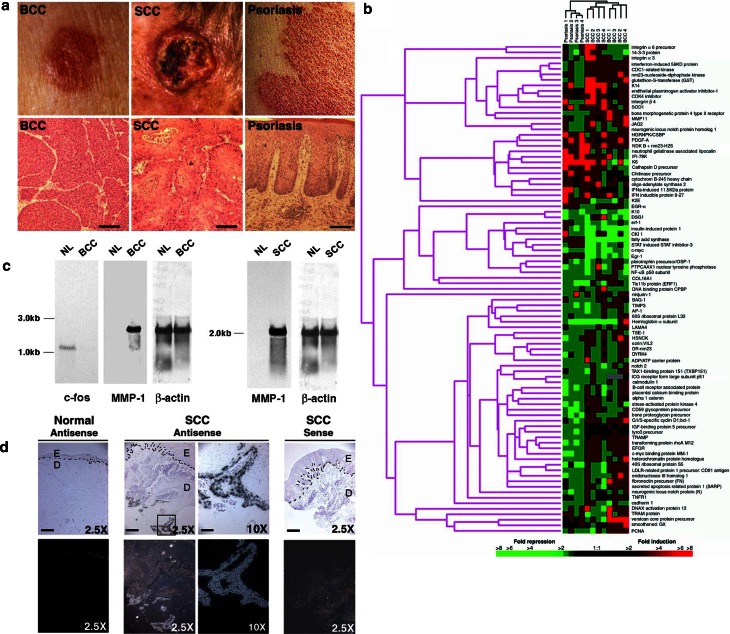
Patients with BCC, SCC and psoriasis display distinctive clinical and histologic features of epidermal hyperproliferation. We isolated mRNA from freshly excised lesional tissue in four patients per disorder. Lesional site prior to biopsy was photographed. Histopathological confirmation of the diagnosis was performed on the excised specimen (Fig. 1a). Site-matched uninvolved skin from the same patient was used as an internal control so that factors such as age or sun exposure would not account for the differences in the expression levels. Microarray analysis was performed in triplicate 1,200 sequence-verified gene microarrays. We employed hierarchical clustering software to analyze these disorders by similarity of gene expression profile (Fig. 1b) using an algorithm shown to group genes of similar biological function (Eisen et al. 1998). Analysis showed that each disease class clustered with itself, demonstrating that distinct genetic expression signatures distinguish BCC, SCC, and psoriasis.
Fig. 1.
Gene expression profiling of BCC, SCC, and psoriasis. (a) Clinical and histological features of the samples. Clinical appearance (upper panel): Translucent papule of nodular-type BCC (left), ulcerated SCC tumor (middle), and sharply demarcated erythematous plaque of psoriasis with micaceous scale (right). Histology (lower panel): Tumor nests with basaloid cells of BCC (left), invasive squamous tumor cells of SCC (middle) and hyperplastic epidermis with elongated rete ridges and inflammation in psoriasis (right). (b) Hierarchical clustering analysis. All genes shown have >2-fold changes in gene expression levels in at least one of the disorders studied. Rows represent genes on the microarray with their corresponding names to the right. Columns represent individual patients. Relative pseudo-colored intensities demonstrate gene expression changes such that red color indicates an increase in gene expression whereas green color indicates a decrease. Color scale shown at bottom. The hierarchical clustering dendrogram of relatedness of gene function is shown on the left. (c) Confirmatory Northern analysis. NL = normal site-matched control from the same patient. (d) Confirmatory in situ hybridization analysis. Dotted lines define the junction between epidermis (E) and dermis (D). The 10× field (boxed in the 2.5× field) shows strong MMP-1 expression in deeply invasive tumor islands. Scale bars = 200 μm for 2.5× low power fields and 50 μm for 10× fields
Over 100 genes displayed >2-fold differential expression in disease tissue compared to the patient’s own site-matched normal skin in all three disorders. These included genes involved in cell cycle promotion, signal transduction, matrix degradation, differentiation and inflammation (Fig. 1b). Among genes strongly induced in psoriasis are Egr-1, cathepsin D precursor, K6, neutrophil gelatinase associated lipocalin, PDGF-A, and FA synthase. Many genes displayed decreased expression in psoriasis, including those encoding rhoA, CD59, notch 2, c-myc binding protein MM-1, EGF receptor, and NF-κB p50. In all 3 diseases, PDGF-A and nm23-H2 were upregulated whereas c-myc was downregulated. We corroborated selected genes identified by microarray analysis by northern blot and in situ hybridization (Fig. 1c and d). Microarray analysis commonly underestimated changes in transcript expression by 6-fold or more when compared to quantitated northern blotting normalized to an actin loading control.
Expression of Egr-1, PDGF-A, and FA synthase
We focused our analysis on distinctive expression patterns displayed by PDGF-A, Egr-1, and FA synthase because the expression patterns of these genes were most consistent throughout samples within a disease category. PDGF-A displayed a 2–4-fold induction in all disorders. In microarray analysis, Egr-1 and FA synthase were increased in psoriasis (2.3 ± 0.7 and approximately 2.0 ± 0.5 fold, respectively), but were decreased in both BCC (2.6 ± 1.2 and 4.6 ± 2.6 fold) and SCC (6.6 ± 4.7 and 3.3 ± 1.2 fold) (Fig. 2a). To confirm these expression differences at the protein level in a larger number of patient tissues, we performed immunohistochemical analysis of 126 specimens of hyperproliferative epidermal diseases (Table 1). Detected predominantly in the basal layer within normal skin, both Egr-1 and PDGF-A were strongly expressed throughout psoriatic epidermis. Egr-1, however, displayed decreased expression in BCC and SCC while PDGF-A retained expression. FA synthase was expressed at minimal levels in normal and malignant tissue, but was strongly induced in psoriasis (Fig. 2b). A portion of the psoriasis samples did not demonstrate Egr-1 expression (Table 1). It is not clear whether it was due to antigen retrieval difficulties or whether other factors, such as active treatment, interfered with Egr-1 expression in these tissues.
Fig. 2.
Expression differences in selected genes. (a) Each data point represents the fold change in gene expression for a given patient in the disease tissue compared to their site-matched normal control skin for PDGF-A, Egr-1, and FA synthase. (b) Representative in situ protein expression in normal and lesional skin. Scale bar shown = 200 μm for all panels
Table 1.
Egr-1, PDGF-A, and FA synthase expression in human diseases of epidermal proliferation
| Human Tissue | No. specimens | ++ (% ) | + (%) | − (%) |
|---|---|---|---|---|
| Egr-1 | ||||
| Psoriasis | 20 | 70 | 6 | 24 |
| BCC, nodular | 21 | 0 | 10 | 90 |
| BCC, superficial | 16 | 0 | 12 | 88 |
| BCC, morpheic | 18 | 0 | 17 | 83 |
| SCC | 24 | 0 | 17 | 83 |
| SCC in situ | 17 | 0 | 47 | 53 |
| PDGF-A | ||||
| Psoriasis | 20 | 100 | 0 | 0 |
| BCC, nodular | 21 | 90 | 10 | 0 |
| BCC, superficial | 16 | 100 | 0 | 0 |
| BCC, morpheic | 18 | 89 | 11 | 0 |
| SCC | 24 | 100 | 0 | 0 |
| SCC in situ | 17 | 100 | 0 | 0 |
| FA synthase | ||||
| Psoriasis | 20 | 100 | 0 | 0 |
| BCC, nodular | 21 | 0 | 5 | 95 |
| BCC, superficial | 16 | 0 | 15 | 85 |
| BCC, morpheic | 18 | 0 | 0 | 100 |
| SCC | 24 | 4 | 8 | 88 |
| SCC in situ | 17 | 6 | 0 | 94 |
BCC = basal cell carcinoma, SCC = squamous cell carcinoma
Expression levels: ++ = strong, + = moderate, − = undetectable
Egr-1 exerts growth regulatory effects in epidermal cells
Strong Egr-1 expression in benign but not malignant epidermal hyperproliferation raised the possibility that Egr-1 may influence epidermal growth. To study this, we expressed both wild-type Egr-1 and a DNA binding domain deletion (DBD) Egr-1 mutant along with marker gene controls in primary keratinocytes by high efficiency retroviral transduction. Egr-1 inhibited growth of sub-confluent, rapidly proliferating normal human keratinocytes, HPV-immortalized cells, and malignant SCC-25 cells. This growth inhibition was dependent on the Egr-1 DNA binding domain as the DBD mutant supported increased proliferation (Fig. 3a). These in vitro growth data suggested that Egr-1 limited proliferation of normal and transformed epidermal cells.
Fig. 3.
Egr-1 limits epidermal cell hyperproliferation. (a) Proliferative output of sub-confluent epidermal cells in growth media. The effect of wild-type Egr-1, the Egr-1 DNA binding domain deletion mutant (DBD) compared to marker gene control is shown in normal human keratinocytes (NL), in keratinocytes immortalized with HPV 18 E6/E7 and in malignant SCC-25 cells. Total cell numbers 6 days after transduction are shown from triplicate independent transductions initiated with identical cell numbers. # = P < 0.01; * = P < 0.05 for differences between Egr-1 transduced cells and control cells. (b) Reactive epidermal hyperplasia in vivo. Murine epidermis transgenic for Egr-1 (K14-Egr-1) resists PMA-induced epidermal hyperplasia; scale bar = 30 μm. (c) Quantitative comparison of epidermal cell numbers/100 μm of basement membrane zone (BMZ) in PMA treated and ethanol control Egr-1 transgenic and littermate skin (n = 4 mice per group). ** = P < 0.001 for differences between PMA treated transgenic and control skin
Egr-1 transgenic murine epidermis is resistant to PMA-induced hyperplasia
To investigate epidermal growth restraint by Egr-1 in vivo, we generated transgenic mice expressing Egr-1 targeted to the basal layer using the K14 promoter. F1 progenies from six transgenic founder lines were characterized for epidermal Egr-1 expression. Four F1 mice that demonstrated significant increase in Egr-1 expression by RT-PCR and immunohistochemistry (data not shown) were used for further analysis along with their non-transgenic littermates. Egr-1 transgenic mice were viable and fertile, displaying no evidence of impaired epidermal growth control under normal conditions. To determine if Egr-1 limits induced epidermal hyperplasia in vivo, we used topical phorbol ester, known to induce epidermal hyperproliferation (Wang et al. 1999). Epidermal cell numbers per 100 μm of basement membrane were similar in unstimulated Egr-1 transgenic epidermis to that of non-transgenic littermates (36.7 ± 4.5 and 36.6 ± 3.0 cells/100 μm, respectively; n = 4 mice per group, P > 0.05). In response to PMA, however, Egr-1 transgenic epidermis showed decreased reactive hyperplasia compared to PMA-treated control (35.3 ± 3.2 and 68.7 ± 6.0 cells/100 μm, respectively; n = 4 mice per group, P < 0.001) (Fig. 3b). A similar response was observed when SDS was used instead of PMA (data not shown). These results suggest that Egr-1 confers resistance to epidermal hyperplasia in vivo.
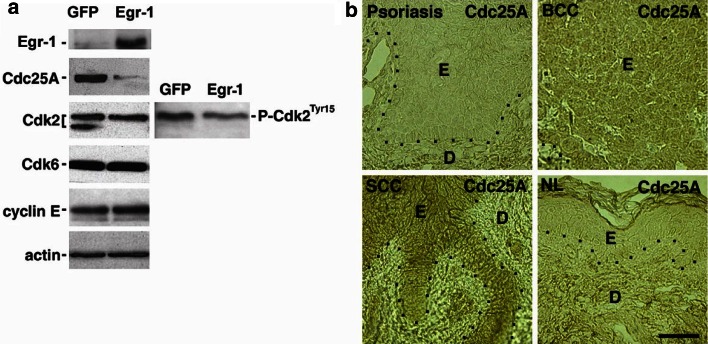
Egr-1 expression is associated with suppression of Cdc25A
To explore how Egr-1 exerts its growth regulatory effects in epidermal cells, we examined levels of G1 phase cell cycle regulators as a function of Egr-1 expression. Among the G1 phase cell cycle regulators studied, including Cdk6, cyclin A, p21, p53, p107, Rb, p130, cyclin D1, cyclin E, and p27KIP1 , a dramatic reduction of Cdc25A expression was observed in Egr-1 transduced cells (Fig. 4a). Because Cdc25A dephosphorylates Cdk2 on Tyr15/Thr14 to activate the Cdk2/cyclin E complex, we examined Cdk2 expression (Nilsson and Hoffmann 2000). Consistent with decreased Cdc25A, Egr-1 induction decreased levels of dephosphorylated active Cdk2. Inactive phosphorylated Cdk2 predominated in Egr-1 expressing cells, as confirmed with an antibody specifically recognizing the Tyr15-phosphorylated residue of Cdk2 (Fig. 4a). Persistent phosphorylation of Cdk2 is thus correlated with the reduced Cdc25A in Egr-1 transduced keratinocytes and therefore represents a potential mechanism for the proliferative restraint by Egr-1. Suppression of Cdc25A in cultured cells by Egr-1 suggested that its loss in epidermal malignancies would lead to increased Cdc25A and that Egr-1 overexpression in psoriasis could contribute to the converse. Indeed, we observed that Cdc25A expression was elevated in epidermal cells of both BCC and SCC but not in psoriasis (Fig. 4b), consonant with a potential role for regulation of Cdc25A in these conditions.
Fig. 4.
Expression of selected G1 phase cell cycle regulators as a function of Egr-1. (a) Protein extracts were prepared 36 h after high efficiency retroviral transduction. Note the decrease in Cdc25A with Egr-1 expression. Note also the decrease in the lower Cdk2 band which represents the phosphorylated less active form as confirmed in the right Cdk2 panel using antibody specific for Cdk2Tyr15. (b) Cdc25A expression in hyperproliferative skin disorders. Note increased expression in BCC and SCC and lack of increase in psoriasis compared to normal skin (NL). E = epidermis, D = dermis, dotted line = basement membrane zone. Scale bar = 100 μm
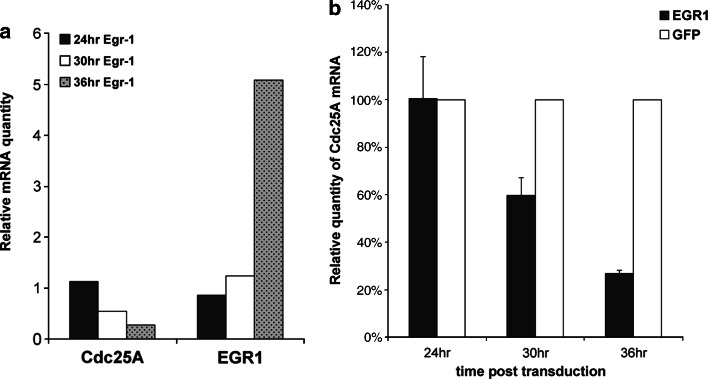
To further investigate the mechanism of Cdc25A regulation by Egr-1, we examined changes in mRNA levels of Cdc25A in primary human keratinocytes upon Egr-1 overexpression using real-time quantitative PCR (Q-PCR). Cdc25A level was decreased correspondent to an increase in Egr-1 mRNA level (Fig. 5). This result suggested that modulation of Cdc25A gene expression by Egr-1 occurred at the transcription level although it did not help to determine a direct or indirect effect of Egr-1 on Cdc25A.
Fig. 5.
Egr-1 modulates Cdc25A at the transcription level. (a) Representative expression of Cdc25A and Egr-1 in normal human keratinocytes transduced with Egr-1 and marker control gene assessed by real-time quantitative PCR (Q-PCR). Total RNA was extracted from cells harvested at three time-points post transduction (12 h, 24 h and 36 h), reverse transcribed to cDNA and subjected to Q-PCR. Relative quantification was performed in triplicates, normalized with endogenous control gene GAPDH, and calibrated against 12 h GFP-transduced cells. (b), Data summarized from three independent Q-PCR assays comparing changes in Cdc25A mRNA levels in Egr-1-transduced versus GFP-transduced cells
Discussion
We have performed gene expression profiling in three common human epidermal hyperproliferative disorders and have demonstrated that BCC, SCC, and psoriasis can be distinguished from each other on the basis of their gene expression patterns. Furthermore, we have identified specific genes that are selectively altered in these disorders, including Egr-1. Finally, functional studies suggest that Egr-1 might restrain hyperproliferation of epidermal cells and tissue.
The gene expression patterns identified by microarray analysis are consistent with known biology of the diseases studied. A number of genes are altered in the same manner. PDGF-A was increased in all 3 disorders compared to controls, suggesting that the PDGF axis may be a common mediator of epidermal hyperproliferation. This is in agreement with increased PDGFRα expression in murine and human BCCs and the proposed role of PDGFRα activation by Gli1 as an important mechanism by which mutations in the SHH pathway cause BCCs (Xie et al. 2001). Molecular profiling of mouse and human SCC also revealed increased gene expression of PDGFA((Dong et al. 2001; Marionnet et al. 2003). Increased PDGFRα had been demonstrated in psoriasis as well (Nilsson and Hoffmann 2000). Nm23-H2 was also upregulated in all three diseases, consistent with its overexpression in many hyperproliferative conditions including colorectal carcinomas (Marionnet et al. 2003; Postel et al. 2000). On the other hand, c-myc and K10 are down regulated in all three diseases, highlighting the distorted balance between cellular proliferation and differentiation within the epidermis in these conditions.
A number of genes are differentially expressed in the three disease states. For example, Smoothened was shown to be induced in all BCC samples but not in psoriasis or SCC, which agrees with known Shh pathway activation in BCC (Bale and Yu 2001). Integrin α6 precursor was downregulated in BCC but upregulated in SCC, as seen in prior immunohistochemical analysis (Kore-eda et al. 1998; Tennenbaum et al. 1996) and consistent with its elucidated biological function in invasive carcinomas (Dajee et al. 2003; Mercurio 2003). Among differentially regulated genes, FA synthase, along with Egr-1, was strongly induced in psoriasis but repressed in BCC and SCC. This is in agreement with reports of increased FA synthase protein expression in normal and psoriatic epidermis (Uchiyama et al. 2000), but contrasts with findings in a variety of other human malignancies, including carcinoma of the colon, prostate, ovary, endometrium, and breast (Kuhajda et al. 2000), where high levels of FA synthase have been observed. FA synthase downregulation in BCC and SCC may thus represent a major difference between cutaneous malignancies and visceral cancers. Further characterization of FA synthase is worthwhile for understanding epidermal tumorigenesis.
Of particular interest is Egr-1, a zinc finger transcription factor expressed in response to diverse stimuli such as mitogens, tissue injury and developmental cues. Depending on the setting, Egr-1 has been associated with both negative and positive growth regulatory effects. In PDGF-transformed murine fibroblasts, Egr-1 reduced tumorigenesis in nude mice (Huang et al. 1994). Egr-1 also suppressed tumorigenesis by a variety of human tumor cell lines including osteosarcomas, fibrosarcomas (Huang et al. 1995), hepatocellular carcinoma, and esophageal carcinoma (Hao et al. 2002). Lung and breast carcinoma can be entirely lacking in Egr-1 expression (Huang et al. 1997; Levin et al. 1995), and deletion of the Egr-1 containing locus 5q31 has been observed in acute myelogenous leukemia (Fairman et al. 1995) and breast cancer (Ronski et al. 2005). In contrast, prostate cancer and metastatic gastric cancer can exhibit high levels of Egr-1 (Kobayashi et al. 2002; Thigpen et al. 1996). The mechanisms underlying these tissue-specific effects of Egr-1 are under active investigation by many laboratories.
In mouse skin, Egr-1 mRNA expression was induced by TPA treatment in multistage carcinogenesis though its role in this process is undefined (Riggs et al. 2000). In human epidermis, we observed that Egr-1 was strongly increased in psoriasis but was decreased in both BCC and SCC, raising the possibility that Egr-1 may contribute to differences between benign and malignant proliferation in epidermis. Consistent with this, Egr-1 inhibited growth of actively proliferating normal human keratinocytes as well as HPV18 E6/E7 immortalized cells and malignant SCC-25 cells. In contrast, the DBD Egr-1 deletion mutant displayed no growth inhibition, implicating DNA binding by Egr-1 in epidermal growth suppression. Because Egr-1 inhibits unrestrained growth in actively proliferating cells in culture as well as PMA-induced hyperplasia but not normal epidermal growth in vivo, Egr-1-mediated growth inhibition appears to be limited to settings of hyperproliferation. Our data raise the possibility that Egr-1 exerts compensatory effects in psoriasis to prevent uncontrolled hyperproliferation that might lead to malignant transformation. The hyperplasia in psoriasis probably results from concerted effects of multiple genes, including PDGF-A and nm23-H2, among others. By maintaining a high level of Egr-1 in the psoriatic epidermis, uncontrolled malignant transformation could be held in check even though hyperproliferation could not be entirely prevented. In BCC and SCC, however, decreased Egr-1 may permit neoplastic growth, especially under external stimuli such as UV irradiation. This model is supported by two lines of evidence. First, Egr-1 transgenic epidermis resisted PMA-induced hyperplasia. Second, a two-stage skin carcinogenesis study demonstrated a uniformly accelerated tumor development in EGR1-null mouse epidermis, consistent with the tumor suppressor characteristics of Egr-1(Krones-Herzig et al. 2005). However, further investigation is necessary to prove this model.
In studying the mechanisms of Egr-1 growth inhibition in epidermal cells, we observed a dramatic reduction in Cdc25A phosphatase levels in cells overexpressing Egr-1. Based on the Q-PCR results, approximately 5-fold increase of Egr-1 leads to ∼4-fold decrease in Cdc25A (Fig. 5). The profiling analysis demonstrated an average of 2.3-fold increase of Egr-1 mRNA in psoriatic tissue (Fig. 2a). Therefore, after normalization against Egr-1 mRNA levels from the profiling analysis, the overall difference of Cdc25A mRNA did not exceed two-fold (∼1.84-fold). This explains why Cdc25A was not identified from the profiling analysis as a differentially expressed gene. Nevertheless, we did observe by immunohistochemistry that Cdc25A protein is less abundant in psoriatic tissue than in BCC and SCC, consistent with prior reports of Cdc25A overexpression in SCCs (Gasparotto et al. 1997).
Cdc25A is a p53-independent cell cycle regulator that removes inhibitory phosphates on residues Tyr15/Thr14 of Cdk2, thereby promoting Cdk2 activity important for G1/S progression (Nilsson and Hoffmann 2000). A role for Egr-1 in Cdc25A regulation has not been recognized nor has Cdc25A expression been systematically analyzed in skin diseases. Egr-1 suppression of Cdc25A in this setting could lead to persistent phosphorylation of Cdk2 with failure to activate cyclin E/Cdk2 and resultant inhibition of the G1/S transition. Indeed, we observed an absence of dephosphorylated Cdk2 with Egr-1 expression. Our data thus suggest that Cdc25A repression may represent a potential mechanism contributing to the growth inhibitory role of Egr-1 in proliferating epidermal cells. Although it is not clear whether Egr-1 regulates Cdc25A in a direct or indirect manner, our data suggest that this function occurs at the transcription level. In addition, in cells overexpressing Egr-1, we did not observe any changes of c-myc (data not shown), which has been shown to modulate Cdc25 phosphatases (Galaktionov et al. 1996), suggesting that Egr-1’s effect on Cdc25A is likely independent of c-myc. The fact that our gene expression profiling showed c-myc reduction in BCC, SCC and psoriasis whereas Egr-1 was differentially expressed also argues against the possible mechanism of Egr-1 acting through c-myc. Furthermore, Egr-1 has been shown to control p21 expression by direct interaction with the p21 promoter in erythroleukemic K562 cells (Ragione et al. 2003). We assessed p21 mRNA and protein expression levels upon Egr-1 expression in normal human keratinocytes but observed no changes (data not shown), suggesting a different target of Egr-1 for growth control in epidermis from that in erythroleukemic cells.
In summary, we identified Egr-1 as a differentially regulated gene among three epidermal hyperproliferative disorders from gene expressing profiling using microarray analysis and further studied the growth regulatory role of Egr-1 on epidermal cells in vitro and in vivo. Egr-1 appeared to restrain uncontrolled epidermal proliferation by modulating cell cycle regulator Cdc25A. Besides increased proliferation, tumorigenesis may also be due to failure to differentiation and/or apoptosis. Whether Egr-1 plays a role in these aspects of the cellular function warrants further investigation.
Acknowledgments
We thank the Univeristy of Connecticut Health Center HCRAC Small Grant Program for continued support of this study. We thank C. Barry and P. Brown for helpful early discussions, V.P. Sukhatme and A. Gashler for Egr-1, DBD[-]-Egr-1 plasmids and M.P. Marinkovich for HPV E6/E7 immortalized keratinocytes and SCC-25 cells. We also thank M. Murphy for his expert help on histopathology of the disorders and C. Guo for her expertise and assistance in transgenic mouse work. Special thanks to Dr. Paul Khavari (Stanford University), whose guidance and funding resources made the initiation of this project possible.
Abbreviations
- BCC
Basal cell carcinoma
- SCC
Squamous cell carcinoma
- EGR1
Early growth response gene 1
- Q-PCR
Real-time quantitative PCR
- HPV
Human papilloma virus
- DBD
DNA binding domain deletion
- FA
Fatty acid
- PDGF-A
Platelet derived growth factor A
- EGR
Epidermal growth factor
References
- Bale AE, Yu KP. The hedgehog pathway and basal cell carcinomas. Hum Mol Genet. 2001;10:757–762. doi: 10.1093/hmg/10.7.757. [DOI] [PubMed] [Google Scholar]
- Barbosa MS, Schlegel R. The E6 and E7 genes of HPV-18 are sufficient for inducing two-stage in vitro transformation of human keratinocytes. Oncogene. 1989;4:1529–1532. [PubMed] [Google Scholar]
- Byrne C, Fuchs E. Probing keratinocyte and differentiation specificity of the human K5 promoter in vitro and in transgenic mice. Mol Cell Biol. 1993;13:3176–3190. doi: 10.1128/MCB.13.6.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon F, Dallapiccola B, Novelli G. Advances in the search for psoriasis susceptibility genes. Mol Genet Metab. 2000;71:250–255. doi: 10.1006/mgme.2000.3031. [DOI] [PubMed] [Google Scholar]
- Choate KA, Medalie DA, Morgan JR, Khavari PA. Corrective gene transfer in the human skin disorder lamellar ichthyosis. Nat Med. 1996;2:1263–1267. doi: 10.1038/nm1196-1263. [DOI] [PubMed] [Google Scholar]
- Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, Marinkovich MP, Tao S, Lin Q, Kubo Y, Khavari PA. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639–643. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- Deng H, Choate KA, Lin Q, Khavari PA. High-efficiency gene transfer and pharmacologic selection of genetically engineered human keratinocytes. Biotechniques. 1998;25:274–280. doi: 10.2144/98252gt02. [DOI] [PubMed] [Google Scholar]
- Dong G, Loukinova E, Chen Z, Gangi L, Chanturita TI, Liu ET, Van Waes C. Molecular profiling of transformed and metastatic murine squamous carcinoma cells by differential display and cDNA microarray reveals altered expression of multiple genes related to growth, apoptosis, angiogenesis, and the NF-kappaB signal pathway. Cancer Res. 2001;61:4797–4808. [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairman J, Chumakov I, Chinault AC, Nowell PC, Nagarajan L. Physical mapping of the minimal region of loss in 5q- chromosome. Proc Natl Acad Sci U S A. 1995;92:7406–7410. doi: 10.1073/pnas.92.16.7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaktionov K, Chen X, Beach D. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature. 1996;382:511–517. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- Gashler AL, Swaminathan S, Sukhatme VP. A novel repression module, an extensive activation domain, and a bipartite nuclear localization signal defined in the immediate-early transcription factor Egr-1. Mol Cell Biol. 1993;13:4556–4571. doi: 10.1128/MCB.13.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparotto D, Maestro R, Piccinin S, Vukosavljevic T, Barzan L, Sulfaro S, Boiocchi M. Overexpression of Cdc25A and CDC25B in head and neck cancers. Cancer Res. 1997;57:2366–2368. [PubMed] [Google Scholar]
- Hao MW, Liang YR, Liu YF, Liu L, Wu MY, Yang HX. Transcription factor Egr-1 inhibits growth of hepatocellular carcinoma and esophageal carcinoma cell lines. World J Gastroenterol. 2002;8:203–207. doi: 10.3748/wjg.v8.i2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RP, Darland T, Okamura D, Mercola D, Adamson ED. Suppression of v-sis-dependent transformation by the transcription factor, Egr-1. Oncogene. 1994;9:1367–1377. [PubMed] [Google Scholar]
- Huang RP, Fan Y, de Belle I, Niemeyer C, Gottardis MM, Mercola D, Adamson ED. Decreased Egr-1 expression in human, mouse and rat mammary cells and tissues correlates with tumor formation. Int J Cancer. 1997;72:102–109. doi: 10.1002/(SICI)1097-0215(19970703)72:1<102::AID-IJC15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Huang RP, Liu C, Fan Y, Mercola D, Adamson ED. Egr-1 negatively regulates human tumor cell growth via the DNA-binding domain. Cancer Res. 1995;55:5054–5062. [PubMed] [Google Scholar]
- Kinsella TM, Nolan GP. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Yamada M, Kamagata C, Kaneko R, Tsuji N, Nakamura M, Yagihashi A, Watanabe N. Overexpression of early growth response-1 as a metastasis-regulatory factor in gastric cancer. Anticancer Res. 2002;22:3963–3970. [PubMed] [Google Scholar]
- Kore-eda S, Horiguchi Y, Ueda M, Toda K, Imamura S. Basal cell carcinoma cells resemble follicular matrix cells rather than follicular bulge cells: immunohistochemical and ultrastructural comparative studies. Am J Dermatopathol. 1998;20:362–369. doi: 10.1097/00000372-199808000-00007. [DOI] [PubMed] [Google Scholar]
- Krones-Herzig A, Mittal S, Yule K, Liang H, English C, Urcis R, Soni T, Adamson ED, Mercola D. Early growth response 1 acts as a tumor suppressor in vivo and in vitro via regulation of p53. Cancer Res. 2005;65:5133–5143. doi: 10.1158/0008-5472.CAN-04-3742. [DOI] [PubMed] [Google Scholar]
- Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci U S A. 2000;97:3450–3454. doi: 10.1073/pnas.97.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin WJ, Press MF, Gaynor RB, Sukhatme VP, Boone TC, Reissmann PT, Figlin RA, Holmes EC, Souza LM, Slamon DJ. Expression patterns of immediate early transcription factors in human non-small cell lung cancer. The Lung Cancer Study Group. Oncogene. 1995;11:1261–1269. [PubMed] [Google Scholar]
- Marionnet C, Lalou C, Mollier K, Chazal M, Delestaing G, Compan D, Verola O, Vilmer C, Cuminet J, Dubertret L, Basset-Seguin N. Differential molecular profiling between skin carcinomas reveals four newly reported genes potentially implicated in squamous cell carcinoma development. Oncogene. 2003;22:3500–3505. doi: 10.1038/sj.onc.1206571. [DOI] [PubMed] [Google Scholar]
- Mercurio AM. Invasive skin carcinoma—Ras and alpha6beta4 integrin lead the way. Cancer Cell. 2003;3:201–202. doi: 10.1016/S1535-6108(03)00049-7. [DOI] [PubMed] [Google Scholar]
- Miller DL, Weinstock MA (1994) Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol 30:774–778 [DOI] [PubMed]
- Nilsson I, Hoffmann I. Cell cycle regulation by the Cdc25 phosphatase family. Prog Cell Cycle Res. 2000;4:107–114. doi: 10.1007/978-1-4615-4253-7_10. [DOI] [PubMed] [Google Scholar]
- Nomura I, Gao B, Boguniewicz M, Darst MA, Travers JB, Leung DY. Distinct patterns of gene expression in the skin lesions of atopic dermatitis and psoriasis: a gene microarray analysis. J Allergy Clin Immunol. 2003;112:1195–1202. doi: 10.1016/j.jaci.2003.08.049. [DOI] [PubMed] [Google Scholar]
- O’Connor DP, Kay EW, Leader M, Murphy GM, Atkins GJ, Mabruk MJ. A high degree of chromosomal instability at 13q14 in cutaneous squamous cell carcinomas: indication for a role of a tumour suppressor gene other than Rb. Mol Pathol. 2001;54:165–169. doi: 10.1136/mp.54.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreicher JL, Walters IB, Kikuchi T, Gilleaudeau P, Surette J, Schwertschlag U, Dorner AJ, Krueger JG, Trepicchio WL. Molecular classification of psoriasis disease-associated genes through pharmacogenomic expression profiling. Pharmacogenomics J. 2001;1:272–287. doi: 10.1038/sj.tpj.6500067. [DOI] [PubMed] [Google Scholar]
- Postel EH, Abramczyk BM, Levit MN, Kyin S. Catalysis of DNA cleavage and nucleoside triphosphate synthesis by NM23-H2/NDP kinase share an active site that implies a DNA repair function. Proc Natl Acad Sci U S A. 2000;97:14194–14199. doi: 10.1073/pnas.97.26.14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn AG, Sikkink S, Rees JL. Basal cell carcinomas and squamous cell carcinomas of human skin show distinct patterns of chromosome loss. Cancer Res. 1994;54:4756–4759. [PubMed] [Google Scholar]
- Ragione FD, Cucciolla V, Criniti V, Indaco S, Borriello A, Zappia V. p21Cip1 gene expression is modulated by EGR1: a novel regulatory mechanism involved in the resveratrol antiproliferative effect. J Biol Chem. 2003;278:23360–23368. doi: 10.1074/jbc.M300771200. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultures from human squamous cell carcinomas. Cancer Res. 1981;41:1657–1663. [PubMed] [Google Scholar]
- Riggs PK, Rho O, DiGiovanni J. Alteration of Egr-1 mRNA during multistage carcinogenesis in mouse skin. Mol Carcinog. 2000;27:247–251. doi: 10.1002/(SICI)1098-2744(200004)27:4<247::AID-MC1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Ronski K, Sanders M, Burleson J, Benn P, Fang M. EGR1 gene is deleted in estrogen receptor-negative human breast cancer. Cancer. 2005;104:925–930. doi: 10.1002/cncr.21262. [DOI] [PubMed] [Google Scholar]
- Tennenbaum T, Belanger AJ, Quaranta V, Yuspa SH. Differential regulation of integrins and extracellular matrix binding in epidermal differentiation and squamous tumor progression. J Investig Dermatol Symp Proc. 1996;1:157–161. [PubMed] [Google Scholar]
- Thigpen AE, Cala KM, Guileyardo JM, Molberg KH, McConnell JD, Russell DW. Increased expression of early growth response-1 messenger ribonucleic acid in prostatic adenocarcinoma. J Urol. 1996;155:975–981. doi: 10.1016/S0022-5347(01)66361-4. [DOI] [PubMed] [Google Scholar]
- Uchiyama N, Yamamoto A, Kameda K, Yamaguchi H, Ito M. The activity of fatty acid synthase of epidermal keratinocytes is regulated in the lower stratum spinousum and the stratum basale by local inflammation rather than by circulating hormones. J Dermatol Sci. 2000;24:134–141. doi: 10.1016/S0923-1811(00)00088-8. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Liefer KM, Tsai S, O’Malley BW, Roop DR. Development of gene-switch transgenic mice that inducibly express transforming growth factor beta1 in the epidermis. Proc Natl Acad Sci U S A. 1999;96:8483–8488. doi: 10.1073/pnas.96.15.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welss T, Papoutsaki M, Michel G, Reifenberger J, Chimenti S, Ruzicka T, Abts HF. Molecular basis of basal cell carcinoma: analysis of differential gene expression by differential display PCR and expression array. Int J Cancer. 2003;104:66–72. doi: 10.1002/ijc.10912. [DOI] [PubMed] [Google Scholar]
- Xie J, Aszterbaum M, Zhang X, Bonifas JM, Zachary C, Epstein E, McCormick F. A role of PDGFRalpha in basal cell carcinoma proliferation. Proc Natl Acad Sci U S A. 2001;98:9255–9259. doi: 10.1073/pnas.151173398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Krueger JG, Kao MC, Lee E, Du F, Menter A, Wong WH, Bowcock AM. Novel mechanisms of T-cell and dendritic cell activation revealed by profiling of psoriasis on the 63,100-element oligonucleotide array. Physiol Genomics. 2003;13:69–78. doi: 10.1152/physiolgenomics.00157.2002. [DOI] [PubMed] [Google Scholar]