Abstract
Understanding risk factors for antimicrobial resistance requires knowledge of antimicrobial selection pressure. The objectives of this research were to develop methodology for collecting quantitative antimicrobial use information from beef producers in Ontario, to document the types and quantities of antimicrobials reported (for a minimum of 12 mo), and to compare 2 metrics for injectable use reporting. Twenty-four volunteer beef producers were asked to complete a questionnaire, document drug use in a treatment diary, and retain empty medication containers. For injectable antimicrobials, producers recorded approximately 60% of the total use in the treatment diaries; oxytetracycline, penicillin, macrolides, florfenicol, and spectinomycin were used in the greatest quantities. Based on estimated weights of active ingredients (calculated according to number of animals exposed, duration, and average dose per day) the antimicrobials most commonly used in feed were monensin, tylosin, lasalocid, and tetracyclines. The antimicrobials most commonly used in water were lincomycin-spectinomycin, chlortetracycline, and oxytetracycline. Based on estimated weights and measured quantities, < 1% of antimicrobials used were in the Canadian category of highest importance to human medicine. A comparison of animal daily dosages to kilograms of active ingredient demonstrated that the relative ranking of use of antimicrobials varied with the chosen metric, and that further investigation into the best measure in relation to antimicrobial resistance is warranted.
Résumé
Pour comprendre les facteurs de risque liés à la résistance antimicrobienne des connaissances sur la pression de sélection des antimicrobiens sont requises. Les objectifs du présent projet étaient de développer une méthodologie pour amasser auprès des producteurs de bovins de l’Ontario des informations quantitatives sur l’utilisation des antimicrobiens, de documenter les types et quantités d’antimicrobiens rapportés (pour une durée minimale de 12 mois) et de comparer deux méthodes de mesure pour rapporter l’utilisation d’injectables. Vingt-quatre producteurs de bovins ont complété volontairement un questionnaire, colligé dans un registre l’information sur les traitements et conservé les contenants vides de médicaments. Pour les antimicrobiens injectables, les producteurs ont enregistré environ 60 % de l’utilisation totale dans les registres; l’oxytétracycline, la pénicilline, les macrolides, le florfénicol et la spectinomycine représentaient les plus grandes quantités. Sur la base des poids estimés des ingrédients actifs (calculé selon le nombre d’animaux exposés, la durée et la dose quotidienne moyenne), les antimicrobiens les plus couramment utilisés dans la nourriture étaient le monensin, la tylosine, le lasalocide et les tétracyclines. Les antimicrobiens les plus couramment utilisés dans l’eau étaient la lincomycine-spectinomycine, la chlortétracycline et l’oxytétracycline. Basé sur les poids estimés et les quantités mesurées, < 1 % des antimicrobiens utilisés se retrouvaient dans la catégorie canadienne «haute importance» pour la médecine humaine. Une comparaison des dosages quotidiens par animal avec le nombre en kg d’ingrédient actif a démontré que le classement relatif d’utilisation d’antimicrobiens variait avec la méthode de mesure choisie, et que des études ultérieures sur la meilleure mesure en relation avec la résistance antimicrobienne sont requises.
(Traduit par Docteur Serge Messier)
Introduction
Knowledge of antimicrobial selection pressures will greatly assist the understanding of risk factors for antimicrobial resistance (AMR) in both pathogenic and nonpathogenic bacteria. Many international organizations, such as the World Health Organization (1) have requested that countries document antimicrobial use in food animals, preferably in quantitative terms; however, few nations have actually implemented use-monitoring programs (2–4). In Canada, there is national recognition of the value of this type of information (5,6), and the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) is currently exploring and testing options for data collection (7). The antimicrobial distribution system in Canada is complex (8), and end-user studies such as those “on-farm” are needed in conjunction with information collected from veterinarians, feedmills, over-the-counter outlets, and wholesalers to provide a comprehensive understanding of agricultural antimicrobial use. End-user studies are particularly necessary as they can provide information on the dose, duration, route of administration, species, and age-class of treated animals. Also important is the development of the optimal standardized reporting metric (a standard of measurement) that best reflects AMR selection pressures.
The objectives of this research were to develop and evaluate methodology for collecting quantitative use information from 24 volunteer beef producers in Ontario, to document the types, overall quantities, and doses of antimicrobials used (for a minimum of 12 mo), and to compare 2 metrics for injectable use reporting.
Materials and methods
Study design
This study is part of another study looking at antimicrobial resistance in generic Escherichia coli on the same farms (9).
Producers were invited to participate in the study through presentations to the Ontario Cattlemen’s Association (August 1999) and the Ontario Cattle Feeders Association (September 1999), and through a poster presentation at the Agriculture’s Role in Managing Antimicrobial Resistance Conference in 1999 (10). Eleven feedlot operations, 8 cow-calf operations, and 5 “combination” farms (farms with both operation types, but owned by one producer) were voluntarily enrolled in the prospective longitudinal study (a total of 24 farms). For parts of the study, the combination farms were separated into their cow-calf and feedlot components yielding a total of 29 operations (16 feedlots, and 13 cow-calf farms). Veal farms and farms that kept young cattle only for short periods prior to entry to feedlots (backgrounded cattle) were excluded from the project.
On the enrolment visit, 12- and 15-page questionnaires were administered to feedlot and cow-calf producers, respectively to collect information on housing, management, and routine use of antimicrobials. The cow-calf questionnaire was longer due to specific questions for both cows and calves. Two questionnaires were administered to combination farms; 1 for the cow-calf part of their operation, and 1 for the feedlot portion. The producers were also asked to record antimicrobial use in a treatment diary, for a minimum of 12 mo.
The treatment diary developed was based on producer input, as well as concepts from the Canadian Cattlemen’s Association Quality Starts HereTM information manuals (11,12), a treatment notebook produced by the Canadian Pork Council (13), and from a use-recording manual developed in previous research (14). The following information was requested in the treatment diary: date, number of animals treated, animal identification(s), weight (or weight range), location, reason for treating (growth promotion, disease prevention, or treatment of health problem), drug, dose, route, duration, withdrawal time, clinical signs, and treatment outcome. The questionnaire and the treatment diary (both available upon request) were pre-tested on 3 beef producers, 4 veterinary researchers, and 1 executive of the Ontario Cattlemen’s Association; improvements were made prior to field use.
The producers were asked to dispose of empty medication containers in designated “garbage cans,” the contents of which were collected at each visit to audit the quantities of medications recorded (hereafter called “audit data”). Inventories of stocked antimicrobials were conducted both at the beginning and end of the study period, and the producers were asked to track any herd changes such as births, purchases, deaths, sales, or culls. The producers were asked to keep receipts or invoices for any purchased medications or medicated feeds. Each farm was visited at least 4 times to verify treatment recording, collect empty medication bottles, and to document changes in routine uses of antimicrobials. Enrolment of farms began in November 1999 and the data collection period ended in May 2002.
Data management and statistical analysis
Data were entered into a Microsoft Office Excel 2000 spreadsheet (Microsoft; Redmond, Washington, USA), and screened for missing or inappropriate entries. Microsoft Office Excel 2000, Statistix Version 7 (Analytical Software; Tallahassee, Florida, USA), and SAS Version 8.02 (SAS Institute; Cary, North Carolina, USA) were used to analyze the data. The antimicrobials were classified according to their importance to human health using a system proposed by the Veterinary Drugs Directorate, Health Canada (15).
Treatment diary
At the beginning of the study, producers indicated that they would have difficulty documenting routine uses of antimicrobials in the treatment diaries (routine metaphylaxis). To ensure collection of this information, routine uses of antimicrobials were reviewed at each visit to record any changes in protocol since the time of the administration of the questionnaire on the first visit (field workers filled in a standard form on each subsequent visit regarding changes in protocol and new uses of in-feed/routine antimicrobials). Thus, the main purpose of the treatment diary evolved to record nonroutine antimicrobial treatments (primarily injectable). To evaluate the treatment diary, the quantity of antimicrobials recorded was compared to the audit data (assuming the audit data represented the true quantity of drug used for injectable medications). Diary entries were excluded if they were for nonantimicrobial treatments (such as, administration of vitamins), if the number of animals treated was missing, or if the antimicrobial was not noted and the producer could not provide the missing information. If the dose was missing, the producer was contacted for the information, otherwise prior treatment entries were reviewed for doses commonly administered on that farm for that antimicrobial. If the duration was missing, it was assumed that there was only 1 administration of the antimicrobial.
The treatment diary information was used to summarize the ranges of administered doses or “used daily doses” (16) which were then compared to doses suggested on the product labels. Statistical analysis involved calculation of mean administered doses, ratios of mean administered dose/labelled dose, and standard errors of the means (using SAS Proc Univariate).
Measuring antimicrobial use — injectable
The audit data were reported as kg of active ingredient, and additionally by animal daily dosages (ADD) for the feedlot data. The mean rates of use (for example, kg of active ingredient/1000 animal-days) between the different farm and antimicrobial types were compared using Poisson regression, in which the total population at risk for each farm was used as the denominator (using SAS Proc Genmod). Animal daily dosages could not be generated for the cow-calf or combination farms because it was not possible to attribute the audit data to specific animal classes (calf, bull, or cow).
For trimethoprim-sulfamethazine and ampicillin-sulbactam (Synergistin Rogar/STB; London, Ontario), the ADD were determined for the constituent drug of interest; ampicillin in the case of Synergistin (the main constituent) as per Timmerman et al (16), and trimethoprim in the case of trimethoprim-sulfamethazine (the minor constituent) as per Grave et al (17). The number of ADD were calculated using the following formula [adapted from DANMAP (3)]:
| (Equation 1) |
The labelled daily dose was derived from manufacturers’ product monographs published in the Compendium of Veterinary Products (18) and from the Handbook of Veterinary Drugs (19). When a range of doses was given, the mean dose was used. For penicillin products, active ingredients were converted to IU’s and the mean labelled daily dose (IU/kg) was used. A standard weight of 300 kg was used, as per DANMAP 2001 (3). The antimicrobial products were ranked according to use by kg of active ingredient, mean kg of active ingredient/1000 animal-days, number of ADDs, and mean number of ADDs/1000 animal-days. Mean kg active ingredient/1000 animal-days and mean number of ADDs/1000 animal-days were compared between the different antimicrobials using Poisson regression (using SAS Proc Genmod).
Estimating antimicrobial use — feed and water
Feed tags and invoices for medicated feeds were requested from the producers, but due to poor compliance, quantities of antimicrobials used were estimated as follows [equation adapted from Mellon et al (20)]:
| (Equation 2) |
where: n = number of animals;
d = duration of treatment (days);
c = average dose of antimicrobials delivered per treatment day (mg/head/day)
For farms routinely administering feed antimicrobials, the number of animals medicated was based upon the population at risk for each farm (based on the initial number of animals, number of additions, number of withdrawals, and days in the study). When available, the duration of treatment was directly entered. When not available, for products used for the entire feeding period, the duration was estimated by subtracting the average initial purchase weight from the average slaughter weight, and then dividing by an average daily gain of 1.60 kg/d. For tylosin, the duration was assumed to be 120 d (19) and for chlortetracycline-sulfamethazine the duration was assumed to be 28 d (21). The average dose of antimicrobials delivered per treatment day was based on manufacturers’ product monographs published in the Compendium of Veterinary Products (18) and the Canadian Food Inspection Agency’s Compendium of Medicating Ingredients Brochures (21). The following average doses were used: monensin [200 (mg/head)/day]; lasalocid [350 (mg/head)/day]; oxytetracycline [75 (mg/head)/day]; chlortetracycline [70 (mg/head)/day]; chlortetracycline-sulfamethazine [700 (mg/head)/day]; chlortetracycline [350 (mg/head)/day]; sulfamethazine [350 (mg/head)/day]; and tylosin [80 (mg/head)/day] (18,20,21). The durations and average doses do not reflect all possible situations, but they do provide a rough estimate of quantities of antimicrobials used through the feed or water, and provide a framework which can be updated should other doses or durations be proposed.
Results
Where possible, data from the combination farms were separated into individual feedlot (n = 16 operations) versus cow-calf information (n = 13 operations).
Questionnaire results (nonantimicrobial specific)
From responses to the questionnaire, it was determined that the feedlots (n = 14 farms; two producers did not answer this question) ranged in size from 55 to 10 000 cattle produced per year, with an average size of 2089 cattle produced per year. The cow-calf herds (n = 13 farms) ranged in size from 9 to 170 cows, with an average of 83 cows per herd. Fourteen of the 16 feedlots and 7/13 cow-calf operations had routine veterinary input on the farm, and 11/16 feedlots and 4/12 cow-calf operations kept medication records prior to the study (1 cow-calf producer did not respond to the question).
Treatment diary — evaluation and administered doses
One feedlot and 1 combination farm were excluded due to poor drug use recording and/or poor retention of empty medication containers. A 2nd combination farm was excluded as this producer stopped farming during the study. Data from the remaining 3 combination farms were combined, yielding a total of 21 operations.
Comparing the total quantity of antimicrobials recorded to the audit data, the 21 producers recorded 57% of the injectable antimicrobials (stratified by farm, producers recorded on average from 12% to 30%). Many producers stated that the treatment diary was easy to use; however, some indicated that recording during disease outbreaks was difficult. Some found it difficult to track repeat treatments on the same animal. Many producers had difficulty tracking animal numbers (number of animals, birth, deaths, purchases, or sales), and some producers could only provide estimates of animal numbers. The producers liked collecting empty medication bottles, and many stated that it provided a handy disposal service for them.
Regardless of producers’ opinions of the treatment diary, during the study 4171 animals were treated on the 21 farms with antimicrobials at least once. Records on only 907 animals, however, were complete enough [inclusion of drug, dose, weight of animal(s), duration, number of animals treated] for evaluation of administered doses (Table I). Producers frequently did not record which penicillin product they used (often referred to in the treatment diaries as “pen”) and several stocked more than 1 penicillin product on the farm. The penicillin entries were corrected wherever possible, based on the on-farm inventories, audit data, and producer recollection; however, the results for penicillin products have more uncertainty than the other antimicrobial products.
Table I.
Comparison of administered dosages to recommended dosages of antimicrobials
| Category of importance to human medicinea | Antimicrobial (n; N) | Administered dose range (mg/kg; IU/kg for penicillin) | Mean administered dose (mg/kg; IU/kg for penicillin) | Mean daily labelled doseb (mg/kg; IU/kg for penicillin) | Mean administered dose/labelled dose | 95% Confidence interval |
|---|---|---|---|---|---|---|
| I | Ampicillin-sulbactamc (6; 2) | 3.43–7.11 | 6.19 | 6.6 | 0.94 | 0.77–1.10 |
| Ceftiofur (10; 2) | 0.85–1.22 | 1.11 | 1 | 1.11 | 1.03–1.20 | |
| II | Penicillind | |||||
| (40; 5) | 9 434–42 857 | 22 396 | 9 000 | 2.34 | 2.09–2.59 | |
| (15; 3) | 13 158–34 331 | 19 444 | 12 000 | 1.58 | 1.06–2.10 | |
| (50; 9) | 6 000–33 333 | 18 592 | 21 000 | 0.89 | 0.80–0.97 | |
| Tilmicosin (149; 9) | 5.50–31.25 | 10.35 | 10 | 1.05 | 1.00–1.09 | |
| Trimethoprim-sulfadoxinec (25; 9) | 1.26–37.89 | 5.24 | 2.67 | 1.97 | 0.92–3.01 | |
| III | Florfenicol (39; 9) | 7.89–40.36 | 24.45 | 40 | 0.61 | 0.55–0.68 |
| Oxytetracycline | ||||||
| 100 mg/mL (2; 1) | 0.59–0.59 | 0.59 | 6.6 | 0.09 | 0.09–0.09 | |
| 200 mg/mL (76; 13) | 1.22–27.91 | 17.64 | 20 | 0.88 | 0.83–0.94 | |
| Spectinomycin (13; 2) | 4.40–11.00 | 7.98 | 10 | 0.80 | 0.67–0.93 |
n = number of treatment diary entries for the antimicrobial; N = number of farms with treatment diary entries for the antimicrobial of the 21 farms included in this analysis.
Ranking of importance to human medicine (15).
Based on the label instructions (18).
Doses for combination products were set for the constituent of interest (ampicillin and trimethoprim) and combination products were placed in a category higher than their constituents as per CIPARS 2002 (8).
Producers often did not distinguish which penicillin product they used in the treatment diary; thus penicillin data have more uncertainty than the antimicrobial doses provided here.
In contrast to the information on the individual drug labels, Allen et al (19) recommended the following range for daily penicillin doses: penicillin G procaine 20 000 to 54 000 IU/kg once or twice daily, and penicillin benzathine 40 000 IU/kg; they also noted “Clinically effective doses of penicillin far exceed label doses” (19). The data herein were re-evaluated using an average of 37000 IU/kg for the penicillin G procaine products, and 40 000 IU/kg for the penicillin benzathine products. The mean doses administered [with 95% confidence intervals (CI)] compared to these new doses were 0.50 (45–0.55) and 0.54 (0.49–0.59), respectively.
Types of antimicrobials used
The producers were asked to keep receipts and invoices for any medications purchased or medicated feeds. Compliance with this aspect of the study was very poor; therefore, we were not able to compare the quantities of the disposed medications or treatment diaries to the invoices/receipts. Antimicrobials used during the study period for the 21 farms are listed in Table II (from the questionnaire, follow-up questions regarding routine uses of antimicrobials, treatment diaries, and audit data). Aside from different doses on the label or different indication for use, the treatment diaries documented the extra-label use (not labelled for the species or age class of animals treated) of lincomycin-spectinomycin (L-S 100; Pharmacia Animal Health, Orangeville, Ontario) and tiamulin (Denagard; Boehringer, Burlington, Ontario). As recorded by the producers, lincomycin-spectinomycin was used for treatment of fever, footrot, and sore joints, whereas tiamulin was used to treat unspecified respiratory disease, fever, swollen joints, footrot, and mycoplasma infections. Gentamicin (Gentocin; Schering-Plough, Pointe Claire, Quebec) was disposed on 1 farm, but use was recorded on 2 farms for treatment of scours or for “disease prevention.” This product, however, was licensed for use only in cows at the time of this study (21). The audit data identified enrofloxacin (Bayer; Toronto, Ontario) use, which was not labelled for use in cattle at the time of the study, but has since become approved (22).
Table II.
Descriptive summary of antimicrobial use on feedlots, cow-calf farms, and combination farms
| Number of farms using antimicrobial product
|
|||||
|---|---|---|---|---|---|
| Category of importance to human medicinea | Antimicrobial | Feedlots (n = 10) | Cow-calf farms (n = 8) | Combination farms (n = 3) | Total farms n (= 21) |
| Injectable products | |||||
| I | Ampicillin-sulbactam | 4 | 1 | 0 | 5 |
| Ceftiofur | 6 | 1 | 1 | 8 | |
| Enrofloxacinb | 1 | 0 | 0 | 1 | |
| Lincomycin-spectinomycinb,c | 2 | 1 | 1 | 4 | |
| II | Gentamicin | 0 | 2 | 0 | 2 |
| Penicillin | 9 | 7 | 3 | 19 | |
| Tilmicosin | 9 | 3 | 2 | 14 | |
| Tylosin | 1 | 1 | 0 | 2 | |
| Trimethoprim-sulfadoxinec | 4 | 5 | 3 | 12 | |
| III | Dihydrostreptomycin | 0 | 1 | 0 | 1 |
| Florfenicol | 10 | 6 | 1 | 17 | |
| Oxytetracycline | 10 | 7 | 2 | 19 | |
| Spectinomycin | 6 | 2 | 1 | 9 | |
| Unclassified | Tiamulinb,d | 2 | 0 | 1 | 3 |
| In-feed products | |||||
| II | Chlortetracycline-sulfamethazinec | 1 | 0 | 0 | 1 |
| Tylosin | 5 | 0 | 0 | 5 | |
| III | Oxytetracycline | 1 | 1 | 0 | 2 |
| Chlortetracycline | 2 | 0 | 0 | 2 | |
| IV | Lasalocid | 0 | 3 | 1 | 4 |
| Monensin | 10 | 3 | 3 | 16 | |
| In-water products | |||||
| I | Lincomycin-spectinomycinb,c | 1 | 0 | 1 | 2 |
| III | Chlortetracycline | 0 | 1 | 0 | 1 |
| Tablets/boluses administered to calves | |||||
| I | Neomycin-sulfamethazinec | — | 1 | 1 | 2 |
| II | Chlortetracycline-sulfamethazinec | — | 1 | 0 | 1 |
| III | Unspecified sulfa boluses/tablets | — | 1 | 0 | 1 |
| Tetracycline (intrauterine administration) | — | 1 | 0 | 1 | |
Ranking of importance to human medicine (15).
Not labelled for cattle use at the time of this study.
Combination products were placed in a category higher than their constituents as per CIPARS 2002 (8).
Tiamulin (a pleuromutilin) is not currently listed in Health Canada’s current categorization system.
Routine injectable antimicrobials were given to prevent or control disease as follows: 2 cow-calf farms gave oxytetracycline or penicillin to newborn calves, 3 feedlots gave oxytetracycline to new arrivals, 1 combination farm gave oxytetracycline to both newborns and new arrivals at the feedlot, and 1 combination farm alternated between administering tilmicosin and oxytetracycline to new arrivals at the feedlot. Five feedlots routinely used antimicrobials in-feed (non-ionophore antimicrobials).
Quantities of antimicrobials used — injectable
Audit data for the 21 farms is presented as kg of active ingredient (Table III) and as mean kg active ingredient/1000 animal-days (Table IV). One percent of the audit data, by weight, involved antimicrobials in the category of highest importance to human medicine [ampicillin-sulbactam, ceftiofur, and enrofloxacin (15)].
Table III.
Quantitative antimicrobial use by farm type (audit data for injectable, estimated for feed and water)
| kg of active ingredient
|
|||||
|---|---|---|---|---|---|
| Category of importance to human medicinea | Antimicrobial | Feedlot (n = 10) | Cow-calf farms (n = 8) | Combination farms (n = 3) | Total (kg) |
| Injectable | |||||
| I | Ampicillin-sulbactam | 0.28 | 0.00 | 0.00 | 0.28 |
| Ceftiofur | 0.12 | 0.01 | 0.01 | 0.14 | |
| Fluoroquinolonesb | 0.004 | 0.00 | 0.00 | 0.004 | |
| II | Gentamicin | 0.00 | 0.005 | 0.00 | 0.005 |
| Macrolides | 3.11 | 0.51 | 0.62 | 4.24 | |
| Penicillins | 2.36 | 1.12 | 1.15 | 4.63 | |
| Trimethoprim-sulfadoxinec | 0.56 | 0.17 | 0.28 | 1.01 | |
| III | Dihydrostreptomycin | 0.00 | 0.037 | 0.00 | 0.037 |
| Florfenicol | 3.52 | 0.40 | 0.22 | 4.14 | |
| Oxytetracycline | 12.78 | 4.83 | 1.11 | 18.72 | |
| Spectinomycin | 1.31 | 0.08 | 0.00 | 1.39 | |
| Unclassified | Tiamulinb,d | 0.05 | 0.00 | 0.03 | 0.08 |
| Total | 24.09 | 7.16 | 3.42 | 34.68 | |
| Feed/water | |||||
| I | Lincomycin-spectinomycinb,c | 0.1 | 0.00 | 0.30 | 0.40 |
| II | Chlortetracycline-sulfamethazinec | 2.10 | 0.00 | 0.00 | 2.10 |
| Tylosin | 55.41 | 0.00 | 0.00 | 55.41 | |
| III | Chlortetracycline | 0.77 | 0.02 | 0.00 | 0.79 |
| Oxytetracycline | 6.42 | 0.004 | 0.00 | 6.42 | |
| IV | Lasalocid | 0.00 | 7.66 | 6.72 | 14.38 |
| Monensin | 298.24 | 2.83 | 47.77 | 348.84 | |
| Total | 363.04 | 10.51 | 54.79 | 428.34 | |
| Total overall use | 463.02 | ||||
Ranking of importance to human medicine (15).
Not labelled for cattle use at the time of the study.
Combination products were placed in a category higher than their constituents as per CIPARS 2002 (8).
Tiamulin (a pleuromutilin) is not currently listed in Health Canada’s current categorization system.
Table IV.
Rate of injectable antimicrobial use by mean kg of active ingredient/1000 animal-days (audit data) n = 21 farms
| Category of importance to human medicinea | Antimicrobial | Mean kg of active ingredient disposed per 1000 animal-daysb | 95% Confidence interval |
|---|---|---|---|
| I | Ampicillin-sulbactam | 0.04 × 10−3 | 0.00 × 10−3 to 1.45 × 10−3 |
| Ceftiofur | 0.02 × 10−3 | 0.00 × 10−3 to 3.78 × 10−3 | |
| Enrofloxacinc | 0.00 × 10−3 | 0.00 × 10−3 to 2999281383 | |
| II | Aminoglycosidesd | 0.01 × 10−3 | 0.00 × 10−3 to 75.84 × 10−3 |
| Macrolides | 0.54 × 10−3 | 0.21 × 10−3 to 1.39 × 10−3 | |
| Penicillin | 0.59 × 10−3 | 0.24 × 10−3 to 1.46 × 10−3 | |
| Trimethoprim-sulfadoxinee | 0.13 × 10−3 | 0.02 × 10−3 to 0.90 × 10−3 | |
| III | Florfenicol | 0.53 × 10−3 | 0.20 × 10−3 to 1.38 × 10−3 |
| Oxytetracycline | 2.37 × 10−3 | 1.51 × 10−3 to 3.73 × 10−3 | |
| Spectinomycin | 0.18 × 10−3 | 0.03 × 10−3 to 0.93 × 10−3 | |
| Unclassified | Tiamulinc,f | 0.01 × 10−3 | 0.00 × 10−3 to 11.04 × 10−3 |
Ranking of importance to human medicine (15).
The mean rates were significantly different between tetracycline and the following drugs: ampicillin-sulbactam, florfenicol, macrolides, penicillin, spectinomycin, and trimethoprim-sulfadoxine (pair-wise comparisons all P < 0.05). All other pair-wise comparisons of rates were not significantly different (P > 0.05).
Not labelled for cattle use at the time of the study.
Includes gentamicin and dihydrostreptomycin.
Combination products were placed in a category higher than their constituents as per CIPARS 2002 (8).
Tiamulin (a pleuromutilin) is not currently listed in Health Canada’s current categorization system.
The rate of usage by farm type (mean kg of active ingredient/1000 animal-days) was: feedlots (n = 10) 3.90 × 10−3, cow-calf farms (n = 8) 10.74 × 10−3, and combination farms (n = 3) 3.28 × 10−3. The mean rate for cow-calf farms was significantly higher than feedlots (P = 0.0174), but these farm types were not significantly different from the combination farms.
Tetracycline was disposed (audit data) in significantly greater quantities (on the 21 farms) than the other antimicrobials for which statistical testing was possible (the quantity used for some antimicrobials was too small to test for significance and was assumed to be almost zero (Table IV).
Comparison of use metrics — injectable
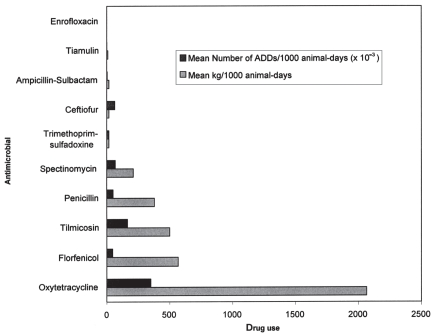
For the feedlot data (n = 10 farms), the kg of active ingredient and estimated number of ADDs for injectable antimicrobials were compared (Table V and Figure 1) and also compared as mean rates/1000 animal-days. In terms of pair-wise comparisons, the only significantly different rates by kg of active ingredient/1000 animal-days were between tetracycline and the following antimicrobials: penicillin, florfenicol, tilmicosin, and spectinomycin (P < 0.05). By kg of active ingredient and by mean kg of active ingredient/1000 animal-days, the top 5 drugs ranked in order of decreasing quantity and rate of use were: oxytetracycline, florfenicol, tilmicosin, penicillins, and spectinomycin. In comparison, the top 5 drugs ranked in order of decreasing quantity and rate of use by number of ADDs and by mean number of ADDs/1000 animal-days were oxytetracycline, tilmicosin, spectinomycin, ceftiofur, and the penicillins. For mean number of ADDs/1000 animal-days, the rates were all significantly different from one another (P < 0.05), with the exception of between rates ceftiofur and spectinomycin, and between florfenicol and penicillin.
Table V.
Animal Daily Dosages (ADDs) for injectable antimicrobial products used on feedlots (feedlot audit data; n = 10 farms)
| Category of importance to human medicinea | Antimicrobial product | Mean daily doseb (mg/kg) or (IU/kg) | Mean dose × standard weight of 300 kg (mg) | Total disposed (mg) | Total number of ADDs |
|---|---|---|---|---|---|
| I | Ampicillin-sulbactam (set for ampicillin) | 6.6 | 1980 | 92 830 | 46.88 |
| Ceftiofur | 1 | 300 | 116 500 | 388.33 | |
| Enrofloxacinc | 3.75 | 1125 | 4 000 | 3.56 | |
| II | Penicillind (IU/kg) total | 347.89 | |||
| Procaine penicillin | 37 000 | 6660 | 1 790 000 | 268.77 | |
| Benzathine + procaine (set for combination) | 40 000 | 7200 | 569 700 | 79.13 | |
| Tilmicosin | 10 | 3000 | 3 106 800 | 1035.60 | |
| Trimethoprim-sulfadoxinee(set for trimethoprim) | 2.67 | 801 | 93 333 | 116.52 | |
| III | Florfenicol (subcutaneous) | 40 | 12000 | 3 526 200 | 293.85 |
| Oxytetracycline Total | 2164.07 | ||||
| 100 mg/mL product | 6.6 | 1980 | 100 000 | 50.51 | |
| 200 mg/mL product | 20 | 6000 | 12 591 400 | 2098.57 | |
| 300 mg/mL product | 20 | 6000 | 90 000 | 15.00 | |
| Spectinomycin | 10 | 3000 | 1 307 600 | 435.87 | |
| Unclassified | Tiamulinc,f (swine dose) | 11 | 3300 | 50 000 | 15.15 |
| Total | 4847.73 |
Ranking of importance to human medicine (15).
Suggested mean daily doses based on Allen et al (19) and the Canadian Animal Health Institute (18).
Not labelled for cattle use at the time of the study.
Allen et al (18) recommended the following range for daily penicillin doses: penicillin G procaine 20 000 to 54 000 IU/kg and penicillin benzathine 40 000 IU/kg. They also noted “Clinically effective doses of penicillin far exceed label doses” (19). Conversion of IU to mg of penicillin: 1 000 000 IU = 600 mg.
Combination products were placed in a category higher than their constituents as per CIPARS 2002 (8).
Tiamulin (a pleuromutilin) is not currently listed in Health Canada’s current categorization system.
Figure 1.
Comparison of kg of active ingredient and ADDs for injectable antimicrobials/1000 animal-days (feedlot audit data; n = 10 farms).
Quantities of antimicrobials used — feed and water
The estimated weights of antimicrobials administered through the feed or water are listed in Table III for the 21 farms. One antimicrobial combination (lincomycin-spectinomycin) was within the category of antimicrobials of highest importance to human medicine (15), and corresponded to 0.09% of the total kg of active ingredient administered through feed or water. Ionophores [category of lowest importance to human medicine (15)] corresponded to 78% of total kg active ingredient (inclusive of all routes of administration).
Most in-feed antimicrobial use occurred on feedlots (Table III); therefore, only the in-feed/in-water antimicrobial use for feedlots (n = 10 farms) was calculated. Rates of use by product (kg of active ingredient/1000 animal-days) were: monensin 0.05, tetracycline 1.50 × 10−3, tylosin 8.97 × 10−3, and lincomycin-spectinomycin 0.02 × 10−3. These means were all significantly different (P < 0.05) in pair-wise comparisons, other than between tetracycline and lincomycin-spectinomycin.
Discussion
This study provided valuable information on the types and quantities of antimicrobials used by the volunteer beef producers. Less than 1% of the antimicrobials used were in the category of highest importance to human medicine (15), whereas 78% were in the category of lowest importance to human medicine (15). While all antimicrobials should be used prudently from a human health perspective, it was encouraging to note that the greatest use involved antimicrobials not used in human medicine and very little of the total use involved the category of highest importance to human medicine.
Ionophores (monensin and lasalocid) are not always classified as antimicrobials, but ionophores were included because Prescott et al (23) classify them as “carboxylic ionophore polyether” antimicrobials derived from Streptomyces, which kill bacteria by changing the pH of the bacterial cell (23). Additionally, both the Veterinary Drugs Directorate and the authors of “Hogging It” include ionophores as antimicrobials (15,20).
At the time of writing, there has been no scientific consensus or decision regarding the best metric of antimicrobial use in relation to antimicrobial resistance. In our study, comparison of numbers of ADDs to kg of active ingredient showed that the reported rank order of various products may change based upon the chosen metric. A standard weight of 300 kg was assumed for the calculation, which could be subject for discussion. A change in the standard weight, however, would not change the relative ranking of the antimicrobial products by ADDs (it is a scaling factor) but rather the absolute total number of ADDs reported per product would change.
Internationally accepted standards for ADDs have not been fully developed, and the ADDs presented in this study reflect current Canadian dosages, which may be subject to change. Neither kg of active ingredient nor ADD provide a direct measure of the selective pressure that antimicrobials apply to bacterial populations because they do not necessarily reflect the administered dose, true exposure of the organism based on site of infection, route or administration, formulation of the product, etc. Limitations to treatment diary over- and under-recording would need to be overcome to use quantities of administered doses as an accurate measure of use. In our study, the treatment diaries did indicate that penicillin products were commonly used at greater than labelled doses, although as previously mentioned, a Canadian veterinary reference states that effective doses exceed label doses (19). Ceftiofur [in an antimicrobial class of the highest importance to human medicine (15)] was used at close to its labelled dose, with a relatively narrow confidence limit [mean administered dose/labelled dose = 1.11; 95% confidence interval (CI) 1.03–1.20]. Timmerman et al (16) proposed looking at the ratio of the administered doses to the ADD and suggested that a range of ± 20% around 1 might be an acceptable margin for appropriate dosing. For Canada, this acceptable margin might vary slightly by product based on ranges in recommended doses. For example, penicillin products that have a large range of suggested/recommended doses might have a wider margin, versus products that have a more consistent suggested dose (ceftiofur). Perhaps narrower margins should be considered for drugs in categories of highest importance to human medicine.
To date, there are few comparable studies of antimicrobial use on beef farms in Canada or the United States. One study that focused on 4 feedlots in southern Alberta documented that all 4 feedlots used the following medications in feed: oxytetracycline, chlortetracycline-sulfamethazine, and monensin (24). In comparison, only 1 of our feedlots (of the 10 feedlot-only operations) used oxytetracycline, 1 used chlortetracycline-sulfamethazine, 2 used chlortetracycline (without sulfamethazine), and all of our feedlots used monensin. In addition, 2 of the Alberta feedlots in that study used lasalocid in-feed (24), whereas only 1 of our combination farms used lasalocid. The Alberta feedlots used oxytetracycline for metaphylaxis, and if the animals were clinically sick upon arrival, they were given florfenicol or tilmicosin (24).
In 1999, the National Animal Health Monitoring System (NAHMS) in the United States collected information on antimicrobial use on 520 feedlots (25). The NAHMS reported similar percentages of feedlots using injectable antimicrobials for metaphylaxis (42%), but they also documented ceftiofur, erythromycin, and tylosin use for metaphylaxis (25). The NAHMS reported fluoroquinolone (not licensed for use in Canada at the time of the study) use on 32% of feedlots to treat respiratory disease (25). They also reported a greater percentage of feedlots using chlortetracycline and oxytetracycline in-feed, but our study showed a greater percentage of feedlots using tylosin. The NAHMS also documented neomycin, virginiamycin, and bacitracin use in-feed (25). The differences between the Alberta study, NAHMS, and our study may reflect differences in product availability, different management practices, different herd sizes, or different prevalence of diseases. Caution should be expressed for comparisons of these studies as ours is a limited small volunteer study (with associated selection bias) and the Alberta study had 4 much larger (from 16 000 to 32 000 animals) feedlots (26).
In terms of cow-calf operations, Kelch et al (27) determined that antimicrobials “…seldom were used..” for prophylaxis on beef cow-calf farms in Tennessee, and the primary antimicrobial used was chlortetracycline. In contrast, 3 farms in our study reported routine use of injectable antimicrobials (oxytetracycline or penicillin) in newborn calves for prophylaxis.
The study herein had some limitations, such as small number of volunteer producers (selection bias), and the true usage was underestimated because quantities of oral tablets/boluses and intrauterine boluses were not well-documented in treatment diaries and generally, there was little packaging to dispose of in garbage cans. The audit data relied on the compliance of the producers; however, it was assumed that these data were a more accurate measure than the treatment diary data as producers were enthusiastic about having their “garbage” disposed of for them, protection of confidentiality created little reason for dishonesty, and throwing out empty containers did not take much effort. The reasons for overestimates of antimicrobial use in the treatment diaries are unknown (9/21 producers on average over-estimated use), but likely include recall error, overestimation of doses particularly when groups of animals were medicated, or some broken bottles that did not enter the garbage cans.
Also, in comparison with average Ontario beef farms, the study herds herein were generally larger [Ontario cow-calf farms have on average 20 to 25 cows, and feedlots have on average 175 animals (28)]. The affect of these limitations on the overall findings is unknown, and for these reasons caution must be expressed in extending the conclusions of this study to the overall Ontario beef industry.
The weight of antimicrobials used via feed/water was approximate, but did provide estimates for comparison purposes. These could be updated if further information on the inputs becomes available. An average daily gain of 1.6 kg/d regardless of age of calves or gender was assumed; however, if a lower average daily gain (1.25 kg/d) is used, then the animals would be on-feed longer and total drug use would be scaled higher. For example, if the 10 feedlots used a 1.6 kg/d average daily gain, monensin use would be estimated to be 298 kg, whereas if a 1.25 kg/d average daily gain is used, the estimate of monensin use would be 380 kg. In the future, simulation models adjusting for the variability and uncertainty in these model inputs will be able to provide more information on the nature of the total use.
Our prior belief was that the volunteer producers, since they were interested in the drug use study, might be more likely to record drug use accurately and perhaps use antimicrobials more prudently than producers who did not choose to participate. Our experience implementing and evaluating the treatment diary, however, emphasizes the difficulties in collecting, validating, and interpreting the recorded drug use data. In our small study of volunteer producers 3 producers (which included two combination farms) had to be excluded because of poor compliance or changes in farming practice during the short course of the study. The drug use recording methods had to be changed because of producer difficulties in recording routine treatments in the treatment diary. Of the remaining 21 farms, only 57% of the injectable antimicrobials were recorded in the treatment diaries, only 22% of the animals treated had records complete enough to provide information on administered doses, and our producers indicated that they had a difficult time recording during disease outbreaks. Additionally, tracking animal numbers throughout the study was very difficult. It was unknown what effect the missing treatment diary entries would have on the findings. This would not likely have a significant effect on the ranges of administered doses within a given farm (assuming producers would be consistent in how they administered antimicrobials). It would, however, affect the measurement of frequencies of treatment particularly if the missing treatment diary entries are related to disease outbreaks, as the producers had indicated.
The overall feedback from the producers on the “ease of use” of the treatment dairy was favorable; however, if used in the future, the treatment diaries should be modified to reflect the difficulties in following individuals over time and recording large numbers of treatments on the same day. Two producers indicated that they would have preferred hand-held computers to facilitate the recording of AMU. While this would decrease recall errors, it might be difficult to implement on all farms due to producer compliance, but could be considered for future research. To improve recording of routine treatments, future treatment diaries could incorporate a simple check system, whereby producers could document the number of animals and date on which a previously identified “treatment protocol” was administered, with protocol changes noted accordingly.
In summary, future studies for documenting on-farm AMU should primarily make use of an audit system to collect quantitative estimates of use, and another system to record routine protocols or protocol changes. This secondary system could take the form of a questionnaire, and a modified short-term treatment diary to document administered doses that are perhaps strategically administered throughout the year to catch seasonal differences.
Acknowledgments
The authors sincerely thank the volunteer beef producers for participating in this study. Brent Avery, Graham Thatcher, Susan Willick, and Pat Pentney are also thanked for their assistance with the farm visits and/or data entry.
Footnotes
This research was supported by the Public Health Agency of Canada (formerly Health Canada), the Ontario Cattlemen’s Association, the Ontario Ministry of Agriculture, Food and Rural Affairs, and the Ontario Veterinary College PhD Fellowship Program.
This paper is part of a PhD thesis, University of Guelph, Guelph, Ontario.
References
- 1.World Health Organization (WHO) Global strategy for containment of antimicrobial resistance. [Last accessed 17 February 2006]; [homepage on the Internet]. Available from http://www.who.int/drugresistance/guidance/en/index.html.
- 2.National Veterinary Institute. Swedish Veterinary Antimicrobial Resistance Monitoring — 2002. [Last accessed 17 February 2006]; [homepage on the Internet]. Available from http://www.sva.se/pdf/svarm2002.pdf.
- 3.Danish Integrated Antimicrobial Resistance Monitoring and Research Programme (DANMAP) DANMAP 2001 — Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, foods and humans in Denmark [homepage on the Internet] [Last accessed 17 February 2006]; Available from http://www.keepantibioticsworking.com/new/resources_library.cfm?refID=36924.
- 4.NORM/NORM-VET. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. [Last accessed 14 October 2007];2006 [homepage on the Internet]. Available from http://www.vetinst.no/eng/tjenester/publikasjoner/norm_norm_vet_rapporten/norm_norm_vet_rapporten_2006.
- 5.Health Canada and the Canadian Infectious Disease Society. Controlling antimicrobial resistance: An integrated action plan for Canadians. Can Commun Dis Rep. 1997;23(S7):1–32. [PubMed] [Google Scholar]
- 6.Advisory Committee on Animal Uses of Antimicrobials and Impact on Resistance and Human Health. Uses of antimicrobials in food animals in Canada: Impact on resistance and human health. Report Prepared for the Veterinary Drugs Directorate, Health Canada (2002) [Last accessed 12 June 2007]; [homepage on the Internet]. Available from http://www.hc-sc.gc.ca/dhp-mps/pubs/vet/amr-ram_final_report-rapport_06-27_cp-pc_e.html.
- 7.Government of Canada, Public Health Agency of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS)-2003. [Last accessed 20 February 2007]; [homepage on the Internet]. Available from http://www.phac-aspc.gc.ca/cipars-picra/2003_e.html.
- 8.Government of Canada, Health Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS)-2002. [Last accessed 20 February 2006]; [homepage on the Internet]. Available from http://www.phac-aspc.gc.ca/cipars-picra/index.html.
- 9.Carson CA, Reid-Smith R, Irwin RJ, Martin SW, McEwen SA. Antimicrobial resistance in generic fecal Escherichia coli from 29 beef farms in Ontario. Can J Vet Res. 2008;72:119–128. [PMC free article] [PubMed] [Google Scholar]
- 10.Bair C, Reid-Smith R, Irwin R, McEwen S. Antimicrobial Use in the Ontario Beef Industry. Proceedings of the Agriculture’s Role in Managing Antimicrobial Resistance Conference; Toronto, Ontario. October 24–26, 1999. [Google Scholar]
- 11.Van Donkersgoed J, Dubeski P, Strankman P. Quality Starts HereTM. Good Production Practices for Cow-Calf Producers; Calgary, Alberta. 1995. [Google Scholar]
- 12.Crandall J, Van Donkersgoed J. Quality Starts HereTM — Alberta Cattle Feeders Association Beef Quality Improvement. Recommended Operating Procedures for Feedlot Animal Health; Calgary, Alberta. 1997. [Google Scholar]
- 13.Canadian Pork Council, Canadian Quality Assurance (CQATM) Swine medication withdrawal times and recording notebook. Hensall, Ontario: North American Compendiums Ltd; 1998. [Google Scholar]
- 14.Dunlop R, McEwen S, Meek A, Black W, Clarke R, Friendship R. Individual and group antimicrobial usage rates on 34 farrow-to-finish swine farms in Ontario, Canada. Prev Vet Med. 1998;34:247–264. doi: 10.1016/s0167-5877(97)00093-7. [DOI] [PubMed] [Google Scholar]
- 15.Veterinary Drugs Directorate (VDD), Health Canada. Categorization of antimicrobial drugs based on importance in human medicine (date modified Nov. 30, 2006) [Last accessed 19 March 2007]; [homepage on the Internet]. Available from http://www.hc-sc.gc.ca/dhp-mps/consultation/vet/consultations/amr_ram_hum-med_e.html.
- 16.Timmerman T, Dewulf J, Catry B, et al. Quantification and evaluation of antimicrobial drug use in group treatments for fattening pigs in Belgium. Prev Vet Med. 2006;74:251–263. doi: 10.1016/j.prevetmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Grave K, Greko C, Nilsson L, Odensvik K, Mørk T, Rønning M. The usage of veterinary antibacterial drugs for mastitis in cattle in Norway and Sweden during 1990–1997. Prev Vet Med. 1999;42:45–55. doi: 10.1016/s0167-5877(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 18.Canadian Animal Health Institute (CAHI) Compendium of Veterinary Products. 7. Hensall, Ontario: North American Compendiums Ltd; 2001. [Google Scholar]
- 19.Allen DG, Pringle JK, Smith D, Conlon P. Handbook of Veterinary Drugs. Philadelphia, Pennsylvania: J.B. Lippincott Company; 1993. [Google Scholar]
- 20.Mellon M, Benbrook C, Benbrook K. Hogging it! Estimates of antimicrobial abuse in livestock. Cambridge, Massachusetts: UCS Publications; 2001. [Google Scholar]
- 21.Canadian Food Inspection Agency. Compendium of medicating ingredients brochures [homepage on the Internet] [Last accessed 20 February 2006]; Available from http://www.inspection.gc.ca/english/toce.shtml.
- 22.Health Canada. Drug Product Database — Baytril 100. [Last accessed 20 February 2006]; [homepage on the Internet]. Available from http://www.hc-sc.gc.ca/drug2/product/p73409.html.
- 23.Prescott J, Baggot J, Walker R, editors. Antimicrobial Therapy in Veterinary Medicine. 3. Ames, Iowa: Iowa State Univ Pr; 2000. [Google Scholar]
- 24.Read R. Antimicrobial resistance of human health importance in Alberta feedlots. Proceedings of the Agriculture’s Role in Managing Antimicrobial Resistance: The Road to Prudent Use Conference; October 23–26, 2005; Toronto, Ontario. [Google Scholar]
- 25.United States Department of Agriculture (USDA). National Animal Health Monitoring System (NAHMS) Feedlot 1999, Part III: health management and biosecurity in U.S. feedlots. Animal and Plant Health Inspection Service (APHIS). Fort Collins, Colorado. [Last accessed 12 October 2007]; [homepage on the Internet]. Available from http://www.aphis.usda.gov/vs/ceah/ncahs/nahms/feedlot/feedlot99/FD99pt3.pdf.
- 26.Inglis GD, Morck DW, McAllister TA, et al. Temporal prevalence of antimicrobial resistance in Campylobacter spp. from beef cattle in Alberta feedlots. Appl Environ Microbiol. 2006;72:4088–4095. doi: 10.1128/AEM.02830-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelch W, New J. The reported use of drugs to prevent diseases in beef cattle in Tennessee. Prev Vet Med. 1993;15:291–302. [Google Scholar]
- 28.Farm Issues.com. Media Resource Centre. Housing: Where do beef cattle live? [Last accessed 21 February 2006]; [homepage on the Internet]. Available from http://www.farmissues.com/mediaPortal/beef/beef_basics.asp.