Abstract
The cell death response known as the hypersensitive response (HR) is a central feature of gene-for-gene plant disease resistance. A mutant line of Arabidopsis thaliana was identified in which effective gene-for-gene resistance occurs despite the virtual absence of HR cell death. Plants mutated at the DND1 locus are defective in HR cell death but retain characteristic responses to avirulent Pseudomonas syringae such as induction of pathogenesis-related gene expression and strong restriction of pathogen growth. Mutant dnd1 plants also exhibit enhanced resistance against a broad spectrum of virulent fungal, bacterial, and viral pathogens. The resistance against virulent pathogens in dnd1 plants is quantitatively less strong and is differentiable from the gene-for-gene resistance mediated by resistance genes RPS2 and RPM1. Levels of salicylic acid compounds and mRNAs for pathogenesis-related genes are elevated constitutively in dnd1 plants. This constitutive induction of systemic acquired resistance may substitute for HR cell death in potentiating the stronger gene-for-gene defense response. Although cell death may contribute to defense signal transduction in wild-type plants, the dnd1 mutant demonstrates that strong restriction of pathogen growth can occur in the absence of extensive HR cell death in the gene-for-gene resistance response of Arabidopsis against P. syringae.
Gene-for-gene resistance is a form of plant disease resistance that is exploited widely by plant breeders, forming a cornerstone of disease control in crop plants (1–4). The name “gene-for-gene” denotes the dependence of this resistance on matched specificity between a plant disease resistance gene and a pathogen avirulence gene (5). In a process that is reminiscent of mammalian antibody–antigen interactions, these genes apparently control receptor–ligand interactions that activate a complex defense response (4, 6, 7). There are thousands of resistance genes that mediate the recognition of specific fungal, bacterial, viral, or nematode pathogen strains. The strong defense response that is triggered after a gene-for-gene interaction includes synthesis of antimicrobial enzymes and metabolites, generation of signaling molecules that activate defense in neighboring cells, and reinforcement of plant cell walls surrounding the site of infection (4, 7, 8). One of the most prominent features of gene-for-gene defense is the death of infected plant cells within hours after initial contact with pathogen, a process known as the hypersensitive response (HR) (9, 10). HR cell death is a programmed cell death response that bears features of the apoptotic cell death processes that occur in other metazoan organisms (8). Although HR cell death is a hallmark of gene-for-gene disease resistance, the relative importance of cell death in this form of disease resistance is not clear and may vary depending on the target pathogen species (7–10).
Proposed roles for cell death in the resistance response include mass release of antimicrobial enzymes and metabolites into the extracellular matrix, the elimination of a cell that the pathogen is exploiting for life support, and the release of signals that activate defense in neighboring and distant cells (7–10). Alternatively, HR cell death may be a side effect caused by exceptionally strong activation of signaling responses such as ion channel gating or oxidative burst or by the extensive build-up of toxic antimicrobial compounds within the cell. In forms of plant disease resistance other than gene-for-gene resistance, defense responses often are activated at a lower level and host cells typically do not undergo programmed cell death (11, 12). However, these other forms of disease resistance are less effective at blocking pathogen growth.
It has been difficult to assess experimentally the utility of cell death in gene-for-gene disease resistance because cell death is usually a central feature of this response. However, prior studies have provided some evidence that the HR is not always required for gene-for-gene resistance. In normal gene-for-gene reactions, where cell death is observed, components of gene-for-gene resistance such as an oxidative burst, salicylate production, or induction of PR gene expression are activated before HR cell death (7, 12). Components of the plant defense response also have been observed in plants in which HR cell death was delayed artificially by incubation in very low oxygen or in high humidity (13, 14). Rare examples have been reported of avirulence gene-specific resistance genes that do not provoke cell death during the restriction of pathogen growth (15, 16). As an additional example, reduced growth of an avirulent race of the obligate biotroph Erysiphe graminis f. sp. hordei, in the absence of an HR, was observed when barley tissue was treated with the transcriptional inhibitor cordycepin (17). Finally, Arabidopsis ndr1 mutants exhibit the converse phenotype of susceptibility to avirulent Pseudomonas syringae despite retention of the HR phenotype in response to a subset of those pathogens (18). Although these examples suggest that cell death may not be essential for gene-for-gene resistance, there is other evidence that cell death is essential for successful restriction of pathogen growth in some gene-for-gene interactions. For example, separate experiments from the above-cited studies of Schiffer et al. provide evidence that Mla-type resistance is rendered ineffective by inhibition of HR cell death (17). The widespread association of HR cell death with gene-for-gene disease resistance in vascular plants suggests that it confers an adaptive benefit.
Here we report the identification and characterization of an Arabidopsis mutant, dnd1, that does not develop the HR in response to avirulent P. syringae pathogens. The dnd1 mutant exhibits gene-for-gene restriction of pathogen growth in the absence of extensive HR cell death and also exhibits a constitutive systemic acquired resistance phenotype.
MATERIALS AND METHODS
Inoculations with P. syringae.
Original mutants and their progeny were tested for the HR by pipet inoculation of individual leaves with P. syringae pv. glycinea Race 4 pV288 (avrRpt2+) or Race 4 pVSP61 (no avr gene) at ≈2 × 108 colony forming units (cfu)/ml (19, 20). Additional P. syringae strains used to test for gene-for-gene HR included P. syringae pv. glycinea Race 4 pAvrRpm1 (avrRpm1+) and Race 4 pVB01 (avrB+) (19, 21). Positive and negative Arabidopsis controls included the use of wild-type Col-0, Col-0 rps2–201/rps2–201, and Col-0 rpm1/rpm1 (“rps3–1”) mutants (19, 21). For bacterial growth experiments and for gene expression studies, P. syringae pv. tomato strain DC3000 and P. syringae pv. maculicola strain 4326 were used with the above plasmids or with pKec218 (avrRps4+) (22). Quantitative determinations of bacterial growth in leaves were performed by dilution plating of homogenized leaf tissue on selective media, as described in ref. 23.
Mutant Screen and Crossing.
Arabidopsis thaliana ecotype Col-0 seeds were mutagenized with ethyl methane sulfonate; M2 populations were obtained from Lehle Seeds (Round Rock, TX). To test for activation of the HR, P. syringae pv. glycinea Race 4 pV288 (avrRpt2+), at a concentration of ≈2 × 108 cfu/ml in 10 mM MgCl2, was introduced by vacuum infiltration into leaf mesophyll tissue of ≈11,000 M2 seedlings. Leaves were observed 24 and 40 h after infiltration, and plants with reduced, delayed, or no leaf collapse were saved for further analysis. Lines of potential interest were crossed with the wild-type Col-0 parent to initiate backcrossing and with ecotype No-0 to initiate genetic mapping. For complementation tests, Arabidopsis Col-0 dnd1/dnd1 plants were crossed to homozygous cpr1 and cpr5 mutants, which also display a reduced rosette size (24, 25). Dominance/recessiveness and genetic complementation were deduced by observation that all F1 plants were wild-type in appearance and displayed the HR after inoculation with P. syringae pv. glycinea Race 4 pV288.
Microscopy.
To monitor HR cell death at the cellular level, pipet infiltration was used to introduce P. syringae pv. glycinea Race 4 pV288 (avrRpt2+) or Race 4 pVSP61 (no avr gene) into 40–70% of the mesophyll space of individual leaves, at the bacterial concentrations indicated. Leaves were removed from plants after 24 h, fixed in 2% formaldehyde, 5% acetic acid, and 40% ethanol for 30 min, and then cleared sequentially in 50% ethanol and 95% ethanol (20). Leaf parenchyma cells then were examined for HR-associated autofluorescence by using fluorescence microscopy with a fluorescein filter set (Ex 495 ± 20 nm, Em > 505 nm) (26). Alternatively, Evan’s Blue (Sigma) was infiltrated into leaves as a 1% aqueous solution 22–26 h after pathogen inoculation (26). After at least 10 min of staining, leaves were removed from plants, a portion of the epidermis was peeled back, and leaves were rinsed in H2O, mounted in H2O, and observed by light microscopy. Leaf areas damaged by physical handling were not considered when evaluating the proportion of dead and living cells.
Genetic Mapping.
F2 populations from a No-0 × Col-0 dnd1/dnd1 cross were used for mapping. The HR phenotype was assessed visually 24 and 48 h after pipet inoculation of leaves with P. syringae pv. glycinea Race 4 pV288 (avrRpt2+) resuspended to ≈1 × 108 cfu/ml in 10 mM MgCl2. Informative F2 lines were retested for HR in selfed F3 families. PCR-based cleaved amplified polymorphic sequence and microsatellite markers were used as described in refs. 27 and 28; a set of 17 markers spanning all five Arabidopsis chromosomes was used for initial linkage analysis.
Inoculations with Other Pathogens.
Tobacco ringspot virus grape strain was applied to plants, and virus multiplication was monitored by using ELISA as described in ref. 29. Xanthomonas campestris pv. campestris strain 2669 (30) and X. c. pv. raphani strains 1946, 2345, and 2586 (31) were applied at a concentration of ≈1 × 107 cfu/ml and monitored as described in ref. 31. Peronospora parasitica isolate Noco2 was applied and monitored as described in ref. 32. For all experiments, Arabidopsis ecotype Col-0 served as a susceptible control for pathogen multiplication and virulence.
Gene Expression Studies.
P. syringae pv. tomato strains DC3000 (pV288) or DC3000 (pVSP61) were introduced into leaf mesophyll of intact plants by vacuum infiltration (as above), typically at a dose of 5 × 104 cfu/ml. Total RNA was extracted from leaf material and equal quantities of RNA from each sample were separated in agarose–formaldehyde gels, blotted, and hybridized with 32P-radiolabeled probe essentially as described in ref. 33. DNA probes were from Cao et al. (25). Hybridization was quantified by using a storage phosphor imaging system according to the manufacturer’s instructions (Molecular Dynamics). Signal for PR-1 or β-glucanase in each lane was normalized to the control β-ATPase signal for that lane to correct for slight differences in gel loading, and normalized signals then were divided by the signal for the Col-0/no-pathogen sample to establish a relative scale.
Salicylic Acid Determinations.
Salicylic acid determinations were performed as described in ref. 34 on leaf material from uninoculated 6-week-old plants.
RESULTS
Mutant Screen and Initial Analysis.
To address the relationship between HR cell death, resistance gene-mediated defense signal transduction, and the actual restriction of pathogen growth, we sought to isolate and characterize mutants of Arabidopsis thaliana that are deficient in the HR. A mutagenized M2 population of Arabidopsis line Col-0, which expresses the RPS2 resistance gene, was screened by inoculating plants with a strain of the bacterial plant pathogen P. syringae pv. glycinea expressing the RPS2-complementary avirulence gene avrRpt2 (19). An extremely high titer of pathogen, 2 × 108 cfu/ml, was used so that plants undergoing a wild-type HR would exhibit visible collapse of leaf tissue. The dnd1 mutant was recovered from this screen as a line displaying reduced rosette size and a clear HR− phenotype. Progeny lines derived from the dnd1 mutant failed to produce an HR not only when inoculated with pathogens expressing avrRpt2 but also in response to P. syringae that express avirulence genes avrRpm1 or avrB (19, 21). Two separate resistance genes (RPS2 and RPM1) control responsiveness to these three separate avirulence genes. Accordingly, we hypothesize that the dnd1 line is disrupted in a common component of the plant defense response that is shared by initially distinct gene-for-gene signal transduction pathways.
Microscopic Analysis of HR Cell Death.
To confirm the absence of hypersensitive cell death in response to avirulent pathogens in the dnd1 mutant, fluorescence microscopy was used to monitor cells within inoculated leaf tissue (26). Plant cells that undergo the HR display a marked increase in fluorescence due primarily to the production and release of phenolic compounds upon cell death. In “low titer” experiments, P. syringae pv. glycinea expressing avrRpt2 were introduced into leaf mesophyll tissue at a concentration of ≈5 × 105 cfu/ml, a dose at which a majority of the plant cells are not initially in contact with pathogen. As expected, leaves from the wild-type parental line infected at this dose with P. syringae expressing avrRpt2 contained numerous isolated autofluorescent cells. In contrast, very few autofluorescent foci were present in dnd1 leaves inoculated with the same avirulent strain. The dnd1 leaves instead resembled uninoculated leaves or leaves inoculated with the nonavirulent P. syringae control.
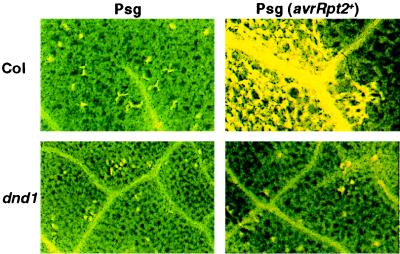
When leaves of the parental Col-0 line were inoculated with an extremely high titer of avirulent P. syringae (2 × 108 cfu/ml), the expected confluent collapse of host cells was observed (Fig. 1) (19, 20). However, even at this high pathogen dose, very little cell death above that seen in negative controls was detected in dnd1 plants (Fig. 1). Separate experiments that used Evans Blue to stain dead or dying cells gave similar results. The autofluorescence assay method was preferred because of greater clarity and less laborious tissue preparation. With the autofluorescence assay, absence of HR cell death in dnd1 plants was observed in multiple experiments, including experiments that used initial bacterial titers as high as 2 × 109 cfu/ml. A slight increase in cell death was observed in ≈5–8% of the dnd1 leaves inoculated with 2 × 108 cfu/ml of avirulent P. syringae but only in isolated areas that represented a fraction of the inoculated tissue. Cell death in these small areas was patchy rather than confluent, and similar small patches of cell death could be observed at a lower frequency in control Col-0 plants inoculated with the nonavirulent P. syringae strain. Because of this isolated cell death in a small minority of inoculated leaves, we cannot absolutely conclude that the HR is abolished. However, no stimulation of cell death by avirulent P. syringae could be detected in the vast majority of the inoculated dnd1 leaves.
Figure 1.
HR cell death defect in dnd1 mutant. Leaves of wild-type parent (Col) and dnd1 mutant (dnd1) plants were inoculated with a high dose (2 × 108 cfu/ml) of avirulent, HR-stimulating P. syringae pv. glycinea Race 4 pV288 (Psg avrRpt2+) or the isogenic, nonavirulent control strain P. syringae pv. glycinea Race 4 pVSP61 (Psg). At 24 h postinoculation, leaves were harvested, fixed, and examined for autofluorescent dead cells by using a fluorescence microscope. (Upper Right) The edge of an inoculated zone, revealing confluent cell death in response to bacteria only on the left (inoculated) side.
Restriction of Avirulent Pathogen Growth.
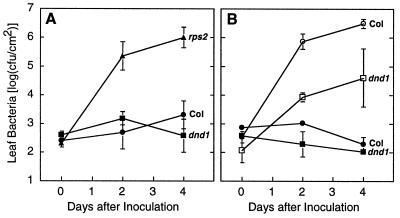
To determine whether the absence of the HR in the Arabidopsis dnd1 mutant is associated with compromised disease resistance, growth of P. syringae pv. tomato within plants was monitored quantitatively over time (23). Pathogenic strains that express an avirulence gene are virulent on plants that do not express the corresponding resistance gene, but their growth is reduced severely on plants with the appropriate resistance gene. Fig. 2A shows the growth of P. syringae pv. tomato expressing avrRpt2 in wild-type Arabidopsis Col-0 (RPS2/RPS2), in a Col-0 line lacking functional RPS2 (rps2–201/rps2–201), and in the Col-0 dnd1 mutant (dnd1/dnd1). Despite the absence of the HR, dnd1 was very similar to wild type in successfully restricting the growth of P. syringae expressing avrRpt2. Strong avirulence and resistance gene-dependent restriction of pathogen growth also was observed in quantitative experiments with P. syringae expressing avrRpm1, avrRps4, or avrB (Fig. 2B; data not shown). These results demonstrate that extensive HR cell death is not always required for resistance gene/avirulence gene-dependent plant disease resistance.
Figure 2.
Growth of bacteria within plant leaves. (A) Arabidopsis lines Col (Col-0 wild-type, RPS2/RPS2; DND1/DND1), rps2 (Col-0 rps2–201/rps2–201; DND1/DND1), and dnd1 (Col-0 RPS2/RPS2; dnd1/dnd1) inoculated with P. syringae pv. tomato DC3000 pV288 (avrRpt2+). (B) Arabidopsis lines Col-0 and dnd1 inoculated with isogenic P. syringae pv. tomato DC3000 differing by the presence (pAvrRpm1, filled symbols) or absence (pVSP61, open symbols) of avirulence gene avrRpm1 carried on plasmid pVSP61. Both plant lines are RPM1/RPM1 genotype. All data points are mean ± SD.
Genetic Mapping.
To determine the genetic basis of the dnd1 phenotype, segregation analysis and gene mapping studies were carried out. Crosses of dnd1 to wild-type Col-0 and No-0 ecotypes yielded F1 individuals that display the wild-type HR+ phenotype, demonstrating the recessive nature of the mutant phenotype. F2 of a Col-0 × dnd1 cross segregated 24:7 for HR+:HR− , F2 of a No-0 × dnd1 cross segregated 154:55, and F2 of a reciprocal dnd1 × No-0 cross segregated 132:45. These data are consistent with a 3:1 ratio (for χ2 test, P = 0.59, 0.66, and 0.90, respectively), indicating that a single mutant locus controls the observed phenotypes. The reduced rosette size phenotype was also recessive, and absolutely co-segregated with the HR− phenotype in these and all other F2 plants analyzed. The gene symbol DND1 was chosen for this locus, reflecting the mutant phenotype of Defense with No HR cell Death. PCR-based microsatellite and cleared amplified polymorphic sequence genetic markers were used to map the mutated locus. No linkage was detected except to markers for the top arm of chromosome 5. Fine-structure mapping with 536 F2 individuals from No-0 × dnd1 crosses yielded only six recombinant chromosomes between dnd1 and nga106 and a different 11 recombinant chromosomes between dnd1 and CHS1. These experiments placed DND1 within the ≈1.6-cM interval between CHS1 and nga106 on the upper arm of Arabidopsis Chromosome 5. This location defines a map position that has not been associated previously with defense-related genes.
Response to Virulent Pathogens.
Having established that dnd1 plants are resistant to avirulent P. syringae despite the absence of the HR, the response of the dnd1 mutant to virulent P. syringae was examined. Fig. 2B shows the growth of the virulent P. syringae pv. tomato strain DC3000 (pVSP61) in wild-type Col-0 and in Col-0 dnd1/dnd1 plants (open symbols). This strain does not trigger gene-for-gene resistance in plants of the Col-0 genotype (19, 23), yet leaf populations of this strain were reduced 10- to 100-fold in experiments with the dnd1 mutant. Similar results were obtained in multiple experiments and in studies with the virulent P. syringae pv. maculicola strain 4326 (data not shown). The dnd1 plants express a level of resistance to virulent P. syringae that is typical of plants exhibiting systemic acquired resistance, induced systemic resistance, or other forms of resistance gene-independent disease resistance (11, 35). This broad spectrum resistance phenotype co-segregated with the other dnd1 mutant phenotypes in all cases tested.
Important to note, Fig. 2B also shows that growth of populations of P. syringae that do express avrRpm1 (closed symbols) was restricted to a much greater extent than was growth of the virulent pathogen strain. A 1,000- to 10,000-fold reduction of pathogen growth was observed if the otherwise-virulent P. syringae strains DC3000 or 4326 expressed avirulence genes avrRpm1 or avrRpt2 (Fig. 2B; data not shown). These experiments demonstrated that gene-for-gene resistance can be induced over and above the weaker resistance gene-independent resistance in dnd1 plants.
To examine the extent of the lower level resistance to virulent pathogens in the dnd1 mutant, plants were inoculated with virulent strains of other pathogen species (29, 30, 36, 37). Tobacco ringspot virus spread systemically in only 9% of dnd1 plants as opposed to 71% for wild-type Col-0. Xanthomonas campestris pv. campestris and X. c. pv. raphani (bacteria) only produced mild yellowing on dnd1 rather than the necrotic lesions produced on Col-0. Peronospora parasitica (oomycete) produced three-fold fewer spores on dnd1 as opposed to Col-0 [3.0 ± 2.2 vs. 10.7 ± 3.1; mean ± SE of (spores × 103) per leaf]. Microscopy of leaves infected with virulent P. parasitica confirmed that restriction of mycelial growth was not associated with HR-like host cell necrosis or autofluorescence. At 3 days postinoculation, mycelia of virulent P. parasitica strain Noco2 typically had formed haustoria on 2–10 host cells in dnd1 plants, whereas in wild-type Col-0 plants a typical mycelium ramified extensively and formed haustoria on 15–30 host cells. Significantly reduced growth of Erysiphe orontii (fungus) in dnd1 plants also has been observed (T. L. Reuber and F. M. Ausubel, personal communication).
PR Gene Expression.
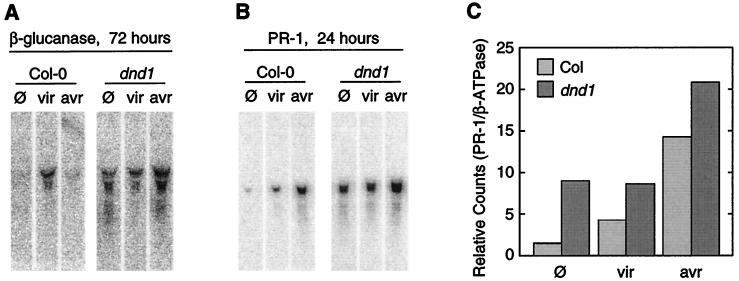
Constitutively elevated broad spectrum resistance has been observed previously in a number of contexts, such as in Arabidopsis cpr, cim, lsd, and acd mutants (8), in hybrid tobacco lines derived from crosses between disparate Nicotiana species (38), and in plants expressing systemic acquired resistance in response to prior pathogen infection or treatment with salicylic acid or synthetic salicylic acid mimics (11). Elevated resistance often is associated with increased expression of pathogenesis-related (PR) genes (11), and examination of uninoculated dnd1 plants revealed constitutively increased expression of the PR genes β-glucanase and PR-1 (Fig. 3 A and B) (25, 33). Although plants infected by virulent P. syringae pv. tomato displayed elevated levels of β-glucanase or PR-1 mRNA, inoculation of dnd1 or wild-type Col-0 with avirulent P. syringae expressing avrRpt2 caused an even greater elevation in PR-1 mRNA (Fig. 3C) (25, 33). Similar or more pronounced results were obtained with four distinct RNA sets prepared, blotted, and probed in entirely separate experiments. These results demonstrate, at the level of gene expression, that gene-for-gene signal transduction and defense response activation are functional in dnd1 plants and are inducible over and above constitutive broad spectrum resistance.
Figure 3.
Pathogenesis-related gene expression monitored by RNA blot analysis of Col-0 wild-type (Col) and Col-0 dnd1/dnd1 mutant (dnd1) plants. (A) β-glucanase expression 72 h after treatment of leaves with 10 mM MgCl2 containing no pathogen (φ), the nonavirulent control strain P. syringae pv. tomato DC3000 pVSP61 (vir), or the isogenic avrRpt2-expressing strain P. syringae pv. tomato DC3000 pV288 (avr). (B) PR-1 expression 24 h after treatment as in A. (C) Phosphorimager quantification of PR-1 expression from blot shown in B, normalized to level of constitutive β-ATPase mRNA. Similar results were obtained in multiple experiments.
Salicylic Acid Levels.
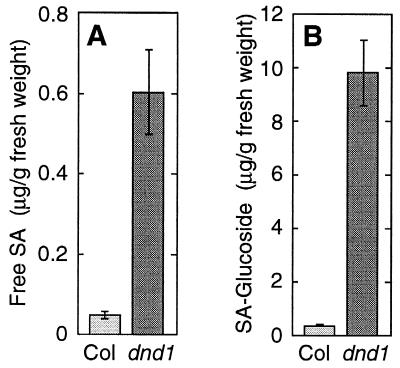
Enhanced PR gene expression and broad spectrum resistance can be induced by elevated levels of endogenous or applied salicylic acid compounds (11). We observed constitutively elevated levels of both free salicylic acid and glucoside-conjugated salicylates in dnd1 plants (Fig. 4). Although salicylates are likely to be a primary mediator of heightened resistance in dnd1 plants, the mechanism by which the dnd1 mutation causes salicylate elevation remains to be discovered.
Figure 4.
Levels of salicylic acid and glucoside-conjugated salicylic acid compounds in Col-0 and mutant Col-0 dnd1–1/dnd1–1 plants.
Comparison to Other Arabidopsis Mutants.
We are not aware of previous reports of plant mutants that display gene-for-gene disease resistance with no HR cell death. However, other Arabidopsis mutants that exhibit constitutively elevated resistance have been isolated, such as the cpr, cim, lsd, and acd mutants (8, 24, 39, 40). Accordingly, dnd1 plants were compared with a number of these lines. In contrast to the acd and lsd mutants, no lesion-mimic phenotype was observed in dnd1 mutants when leaf tissue from uninoculated plants was inspected by naked eye, by autofluorescence microscopy as described in ref. 20, or after trypan blue staining as described in ref. 41. Genetic complementation tests demonstrated that DND1 is a separate locus from the two published cpr loci, CPR1 and CPR5 (see Materials and Methods). In addition, the dnd1 mutant apparently does not resemble many of the other unpublished cpr or cim mutants because the dnd1 mutant does not exhibit traits observed in preliminary analysis of those mutants such as dominant or semi-dominant behavior, very low fertility, glabrousness, or distorted leaf shape (data not shown; S. Bowling and X. Dong, personal communication; K. Maleck and J. Ryals, personal communication). In particular, previously described cpr and cim mutants do not display the dnd phenotype of gene-for-gene defense with no HR cell death. The dnd1 mutant does exhibit a dwarf phenotype, as is observed in Arabidopsis cpr, cim, and other constitutive PR-expression mutants, but dnd1 plants otherwise appear normal in their growth and development.
DISCUSSION
A strong association exists between HR cell death and gene-for-gene resistance. However, there is evidence in the literature that it might be possible for gene-for-gene disease resistance to occur without HR cell death (see examples in Introduction). In the present study, a plant mutant was isolated that directly demonstrates gene-for-gene-mediated restriction of pathogen growth despite virtual elimination of HR cell death.
Models for the induction of plant defense appropriately place cell death downstream of resistance gene product/avirulence gene product interaction (4, 7, 8). Cell death can be placed upstream of defense induction, given that cell death is a known inducer of responses such as PR gene expression or local and systemic acquired resistance (8, 11). However, the role of cell death in defense induction is apparently supplementary or reinforcing rather than essential. Many forms of plant disease resistance other than gene-for-gene resistance do not involve cell death (3). Even in gene-for-gene resistance, where cell death is so prevalently observed, components of gene-for-gene resistance such as an oxidative burst, salicylate production, or induction of PR gene expression are activated before HR cell death (7, 12). The fact that cell death also can activate these responses suggests that a function of HR cell death may be to reinforce or strengthen the induction of defenses (8, 11, 42). Enhanced stimulation of defense responses by host cell death may account in part for the observation that gene-for-gene resistance often provides more complete restriction of pathogen growth than systemic acquired resistance [(Fig. 2 and ref. 43; compare also refs. 19, 21, 44, and 45)]. In particular, HR cell death causes elevation of salicylic acid levels, and salicylic acid is known to potentiate enhanced responsiveness of the host to subsequent pathogen infections (8, 11, 46). The constitutive elevation of salicylate observed in dnd1 plants may substitute for extensive HR cell death in potentiating the strong, gene-for-gene-mediated defense response. Alternatively, the dnd1 mutation may alter production of or sensitivity to other potentiators of gene-for-gene resistance. It is also possible that the HR cell death response is unnecessary for resistance signaling. Even if cell death does contribute to defense signal transduction in wild-type plants, our data with the dnd1 mutant demonstrate that cell death itself is not directly essential for the strong restriction of pathogen growth observed in gene-for-gene resistance of Arabidopsis against P. syringae.
In building models that account for the effect of the dnd1 mutation, a number of defense-related plant phenotypes must be considered. Because recessive mutation of DND1 caused constitutive activation of defense responses, it can be stated formally that the product of the wild-type DND1 locus suppresses constitutive elevation of defenses. This effect may be direct or quite indirect, however, because absence or misfunction of DND1 may cause any of a variety of perturbations that trigger defenses. It is likely that this perturbation acts upstream of salicylic acid production because salicylate levels are elevated in dnd1 mutants. The elevated salicylate would seem to account for the observed elevation of PR gene expression and the enhancement of resistance against virulent pathogens. The dnd1 defect does not significantly inhibit initial resistance gene-dependent recognition of avirulent pathogens nor does it block the defense signaling that leads to strong PR gene expression and strong restriction of the growth of avirulent P. syringae.
The defective HR phenotype of dnd1 mutants may result from elevation of cell death-suppressing functions as one component of the constitutively elevated broad spectrum resistance. As early as 1970, Lozano and Sequeira reported suppression of the HR by resistance-inducing pretreatment of tobacco with heat-killed cells of Ralstonia (Pseudomonas) solanacearum (47). Research with many biological systems has provided evidence of programmed cell death pathways that will function unless they are suppressed (48, 49). Study of lesion-mimic plant mutants indicates that such cell death suppression pathways operate in wild-type plants to prevent excessive spread of HR cell death (8, 50, 51). However, if the dnd1 mutants do actively suppress HR cell death, this suppression is not explained simply by constitutive elevation of salicylic acid or PR gene expression. Many other Arabidopsis mutants display these traits and yet do not suppress HR cell death. In addition to elevation of salicylic acid, disruption of DND1 function apparently causes the activation of other plant responses, including processes that contribute to the suppression of HR cell death.
The availability of dnd1 plants should facilitate future study of the mechanisms that control programmed cell death, gene-for-gene disease resistance, and broad spectrum disease resistance in plants. Study of dnd1 mutants may also suggest novel approaches to the engineering of disease resistance in cultivated plant species.
Acknowledgments
We thank Diya Banerjee, Steve Clough, Anna Huttenlocher, and Bernadette Lippok for critical reading of the manuscript, Xinnian Dong, Hui Cao, and Scott Bowling for seed, probes, and communication of results before publication, Kay Lawton, Leslie Friedrich, Novartis Crop Protection, and Novartis Seeds for advice and use of facilities for salicylate assays and for communication of results before publication, Lynne Reuber for sharing unpublished data, Jian-ming Lee for assistance with TRSV experiments, and Lila Vodkin and Lane Rayburn for use of microscopes. Research funded by National Institutes of Health Grant GM53595-03 and U.S. Department of Agriculture Grant 9702699 to A.F.B.; and by support to J.P. from The Gatsby Charitable Foundation.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: HR, hypersensitive response; PR, pathogenesis-related; cfu, colony-forming units.
References
- 1.McIntosh R A, Wellings C R, Park R F. Wheat Rusts: An Atlas of Resistance Genes. Australia, and Kluwer, Dordrecht, The Netherlands: Commonwealth Scientific and Industrial Research Organization; 1995. [Google Scholar]
- 2.Crute I R, Pink D A C. Plant Cell. 1996;8:1747–1755. doi: 10.1105/tpc.8.10.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrios G N. Plant Pathology. San Diego: Academic; 1997. [Google Scholar]
- 4.Bent A F. Plant Cell. 1996;8:1757–1771. doi: 10.1105/tpc.8.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flor H H. Phytopathology. 1942;32:653–669. [Google Scholar]
- 6.Alfano J R, Collmer A. Plant Cell. 1996;8:1683–1698. doi: 10.1105/tpc.8.10.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammond-Kosack K E, Jones J D G. Plant Cell. 1996;8:1773–1791. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dangl J L, Dietrich R A, Richberg M H. Plant Cell. 1996;8:1793–1807. doi: 10.1105/tpc.8.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stakman E C. J Agric Res. 1915;4:193–199. [Google Scholar]
- 10.Goodman R N, Novacky A J. The Hypersensitive Reaction in Plants to Pathogens: A Resistance Phenomenon. St. Paul: Am. Phytopathol. Soc.; 1994. [Google Scholar]
- 11.Ryals J L, Neuenschwander U H, Willits M C, Molina A, Steiner H-Y, Hunt M D. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon R A, Harrison M J, Lamb C J. Annu Rev Phytopathol. 1994;32:479–501. [Google Scholar]
- 13.Hammond-Kosack K E, Silverman P, Raskin I, Jones J D G. Plant Physiol. 1996;110:1381–1394. doi: 10.1104/pp.110.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittler R, Shulaev V, Seskar M, Lam E. Plant Cell. 1996;8:1991–2001. doi: 10.1105/tpc.8.11.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goulden M G, Baulcombe D C. Plant Cell. 1993;5:921–930. doi: 10.1105/tpc.5.8.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehnackers H, Knogge W. Can J Bot. 1990;68:1953–1961. [Google Scholar]
- 17.Schiffer R, Gorg R, Jarosch B, Beckhove U, Bahrenberg G, Kogel K-H, Schulze-Lefert P. Mol Plant-Microbe Interact. 1997;10:830–839. [Google Scholar]
- 18.Century K S, Holub E B, Staskawicz B J. Proc Natl Acad Sci USA. 1995;92:6597–6601. doi: 10.1073/pnas.92.14.6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunkel B N, Bent A F, Dahlbeck D, Innes R W, Staskawicz B J. Plant Cell. 1993;5:865–875. doi: 10.1105/tpc.5.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu G-L, Katagiri F, Ausubel F M. Mol Plant-Microbe Interact. 1993;6:434–443. doi: 10.1094/mpmi-6-434. [DOI] [PubMed] [Google Scholar]
- 21.Bisgrove S R, Simonich M T, Smith N M, Sattler A, Innes R W. Plant Cell. 1994;6:927–933. doi: 10.1105/tpc.6.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinsch M, Staskawicz B. Mol Plant-Microbe Interact. 1996;9:55–61. doi: 10.1094/mpmi-9-0055. [DOI] [PubMed] [Google Scholar]
- 23.Whalen M, Innes R, Bent A, Staskawicz B. Plant Cell. 1991;3:49–59. doi: 10.1105/tpc.3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowling S A, Clarke J D, Liu Y, Klessig D F, Dong X. Plant Cell. 1997;9:1573–1584. doi: 10.1105/tpc.9.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao H, Bowling S A, Gordon A S, Dong X. Plant Cell. 1994;6:1583–1592. doi: 10.1105/tpc.6.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klement Z, Stall R E, Novacky A, Ersek T, Fett W, Huang J, Beckman C. In: Methods in Phytobacteriology. Klement Z, Rudolph K, Sands D C, editors. Budapest: H. Stillman; 1990. pp. 469–473. [Google Scholar]
- 27.Bell C J, Ecker J R. Genomics. 1994;19:137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- 28.Konieczny A, Ausubel F M. Plant J. 1993;4:403–410. doi: 10.1046/j.1365-313x.1993.04020403.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee J-M, Hartman G L, Domier L L, Bent A F. Mol Plant-Microbe Interact. 1996;9:729–735. doi: 10.1094/mpmi-9-0729. [DOI] [PubMed] [Google Scholar]
- 30.Bent A, Innes R, Ecker J, Staskawicz B. Mol Plant-Microbe Interact. 1992;5:372–378. doi: 10.1094/mpmi-5-372. [DOI] [PubMed] [Google Scholar]
- 31.Parker J E, Barber C E, Fan M J, Daniels M J. Mol Plant-Microbe Interact. 1993;6:216–224. doi: 10.1094/mpmi-6-216. [DOI] [PubMed] [Google Scholar]
- 32.Parker J E, Coleman M J. Trends Biochem Sci. 1997;22:291–296. doi: 10.1016/s0968-0004(97)01089-x. [DOI] [PubMed] [Google Scholar]
- 33.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols In Molecular Biology. New York: Wiley; 1997. [Google Scholar]
- 34.Uknes S, Winter A, Delaney T, Vernooij B, Friedrich L, Morse A, Potter S, Ward E, Ryals J. Mol Plant-Microbe Interact. 1993;6:692–698. [Google Scholar]
- 35.Pieterse C M, van Wees S C, Hoffland E, van Pelt J A, van Loon L C. Plant Cell. 1996;8:1225–1237. doi: 10.1105/tpc.8.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parker J E, Barber C E, Fan M J, Daniels M J. Mol Plant-Microbe Interact. 1993;6:216–224. doi: 10.1094/mpmi-6-216. [DOI] [PubMed] [Google Scholar]
- 37.Parker J E, Coleman M J, Szabo V, Frost L N, Schmidt R, van der Biezen E A, Moores T, Dean C, Daniels M J, Jones J D G. Plant Cell. 1997;9:879–894. doi: 10.1105/tpc.9.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahl Goy P, Felix G, Metraux J P, Meins F. Physiol Mol Plant Pathol. 1992;41:11–21. [Google Scholar]
- 39.Bowling S A, Guo A, Cao H, Gordon A S, Klessig D F, Dong X. Plant Cell. 1994;6:1845–1857. doi: 10.1105/tpc.6.12.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawton K, Uknes S, Friedrich L, Gaffney T, Alexander D, Goodman R, Metrauz J-P, Kessmann H, Ahl-Goy P, Gut-Rella M, et al. In: Mechanisms of Defence Responses in Plants. Fritig B, Legrand M, editors. Dordrecht, The Netherlands: Kluwer; 1993. pp. 422–432. [Google Scholar]
- 41.Parker J E, Szabo V, Staskawicz B J, Lister C, Dean C, Daniels M J, Jones J D G. Plant J. 1993;4:821–831. [Google Scholar]
- 42.Alvarez M E, Pennel R I, Meijer P J, Ishikawa A, Dixon R A, Lamb C. Cell. 1998;92:773–784. doi: 10.1016/s0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- 43.Sequeira L. Annu Rev Microbiol. 1983;37:51–79. doi: 10.1146/annurev.mi.37.100183.000411. [DOI] [PubMed] [Google Scholar]
- 44.Cameron R K, Dixon R, Lamb C. Plant J. 1994;5:715–725. [Google Scholar]
- 45.Lawton K, Friedrich L, Hunt M, Weymann K, Staub T, Kessman H, Ryals J. Plant J. 1996;10:71–82. doi: 10.1046/j.1365-313x.1996.10010071.x. [DOI] [PubMed] [Google Scholar]
- 46.Shirasu K, Nakaijma H, Rajasekhar V K, Dixon R A, Lamb C. Plant Cell. 1997;9:261–270. doi: 10.1105/tpc.9.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lozano J C, Sequira L. Phytopathology. 1970;60:875–879. [Google Scholar]
- 48.Meredith J E J, Schwartz M A. Trends Cell Biol. 1997;7:146–150. doi: 10.1016/S0962-8924(97)01002-7. [DOI] [PubMed] [Google Scholar]
- 49.Nagata S. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 50.Gray J, Close P S, Briggs S P, Johal G S. Cell. 1997;89:25–31. doi: 10.1016/s0092-8674(00)80179-8. [DOI] [PubMed] [Google Scholar]
- 51.Dietrich R A, Richberg M H, Schmidt R, Dean C, Dangl J L. Cell. 1997;88:685–694. doi: 10.1016/s0092-8674(00)81911-x. [DOI] [PubMed] [Google Scholar]