Abstract
Salmonella spp. (n = 468), isolated from the feces of sows, nursery, and grow‐finish pigs in 20 farrow‐to‐finish herds in Alberta and Saskatchewan, were tested for susceptibility to 16 antimicrobials. No resistance was identified to amikacin, amoxicillin‐clavulanic acid, ceftiofur, ceftriaxone, ciprofloxacin or nalidixic acid, and less than 1% of the isolates were resistant to cefoxitin and gentamicin. Isolates were most commonly resistant to tetracycline (35%) and sulfamethoxazole (27%). Overall, 59% of the Salmonella were susceptible to all 16 drugs (pansusceptible). Isolates from sows were more likely to be pansusceptible than isolates from nursery or grow‐finish pigs. Resistance to 2 or more drugs occurred in 29% of the isolates and was significantly more likely to occur in Salmonella from nursery pigs than from sows. The odds of resistance to 4 of the drugs, streptomycin, ampicillin, kanamycin and cephalothin, were significantly higher in isolates from nursery pigs than grow‐finish pigs, while the odds of resistance to 2 drugs, tetracycline and streptomycin, were higher in Salmonella from nursery pigs than from sows. More age‐specific risk factor studies are needed to investigate these differences between production phases.
Résumé
Des isolats de Salmonella spp. (n = 468), provenant des fèces de truies, de porcelets en pouponnière et de porcs en croissance-finition prélevées dans 20 troupeaux de naisseur-finisseur en Alberta et Saskatchewan, ont été testés pour leur sensibilité à 16 antimicrobiens. Aucune résistance ne fut détectée envers les antibiotiques suivants : amikacine, amoxicilline-acide clavulanique, ceftiofur, ceftriaxone, ciprofloxacin et acide nalidixique; et moins de 1 % des isolats étaient résistants au cefoxitin et à la gentamycine. Les isolats étaient résistants le plus fréquemment à la tétracycline (35 %) et au sulfaméthoxazole (27 %). Globalement, 59 % des Salmonella étaient sensibles aux 16 antimicrobiens (pansensible). Les isolats provenant des truies étaient plus susceptibles d’être pansensibles que les isolats des animaux en pouponnière ou en croissance-finition. Une résistance à 2 antimicrobiens ou plus a été notée chez 29 % des isolats et étaient significativement plus probable de se produire chez les animaux en pouponnière que chez les truies. Les probabilités de résistance à 4 antimicrobiens (streptomycine, ampicilline, kanamycine et céphalothine) étaient significativement plus élevées chez les isolats provenant des porcs en pouponnière que les animaux en croissance-finition, alors que les probabilités de résistance à 2 antimicrobiens (tétracycline et streptomycine) étaient plus élevées pour les Salmonella provenant des animaux en pouponnière que chez les truies. Des études supplémentaires sur les facteurs de risque reliés à l’âge sont nécessaires pour comprendre ces différences entre les phases de production.
(Traduit par Docteur Serge Messier)
Introduction
Salmonella spp. are the 2nd leading cause of bacterial foodborne disease in Canada. Approximately 6000 to 9000 cases of human salmonellosis are reported annually, and for each reported illness 13 to 37 cases remain unreported (1–3). Antimicrobial resistant Salmonella can cause even greater morbidity than their susceptible counterparts due to treatment failure, increased infection severity, and increased rates of disease in people taking antimicrobials for other reasons (4–7). Although pork is not a major cause of salmonellosis in North America, it has been responsible for disease outbreaks of multi‐resistant Salmonella in humans elsewhere (8–10).
Most Salmonella infections are acquired from contaminated food; therefore, studying antimicrobial resistance (AMR) in live, close‐to‐market pigs indirectly estimates the potential for cross‐contamination at slaughter and the risk to consumers from resistant organisms in pork (11,12). In Canada, Salmonella AMR data are available from pigs at slaughter (13–15); however, these data may differ from those acquired on‐farm because AMR patterns and serovars can change after transport and lairage (16). Extending AMR investigations to other pig categories, such as sows and nursery pigs, might improve our understanding of the occurrence and spread of AMR within pig production systems. This in turn, could lead to the identification of possible control measures. In Canada, on‐farm Salmonella AMR data from healthy pigs are currently limited to a longitudinal study of finishing pigs from Alberta (17). The main goal of this study was to investigate and describe the AMR profiles of Salmonella isolates from apparently healthy nursery pigs, grow‐finish pigs, and sows in 20 herds in Alberta and Saskatchewan.
Materials and methods
Herd and sample selection
Herd selection has been described in detail (18,19). Briefly, a convenience sample of 20 farms participated in this study. Ten herds were enrolled with knowledge of the presumed Salmonella status to address other research objectives (20). Seven herds were enrolled as Salmonella‐positive and 3 Salmonella‐negative based on clinical disease or test results in the previous 12 mo. The principle investigator and laboratory personnel were blind to the presumed herd status. The remaining 10 herds, with an unknown Salmonella status, were selected from as many veterinarians as possible in order to minimize clustering by geography and management practices. Each herd was visited once between May and September 2004. Fresh fecal samples were collected from pens selected using a random numbers table. Pooled pen samples (PPS) consisted of feces from 5 pigs for a pooled sample weight of approximately 75 g. In the 10 herds of unknown Salmonella status, 25 PPS were collected from grow‐finish pigs. In the 10 presumed‐known Salmonella status herds, samples were collected from each phase of pig production: 20 PPS from sows, 30 PPS from nursery pigs, and 30 PPS from grow‐finish pigs. In addition, 30 individual samples were collected from grow‐finish pigs and 10 from sows.
Laboratory methods
Salmonella isolation
All samples were shipped on ice to 1 of 3 laboratories within 24 h of collection. Samples from 10 herds were cultured for Salmonella by Agri‐Food Laboratories Branch (AFLB), Food Safety Division of Alberta Agriculture, Food and Rural Development, Edmonton, Alberta; samples from 4 herds by Prairie Diagnostic Services (PDS), University of Saskatchewan, Saskatoon, Saskatchewan; and samples from 6 herds by Laboratory Services Division, University of Guelph, Guelph, Ontario.
AFLB Food Safety Division of Alberta Agriculture, Edmonton, Alberta
Salmonella Typhimurium ATCC 14028 was used as the quality control organism. Unless otherwise specified, the incubation temperature was 35°C. A 10 g fecal aliquot per sample was pre‐enriched in 90 mL of buffered peptone water (BPW) for 20 to 24 h. From the BPW, 0.1 mL was transferred into 10 mL Rappaport‐Vassiliadis (RV) enrichment broth and incubated at 42°C for 24 h. Concurrently, 1.0 mL was transferred into 10 mL of tetrathionate (TT) enrichment broth containing 0.2 mL of iodine solution and incubated for 24 h. Aliquots from the RV and TT broths (0.15 mL each) were pooled and screened by real‐time polymerase chain reaction (R‐PCR) for the presence of Salmonella (21). Following incubation, 10 μL of RV broth and 10 μL of TT broth were transferred to xylose‐lysine‐tergitol 4 Agar (XLT4) and Rambach agar plates and incubated for 24 to 48 h. The TT broth (0.1 mL) was transferred to 3 sites on modified semi‐solid Rappaport‐Vassiliadis (MSRV) (Difco Laboratories, Oakville, Ontario) media and incubated at 42°C for up to 72 h. Each MSRV plate with a halo was subcultured onto XLT4 and RAM plates. Samples positive by R‐PCR, but culture negative, were tested using immunomagnetic separation (IMS) technology (Dynabeads anti‐Salmonella; Dynal Biotech, ASA, Oslo, Norway). Beads were enriched in TT broth and processed as described previously. In general, 1 suspect Salmonella colony per sample was selected for further characterization unless morphologically different colonies were identified, in which case both were harvested. Isolates were initially screened with triple sugar iron agar (TSI), lysine iron agar (LIA), and urea agar slants, and purity checked using 1/4 MacConkey agar and blood agar plates, respectively. Isolates were further screened with Salmonella Poly 0/01 agglutination (Denka Seiken, Tokyo Japan) and atypical colonies were tested with Vitek GNI or API‐20E (bioMérieux Canada, Montreal, Quebec). Presumptive Salmonella isolates were frozen at − 70°C in defibrinated sheep’s blood and sent to the Office International des Épizooties (OIE) Reference Laboratory for Salmonellosis, in Guelph, Ontario for confirmation by serotyping.
Prairie Diagnostic Services, University of Saskatchewan, Saskatoon, Saskatchewan
The protocol developed by AFLB was utilized by PDS with minor modifications; R‐PCR and IMS were not used. Screening differed in that suspect colonies were incubated for 2 to 4 h in trypticase soy (TS) broth and subsequently tested with a citrate slant rather than LIA. Isolates were sent in TS broth to the Saskatchewan Health Provincial Laboratory for serotyping.
Laboratory Services Division, University of Guelph, Guelph, Ontario
Health Canada’s standard Salmonella isolation protocol (22) was used with the IMS technology (Dynabeads anti‐Salmonella; Dynal Biotech, ASA). Suspect colonies were tested with TSI, LIA, and urea slants to confirm Salmonella status, frozen at − 80°C in glycerol, and sent to the OIE Reference Laboratory for Salmonellosis, Guelph, Ontario for serological testing.
Serotyping and phagetyping
Office International des Épizooties (OIE) Reference Laboratory for Salmonellosis, Guelph, Ontario
The O antigens of the Salmonella isolates were determined by slide agglutination (23). The H antigens were identified using a microtechnique that employs microtitre plates (24). The antigenic formulae of Le Minor and Popoff (25) were used to name the sero‐vars. The standard phagetyping technique described by Anderson and Williams (26) was followed. The phagetyping scheme and phages for Salmonella Typhimurium, developed by Callow (27), and further extended by Anderson (28), Anderson et al (29), and Ward (30), were obtained from the International Centre for Enteric Phage Typing (ICEPT), Central Public Health Laboratory, Colindale, United Kingdom via the National Microbiology Laboratory (NML), Public Health Agency of Canada, Winnipeg, Manitoba. The Salmonella Heidelburg phagetyping scheme and phages were supplied by the NML (31). Isolates that reacted with the phages but did not conform to any recognized phage type were considered atypical. Strains that did not react with any of the typing phages were considered untypable.
Saskatchewan Health Provincial Laboratory, Regina, Saskatchewan
The O antigens of the Salmonella isolates were determined by slide agglutination and the H antigens were identified by a broth culture method (32). The antigenic formulae of Le Minor and Popoff were used to name the serovars (25). Samples identified as Salmonella Typhimurium (including var. Copenhagen), Salmonella Heidelberg or Salmonella Enteriditis were sent to the OIE Reference Laboratory for Salmonellosis, in Guelph, Ontario for phagetyping as described previously.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was conducted by AFLB and PDS using a broth microdilution technique following Clinical and Laboratory Standards Institute (CLSI) guidelines (33,34). Antimicrobial susceptibility testing methods have been described in detail (18,19).
Data comparisons and statistical analysis
Culture, serovar, and minimal inhibitory concentration (MIC) data were maintained in a relational database (Microsoft Access; Microsoft Corporation, Redmond, Washington, USA). Intermediate MIC values were categorized as susceptible for all analyses (33,34). Isolates susceptible to all drugs in the panel were designated ‘pan‐susceptible’. This definition does not imply isolates are susceptible to drugs not on the panel. Isolates resistant to 2 or more drugs were designated ‘multiresistant’ (14,17,35).
Descriptive analyses were conducted using commercially available software (Microsoft Excel; Microsoft Corporation, Redmond, Washington, USA). All statistical analyses accounted for clustering of resistance within herds through generalized estimating equations (GEE) (PROC GENMOD, SAS for Windows version 9.1; SAS Institute, Cary, North Carolina, USA). All models had a binary outcome, logit‐link function, and an exchangeable correlation structure. Null binomial response models estimated the prevalence of pansusceptibility, multiresistance, and resistance to each drug. From each model the intercept (β0) and 95% confidence intervals (CI) were used to calculate population‐average prevalence estimates using the formula [1 + exp(− β0)]− 1 (36). Univariate logistic regression examined the source of isolates (production phase) as a predictor for the following outcomes: resistance to each drug, pansusceptibility, and multiresistance. Four additional sets of univariate analyses explored potential biases introduced by study design: knowledge of herd Salmonella status, herd size, individual sample versus PPS, and laboratory performing isolation. These factors, which were known prior to susceptibility testing, were each considered as predictors for drug resistances observed in more than 5% of the isolates. Associations between variables of interest and outcomes were reported as an odds ratio (OR = expβ ) with 95% CI. All associations with P < 0.05 were reported as significant (36).
Results
Salmonella was identified in 14 herds with a range of 2 to 95 positive samples per herd. Overall, 452 (32%) of the samples were positive for Salmonella and 16 samples had 2 distinct isolates harvested, resulting in 468 isolates for susceptibility testing. Thirty serovars were identified with 9 accounting for 89% of the isolates. These 9 serovars were each identified in at least 2 herds and, except for serovar Banana, were found in every production phase.
The highest prevalence of resistance was to tetracycline. No isolates were resistant to 6 drugs, including amikacin (Table I). However, the amikacin status of 1 isolate was indeterminable because the dilution range did not cross the breakpoint and the MIC was greater than the dilution range. Almost 85% of the isolates from 6 of the top 9 sero‐vars were pansusceptible (Table II). In contrast, only 8% of Salmonella Mbandaka, 20% of Salmonella Typhimurium var. Copenhagen, and 55% of Salmonella Derby were pansusceptible (Table II).
Table I.
Population average prevalence estimates and 95% confidence intervals for resistance to each drug with minimum inhibitory concentrations (MIC) distributions of 468 Salmonella isolates
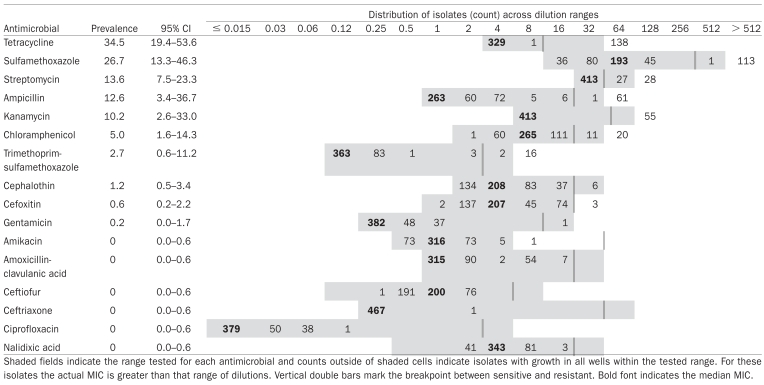 |
Table II.
Frequency of the 9 most common Salmonella serovars, the number of isolates per serovar with any resistance and number of isolates resistant to each antimicrobial (n = 468)
| Salmonella Serovar (any resistance/all isolates) | Number of isolates from each serovar resistant to each antimicrobial
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMP | CEP | CHL | COT | FOX | GEN | KAN | STR | SMX | TET | |
| Derby (56/124) | — | 4 | 22 | 12 | 3 | — | — | 17 | 24 | 31 |
| Typhimurium var. | 58 | 2 | 2 | — | — | — | 54 | 9 | 58 | 61 |
| Copenhagen (65/81) | ||||||||||
| Putten (2/49) | — | — | 1 | 1 | — | — | — | 1 | 2 | 2 |
| Infantis (0/37) | — | — | — | — | — | — | — | — | — | — |
| I:ROUGH-O:d:l,w (3/34) | — | — | — | 2 | — | — | — | 1 | 3 | 2 |
| Banana (0/27) | — | — | — | — | — | — | — | — | — | — |
| Mbandaka (24/26) | 1 | — | — | — | — | — | — | 19 | 12 | 24 |
| Anatum (1/20) | — | — | — | — | — | — | — | 1 | 1 | 1 |
| Give (3/19) | — | — | 1 | — | — | — | — | — | 3 | — |
| All others (21/51) | 3 | — | 5 | 3 | — | 1 | 1 | 7 | 11 | 17 |
AMP — ampicillin, CEP — cephalothin, CHL — chloramphenicol, COT — trimethoprim-sulfamethoxazole, FOX — cefoxitin, GEN — gentamicin, KAN — kanamycin, STR — streptomycin, SMX — sulfamethoxazole, TET — tetracycline.
Salmonella was isolated from 32% of the nursery samples, 28% of the grow‐finish samples, and 47% of the samples from sows (Table III). A higher percent of nursery, compared to grow‐finish or sow, isolates demonstrated resistance. This pattern was evident across all drugs except trimethoprim‐sulfamethoxazole, cefoxitin, and gentamicin (Table IV). The probability of observing resistance to 4 of the drugs was significantly higher in isolates from nursery pigs than from grow‐finish pigs and resistance to tetracycline and streptomycin was more likely in Salmonella from nursery pigs than from sows (Table IV). The odds of resistance to a given drug were not significantly different between Salmonella from grow‐finish pigs and sows.
Table III.
Distribution of production phases from which 1394 samples were collected, 452 Salmonella positive samples were identified and 468 Salmonella isolates were harvested
| Production phase | Sample type | Samples collected | Salmonella positive | Isolates harvested |
|---|---|---|---|---|
| Nursery | PPS | 255 | 81 | 83 |
| Grow-finish | Individual | 295 | 73 | 80 |
| PPS | 545 | 158 | 161 | |
| Sow | Individual | 100 | 38 | 38 |
| PPS | 199 | 102 | 106 | |
| Total | 1394 | 452 | 468 | |
PPS — pooled pen sample.
Table IV.
Percent of Salmonella isolates from nursery pigs (n = 83), grow-finish pigs (n = 241), and sows (n = 144) resistant to each drug
| Percent resistant
|
|||
|---|---|---|---|
| Antimicrobial | Nursery | Grow-finish | Sow |
| Tetracyclineb | 47.0 | 27.4 | 22.9 |
| Sulfamethoxazole | 41.0 | 16.6 | 27.8 |
| Streptomycina,b | 22.9 | 8.7 | 10.4 |
| Ampicillina | 19.3 | 9.1 | 16.7 |
| Kanamycina | 16.9 | 7.9 | 15.3 |
| Chloramphenicol | 7.2 | 7.1 | 5.6 |
| Trimethoprim-sulfamethoxazole | 2.4 | 3.7 | 4.9 |
| Cephalothina | 2.4 | 0.8 | 1.4 |
| Cefoxitinc | 0.0 | 0.4 | 1.4 |
| Gentamicinc | 0.0 | 0.4 | 0.0 |
Significant difference between isolates from nursery and grow-finish pigs (P < 0.05).
Significant difference between isolates from nursery pigs and sows (P < 0.05).
Models did not converge because frequency of resistance was too low.
Overall, 59% (95% CI, 42 to 75) of the Salmonella were pansus‐ceptible. Most isolates from sows (74%; 95% CI, 53 to 88) were pansusceptible, as were approximately half of the isolates from grow‐finish (56%; 95% CI, 36 to 73) and nursery pigs (48%; 95% CI, 26 to 71). The odds of an isolate being pansusceptible were sig‐ nificantly higher in sows compared to the other production phases (Table V). Multiresistance was observed in 29% (95% CI, 16 to 48) of the isolates. The prevalence of multiresistance was higher in isolates from nursery pigs (48%; 95% CI, 26 to 71) than from grow‐finish pigs (26%; 95% CI, 13 to 46) or sows (22%; 95% CI, 10 to 42). The difference between multiresistance in Salmonella from nursery pigs and sows was statistically significant (Table V).
Table V.
Unconditional odds ratios for Salmonella pansusceptibility and multiresistance between isolates from each production phases (n = 468)
| Outcome | Risk factor Source of Salmonella | Odds ratio | 95% Confidence interval | P |
|---|---|---|---|---|
| Pansusceptiblea | Sow compared with nursery | 3.0 | 1.2–7.8 | 0.02 |
| Sow compared with grow-finish | 2.3 | 1.1–4.6 | 0.02 | |
| Grow-finish compared with nursery | 1.3 | 0.7–2.5 | 0.37 | |
| Multiresistantb | Nursery compared with sow | 3.2 | 1.1–9.1 | 0.03 |
| Nursery compared with grow-finish | 2.6 | 1.0–7.0 | 0.05 | |
| Grow-finish compared with sow | 1.2 | 0.8–1.9 | 0.36 |
Pansusceptible defined as susceptible to all 16 drugs considered.
Multiresistant defined as resistant to 2 or more of the 16 drugs considered.
Twenty‐nine resistance patterns were identified and 9 occurred in more than 5 isolates (Table VI). Eight of these 9 were found in more than 1 phase in a herd. Despite this, isolates with the same pattern, from the same phase, and same herd, were not always the same serovar. Unobserved resistance patterns were also notable; resistance to ampicillin, chloramphenicol, streptomycin, sulfamethoxazole, and tetracycline (ACSSuT), suggestive of a chromosomally located gene cluster (37), was not identified in any isolate. Also noteworthy, the 13 Salmonella Typhimurium var. Copenhagen phage type 104 isolates were all pansusceptible.
Table VI.
Salmonella spp. resistance patterns observed in more than 5 isolates; number identified in each production phase, number of herds phenotype was identified in, and number of serovars with phenotype identified
| Number of isolates
|
|||||
|---|---|---|---|---|---|
| Resistance pattern | Nursery | Grow-finish | Sow | Number of herds | Number of serovars |
| AMP KAN SMX TET | 11 | 15 | 19 | 2 | 1 |
| TET | 1 | 31 | 4 | 4 | 6 |
| SMX STR TET | 5 | 4 | 5 | 4 | 3 |
| STR TET | 6 | 8 | 0 | 2 | 3 |
| CHL | 0 | 6 | 1 | 3 | 2 |
| CHL SMX STR TET | 5 | 2 | 0 | 1 | 5 |
| AMP KAN SMX STR TET | 2 | 1 | 3 | 1 | 1 |
| SMX TET | 5 | 0 | 1 | 3 | 3 |
| CHL SMX STR COT | 0 | 3 | 3 | 1 | 1 |
AMP — ampicillin, CHL — chloramphenicol, COT — trimethoprim-sulfamethoxazole, KAN — kanamycin, SMX — sulfamethoxazole, STR — streptomycin, TET — tetracycline.
Herd size was associated with resistance to streptomycin; the odds of resistance increased 1.02 times (95% CI, 1.01 to 1.03; P = 0.003) for every 1000 pigs marketed annually. No other resistance outcome was significantly associated with herd size (P > 0.05). Sample type was a significant predictor of 2 resistance outcomes. The odds of resistance to kanamycin were 1.2 times higher (95% CI, 1.1 to 1.3; P < 0.0001) in isolates from individual animals compared to PPS. In contrast, the odds of streptomycin resistance were decreased 0.47 times (95% CI, 0.26 to 0.86; P = 0.02) in samples collected from individual ani‐ mals. Resistance to ampicillin, chloramphenicol, sulfamethoxazole, and tetracycline were not significantly associated with sample type (P > 0.1). Finally, knowledge of herd Salmonella status and laboratory performing isolation were not significantly associated with resistance to streptomycin, sulfamethoxazole, or tetracycline (P > 0.2). For resistance to ampicillin, chloramphenicol, and kanamycin, the significance of these variables could not be determined because of strong clustering within serovar.
Discussion
Describing AMR in Salmonella from on‐farm studies provides insight into the epidemiology of resistance in pigs prior to transport, slaughter, and processing, which can affect Salmonella serovars and resistance (16,38,39). Different rates of resistance in each production phase shows Salmonella from each pig‐class may pose unique food safety risks and suggests that resistance is dynamic within barns. Hence, future on‐farm studies should investigate risk factors for resistance in each production phase. Identifying variables associated with changes in resistance between phases might lead to interventions to mitigate AMR in Salmonella.
Fluoroquinolones are used to treat invasive salmonellosis, while third‐generation cephalosporins are indicated for Salmonella infections in children. There are few therapeutic alternatives to these antimicrobial classes (12,40). Because humans generally acquire resistant Salmonella, rather than the resistance developing during treatment, resistance to these drugs in isolates from food animals is of utmost importance (12). Like other North American reports, no resistance was noted to ceftriaxone, ciprofloxacin, or nalidixic acid (15,17,41,42). Likewise, no resistance was found to ceftiofur. This differs from a report of frequent resistance to ceftiofur on United States farms (21%) (42). This difference may be partially explained by serotype as over 75% of the ceftiofur resistant Salmonella were Salmonella Derby (42). Canadian swine abattoir surveillance has noted changes in the Salmonella serovar distribution over time (43). Therefore, although no resistance to ceftiofur was reported in this study, common ceftiofur resistance in the United States suggests Canadian authorities and the swine industry should be vigilant for emerging third‐generation cephalosporin resistance in Salmonella from pigs.
Antimicrobial resistance studies typically focus on close‐to‐market animals; however, information on resistance within pig categories provides insight into AMR within farms. Lower rates of AMR in older pigs suggest that resistant Salmonella populations may fluctuate within barns. Investigating why Salmonella resistance declines as pigs age might identify on‐farm interventions to exploit this phenomenon. In a study of 3 farms, Nollet et al (44) observed less resistance in Salmonella from sows compared to growing pigs, but did not corroborate our observation of increased resistance in nursery pigs. However, others have reported relatively more resistance in coliforms from young pigs (45,46). These reports speculate that young animals carry more resistant organisms due to increased antimicrobial exposure and physiological differences (45,46). Identifying risk factors for resistance in nursery pigs is crucial; weaned pigs commonly receive antimicrobials, and in some instances are continuously exposed to drugs, which raises concerns about selection for resistance (19,47,48). The effect of this exposure on resistance should be investigated and accounted for in judicious use guidelines.
Describing how resistance in Salmonella relates to herd was complicated by the relationship with serovar. This study was too small to account for serovar in the statistical analyses. Antimicrobial resistance is known to be associated with serovar and our inability to account for this important confounder is a study weakness (35,49–51). Future studies will need a much larger sample size to address this concern. The descriptive statistics revealed that the most common resistance pattern in this study occurred exclusively in Salmonella Typhimurium var. Copenhagen, and only in 2 herds, although Salmonella Typhimurium var. Copenhagen occurred in 4 other herds without this pattern. Investigating the extent that these isolates are related might indicate if this resistance was due to the spread of a resistant clone or common selective pressures. In numerous situations, Salmonella with the same resistance pattern and serovar were found in different production phases of the same herd. Thus also describing how isolates from different production phases are related could improve the understanding of how resistance spreads and persists within farms. Regardless of how these isolates are related, the apparent clustering of serovars and resistance patterns within herds gives perspective to the food safety risk from these Salmonella: multiresistant Salmonella Typhimurium var. Copenhagen were frequently identified, but the herds with this pattern produced less than 2% of the pigs marketed by study herds. This demonstrates why abattoir‐based monitoring, which can sample farms proportionate to the pork supply, can better estimate the food‐safety risk from resistant Salmonella.
The few associations identified between given resistances and herd characteristics suggest that the study design did not substantially bias the results. Herd size was associated with resistance to streptomycin, so by excluding herds with less than 100 sows, resistance to streptomycin might have been slightly overestimated. However, as most pigs in Saskatchewan and Alberta are raised on large farms, restricting this study to herds with more than 100 sows more realistically reflected the source of market pigs from these provinces (52). Identifying different odds of streptomycin and kanamycin resistance in Salmonella from “individual‐pig” compared with pooled pen fecal samples could be a type I error, because serovar was not accounted for. The biological importance of these associations is also questionable, as for both kanamycin and streptomycin, the predicted resistance from either sample type lies within the confidence intervals for the overall prevalence estimate. Finally, culture methods can affect the serovars isolated, so using 3 isolation methodologies could have introduced bias into these data (35,49). However, AFLB and PDS used similar methods to culture 89% of the samples and “laboratory” was not associated with any given resistance outcome, indicating bias was minimal. Overall, the study design does not appear to have biased the results substantially.
In summary, the resistance of Salmonella isolates from swine farms in Alberta and Saskatchewan is described. A high frequency of pansusceptibility and no resistance to 6 of the 16 antimicrobials are encouraging findings. Even so, the extent of multiresistance in these isolates was a concern. Few on‐farm studies have described AMR in Salmonella from all age categories of pigs (35,44). This project identified many future research needs. Age‐specific risk factor studies are needed to investigate reasons for differences in resistance between production phases. Likewise, further description of the associations between resistance and serovar, and how resistance spreads within herds, are needed before effective intervention strategies can be designed to control AMR in Salmonella from pigs.
Acknowledgments
The authors thank the producers and veterinarians who participated in this project, Dr. Sheryl Gow who contributed to the design, and Dr. Wendy Wilkins for data collection. The authors also acknowledge technical support from Agri‐Food Laboratories Branch (AFLB), Food Safety Division of Alberta Agriculture, Food and Rural Development; Dawn Daku of the Saskatchewan Health Provincial Lab; and Dr. Anne Muckle, Linda Cole, and Betty Wilkie of the Public Health Agency of Canada. Financial support provided by Sask Pork Development Board, the Saskatchewan Department of Agriculture, Food and Rural Revitalization, and the Public Health Agency of Canada is also acknowledged.
References
- 1.Todd ECD. Epidemiology of foodborne diseases: A worldwide review. World Health Statist Quart. 1997;50:30–50. [PubMed] [Google Scholar]
- 2.Thomas MK, Majowicz SE, Sockett PN, et al. Estimated numbers of community cases of illness due to Salmonella, Campylobacter and verotoxigenic Escherichia coli: Pathogen‐specific community rates. Can J Infect Dis Med Microbiol. 2006;17:229–234. doi: 10.1155/2006/806874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Government of Canada. Canada Communicable Disease Report. Ottawa, Ontario: Division of Enteric, Foodborne and Waterborne Diseases, Centre for Infectious Disease Prevention and Control, Public Health Agency of Canada, Health Canada; 2003. [Last accessed March 1, 2007]. Canadian Integrated Surveillance Report Salmonella, Campylobacter, pathogenic E. coli and Shigella, from 1996 to 1999. [homepage on the Internet]. Available from http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/03vol29/29s1/index.html. [PubMed] [Google Scholar]
- 4.Varma JK, Mølbak K, Barrett TJ, et al. Antimicrobial‐resistant nontyphoidal Salmonella is associated with excess bloodstream infections and hospitalizations. J Infect Dis. 2005;191:554–561. doi: 10.1086/427263. [DOI] [PubMed] [Google Scholar]
- 5.Varma JK, Greene KD, Ovitt J, Barrett TJ, Medalla F, Angulo FJ. Hospitalization and antimicrobial resistance in Salmonella outbreaks, 1984–2002. Emerg Infect Dis. 2005;11:943–946. doi: 10.3201/eid1106.041231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin LJ, Fyfe M, Doré K, et al. The Multi‐Provincial Salmonella Typhimurium Case‐Control Study Steering Committee. Increased burden of illness associated with antimicrobial‐resistant Salmonella enterica serotype Typhimurium infections. J Infect Dis. 2004;189:377–384. doi: 10.1086/381270. [DOI] [PubMed] [Google Scholar]
- 7.Barza M. Potential mechanisms of increased disease in humans from antimicrobial resistance in food animals. Clin Infect Dis. 2002;34:123–125. doi: 10.1086/340249. [DOI] [PubMed] [Google Scholar]
- 8.Mølbak K, Baggesen DL, Aarestrup FM, et al. An outbreak of multidrug‐resistant, quinolone‐resistant Salmonella enterica serotype Typhimurium DT104. N Engl J Med. 1999;341:1420–1425. doi: 10.1056/NEJM199911043411902. [DOI] [PubMed] [Google Scholar]
- 9.Maguire HCF, Codd AA, Mackay VE, Rowe B, Mitchell E. A large outbreak of human salmonellosis traced to a local pig farm. Epidemiol Infect. 1993;110:239–246. doi: 10.1017/s0950268800068151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedberg CW. The role of pork as a vehicle for confirmed food‐borne disease outbreaks in the United States, 1990–1997. Proc. Pork Quality Safety Summit; 2002. pp. 159–166. [Google Scholar]
- 11.van den Bogaard AE, Stobberingh EE. Epidemiology of resistance to antibiotics. Links between animals and humans. Int J Antimicrob Agents. 2000;14:327–335. doi: 10.1016/s0924-8579(00)00145-x. [DOI] [PubMed] [Google Scholar]
- 12.Angulo FJ, Johnson KR, Tauxe RV, Cohen ML. Origins and consequences of antimicrobial‐resistant nontyphoidal Salmonella: Implications for the use of fluoroquinolones in food animals. Microb Drug Resist. 2000;6:77–83. doi: 10.1089/mdr.2000.6.77. [DOI] [PubMed] [Google Scholar]
- 13.Sorensen O, McFall M, Rawluk S, Ollis G, Schoonderwoerd M, Manninen K. Salmonella enterica in Alberta slaughter hogs. Int Symp on the Epidemiology and Control of Salmonella and other Foodborne Pathogens in Pork. 2001:183–185. [Google Scholar]
- 14.Larkin C, Poppe C, McNab B, McEwen B, Mahdi A, Odumeru J. Antibiotic resistance of Salmonella isolated from hog, beef, and chicken carcass samples from provincially inspected abattoirs in Ontario. J Food Prot. 2004;67:448–455. doi: 10.4315/0362-028x-67.3.448. [DOI] [PubMed] [Google Scholar]
- 15.Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2004. Guelph, Ontario: Public Health Agency of Canada; 2006. [Last accessed March 17, 2007]. [homepage on the Internet]. Available from http://www.phac-aspc.gc.ca/cipars-picra/2004_e.html. [Google Scholar]
- 16.Erdman MM, Wedel SD, Harris DL. Genotypic and phenotypic comparison of swine Salmonella isolates from farm and abattoir. J Swine Health Prod. 2003;11:169–172. [Google Scholar]
- 17.Rajic A, McFall ME, Deckert AE, et al. Antimicrobial resistance of Salmonella isolated from finishing swine and the environment of 60 Alberta swine farms. Vet Micro. 2004;104:189–196. doi: 10.1016/j.vetmic.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Rosengren LB, Waldner CL, Reid‐Smith RJ, Checkley SL, McFall ME, Rajic A. Antimicrobial resistance of fecal Escherichia coli isolated from grow‐finish pigs in 20 herds in Alberta and Saskatchewan, Canada. Can J Vet Res. 2008 Accepted for publication. [PMC free article] [PubMed] [Google Scholar]
- 19.Rosengren L. Antimicrobial resistance of Salmonella, Escherichia coli and Campylobacter from pigs on‐farm in Alberta and Saskatchewan Canada [PhD dissertation] Saskatoon, Saskatchewan: University of Saskatchewan; 2007. [Google Scholar]
- 20.Wilkins W, Waldner C, Rajic A, McFall M, Chow E, Muckle A. Estimation of sensitivity and specificity of culture and Danish‐mix ELISA for detection of Salmonella in swine using Bayesian methods. The 6th International Symposium on the Epidemiology and Control of Foodborne Pathogens in Pork; 2005. [Google Scholar]
- 21.Bohaychuk V, Gensler G, King R, McFall M, Renter D. A real‐time PCR assay for the detection of Salmonella spp. in food and food‐animal matrices. International Association for Food Protection. 2005:P5–11. doi: 10.4315/0362-028x-70.5.1080. [DOI] [PubMed] [Google Scholar]
- 22.D’Aoust JY, Purvis U. HPB Method. Ottawa: Health Protection Branch, Health Canada; 1998. [Last accessed May 1, 2007]. MFHPB‐20 Isolation and Identification of Salmonella from Foods. [homepage on the Internet]. Available from http://www.hc-sc.gc.ca/fn-an/alt_formats/hpfb-dgpsa/pdf/res-rech/mfhpb20_e.pdf. [Google Scholar]
- 23.Ewing WH. Serological identification of Salmonella. In: Ewing WH, editor. Edwards and Ewing’s Identification of Enterobacteriaceae. 4. New York: Elsevier; 1986. pp. 201–238. [Google Scholar]
- 24.Shipp CR, Rowe B. A mechanized microtechnique for Salmonella serotyping. J Clin Path. 1980;33:595–597. doi: 10.1136/jcp.33.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Minor L, Popoff MY. Antigenic formulas of the Salmonella serovars. 8. Paris, France: WHO Collaborating Centre for Reference and Research on Salmonella; 2001. [Google Scholar]
- 26.Anderson ES, Williams REO. Bacteriophage typing of enteric pathogens and staphylococci and its use in epidemiology. J Clin Path. 1956;9:94–127. doi: 10.1136/jcp.9.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Callow BR. A new phage typing scheme for Salmonella typh‐imurium. J Hyg (Lond) 1959;57:346–359. doi: 10.1017/s0022172400020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson ES. The phagetyping of Salmonella other than S. typhi. In: Van Oye E, editor. The World Problem of Salmonellosis. The Hague, The Netherlands: Dr. W. Junk Publ; 1964. pp. 89–100. [Google Scholar]
- 29.Anderson ES, Ward LR, Saxe MJ, de Sa JDH. Bacteriophage‐typing designations of Salmonella typhimurium. J Hyg (Lond) 1977;78:297–300. doi: 10.1017/s0022172400056187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward LR, de Sa JDH, Rowe B. A phage‐typing scheme for Salmonella enteritidis. Epidemiol Infect. 1987;99:291–294. doi: 10.1017/s0950268800067765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demczuk W, Soule G, Clark C, et al. Phage‐based typing scheme for Salmonella enterica serovar Heidelberg, a causative agent in food poisonings in Canada. J Clin Microbiol. 2003;41:4279–4284. doi: 10.1128/JCM.41.9.4279-4284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH. Manual of Clinical Microbiology. 8. Washington, DC: ASM Pr; 2003. [Google Scholar]
- 33.Clinical and Laboratory Standards Institute: Performance Standards for Antimicrobial Susceptibility Testing; 15th Informational Supplement M100‐S15, in CLSI2005, pp 102–106.
- 34.Clinical and Laboratory Standards Institute: Performance standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Informational Supplement: Updated tables for the NCCLS antimicrobial susceptibility testing standard M31‐A2, in CLSI2004.
- 35.Gebreyes WA, Davies PR, Morrow WEM, Funk JA, Altier C. Antimicrobial resistance of Salmonella isolates from swine. J Clin Microbiol. 2000;38:4633–4636. doi: 10.1128/jcm.38.12.4633-4636.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research. University of Prince Edward Island, Charlottetown, PEI: AVC Inc; 2003. [Google Scholar]
- 37.Cloeckaert A, Schwarz S. Molecular characterization, spread and evolution of multidrug resistance in Salmonella enterica Typhimurium DT104. Vet Res. 2001;32:301–310. doi: 10.1051/vetres:2001126. [DOI] [PubMed] [Google Scholar]
- 38.Gebreyes WA, Davies PR, Turkson P‐K, et al. Characterization of antimicrobial‐resistant phenotypes and genotypes among Salmonella enterica recovered from pigs on farms, from transport trucks, and from pigs after slaughter. J Food Prot. 2004;67:698–705. doi: 10.4315/0362-028x-67.4.698. [DOI] [PubMed] [Google Scholar]
- 39.Hurd HS, McKean JD, Wesley IV, Karriker LA. The effect of lariage on Salmonella isolation from market swine. Salinpork 2001 The 4th International Symposium on Epidemiology and Control of Salmonella and other Foodborne Pathogens in Pork; 2001. pp. 289–291. [Google Scholar]
- 40.World Health Organization. Canberra, Australia: 2005. [Last accessed March 17, 2007]. Critically Important Antibacterial Agents for Human Medicine for Risk Management Strategies of Non‐Human Use: report of a WHO working group consultation. [homepage on the Internet]. Available from http://www.who.int/foodborne_disease/resistance/amr_feb2005.pdf. [Google Scholar]
- 41.United States Department of Agriculture. National Antimicrobial Monitoring System — Enteric Bacteria (NARMS — EB) Veterinary Isolates Final Report 2003, Salmonella 2003 Report, Table 6. Last Updated Jan 12, 2005. [Last accessed May 1, 2007]; [homepage on the Internet]. Available from http://www.ars.usda.gov/Business/docs.htm?docid=6764.
- 42.United States Department of Agriculture. Collaboration in Animal Health and Food Safety Epidemiology (CAHFSE) Annual Report, July 2004 – June 2005. [Last accessed May 1, 2007]; [homepage on the Internet]. Available from http://www.aphis.usda.gov/cahfse/index.htm.
- 43.Government of Canada. Guelph, Ontario: Public Health Agency of Canada; 2005. [Last accessed November 19, 2007]. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2003. [homepage on the Internet]. Available from http://www.phac-aspc.gc.ca/cipars-picra/index.html. [Google Scholar]
- 44.Nollet N, Houf K, Dewulf J, et al. Variability in antimicrobial resistance among Salmonella enterica strains from fattening pigs and sows. Microb Drug Resist. 2006;12:74–81. doi: 10.1089/mdr.2006.12.74. [DOI] [PubMed] [Google Scholar]
- 45.Mathew AG, Saxton AM, Upchurch WG, Chattin SE. Multiple antibiotic resistance patterns of Escherichia coli isolates from swine farms. Appl Environ Microbiol. 1999;65:2770–2772. doi: 10.1128/aem.65.6.2770-2772.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brun E, Holstad G, Kruse H, Jarp J. Within‐sample and between‐sample variation of antimicrobial resistance in fecal Escherichia coli isolates from pigs. Microb Drug Resist. 2002;8:385–391. doi: 10.1089/10766290260469660. [DOI] [PubMed] [Google Scholar]
- 47.Raji′c A, Reid‐Smith R, Deckert AE, Dewey CE, McEwen SA. Reported antibiotic use in 90 swine farms in Alberta. Can Vet J. 2006;47:446–452. [PMC free article] [PubMed] [Google Scholar]
- 48.Dunlop RH, McEwen SA, Meek AH, Friendship RA, Clarke RC, Black WD. Antimicrobial drug use and related management practices among Ontario swine producers. Can Vet J. 1998;39:87–96. [PMC free article] [PubMed] [Google Scholar]
- 49.Jameson JE. A discussion of the dynamics of salmonella enrichment. J Hyg (Lond) 1962;60:193–207. doi: 10.1017/s0022172400039462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poppe C, McFadden KA, Demczuk W. Drug resistance, plasmids, biotypes and susceptibility to bacteriophages of Salmonella isolated from poultry in Canada. Int J Food Microbiol. 1996;30:325–344. doi: 10.1016/0168-1605(96)00960-9. [DOI] [PubMed] [Google Scholar]
- 51.Poppe C, Ayroud M, Ollis G, et al. Trends in antimicrobial resistance of Salmonella isolated from animals, foods of animal origin, and the environment of animal production in Canada, 1994–1997. Microb Drug Resist. 2001;7:197–212. doi: 10.1089/10766290152045084. [DOI] [PubMed] [Google Scholar]
- 52.Canadian Pork Council. Statistics: Description of Canadian hog farms. Last Updated June 2006. [Last accessed March 17, 2007]; [homepage on the Internet]. Available from http://www.cpc-ccp.com/stats.html.


