Abstract
Escherichia coli (n = 1439), isolated from the feces of apparently healthy grow-finish pigs in 20 herds in Alberta and Saskatchewan, were tested for susceptibility to 16 antimicrobials. All isolates were susceptible to amikacin, ceftriaxone, and ciprofloxacin and less than 1% was resistant to amoxicillin-clavulanic acid, cefoxitin, ceftiofur, gentamicin, and nalidixic acid. Resistance was most common to tetracycline (66.8%), sulfamethoxazole (46.0%) and streptomycin (33.4%). Twenty-one percent of the isolates were susceptible to all drugs, while 57% were resistant to 2 or more antimicrobials. Unconditional associations between resistances provided insight into the potential for co-selection. Every resistance-outcome was associated with at least 2 other drug-resistances. These associations illustrate the propensity for resistance phenotypes to occur together and the importance of considering co-selection in antimicrobial use decisions. A 2nd analysis explored the associations between resistance phenotypes in E. coli and Salmonella spp. from the same herd. Only 2 resistances in Salmonella were associated with herd-level E. coli resistance, indicating that E. coli is a poor sentinel for Salmonella AMR within herds. Herd-level management, including antimicrobial use, could affect antimicrobial resistance. The intra-class correlation between isolates within herds ranged from 0.1 to 0.46, which confirmed resistance clustered within herds. This suggests herd-level interventions might mitigate antimicrobial resistance. Overall, these results reflect the on-farm selection pressure for resistance and the potential food-safety risk from near-market animals. These data provide a baseline for comparisons with future on-farm monitoring of antimicrobial resistance in E. coli.
Résumé
Des isolats d’Escherichia coli (n = 1439), provenant des fèces de porcs en santé en croissance-finition prélevées dans 20 troupeaux de l’Alberta et de la Saskatchewan, ont été testés pour leur sensibilité à 16 agents antimicrobiens. Tous les isolats étaient sensibles aux antimicrobiens suivants : amikacine, ceftriaxone et ciprofloxacin; et moins de 1 % était résistant à : amoxicilline-acide clavulanique, cefoxitin, ceftiofur, gentamycine et acide nalidixique. De la résistance était fréquente à la tétracycline (66,8 %), au sulfaméthoxazole (46,0 %) et à la streptomycine (33,4 %). Une sensibilité envers tous les antimicrobiens était notée pour 21 % des isolats, alors que 57 % étaient résistants à 2 antimicrobiens ou plus. Des associations inconditionnelles entre des résistances ont fourni des informations sur le potentiel de co-sélection. Chaque résultat de résistance était associé à au moins 2 autres résistances. Ces associations démontrent la propension pour les phénotypes de résistance à se produire ensemble et l’importance de considérer la co-sélection lors des décisions dans l’utilisation des antimicrobiens. Une deuxième analyse a exploré les associations entre les phénotypes de résistance d’E. coli et de Salmonella spp. provenant d’un même troupeau. Seulement 2 résistances chez Salmonella étaient associées à de la résistance chez E. coli au niveau du troupeau, indiquant ainsi qu’E. coli n’est pas un bon indicateur de l’antibiorésistance de Salmonella dans un même troupeau. La gestion du troupeau, incluant l’utilisation des antimicrobiens, pourrait affectée la résistance aux antimicrobiens. La corrélation intra-classe entre les isolats à l’intérieur d’un troupeau variait de 0,1 à 0,46, ce qui confirme que la résistance était regroupée à l’intérieur des troupeaux. Ceci suggère que les interventions au niveau du troupeau pourraient limiter la résistance aux antimicrobiens. De manière globale, ces résultats reflètent la sélection de pression pour la résistance sur la ferme et le risque potentiel pour la santé publique que représente les animaux prêts à être commercialisés. Ces résultats fournissent des données de base comparatives pour la surveillance à la ferme de la résistance aux antimicrobiens chez E. coli.
(Traduit par Docteur Serge Messier)
Introduction
Antimicrobial resistance (AMR) in bacteria from food animals is a worldwide public health issue. Zoonotic infections and the transmission of resistance genes to commensal and pathogenic bacteria of humans are frequently cited concerns (1–3). The frequency of AMR in commensal organisms reflects the selective pressure exerted on bacteria to develop resistance and the potential reservoir of resistance genes available for dissemination to pathogens (2,4). For these reasons, the World Health Organization (WHO) and World Organization for Animal Health (OIE) recommend monitoring AMR in commensal organisms including Escherichia coli (5,6). Escherichia coli are highly prevalent in healthy animals, facilitating comparisons of AMR within and between species (2,7,8).
Pork is among the 3 most commonly consumed animal proteins in Canada and is the most common worldwide (9,10). The frequency of resistance in E. coli from pigs sampled at Canadian abattoirs, and pork sampled at retail, has been intermediate between that of chicken and beef (7). This resistance, combined with the extensive use of antimicrobials in pork production, (11–13), has created interest in antimicrobial resistant bacteria in pigs.
Previous Canadian studies have considered E. coli from swine farms in Ontario and British Columbia and on-farm surveillance has been initiated (7,14,15). This study investigated AMR in E. coli from apparently healthy grow-finish pigs on farms in Alberta and Saskatchewan. Three analyses described the resistance in these isolates. First, unconditional associations between resistance phenotypes described the potential for co-selection in these isolates. Second, E. coli was evaluated as a farm-level sentinel for AMR in Salmonella. Finally, others have observed that resistance clusters within pigs, pens, and herds (16–19). This project quantified the variation in resistance between isolates within herds, to determine the value of investigating farm-level risk factors for AMR.
Materials and methods
Herd and sample selection
A convenience sample of 20 farms was allocated to 8 swine veterinarians in Saskatchewan (13 farms) and Alberta (7 farms). Farms were selected by veterinarians based on a minimum herd size of 100 sows and enrollment with the Canadian Quality Assurance (CQA) Program (20). The number of farms per veterinarian ranged from 2 to 4. Half of the veterinarians were asked to identify the presumed Salmonella status of the herds; 10 herds were enrolled with knowledge of the presumed Salmonella status (21). Each herd was sampled once between May and September of 2004. Fecal samples were collected from 20 randomly selected pens per herd. Samples were pooled at the pen level and each consisted of feces from 5 grow-finish pigs.
Laboratory methods
Samples were manually mixed, shipped on ice to a commercial veterinary laboratory [Prairie Diagnostic Services (PDS), University of Saskatchewan, Saskatoon, Saskatchewan], and cultured for E. coli within 24 h of collection. Each sample was streaked onto a whole Blood Agar and MacConkey plate with a heavily coated cotton swab and incubated at 37°C for 18 h. Three lactose-positive colonies were selected from each MacConkey plate unless distinct colonies, such as hemolytic and nonhemolytic or mucoid and dry were identified; up to 6 colonies were harvested from those samples. Selected colonies were incubated in trypticase soy (TS) broth for 3 to 4 h at 37°C. Each TS broth was inoculated onto urea and citrate slants with a 1 μL loop, and a blood agar plate was concurrently inoculated to ensure sample purity. These were incubated at 37°C for 18 h. Samples requiring further confirmation were tested with triple sugar iron/indole. Pure, confirmed E. coli cultures were frozen in 25% glycerol at −80°C until tested for antimicrobial susceptibility.
Antimicrobial susceptibility testing was conducted by the Agri-Food Laboratories Branch (AFLB), Food Safety Division of Alberta Agriculture, Food and Rural Development, Edmonton, Alberta and PDS, Saskatoon, Saskatchewan. Isolates were tested using a broth micro-dilution technique following Clinical and Laboratory Standards Institute (CLSI) guidelines (22,23). National Antimicrobial Resistance Monitoring System (NARMS) minimum inhibitory concentration (MIC) CMV7CNCD susceptibility panels (Sensititre; TREK Diagnostic Systems, Westlake, Ohio, USA) were used to test isolates for susceptibility to 16 antimicrobials across a standard range of dilutions (Table I). Each isolate was grown up on a non-selective media. A 0.5 McFarland standard was made in 5 mL of demineralized water, 10 μL of which was transferred into 11 mL of cation-adjusted Mueller-Hinton broth with TES buffer. A 50-μL aliquot was inoculated into each of the 96 wells on the panel. Inoculated plates were incubated and read by the Sensititre ARIS (Automated Reading and Incubation System; TREK Diagnostic Systems). Readings were transferred to Sensititre Automated Microbiology Systems (SAMS) computer software (TREK Diagnostic Systems) and interpreted according to CLSI breakpoints for animals or humans (22,23) (Table I). The MIC breakpoint for streptomycin, which does not have a CLSI guideline, was taken from the National Antimicrobial Resistance Monitoring System (NARMS) 2000 E. coli report (24). Quality control organisms used were Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212, and Pseudomonas aeruginosa ATCC 27853.
Table I.
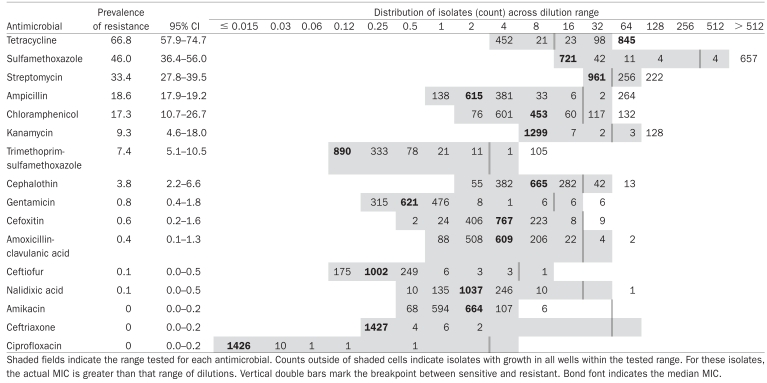
Minimum inhibitory concentrations distributions, population average prevalence estimates, and 95% confidence intervals for resistance to each drug in E. coli isolates (n = 1439)
 |
Data comparisons and statistical analysis
Intermediate MIC values were categorized as susceptible (22,23). Descriptive analyses were calculated with commercially available software (Microsoft Excel; Microsoft Corporation, Redmond, Washington, USA) and statistical analyses were performed using generalized estimating equations (GEE) (PROC GENMOD, SAS version 9.1; SAS Institute, Cary, North Carolina, USA) to adjust for clustering of resistance within herds. All models had a logit-link function, binomial distribution, and exchangeable correlation structure. Unless stated otherwise, the outcome variable was ‘any resistance’ versus ‘no resistance’ (dichotomous) at the isolate level. The association between each variable of interest and outcome was reported as an odds ratio (OR = expβ) with 95% confidence intervals (CI) (25).
The population average prevalence for resistance to each antimicrobial was calculated using the intercept (β0) and 95% CI from a null binomial response model in [1 + exp(−β0)]−1 (25). Beyond these prevalence estimates, analyses were restricted to drugs with more than 5% prevalence of resistance to minimize problems with power, model stability, and convergence.
In the 1st set of analyses, the associations between different resistance phenotypes were considered. As there were 7 drugs with more than 5% prevalence of resistance, each outcome was unconditionally considered against 6 other drug-resistances. Based on this, the cutoff for statistical significance was adjusted with a Bonferroni correction and reported significant if P > 0.007 (0.05/7) (25).
The 2nd set of analyses explored potential biases introduced by study design. The unconditional associations between E. coli resistance phenotypes and 2 herd characteristics known at selection, herd size and knowledge of the presumed-herd-Salmonella status, were evaluated. These associations were reported as being significant if P > 0.05.
The associations between resistances observed in Salmonella spp. and E. coli were measured to assess the potential for herd-level E. coli AMR data to predict resistance in Salmonella. Susceptibility data were generated concurrently in these herds (21,26). The outcome variables represented Salmonella resistance to each drug, and were modeled as the number of resistant isolates in the herd as the numerator and the number of isolates tested in each herd as the denominator. The predictor variable was the proportion of E. coli in the herd that were resistant to the same drug. These associations were reported as being significant if P > 0.05.
A different approach to modeling AMR estimated the extent that resistance clustered within herds, and within the veterinarians servicing the herds. The variance at each level was determined using restricted maximum likelihood estimation. The resistance phenotype outcome was modeled as the proportion of resistant isolates in the herd as the numerator and the number of isolates tested in each herd as the denominator. The restricted generalized iterative least-squares (RIGLS) algorithm in MLwiN (MLwiN version 2.0r; Centre for Multilevel Modelling, Institute of Education, University of London, London, England) was used and 2nd-order penalized quasi-likelihood (PQL-2) estimates were reported. Under-dispersion was accounted for by allowing random variation at the lowest level (27). Nonsignificant hierarchical levels, based on the liberal criteria of the standard error being larger than the variance estimate, were excluded from further consideration. The intra-class correlation (ICC) between isolates within herds was approximated by the latent variable approach (25,27). Specifically, the herd variance was divided by the total variance after fixing the error variance at π2/3.
Results
Four hundred and five samples were cultured and 1439 E. coli harvested for an average of 63 isolates per herd (range, 60 to 88). The prevalence of resistance was highest to tetracycline, sulfamethoxazole, and streptomycin, while no resistance was observed to ceftriaxone or ciprofloxacin (Table I). For amikacin, 6 isolates had an MIC greater than the dilution range tested, thus the status of these isolates was indeterminable.
Twenty-one percent (95% CI, 15.0 to 28.3) of the E. coli were susceptible to all drugs, while 57.0% (95% CI, 47.2 to 66.2) were resistant to 2 or more. Two isolates were resistant to 9 antimicrobials. Ninety-two unique resistance patterns were identified. Combinations of resistance to tetracycline, sulfamethoxazole, and streptomycin accounted for the 4 most common patterns, and these antimicrobials were involved in all of the 10 most common resistance patterns (Table II).
Table II.
Ten most common antimicrobial resistance patterns observed in E. coli (n = 1439)
| AMR Pattern | Frequency | Percent |
|---|---|---|
| TET | 235 | 16.3 |
| SMX-TET | 98 | 6.8 |
| STR-SMX-TET | 97 | 6.7 |
| STR-TET | 94 | 6.5 |
| CHL-STR-SMX-TET | 56 | 3.9 |
| CHL-SMX-TET | 45 | 3.1 |
| STR | 36 | 2.5 |
| SMX | 35 | 2.4 |
| AMP-STR-TET | 34 | 2.4 |
| AMP-TET | 31 | 2.2 |
AMP — ampicillin; CHL — chloramphenicol; SMX — sulfamethoxazole; STR — streptomycin; TET — tetracycline.
Among E. coli, resistance to each drug was significantly associated with resistance to at least 2 other drugs. The odds of an isolate being resistant to sulfamethoxazole increased significantly if it was resistant to any other drug considered (Table III). Because only 1 isolate was both resistant to trimethoprim-sulfamethoxazole and susceptible to sulfamethoxazole, the associations between these resistances were very strong. The next strongest associations were between resistance to sulfamethoxazole and chloramphenicol.
Table III.
Significant univariate associations between antimicrobial resistances (P > 0.007), (n = 1439)
| Variable
|
||||
|---|---|---|---|---|
| Outcome | Predictor | Odds ratio | 95% Confidence Interval | P |
| Ampicillin | Kanamycin | 3.1 | 1.6–6.0 | 0.001 |
| Streptomycin | 2.0 | 1.2–3.1 | 0.004 | |
| Tetracycline | 2.5 | 1.8–3.5 | ≤ 0.001 | |
| Sulfamethoxazole | 2.3 | 1.4–3.7 | ≤ 0.001 | |
| Trimethoprim-sulfamethoxazole | 5.7 | 2.6–12.6 | ≤ 0.001 | |
| Chloramphenicol | Sulfamethoxazole | 34.6 | 12.6–95 | ≤ 0.001 |
| Trimethoprim-sulfamethoxazole | 3.3 | 1.7–6.4 | ≤ 0.001 | |
| Kanamycin | Ampicillin | 2.8 | 1.5–5.2 | 0.001 |
| Streptomycin | 4.2 | 1.9–8.9 | ≤ 0.001 | |
| Sulfamethoxazole | 5.9 | 3.2–10.9 | ≤ 0.001 | |
| Trimethoprim-sulfamethoxazole | 1.4 | 1.1–1.7 | 0.005 | |
| Streptomycin | Ampicillin | 2.1 | 1.3–3.4 | 0.002 |
| Kanamycin | 5.2 | 2.1–12.5 | ≤ 0.001 | |
| Tetracycline | 5.6 | 3.9–7.9 | ≤ 0.001 | |
| Sulfamethoxazole | 2.7 | 1.9–3.8 | ≤ 0.001 | |
| Sulfamethoxazole | Ampicillin | 2.2 | 1.4–3.5 | 0.001 |
| Kanamycin | 5.6 | 2.7–11.5 | ≤ 0.001 | |
| Streptomycin | 2.4 | 1.8–3.2 | ≤ 0.001 | |
| Tetracycline | 2.9 | 1.9–4.5 | ≤ 0.001 | |
| Trimethoprim-sulfamethoxazole | 118 | 14–968 | ≤ 0.001 | |
| Chloramphenicol | 31 | 13–73 | ≤ 0.001 | |
| Tetracycline | Ampicillin | 2.3 | 1.7–3.2 | ≤ 0.001 |
| Streptomycin | 4.6 | 3.3–6.5 | ≤ 0.001 | |
| Sulfamethoxazole | 3.1 | 2.1–4.7 | ≤ 0.001 | |
| Trimethoprim-sulfamethoxazole | Ampicillin | 6.5 | 3.0–14.2 | ≤ 0.001 |
| Sulfamethoxazole | 215 | 9.0–5170 | ≤ 0.001 | |
| Chloramphenicol | 3.6 | 1.8–7.2 | ≤ 0.001 | |
The odds of an isolate being resistant to streptomycin, sulfamethoxazole, and tetracycline each decreased by 0.99 times for every 1000 pigs finished annually (P = > 0.0001, 0.007, and 0.049, respectively). Escherichica coli resistance to ampicillin, chloramphenicol, kanamycin, and trimethoprim-sulfamethoxazole were not significantly associated with herd size (P > 0.23). No resistance was significantly associated with knowledge of the presumed Salmonella status (P > 0.09).
The frequency of E. coli resistance in the herd was a significant predictor of Salmonella resistance to 2 drugs. For each 1% increase in E. coli resistance to kanamycin the odds of Salmonella resistance to kanamycin increased 1.24 times (95% CI, 1.15 to 1.34; P = 0.001). Similarly, for trimethoprim-sulfamethoxazole, a 1% increase in E. coli resistance increased the odds of Salmonella resistance by 1.32 times (95% CI, 1.10 to 1.58, P = 0.003). The associations for the remaining 5 drugs were not significant (P > 0.19).
For all resistance phenotypes, the variance at the veterinary practitioner level was not significant (P > 0.31) while the variance at the herd level was highly significant (P = 0.003 to 0.017). Hence, variance at the veterinary practitioner level was not considered when calculating the ICCs. The ICC between isolates within herds was smallest for streptomycin and largest for kanamycin (Table IV).
Table IV.
Distribution of within herd prevalence of resistance, variance attributed to clustering within herds and intra-class correlation between isolates within herds (N = 20 herds; n = 1439 E. coli)
| Within herd prevalence
|
||||
|---|---|---|---|---|
| Antimicrobial | Median | IQR | Herd variance (standard error) | ICC |
| Ampicillin | 14.2 | 11.1–27.6 | 1.0 (0.35) | 0.23 |
| Chloramphenicol | 10.5 | 4.2–20.9 | 2.0 (0.71) | 0.37 |
| Kanamycin | 4.6 | 0.9–11.2 | 2.8 (1.1) | 0.46 |
| Streptomycin | 34.0 | 22.5–43.2 | 0.4 (0.14) | 0.10 |
| Sulfamethoxazole | 42.3 | 28.6–64.7 | 1.2 (0.39) | 0.26 |
| Tetracycline | 62.1 | 49.9–82.0 | 1.4 (0.48) | 0.30 |
| Trimethoprim-sulfamethoxazole | 8.1 | 1.4–11.3 | 1.1 (0.04) | 0.24 |
ICC — Intra-class correlation coefficient between isolates within herds.
IQR — Interquartile range.
Discussion
Antimicrobial resistance in E. coli from pigs has been well described in North America. Nationally representative, abattoir-based monitoring in Canada and the United States is ongoing, while an on-farm monitoring program has occurred in the United States and a similar program was initiated in 2006 in Canada (7,24,28). Targeted cross-sectional studies in Ontario and British Columbia have described E. coli AMR on Canadian swine farms (14,15). This study differed from these previous reports by considering the ability for E. coli to predict Salmonella AMR within herds and the extent resistance clustered within herds.
The frequency of resistance to at least 1 drug, and to each individual drug, was comparable to other North American reports (7,14,24,28). Therefore, despite describing resistance in a small number of herds, the findings herein are consistent with other regions in North America. Slight differences between these findings and previous reports were a lower prevalence of resistance to ampicillin (19% versus 22% to 35%) and a higher prevalence to chloramphenicol (17% versus 8% to 13%) (7,14,28).
Resistance to chloramphenicol was particularly interesting because the use of this drug in Canadian food animals was banned in 1985 (29). Florfenicol was not used in study herds in the 12 mo prior to sample collection (26); therefore, direct selection for the floR gene, which confers resistance to both florfenicol and chloramphenicol, is unlikely (30). Rather, chloramphenicol resistance likely persisted due to co-selection, which occurs with transmission of linked AMR genes on plasmids, transposons, and integrons. Bacteria resistant to multiple drugs have a competitive advantage in a wider range of environments (4,31,32). The strong associations between chloramphenicol and sulfamethoxazole resistance suggests co-selection may be occurring between resistance genes encoding for these drugs. This hypothesis is supported by reports of co-transmission of chloramphenicol and sulfonamide genes on transmissible plasmids, and significant odds ratios between genes encoding for resistance to these drugs (30,33). However, further molecular study of these isolates is required to confirm this hypothesis.
In addition to being associated with chloramphenicol, sulfamethoxazole resistance was associated with every other drug resistance. The sul1 gene, which encodes for sulfamethoxazole resistance, is a component of class I integrons; genetic elements that acquire and link resistance gene cassettes (31). Thus, integrons carrying resistance genes could explain the associations between sulfamethoxazole and other drug resistances. Sulfonamide use has been associated with sulfamethoxazole resistance (14). By extension, the findings herein suggest that it could be influencing resistance to many other antimicrobials. This is important considering sulfonamides are commonly administered to Canadian pigs (12,13). Overall, identifying numerous associations between resistances means co-selection between unrelated antimicrobials must be considered when making drug use decisions.
Antimicrobial resistant Salmonella are a more obvious food safety hazard than E. coli, given that outbreaks of resistant salmonellosis from pork have occurred (34,35). However, monitoring Salmonella AMR is challenging because subclinically infected pigs shed intermittently, barns fluctuate between a Salmonella-positive and apparent-negative status, and isolation techniques can have poor sensitivity (36,37). In contrast, E. coli, another member of the Enterobacteriaceae family, is highly prevalent and easily isolated. Salmonella and E. coli from pigs can share resistance genes in vitro and molecular evidence suggests transmission occurs in vivo (38,39). Despite this, herd-level resistance in E. coli was not associated with Salmonella resistance for 5 of 7 resistance phenotypes. Although this appears inconsistent with using E. coli as an indicator bacterium, the findings herein could be due to low study power, describing resistance by phenotype rather than genotype, clonal spread of resistance, and unaccounted for associations between AMR and serotype. These factors could easily overshadow any shared resistance genes and create substantial differences between E. coli and Salmonella resistance within herds.
Swine barns have a hierarchical structure; pigs are grouped in pens, pens in rooms, rooms in barns, and barns in production companies. The variation in resistance between each of these levels, as well as between pigs at different time points and between herds, has been described (15–19,40). Describing the extent that resistance varies within herds is valuable because many management decisions, including antimicrobial use, occur at this level. Identifying substantial variation suggests that these practices may influence resistance (25). The intra-class correlations between isolates within farms demonstrated that on-farm risk factor studies for resistances should be undertaken, and statistical analyses should account for the lack of independence in AMR data (25). In contrast, the lack of variation between veterinary practitioners indicated that interventions targeted at veterinary practitioners would likely have a negligible impact on resistance compared to those targeted at farms.
Antimicrobial susceptibility testing methods were chosen to facilitate comparisons with existing national surveillance data from Canada and the United States (7,24,28). This benefit outweighed the limited dilution ranges examined for streptomycin and amikacin. Streptomycin resistance may have been underestimated: a wider MIC distribution might have distinguished between truly susceptible isolates and those expressing aadA (MIC > 64) (41,42). To help address this weakness, AMR genes were examined in a subset of these E. coli. This confirmed that some misclassification occurred and indicates a wider dilution range should be examined in future studies (26). Additional testing could determine if the 6 E. coli with an amikacin MIC > 4 have a true MIC above the resistance breakpoint (> 32). If resistant, this would provide important data about a rare resistance. Differences between studies can arise due to geographical, temporal, or management factors. Although comparisons with other AMR data should be made cautiously because selection criteria and sampling strategy could also influence results, the similarity between AMR in E. coli from this study and other North American reports indicates that this regional study provides useful data for understanding AMR in E. coli from healthy pigs (7,14,15,24,28).
Exploring herd characteristics influenced by the selection strategy found no significant associations between any resistance and knowledge of the herd Salmonella-status. This indicates that participation bias, due to the more intensive sampling in known-Salmonella-status herds, was unlikely present. In contrast, herd size was significantly associated with 3 resistance outcomes. For these drug-resistances, limiting the study to herds with more than 100 sows may have resulted in slightly less observed resistance than if all herds had been eligible. In Saskatchewan, 70% of pig farms market less than 1000 pigs annually (which roughly corresponds to herds of 50 sows or less); however, they produce less than 3% of Saskatchewan’s pigs (43). Restricting the study to larger herds (> 100 sows); therefore, improved study representation of market hog production in western Canada. Although this study utilized a convenience sample of herds, the investigators had no knowledge at selection of the antimicrobial use patterns in the herds. This was presumed to be the primary influence on resistance. Participation in this study was restricted to herds enrolled in the CQA program to ensure adequate antimicrobial use records for other aspects of the study (20). Although participation in the CQA program may influence antimicrobial use practices, it is unlikely to have biased the results, as more than 98% of production units in Alberta and Saskatchewan participate in this program (personal communication Sarah Turner, Alberta Pork and Harvey Wagner, Sask Pork).
The findings of this study reflect the on-farm selection pressure for AMR and the potential food-safety risk from near-market animals. Describing E. coli AMR in Alberta and Saskatchewan herds provides baseline information for monitoring on-farm AMR in E. coli. With further study on swine farms, there is potential to identify risk-factors for antimicrobial resistance.
Acknowledgments
The authors thank the swine producers and veterinarians who participated in this project as well as Dr. Sheryl Gow for help in study design and implementation and Dr. Wendy Wilkins for help with data collection. The authors gratefully acknowledge the support of Sask Pork Development Board, the Saskatchewan Department of Agriculture, Food and Rural Revitalization, the Public Health Agency of Canada, and the technical support of the Food Safety Division, Alberta Agriculture, Food and Rural Development.
References
- 1.Barza M. Potential mechanisms of increased disease in humans from antimicrobial resistance in food animals. Clin Infect Dis. 2002;34(S3):S123–125. doi: 10.1086/340249. [DOI] [PubMed] [Google Scholar]
- 2.van den Bogaard AE, Stobberingh EE. Epidemiology of resistance to antibiotics. Links between animals and humans. Int J Antimicrob Agents. 2000;14:327–335. doi: 10.1016/s0924-8579(00)00145-x. [DOI] [PubMed] [Google Scholar]
- 3.Tollefson L, Karp BE. Human health impact from antimicrobial use in food animals. Med Mal Infect. 2004;34:514–521. [PubMed] [Google Scholar]
- 4.Summers AO. Generally overlooked fundamentals of bacterial genetics and ecology. Clin Infect Dis. 2002;34(S3):S85–92. doi: 10.1086/340245. [DOI] [PubMed] [Google Scholar]
- 5.Franklin A, Acar J, Anthony F, et al. Antimicrobial resistance: Harmonisation of national antimicrobial resistance monitoring and surveillance programmes in animals and in animal-derived food. Rev Sci Tech. 2001;20:859–870. doi: 10.20506/rst.20.3.1315. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO Global Principles For The Containment of Antimicrobial Resistance in Animals Intended for Food. Geneva Switzerland: World Health Organization; 2000. [Last accessed May 1, 2007]. [homepage on the Internet]. Available from http://whqlibdoc.who.int/hq/2000/WHO_CDS_CSR_APH_2000.4.pdf. [Google Scholar]
- 7.Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2004. Guelph, Ontario: Public Health Agency of Canada; 2006. [Last Accessed March 17, 2007]. [homepage on the Internet]. Available from http://www.phac-aspc.gc.ca/cipars-picra/2004_e.html. [Google Scholar]
- 8.Sørum H, Sunde M. Resistance to antibiotics in the normal flora of animals. Vet Res. 2001;32:227–241. doi: 10.1051/vetres:2001121. [DOI] [PubMed] [Google Scholar]
- 9.Government of Canada. Canada Food Statistics. Ottawa, Ontario: Statistics Canada; 2006. [Last accessed May 1, 2007]. Food Statistics, 2005. [homepage on the Internet]. Available from http://www.statcan.ca/english/freepub/21-020-XIE/21-020-XIE2005001.pdf. [Google Scholar]
- 10.United States Department of Agriculture. Livestock and Poultry: World Markets and Trade Circular Series 2-06. [Last accessed May 1, 2007];Foreign Agriculture Service. 2006 [homepage on the Internet]. Available from http://www.fas.usda.gov/dlp/circular/2006/2006%20Annual/Livestock&Poultry.pdf.
- 11.McEwen SA, Fedorka-Cray PJ. Antimicrobial use and resistance in animals. Clin Infect Dis. 2002;34(S3):S93–106. doi: 10.1086/340246. [DOI] [PubMed] [Google Scholar]
- 12.Rajić A, Reid-Smith R, Deckert AE, Dewey CE, McEwen SA. Reported antibiotic use in 90 swine farms in Alberta. Can Vet J. 2006;47:446–452. [PMC free article] [PubMed] [Google Scholar]
- 13.Dunlop RH, McEwen SA, Meek AH, Friendship RA, Clarke RC, Black WD. Antimicrobial drug use and related management practices among Ontario swine producers. Can Vet J. 1998;39:87–96. [PMC free article] [PubMed] [Google Scholar]
- 14.Akwar TH. Prevalence and risk factors of antimicrobial resistance of fecal Escherichia coli and Enterococci of pigs and farm residents [PhD dissertation] Guelph, Ontario: University of Guelph; 2003. [Google Scholar]
- 15.Dunlop RH, McEwen SA, Meek AH, Black WD, Friendship RM, Clarke RC. Prevalences of resistance to seven antimicrobials among fecal Escherichia coli of swine on thirty-four farrow-to-finish farms in Ontario, Canada. Prev Vet Med. 1998;34:265–282. doi: 10.1016/s0167-5877(97)00094-9. [DOI] [PubMed] [Google Scholar]
- 16.Dunlop RH, McEwen SA, Meek AH, Friendship RM, Black WD, Clarke RC. Sampling considerations for herd-level measurement of faecal Escherichia coli antimicrobial resistance in finisher pigs. Epidemiol Infect. 1999;122:485–496. doi: 10.1017/s0950268899002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brun E, Holstad G, Kruse H, Jarp J. Within-sample and between-sample variation of antimicrobial resistance in fecal Escherichia coli isolates from pigs. Microb Drug Resist. 2002;8:385–391. doi: 10.1089/10766290260469660. [DOI] [PubMed] [Google Scholar]
- 18.Mathew AG, Saxton AM, Upchurch WG, Chattin SE. Multiple antibiotic resistance patterns of Escherichia coli isolates from swine farms. Appl Environ Microbiol. 1999;65:2770–2772. doi: 10.1128/aem.65.6.2770-2772.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sunde M, Fossum K, Solberg A, Sørum H. Antibiotic resistance in Escherichia coli of the normal intestinal flora of swine. Microb Drug Resist. 1998;4:289–299. doi: 10.1089/mdr.1998.4.289. [DOI] [PubMed] [Google Scholar]
- 20.Canadian Pork Council. Canadian Quality Assurance Producer Materials. [Last accessed March 17, 2007.];CQA For Canadian Hog Producers. Last Updated 2007. [homepage on the Internet]. Available from http://www.cqa-aqc.ca/home_e.cfm.
- 21.Rosengren LB, Waldner CL, Reid-Smith RJ, Checkley SL, McFall ME, Rajić A. Antimicrobial resistance of fecal Salmonella spp. isolated from all phases of pig production in 20 herds in Alberta and Saskatchewan. Can J Vet Res. 2008;72:151–159. [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute: Performance standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Informational Supplement: Updated tables for the NCCLS antimicrobial susceptibility testing standard M31-A2, in CLSI2004.
- 23.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 15th Informational Supplement M100-S15, in CLSI. 2005:102–106. [Google Scholar]
- 24.United States Department of Agriculture. National Antimicrobial Resistance Monitoring System, Escherichia coli — 2000. [Last accessed May 1, 2007];Bacterial Epidemiology and Antimicrobial Resistance. [homepage on the Internet]. Last Updated Jan. 14, 2005. Available from http://www.ars.usda.gov/Business/docs.htm?docid=6770.
- 25.Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research. Charlottetown, PEI: AVC Inc; 2003. [Google Scholar]
- 26.Rosengren L. Antimicrobial resistance of Salmonella, Escherichia coli and Campylobacter from pigs on-farm in Alberta and Saskatchewan Canada [PhD dissertation] Saskatoon, Saskatchewan: University of Saskatchewan; 2007. [Google Scholar]
- 27.Browne WJ, Subramanian SV, Jones K, Goldstein H. Variance partitioning in multilevel logistic models that exhibit overdispersion. J R Statist Soc A. 2005;168:599–613. [Google Scholar]
- 28.United States Department of Agriculture. Collaboration in Animal Health and Food Safety Epidemiology (CAHFSE) Annual Report, July 2004–June 2005. [Last accessed May 1, 2007]; [homepage on the Internet]. Available from http://www.aphis.usda.gov/cahfse/index.htm.
- 29.Government of Canada. Consolidation of the Food and Drugs Act and the Food and Drugs Regulations. [Last accessed Oct. 25, 2006];Health Canada. Last Updated Jan. 27, 2006. [homepage on the Internet] Available from http://www.hc-sc.gc.ca/vetdrugs-medsvet/pub_banned_drugs_e.html.
- 30.Bischoff KM, White DG, Hume ME, Poole TL, Nisbet DJ. The chloramphenicol resistance gene cmlA is disseminated on transferable plasmids that confer multiple-drug resistance in swine Escherichia coli. FEMS Microbiol Lett. 2005;243:285–291. doi: 10.1016/j.femsle.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Carattoli A. Importance of integrons in the diffusion of resistance. Vet Res. 2001;32:243–259. doi: 10.1051/vetres:2001122. [DOI] [PubMed] [Google Scholar]
- 32.Smith JT, Lewin CS. Mechanisms of antimicrobial resistance and implications for epidemiology. Vet Microbiol. 1993;35:233–242. doi: 10.1016/0378-1135(93)90148-z. [DOI] [PubMed] [Google Scholar]
- 33.Travis RM, Gyles CL, Reid-Smith R, et al. Chloramphenicol and kanamycin resistance among porcine Escherichia coli in Ontario. J Antimicrob Chemother. 2006;58:173–177. doi: 10.1093/jac/dkl207. [DOI] [PubMed] [Google Scholar]
- 34.Mølbak K, Baggesen DL, Aarestrup FM, et al. An outbreak of multidrug-resistant, quinolone-resistant Salmonella enterica sero-type Typhimurium DT104. N Engl J Med. 1999;341:1420–1425. doi: 10.1056/NEJM199911043411902. [DOI] [PubMed] [Google Scholar]
- 35.Maguire HCF, Codd AA, Mackay VE, Rowe B, Mitchell E. A large outbreak of human salmonellosis traced to a local pig farm. Epidemiol Infect. 1993;110:239–246. doi: 10.1017/s0950268800068151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurd HS, McKean JD, Griffith RD, Rostagno MH. Estimation of the Salmonella enterica prevalence in finishing swine. Epidemiol Infect. 2004;132:127–135. doi: 10.1017/s0950268803001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajić A, Keenliside J, McFall ME, et al. Longitudinal study of Salmonella species in 90 Alberta swine finishing farms. Vet Microbiol. 2005;105:47–56. doi: 10.1016/j.vetmic.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Winokur PL, Vonstein DL, Hoffman LJ, Uhlenhopp EK, Doern GV. Evidence for transfer of CMY-2 AmpC beta-Lactamase humans. Antimicrob Agents Chemother. 2001;45:2716–2722. doi: 10.1128/AAC.45.10.2716-2722.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blake DP, Hillman K, Fenlon DR, Low JC. Transfer of antibiotic resistance between commensal and pathogenic members of the Enterobacteriaceae under ileal conditions. J Appl Microbiol. 2003;95:428–436. doi: 10.1046/j.1365-2672.2003.01988.x. [DOI] [PubMed] [Google Scholar]
- 40.Funk JA, LeJeune JT, Wittum TE, Rajala-Schultz PJ. The effect of subtherapeutic chlortetracycline on antimicrobial resistance in the fecal flora of swine. Microb Drug Resist. 2006;12:210–218. doi: 10.1089/mdr.2006.12.210. [DOI] [PubMed] [Google Scholar]
- 41.Boerlin P, Travis R, Gyles CL, et al. Antimicrobial resistance and virulence genes of Escherichia coli isolates from swine in Ontario. Appl Environ Microbiol. 2005;71:6753–6761. doi: 10.1128/AEM.71.11.6753-6761.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sunde M, Norström M. The genetic background for streptomycin resistance in Escherichia coli influences the distribution of MICs. J Antimicrob Chemother. 2005;56:87–90. doi: 10.1093/jac/dki150. [DOI] [PubMed] [Google Scholar]
- 43.Sask Pork. Annual Report 2004–2005. [Last accessed March 19, 2007];Sask Pork. 2005 [home-page in the Internet]. Available from http://www.agr.gov.sk.ca/agrifood/boards/SKPorkAnnualRpt2005.pdf.


