Abstract
The primary aim of this study was to evaluate the level of agreement of the E-test for in vitro antimicrobial susceptibility testing of Campylobacter coli using the agar dilution technique, which is the approved method. A convenience sample of 80 Ontario swine farms was chosen for this study; each farm was visited from January to June 2004. A total of 233 isolates of C. coli were tested for susceptibility to 10 antimicrobials by agar dilution and the E-test. Performance of the tests was evaluated using 7 quality control strains: Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 29213, Campylobacter jejuni ATCC 33560, and Campylobacter coli ATCC 33559 for the E-test and E. coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and C. jejuni ATCC 33560 for the agar dilution test. Weighted Cohen’s kappa and prevalence-adjusted bias-adjusted kappa (PABAK) tests were used for statistical analysis. The E-test and agar dilution test results had a strong agreement when resistance to streptomycin and tetracycline were evaluated (weighted kappa: 0.68 and 0.66, respectively). However, marked disagreement was detected when testing susceptibility to nalidixic acid and ampicillin (0.15 and 0.22, respectively). Almost perfect agreement was detected by PABAK when testing susceptibility to gentamicin (0.99). Agreement was found to be moderate for ciprofloxacin, azithromycin, clindamycin, erythromycin, and chloramphenicol. Although the level of agreement between the E-test and agar dilution depended on the antimicrobial being tested, the E-test always detected a lower proportion of resistant isolates compared to agar dilution.
Résumé
L’objectif principal de cette étude était d’évaluer le niveau d’agrément de deux méthodes in vitro de détermination de la sensibilité aux antibiotiques des Campylobacter, soit le E-test et la méthode de dilution en gélose qui est la méthode approuvée. Un échantillon de convenance de 80 fermes porcines ontariennes a été choisi pour cette étude; chaque ferme a été visitée durant la période de janvier à juin 2004. Un total de 233 isolats de C. coli a été testé pour leur sensibilité à 10 antimicrobiens par dilution en gélose et E-test. La performance des tests a été évaluée à l’aide de 7 souches de contrôle de qualité : Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 29213, Campylobacter jejuni ATCC 33560 et Campylobacter coli ATCC 33559 pour le E-test et E. coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 et C. jejuni ATCC 33560 pour la méthode de dilution en gélose. Pour les analyses statistiques, un test de kappa de Cohen pondéré et un test de kappa ajusté pour la prévalence et ajusté pour le biais (PABAK) ont été utilisés. Le E-test et le test de dilution en gélose avaient un accord marqué lorsque la résistance à la streptomycine et la tétracycline était évaluée (kappa pondéré : 0,68 et 0,66, respectivement). Toutefois, un désaccord marqué a été détecté lorsque la sensibilité à l’acide nalidixique et à l’ampicilline était testée (0,15 et 0,22, respectivement). Un accord presque parfait a été détecté par PABAK pour la sensibilité à la gentamycine (0,99). L’accord s’est avéré modéré pour ce qui est des antimicrobiens suivants : ciprofloxacin, azithromycine, clindamycine, érythromycine et chloramphénicol. Bien que le degré d’accord entre le E-test et la méthode de dilution en agar dépende de l’antimicrobien testé, le E-test détectait toujours une plus faible proportion d’isolats résistants comparativement à la méthode de dilution en gélose.
(Traduit par Docteur Serge Messier)
Introduction
The emergence of antimicrobial resistance is a global problem that encouraged the establishment of the National Antimicrobial Resistance Monitoring System (NARMS), proposed by the United States Food and Drug Administration (FDA), to monitor the occurrence of antimicrobial resistance. Resistance has been observed and reported among many bacterial species, including Campylobacter spp. The agar dilution procedure is a technique approved by the Clinical and Laboratory Standards Institute (CLSI), for antimicrobial susceptibility testing of Campylobacter spp. However, there are no internationally agreed upon susceptibility criteria for Campylobacter spp. and breakpoints used by NARMS are provisional. For this reason, other techniques for testing antimicrobial susceptibility in Campylobacter spp. have been performed, including disk diffusion, broth microdilution, and the E-test.
Disk diffusion refers to the diffusion of an antimicrobial agent of a specified concentration from disks, tablets, or strips, into solid culture media seeded with a standardized bacterial inoculum; results are based on the size of the inhibition zone (1). Disk diffusion has been reported to be the simplest and most cost-effective method for testing antimicrobial susceptibility; however, it requires standardization and involves the manual measurement of zones of inhibition making it impractical for some laboratories (1,2).
Broth microdilution is a technique in which standardized suspensions of bacteria are tested against varying concentrations of an antimicrobial agent in a standardized liquid medium (1). Although this technique has been recommended for antimicrobial susceptibility testing of Campylobacter coli (3), it involves the use of special equipment and commercially prepared antimicrobial panels that make it a costly option (1).
In order to detect emergence of antimicrobial resistance it is important to use a practical, consistent, and standardized method that will allow comparison with national or international monitoring data. Agar dilution involves the incorporation of an antimicrobial agent into an agar medium in a geometrical progression of concentrations, followed by the application of a defined bacterial inoculum to the agar surface of the plate (1). Some of the advantages offered by the agar dilution technique include: accurate determination of minimum inhibitory concentrations (MICs) when a full dilution range of the antimicrobial is used; the ability to test many organisms against a series of dilutions of a single antimicrobial at the same time; the potential to extend the antimicrobial concentration as far as required; and the possibility to be adapted to semi-automation (1,3). The agar dilution method has been known as the gold standard technique of antimicrobial susceptibility testing; however, it is rarely performed in routine laboratories due to the large amount of manual handling needed to run it. This technique requires extensive training of personnel and may be more expensive and labor-intensive, when testing many organisms against many antimicrobials, than other testing methods (1,3). The E-test might be a reliable alternative method for performing antimicrobial susceptibility of Campylobacter spp. (4,5) since quantitative data may be obtained. Plastic strips carrying a continuous gradient of antimicrobial agent are placed on the surface of an inoculated agar plate; the antimicrobial diffuses into the agar generating the MIC values of a given antimicrobial. The E-test method is less laborious, less expensive when testing a limited number of antimicrobials (≤ 3) per microorganism, and easier to perform than the agar dilution technique, thus making it an attractive alternative for antimicrobial susceptibility testing (3). However, it is important to validate this method before making any recommendations for testing antimicrobial susceptibility of C. coli.
Campylobacter spp., primarily C. jejuni and C. coli, account for the highest annual average incidence rate of human gastroenteritis worldwide (6), generally, the disease is self-limiting but in severe cases antimicrobial therapy is required. Since an increased number of Campylobacter strains that are resistant to several antimicrobials have been isolated from clinical samples in many countries (5), there is a concern that antimicrobial use in food animals may select for resistance. Pork is considered a possible source of Campylobacter, especially C. coli, as a result of carcass contamination at slaughter. Previous studies have demonstrated that in Ontario almost 100% of the pigs carry Campylobacter coli as a normal inhabitant of their gastrointestinal system (7). For this reason, it is important to determine the existing profile of antimicrobial susceptibility of Campylobacter isolates from healthy pigs in Ontario through surveillance programs. However, the lack of a standardized technique to measure antimicrobial susceptibility in Campylobacter isolates has been a limitation in achieving this goal. The objective of this study was to evaluate the level of agreement between the E-test and the agar dilution procedure using isolates from commercial farms.
Materials and methods
A convenience sample of 80 Ontario swine farms was selected for collection of fecal samples; an attempt was made to include a wide variety of farm type, size, and geographical distribution. All the farms were visited between January and June 2004. The 1200 fecal samples collected from hogs that were close to market weight were used to establish the pattern of antimicrobial resistance in Campylobacter isolates recovered from swine in Ontario (7). Campylobacter was isolated from 1194 of the 1200 samples; those identified as C. coli (n = 1185) were stored at −70°C for further susceptibility testing.
For the E-test, 240 isolates were randomly selected from the 1185 frozen isolates using a phone book as random number table. The phone book was randomly opened and the last 3 digits from the 1st number on the right page were used as the starting point. Appropriate numbers were then selected by moving down the columns in the phone book; these numbers were used to identify the 240 isolates.
The original results from the main study were used for the agar dilution testing (7). Isolates were grown on Mueller-Hinton Blood Agar (MHBA) plates with antimicrobials (cefoperazone and VTP: vancomycin, trimethoprim, polymyxin B solution). The isolates were supplemented with 5% defibrinated sheep blood at 37°C for 48 h in a microaerophilic environment for 2 passages and adjusted to turbidity equal to a 0.5 McFarland standard: 233 isolates were recovered. Antimicrobial breakpoint concentrations were based on the National Committee for Clinical Laboratory Standards (NCCLS) approved standard for members of the Enterobacteriaceae family (M31-A), listed by the FDA, and used by NARMS. Susceptibility of C. coli was tested against a range of antimicrobials: ampicillin, azithromycin, chloramphenicol, ciprofloxacin, clindamycin, erythromycin, gentamicin, nalidixic acid, streptomycin, and tetracycline.
Agar dilution test
Suspensions were dispensed into the wells of a cold Cathra Replicator 36 well plate (Oxoid, Nepean, Ontario) beginning by filling the 1st well with 300 μL of India ink. Each of the next 3 wells was filled with 300 μL of 1 of 3 reference strains (E. coli ATCC 25922, P. aeruginosa ATCC 27853, and C. jejuni ATCC 33560), and the last 33 wells were filled with C. coli isolates. Isolates were spotted onto Mueller Hinton Blood Agar (MHBA) plates containing the antimicrobial agents tested (Oxoid). Determination of the MICs for this test was based on plates holding concentrations of antimicrobials close to the resistance breakpoints, rather than a full dilution range (Table I). Each of the inoculated agar plates were dried at room temperature and microaerophilically incubated at 42°C for 48 h. The MICs were read as the lowest concentration of the antimicrobial at which there was no visible growth.
Table I.
Minimum inhibitory concentration (MIC) breakpoints used to test agreement between agar dilution (AD) and E-test for 10 antimicrobials for 233 Campylobacter coli isolates recovered from 80 swine farms in Ontario
| Minimum inhibitory concentration (MIC)a breakpoints
|
|||||
|---|---|---|---|---|---|
| Antimicrobial | Codes | Susceptible ≤ μg/mL | Intermediate = μg/mL | Resistant ≥ μ/mL | |
| I | Ciprofloxacin | CIP | 1 | 2 | 4 |
| II | Azithromycin | AZM | 0.25 | 2 | |
| Clindamycin | CLI | 0.5 | 2 | 4 | |
| Erythromycin | ERY | 0.5 | 4 | 8 | |
| Nalidixic acid | NAL | 4 | 16 | 32 | |
| III | Ampicillin | AMP | 8 | 16 | 32 |
| Chloramphenicol | CHL | 8 | 16 | 32 | |
| Gentamicin | GEN | 4 | 8 | 16 | |
| Streptomycin | STR | 32 | 64 | ||
| Tetracycline | TCY | 4 | 8 | 16 | |
Minimum inhibitory concentration (MIC) specified by the CLSI standards.
E-test
Following suspension of C. coli colonies in Mueller Hinton Broth (MHB) to produce the required turbidity of 0.5 McFarland standard, the suspension was swabbed onto Mueller-Hinton Blood Agar plates supplemented with 5% laked horse blood (MHLHB) 3 times by rotating the plate approximately 45° each time. Plates were dried in a biohood at room temperature, and 2 E-test strips containing a predefined gradient of the antimicrobials were placed on each plate. The E-test strips were placed onto inoculated agar surfaces with the minimum inhibitory concentration (MIC) scales facing upwards. The 1st strip was placed on one half of the surface, and the 2nd was placed in an anti-parallel orientation to the 1st strip. Plates were incubated in an inverted position at 37°C under microaerophilic conditions for 48 h. The MICs were read according to the E-test reading guide: where the edge of the inhibition ellipse intersects the side of the strip (AB Biodisk, Solna, Sweden).
For each set of samples, the following quality control strains were tested to check the performance of the E-test: E. coli ATCC 25922, S. aureus ATCC 29213, C. jejuni ATCC 33560, and C. coli ATCC 33559. For E. coli and S. aureus, 1 set of MHLHB plates was inoculated at 37°C under aerobic conditions for 24 h, and a 2nd set was incubated microaerophilically at 37°C for 48 h. For C. jejuni and C. coli, 1 set of inoculated MHLHB plates containing the E-test strips was incubated at 37°C under microaerophilically conditions for 48 h.
Data handling and statistical analysis
Data were entered into an Excel spreadsheet (Microsoft Excel 2000; Microsoft, Redmond, Washington, USA) and subsequently imported into Intercooled STATA 8.2 (Stata, College Station, Texas, USA) for statistical analysis. Percentage of agreement or exact agreement was calculated for 3 different tables, by dividing the sum of the diagonal matrix by the total number of observations in each table. The 1st table maintained the 3 original categories [S, I, R]; afterwards, data were collapsed into 2 × 2 tables in 2 different ways: intermediate susceptibility was 1st classified as resistant [S, (I + R)], and then as susceptible [(S + I), R]. To assess the degree of agreement between the tests, and correct for any chance-expected agreement, a Cohen’s weighted kappa statistic was calculated. Weighted kappa is an appropriate measure of agreement for ordinal data because it attaches greater emphasis to large differences between ratings than to small differences.
One problem of the kappa coefficient, however, is that its interpretation is not straightforward. There are some factors such as prevalence and bias effects that may influence the magnitude of kappa; if bias and prevalence effects are present, the kappa coefficient may be larger or smaller than the PABAK, depending of the size of PI and BI (8,9). Kappa was adjusted for high or low prevalence using the 2 × 2 tables by computing the average of the 2 concordant cells and substituting these values for the actual values in those cells (9). Similarly, an adjustment for bias was achieved by substituting the mean of the disconcordant cells for those actual values. The kappa coefficient that results is referred to as prevalence-adjusted bias-adjusted kappa (PABAK) (9). The PABAK coefficient was used to investigate the likely effects of prevalence and bias alongside the true value of kappa. The PABAK coefficient chosen for each antimicrobial was that from the 2 × 2 table showing the closer percentage to that detected by weighted kappa (Table II).
Table II.
Adjusted reliability measures to test agreement between agar dilution (AD) and E-test for 10 antimicrobials for 233 Campylobacter coli isolates recovered from 80 swine farms in Ontario
| Categories
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agreement (%)
|
S, I, R
|
S, (I + R)a |
(S + I), Rb |
|||||||||
| Antimicrobial | A | Ba | Cb | Weighted kappa | Kappa | PI | BI | PABAK | Kappa | PI | BI | PABAK |
| Ciprofloxacin | 96 | 96 | 97 | 0.45 | 0.36 | 0.93 | 0.04 | 0.92 | 0.49 | 0.95 | 0.03 | 0.95 |
| Azithromycin | 73 | 73 | 73 | 0.25 | 0.25 | 0.59 | 0.27 | 0.46 | 0.25 | 0.59 | 0.27 | 0.46 |
| Clindamycin | 73 | 81 | 83 | 0.50 | 0.18 | 0.75 | 0.19 | 0.62 | 0.57 | 0.45 | 0.14 | 0.67 |
| Erythromycin | 78 | 91 | 85 | 0.58 | 0.13 | 0.89 | 0.09 | 0.82 | 0.62 | 0.49 | 0.13 | 0.71 |
| Nalidixic Acid | 15 | 18 | 82 | 0.15 | 0.02 | 0.07 | 0.82 | −0.62 | 0.34 | 0.70 | 0.14 | 0.65 |
| Ampicillin | 27 | 33 | 79 | 0.22 | 0.05 | 0.03 | 0.67 | −0.34 | 0.35 | 0.66 | 0.17 | 0.58 |
| Chloramphenicol | 75 | 75 | 97 | 0.17 | 0.03 | 0.64 | 0.25 | 0.5 | −0.007 | 0.97 | 0.02 | 0.95 |
| Gentamicin | 99 | 99 | ~100 | 0.00 | 0.00 | 0.99 | 0.00 | 0.98 | 0.00 | 0.99 | 0.00 | 0.99 |
| Streptomycin | 84 | 86 | 85 | 0.68 | 0.66 | 0.41 | 0.14 | 0.72 | 0.66 | 0.37 | 0.09 | 0.71 |
| Tetracycline | 76 | 84 | 79 | 0.66 | 0.67 | 0.25 | 0.16 | 0.68 | 0.58 | 0.12 | 0.20 | 0.57 |
Intermediate isolates were categorized as resistant.
Intermediate isolates were classified as susceptible.
A — susceptible, intermediate and resistant categories; PI — preference index; BI — bias index; PABAK — prevalence-adjusted bias-adjusted kappa.
The interpretation used for Cohen’s kappa coefficient was as follows: poor agreement below 0.20, fair from 0.21 to 0.40, moderate from 0.41 to 0.60, substantial from 0.61 to 0.80, and almost perfect agreement from 0.81 to 1.00 (8).
Results
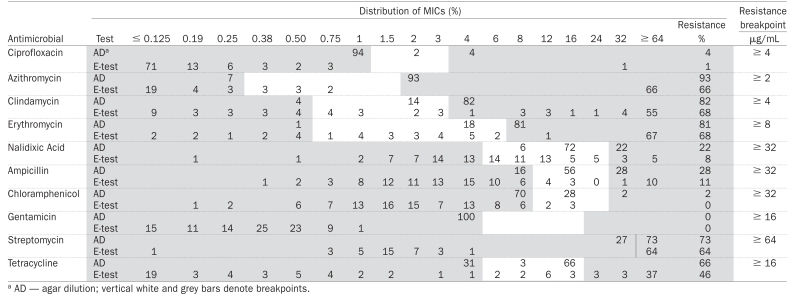
The prevalence of resistance detected by each test, weighted kappa indices with their respective 95% confidence intervals (CI) and classification by categories are presented for each of the 10 antimicrobials in Table III. Although the agar dilution test classified a higher proportion of isolates as resistant compared to the E-test, the E-test produced results highly comparable to the agar dilution method. In general, near perfect agreement between the E-test and agar dilution was observed when testing C. coli susceptibility against gentamicin. Agreement on resistance was substantial for streptomycin and tetracycline, moderate for ciprofloxacin, clindamycin, erythromycin, and chloramphenicol, and fair for azithromycin. The 2 tests disagreed when testing nalidixic acid and ampicillin. The percentage of resistance and MICs detected by both the E-test and the agar dilution method are presented in Table IV.
Table III.
Agreement between agar dilution (AD) and E-test for 10 antimicrobials for 233 Campylobacter coli isolatesa
| % Resistance
|
Test
|
Number of isolates in the following Categories
|
||||
|---|---|---|---|---|---|---|
| Antimicrobial | AD | E-test | Weighted kappa | 95% CI | Similar | Different |
| Ciprofloxacin | 4% (9) | 1% (3) | 0.45 | 0.12–0.78 | 223 | 10 |
| Azithromycin | 93% (217) | 66% (154) | 0.25 | 0.15–0.36 | 170 | 63 |
| Clindamycin | 82% (190) | 67% (157) | 0.50 | 0.39–0.60 | 169 | 64 |
| Erythromycin | 81% (189) | 68% 159) | 0.58 | 0.48–0.68 | 182 | 51 |
| Nalidixic Acid | 22% (52) | 8% (19) | 0.15 | 0.08–0.21 | 35 | 198 |
| Ampicillin | 28% (64) | 11% (25) | 0.22 | 0.14–0.30 | 62 | 171 |
| Chloramphenicol | 2% (5) | 0.4% (1) | 0.17 | 0.07–0.28 | 175 | 58 |
| Gentamicin | 0% | 0.4% (1) | 0.00 | — | 231 | 2 |
| Streptomycin | 73% (170) | 64% (150) | 0.68 | 0.58–0.77 | 195 | 38 |
| Tetracycline | 66% (154) | 46% (108) | 0.66 | 0.57–0.75 | 176 | 57 |
Isolates were recovered from 80 swine farms in Ontario.
Table IV.
Distribution of antimicrobial minimum inhibitory concentrations (MICs) in 233 Campylobacter coli isolates from swine in Ontario
 |
Discussion
It has been suggested that a McNemar’s X2 test should be used to evaluate any evidence of agreement between tests before assessing the magnitude of such agreement using kappa. A significant result from the McNemar’s X2 test would indicate serious disagreement and thus kappa would be of little value (8). However, a correlation coefficient, like McNemar’s X2 test, is a measure of association that measures the strength of a relation between 2 variables and not the agreement between them (10,11). For this reason, the significance of McNemar’s X2 was not considered in our analysis.
The Cohen’s kappa coefficient is an adequate method to measure agreement because it measures not only the percentage of agreement, but also the percentage of agreement beyond chance (12). One of the advantages is that the kappa coefficient can be used for scales with more than 2 categories; however, unweighted kappa is inappropriate for ordinal scales because it treats all disagreement equally. A weighted kappa coefficient was chosen as the most appropriate measure of agreement in our analysis (ordinal data: S, I, R) because it attaches greater emphasis to large differences between categories (S vs R) rather than to small differences (S vs I; I vs R) (9,11).
There are limitations to the interpretation of the kappa coefficient if the prevalence of resistance is either lower than 20% or higher than 80% (6). In this study, the prevalence of resistance, determined by at least one test, was either lower than 20% or higher than 80% for all antimicrobials tested except streptomycin and tetracycline. The strong dependence of kappa on the prevalence complicates its interpretation as an index of agreement for most of the antimicrobials tested. The prevalence of resistance detected for streptomycin and tetracycline using either the E-test or agar dilution was categorized as moderate (lower than 80% but higher than 20%), and therefore, for these 2 antimicrobials, assumptions can be made for the use of the E-test and the agar dilution method interchangeably for most practical purposes.
Since the degree of resistance was categorized as high to moderately high for several antimicrobials agents including ciprofloxacin, azithromycin, clindamycin, erythromycin, nalidixic acid, ampicillin, chloramphenicol, and gentamicin, it is important to consider the factors that may influence the magnitude of kappa such as prevalence and bias effects, to evaluate the performance of the test. Prevalence effect occurs when the overall proportion of positive (resistant) results is substantially different from 50%, and it can be expressed as prevalence index (PI); a large PI indicates that the kappa coefficient has been lowered (13). The bias effect expressed as bias index (BI) is defined as the extent to which the tests disagree on the proportion of resistant (or susceptible) isolates; a large BI value will indicate an inflated kappa coefficient (9). A large PI was observed for ciprofloxacin, clindamycin, chloramphenicol, and gentamicin, indicating that the values of kappa may be underestimated. Adjusted kappa (PABAK) indicates that the agreement observed for these antimicrobials is in fact affected by PI and for this reason, it can be assumed that real agreement is higher than that detected by weighted kappa. However, the PABAK coefficient is presented in addition to, rather than in place of, the obtained value of kappa, because it gives an indication of the likely effects of prevalence and bias index along the true kappa value (9). A moderate agreement was declared between the tests with ciprofloxacin, clindamycin, and chloramphenicol. The PABAK coefficient gave an indication that the apparently poor agreement observed for chloramphenicol was underestimated.
Poor agreement was observed for nalidixic acid and ampicillin; although large BIs were detected for these 2 antimicrobials, adjusted kappa confirmed this observation.
Since PI observed for azithromycin and erythromycin were not significantly different from 50% (0.59 and 0.49, respectively), it was assumed that the weighted kappa coefficient, which indicated fair and moderate agreement, respectively was an appropriate interpretation.
The CSLI recently approved the agar dilution test as a standardized in vitro antimicrobial susceptibility testing method for Campylobacter for the following antimicrobials: ciprofloxacin, erythromycin, and gentamicin (14). Moderate agreement was observed in this study when antimicrobial susceptibility was tested using these antimicrobials. The lack of standards for testing accepted resistance breakpoints for Campylobacter spp. for some antimicrobials used in this study makes it difficult to declare a best method for testing antimicrobial susceptibility of Campylobacter isolates.
The E-test is considered to be an acceptable alternative for testing the antimicrobial susceptibility of Campylobacter spp. depending on the antimicrobial being tested. It has been reported that the E-test is a less tedious, time-consuming, and expensive method than the agar dilution (15), because multiple antimicrobials can be evaluated on a single plate containing a single isolate. The results of this study validate the E-test as an optional method for testing the antimicrobial susceptibility of Campylobacter spp. for all the antimicrobials used except for nalidixic acid and ampicillin. However, it is important to remember not only that the E-test underestimates the prevalence of antimicrobial resistance when compared to the agar dilution test, but also that not all antibiotic-containing E-test strips are approved by the FDA.
A high level of disagreement between the E-test and agar dilution was seen with nalidixic acid and ampicillin; therefore, the agar dilution test should be the preferred method for testing antimicrobial susceptibility of Campylobacter against these 2 antimicrobials. The major disadvantage of the agar dilution test is the preparation of serial dilutions of antimicrobials incorporated into agar plates. However, the endpoint of the agar dilution test is easier to interpret than the E-test endpoint. The E-test endpoints may be difficult to interpret because some strains produce feathery or swarming edges at the intersection with the E-test strip (16).
The MICs obtained in this study with the agar dilution method were only estimates, as plates with concentrations of antimicrobials close to the resistance breakpoints were used instead of a specific MIC value generated by the E-test. Our study showed that the E-test tends to define lower MICs than the agar dilution test, as in other studies (3,5,17). Further multicenter laboratory evaluations of the E-test have been recommended in order to establish the efficiency of this technique for Campylobacter susceptibility testing (5).
Since there is international concern regarding the transfer of bacterial resistance from animals to humans, susceptibility data from laboratories in different countries should be comparable. However, different regulatory agencies utilize different methods with different breakpoints. Standardization of antimicrobial susceptibility testing methodologies, and accepted breakpoints for determining resistance of Campylobacter spp. offers the opportunity to obtain higher quality data in epidemiological surveillance of antimicrobial drug resistance, as MIC values may be monitored over the years to detect trends and changes. Likewise, different mechanisms producing resistance could be detected by evaluating the MIC distribution of the bacteria against different antimicrobials. Since results from the agar dilution method were only estimates, our results only provided qualitative information, such as whether the organism was susceptible or resistant to an antimicrobial agent and did not provide any kind of quantitative data concerning the level of susceptibility. Interpretative issues regarding the comparison of susceptibility data may arise as a result of using qualitative data or dichotomization rather than MICs. Although qualitative data are clinically valuable, they cannot be use for the purpose of monitoring shifts in susceptibility to antimicrobial agents. The aim of surveillance programs is to monitor antimicrobial susceptibility changes in target bacterial pathogens; however, since there is no standardization and harmonization of laboratory methodologies for the detection of antimicrobial resistance, susceptibility testing data between laboratories within and between countries cannot be compared. Comparable results from antimicrobial susceptibility tests (AST) may arise from quantitative data. Results from AST should be reported quantitatively rather than qualitatively, providing the minimal concentration of an antimicrobial required to inhibit the growth of the microorganism (MIC). This approach would facilitate the detection of small changes in antimicrobial susceptibility over time. The agar dilution test provides quantitative results when all serial dilutions of antimicrobials are used, improving the identification of MIC endpoints and the possibility of extending the antibiotic concentration range as far as necessary. Further studies are needed to determine whether agar dilution and E-test produce more comparable results when all the antimicrobial dilution ranges available for the agar dilution test are used.
In conclusion, the level of agreement between the E-test and agar dilution depends on the antimicrobial being tested. The E-test consistently detects a lower proportion of resistant isolates than agar dilution. Either the agar dilution test or the E-test may be used for testing Campylobacter spp. susceptibility to certain antimicrobials, but under conditions of this study there was marked disagreement between the 2 tests for ampicillin and nalidixic acid.
Acknowledgments
This work was supported by Ontario Pork, Ontario Ministry of Agriculture Food and Rural Affairs (OMAFRA), the Public Health Agency of Canada, and the University of Guelph-OMAFRA Animal Research Program.
Footnotes
This article is part of a master’s thesis, University of Guelph, 2005.
References
- 1.White DG, Acar J, Anthony F, et al. Antimicrobial resistance: Standardisation and harmonisation of laboratory methodologies for the detection and quantification of antimicrobial resistance. Rev Sci Tech. 2001;20:849–858. doi: 10.20506/rst.20.3.1316. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen JH. Selection criteria for an antimicrobial susceptibility testing system. J Clin Microbiol. 1993;31:2841–2844. doi: 10.1128/jcm.31.11.2841-2844.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luber P, Bartelt E, Genschow E, Wagner J, Hahn H. Comparison of broth microdilution, E-test, and agar dilution methods for antibiotic susceptibility testing of Campylobacter jejuni and Campylobacter coli. J Clin Microbiol. 2003;41:1062–1068. doi: 10.1128/JCM.41.3.1062-1068.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker CN. The E-test and Campylobacter jejuni. Diagn Microbiol Infect Dis. 1992;15:469–472. doi: 10.1016/0732-8893(92)90092-8. [DOI] [PubMed] [Google Scholar]
- 5.Ge B, Bodeis S, Walker RD, et al. Comparison of the E-test and agar dilution for in vitro antimicrobial susceptibility testing of Campylobacter. J Antimicrob Chemother. 2002;50:487–494. doi: 10.1093/jac/dkf162. [DOI] [PubMed] [Google Scholar]
- 6.Lee M, Middleton D. Enteric illness in Ontario, Canada, from 1997 to 2001. J Food Prot. 2003;66:953–961. doi: 10.4315/0362-028x-66.6.953. [DOI] [PubMed] [Google Scholar]
- 7.Varela N, Friendship R, Dewey C. Prevalence of resistance to 11 antimicrobials among Campylobacter coli isolates from pigs on 80 grower-finisher farms in Ontario. Can J Vet Res. 2007;71:89–194. [PMC free article] [PubMed] [Google Scholar]
- 8.Dohoo I, Martin W, Stryhn H. Screening and diagnostic tests. In: Dohoo I, Martin W, Stryhn H, editors. Veterinary Epidemiologic Research. Prince Edward Island: Charlottetown, University of Prince Edward Island; 2003. pp. 86–120. [Google Scholar]
- 9.Sim J, Wright CC. The kappa statistic in reliability studies: Use, interpretation, and sample size requirements. Phys Ther. 2005;85:257–268. [PubMed] [Google Scholar]
- 10.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 11.Jakobsson U, Westergren A. Statistical methods for assessing agreement for ordinal data. Scand J Caring Sci. 2005;19:427–431. doi: 10.1111/j.1471-6712.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- 12.Byrt T, Bishop J, Carlin J. Bias, prevalence and kappa. J Clin Epidemiol. 1993;46:423–429. doi: 10.1016/0895-4356(93)90018-v. [DOI] [PubMed] [Google Scholar]
- 13.Martin SW, Meek AH, Willeberg P. Disease causation. In: Martin SW, Meek AH, Willeberg P, editors. Veterinary Epidemiology: Principles and Methods. Ames, Iowa: Iowa State Univ Pr; 1987. pp. 121–148. [Google Scholar]
- 14.McDermott PF, Bodeis SM, Aarestrup FM, et al. Development of a standardized susceptibility test for Campylobacter with quality-control ranges for ciprofloxacin, doxycycline, erythromycin, gentamicin, and meropenem. Microb Drug Resist. 2004;10:124–131. doi: 10.1089/1076629041310064. [DOI] [PubMed] [Google Scholar]
- 15.Valdivieso A, Imgrund R, Deckert A, et al. Cost analysis and antimicrobial susceptibility testing comparing the E-test® and the agar dilution method in Campylobacter spp. Proc 103rd Gen Meet ASM. 2003:15. doi: 10.1016/j.diagmicrobio.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Osato MS. Antimicrobial susceptibility testing for Helicobacter pylori: Sensitivity test results and their clinical relevance. Curr Pharm Des. 2000;6:545–1555. doi: 10.2174/1381612003399059. [DOI] [PubMed] [Google Scholar]
- 17.Båverud V, Gunnarsson A, Karlsson M, Franklin A. Antimicrobial susceptibility of equine and environmental isolates of Clostridium difficile. Microb Drug Resist. 2004;10:57–63. doi: 10.1089/107662904323047817. [DOI] [PubMed] [Google Scholar]


