Abstract
The main objectives of this study were to determine the prevalence and patterns of antimicrobial resistance in pigs on farms that medicated swine ration and those that did not. A total of 940 isolates of Escherichia coli from 188 pooled fecal samples obtained from weaner and finisher pigs on 47 farrow-to-finish swine farms (34 farms used in-feed medication and 13 did not) were tested for susceptibility to 21 antimicrobials using a breakpoint concentration method. The prevalence of resistance varied widely (0.0% to 81.3%) among the antimicrobials tested. Ninety percent of all the isolates tested were resistant to one or more antimicrobials. The most common multi-drug resistance patterns were to 2 to 6 antimicrobials. Resistance was significantly more frequent (P < 0.01) on farms that used in-feed medication compared to those that did not, and significantly more frequent (P < 0.01) in weaner pigs compared to finisher pigs. These findings indicate that resistance to a broad range of antimicrobials was prevalent among fecal E. coli isolates of pigs on study farms, and that this constitutes a potential reservoir for resistance genes that could spread to pathogens. The findings also provide further evidence that use of medication in swine rations provides selective pressure for antimicrobial resistance in E. coli in pigs.
Résumé
L’objectif principal de cette étude était de déterminer la prévalence et les patrons de résistance aux antimicrobiens de bactéries provenant de porcs élevés sur des fermes donnant une ration médicamentée et d’autres une ration non-médicamentée. Un total de 940 isolats d’Escherichia coli provenant de 188 échantillons de fèces combinées obtenus de porcs au sevrage et en finition de 47 fermes de type naisseur-finisseur (34 fermes utilisant une médication dans la nourriture et 13 non) ont été éprouvés pour leur sensibilité envers 21 agents antimicrobiens par une méthode de concentrations limites. La prévalence de résistance aux antimicrobiens testés variait grandement (0 % à 81,3 %). Quatre-vingt-dix pourcents de tous les isolats testés étaient résistants à au moins un antimicrobien. Les patrons de résistance multiple les plus communs indiquaient une résistance envers 2 à 6 antimicrobiens. De la résistance était observée significativement plus fréquemment (P < 0,01) sur les fermes qui utilisaient une ration médicamentée comparativement à celles qui n’en n’utilisaient pas, et était significativement plus fréquente (P < 0,01) chez les porcs au sevrage que chez les porcs en finition. Ces résultats indiquent que la résistance envers un large éventail d’antimicrobiens était prévalente parmi les isolats d’E. coli d’origine fécale provenant des porcs issus des fermes de cette étude, et que ceci constitue un réservoir potentiel de gènes de résistance qui pourraient se répandre à des agents pathogènes. Ces données fournissent également des preuves supplémentaires que l’utilisation de ration médicamentée cause une pression sélective pour la résistance aux antimicrobiens chez les E. coli porcins.
(Traduit par Docteur Serge Messier)
Introduction
The use of in-feed medication to promote growth is widespread in swine production (1) and this use can lead to selection for resistant bacteria (2–4). Antimicrobial resistance among commensal Escherichia coli of swine is important because it may constitute a reservoir of antimicrobial resistance genes (5) that could be transferred to pathogenic bacteria. For example, plasmids conferring resistance to multiple antimicrobials can transfer from E. coli to Salmonella Typhimurium and other pathogens (6,7). The level of antimicrobial resistance among commensals may also be used as an indicator of selection pressure by antimicrobial use (7). Furthermore, E. coli of animal origin may colonize the intestinal tract of humans (8–10).
The prevalence of antimicrobial resistance in commensal E. coli of pigs has been described in several previous studies (5,11–13) in various countries. The purpose of this cross-sectional study was to determine the prevalence of resistance to single and multiple antimicrobials among fecal E. coli from pigs on purposefully selected farms in Ontario and British Columbia that did or did not use in-feed antimicrobials, and also describe the patterns of resistance among E. coli from weaner and finisher pigs.
Materials and methods
Selection of study farms
The study population was pigs from farrow-to-finish pig farms in Ontario and British Columbia (BC). Forty seven farrow-to-finish swine farms were enrolled in the study, 39 from Ontario and 8 from BC. To be eligible, a farm had to be a farrow-to-finish operation, have a minimum of 50 sows on-site, and be willing to participate in the study. A random sample of farms was selected from a pre-existing list of farrow-to-finish swine farms. The number of farms enrolled was determined by the ability to meet the objectives of the study, taking into account financial and logistical considerations. Selected farms were classified into 2 groups based on their in-feed medication practices (farms that used in-feed medication versus those that did not). Antimicrobial use information and other management factors were obtained using a questionnaire during farm visits. To validate the claims of farmers concerning their antimicrobial use practices, a manual check of the feed additive records, and existing antimicrobial stocks in their refrigerators and on shelves was made. Each farm was visited twice from March to September 1999 for Ontario farms, and from May to August, 2000 for those in BC. On each visit, 10 fecal samples were collected from each of the weaner and finisher pig groups and pooled respectively for culture and isolation of E. coli, giving a total of 94 pooled samples per visit × 2 visits per farm = 188 pooled samples.
Processing of fecal samples
Upon receipt at the laboratory, 5 g of each of the 2 pooled fecal samples were placed in a sterile Sterifil bag (Spiral System, Bethesda, Maryland, USA) with a filtered channel on the side. Sterile 0.85% saline solution (200 mL) was added to each bag, then mixed in a mechanical “stomacher” for 20 s. A 10-mL diluted sample was combined and mixed with 10 mL of sterile Tryptic Soy Broth (TSB) (Difco, Becton, Dickinson, Sparks, Maryland, USA) containing 50% glycerol. One mL aliquots of the sample were stored frozen at −70°C.
Isolation of Escherichia coli
Frozen diluted fecal samples were partially thawed and loopfuls were streaked onto MacConkey agar (Difco, Becton, Dickinson) and incubated for 18 to 24 h at 37°C. Five colonies with the typical color and appearance of E. coli were picked and streaked again on MacConkey agar plates, then re-streaked on Luria-Bertani (LB), Miller agar plates (Difco, Becton, Dickinson). Isolates were tested for indole production and use of citrate as the sole carbon source. Lactose positive, indole positive and citrate negative isolates were considered to be E. coli, and they were stored in Mueller Hinton (MH) broth containing 50% glycerol (Difco, Detroit, Michigan, USA) at −70°C. Five isolates of E. coli were obtained from each of the 188 pooled fecal samples for susceptibility testing, for a total of 940 isolates.
Testing of Escherichia coli susceptibility to antimicrobials
Susceptibility of E. coli isolates was tested to 21 antimicrobials at 1 to 3 different concentrations using the Cathra Replicator (14) (Automed, Shoreview, Minnesota, USA). The isolates, grown to a set concentration in Mueller Hinton (MH) broth, were spotted onto MH agar plates (Difco) containing antimicrobials at the concentrations listed in Table I. Aquaflor (Schering-Plough Animal Health, Pointe Claire, Quebec) containing 50% florfenicol was dissolved in dimethylformamide and a stock solution containing 20 mg/mL Aquaflor was added to MH agar to obtain plates with 16 and 32 μg/mL of florfenicol. Antimicrobial resistance testing with the breakpoint concentration method was performed in accordance with the Clinical and Laboratory Standards Institute (CLSI) for antimicrobial susceptibility tests of bacteria isolated from animal (15) and humans (16) and those listed by the FDA/USDA/CDC National Antimicrobial Resistance Monitoring Program — Enteric Bacteria, Final Report, 1997 (17). Resistance was declared when there was growth at the NCCLS (CLSI) defined resistance breakpoint levels, even for antimicrobials for which susceptibility was tested at ≥ 2 concentrations (Table I). The E. coli strain R1022, a bovine isolate resistant to antimicrobials including apramycin, gentamicin, tetracycline, and tobramycin, and P. aeruginosa, America Type Culture Collection (ATCC) strain ATCC27853, E. coli ATCC25922, and E. coli ATCC35218, were used for quality control purposes (15,16).
Table I.
Prevalences of resistance among fecal Escherichia coli isolates from healthy weaner and finisher pigs on 47 farrow-to-finish farms
| Number of isolates growing at different concentrations (μg/mL)a |
% Resistant
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | 0.125 | 1 | 4 | 8 | 16 | 30 | 32 | 64 | 80 | 512 | Weaners (n = 470) | Finishers (n = 470) | Combined |
| Amikacin | 0 | 0 | 0.0 | 0.0 | 0 | ||||||||
| Ampicillin | 331 | 44.68 | 25.74 | 35.21 | |||||||||
| Apramycin | 31 | 4.68 | 1.91 | 3.3 | |||||||||
| Carbadox | 95 | 16.17 | 4.04 | 10.11 | |||||||||
| Ceftiofur | 0 | 0.0 | 0.0 | 0 | |||||||||
| Ceftriaxone | 0 | 3 | 0.21 | 0.43 | 0.32 | ||||||||
| Cephalothin | 30 | 4.04 | 2.34 | 3.19 | |||||||||
| Chloramphenicol | 103 | 15.53 | 6.38 | 10.96 | |||||||||
| Ciprofloxacin | 0 | 0 | 0 | 0.0 | 0.0 | 0 | |||||||
| Cotrimoxazole | 52 | 8.51 | 2.55 | 5.53 | |||||||||
| Florfenicol | 3 | 0.64 | 0.0 | 0.32 | |||||||||
| Gentamicin | 7 | 1.06 | 0.43 | 0.74 | |||||||||
| Kanamycin | 90 | 12.34 | 6.81 | 9.57 | |||||||||
| Nalidixic acid | 4 | 0.64 | 0.21 | 0.43 | |||||||||
| Neomycin | 92 | 12.55 | 7.02 | 9.79 | |||||||||
| Nitrofurantoin | 39 | 5.74 | 2.55 | 4.15 | |||||||||
| Spectinomycin | 517 | 65.53 | 44.47 | 55 | |||||||||
| Streptomycin | 301 | 36.60 | 27.45 | 32.02 | |||||||||
| Sulfamethoxazole | 550 | 70.43 | 46.60 | 58.51 | |||||||||
| Tetracycline | 764 | 89.57 | 72.98 | 81.28 | |||||||||
| Tobramycin | 32 | 4.89 | 1.91 | 3.4 | |||||||||
Vertical lines in the table indicate breakpoint concentrations at which resistance was determined for each antimicrobial.
Statistical analysis
The data from Ontario and BC were collected, collated and analyzed using SAS version 9.1 (SAS Institute, Cary, North Carolina, USA). Fisher’s Exact Test was used to compare resistance proportions. Observed prevalence of resistance to 2 to 11 antimicrobials and to ≥ 2 classes of antimicrobials was assessed. Tabulation of observed patterns of resistance to multiple antimicrobial classes was limited to 4 in the interests of space.
Results
Descriptive statistics
The study population included 47 swine farms: 39 from Ontario and 8 from BC. Thirty-four farms (72.3%) used in-feed medication, while 13 farms (27.7%) did not (Table II). Farm size ranged from 50 to 1400 sows per farm with a mean of 393 and standard deviation of 9. Overall, 188 (94 each from weaner and grower/finish pigs) pooled fecal samples were obtained for culture. Five isolates (independent colonies) were obtained from each pooled fecal sample, giving a total of 940 isolates that were tested for susceptibility to 21 antimicrobials.
Table II.
Prevalences of resistance to at least one of 21 antimicrobials tested among fecal Escherichia coli from pigs on farms that used in-feed antimicrobial medication and those that did not
| Group (Number of isolates) | Farm medication group/Pig-age group | Number of farms | Number of isolates per category | Prevalence of resistance to at least one antimicrobial among isolates (%) |
|---|---|---|---|---|
| Antimicrobial use (940) | Use in-feed medication | 34 | 680 | 68.19a |
| No in-feed medication | 13 | 260 | 21.81 | |
| Age of pigs (940) | Weaner pigs | 47 | 470 | 46.70b |
| Finisher pigs | 47 | 470 | 43.30 |
Significantly greater (P < 0.0001; Fisher’s Exact Test) than prevalence from no in-feed medication farms.
Significantly greater (P = 0.0001; Fisher’s Exact Test) than prevalence from finisher pigs.
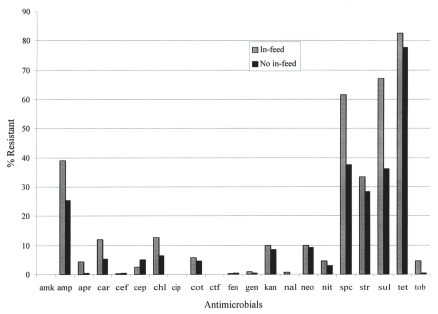
The overall prevalence of resistance to 1 or more antimicrobials among the 940 fecal E. coli isolates was 90%. Figure 1 shows the prevalence of resistance to individual antimicrobials tested by in-feed medication practices on farms of origin. On visual inspection of the data, for most of antimicrobials, resistance was more frequent in E. coli from farms that used in-feed medication compared to farms that did not. Overall, the prevalence of resistance was significantly more frequent (P < 0.01) on farms that used in-feed medication compared to farms that did not (Table II). Also, the prevalence of resistance was significantly higher in E. coli from weaner than finisher pigs (Table II; P < 0.05). Similarly, Table I shows the prevalence of resistance to individual antimicrobials. In nearly all cases, the point prevalence was higher in E. coli from weaners than finishers, and the highest prevalence of resistance was to tetracycline (89.6% in weaners; Table I). There was no resistance found to amikacin, ceftiofur, and ciprofloxacin at the CLSI breakpoint concentrations, nor was there decreased susceptibility to amikacin and ciprofloxacin at concentrations lower than the CLSI resistance breakpoints.
Figure 1.
Prevalence of resistance to antimicrobials among fecal Escherichia coli from pigs on farrow-to-finish farms that used in-feed medication compared to those from farms that did not use in-feed medication.
Resistance to ≥ 1 antimicrobials in each class of antimicrobials studied was computed and the following prevalences of resistance to classes of antimicrobials were observed among the 940 isolates; tetracyclines 81.3%, aminoglycosides/aminocyclitols 62.9%, sulfonamides 58.5%, β-lactams (penicillins and cephalosporins) 36.8%, phenicols (chloramphenicol and florfenicol) 11.1%, quinoxalines (carbadox) 10.1%, nitrofurans 4.2%, and quinolones/fluoroquinolones 0.4%.
Patterns of resistance to ≥ 2 antimicrobials are presented in Table III. One isolate was resistant to 11 antimicrobials (0.1%; the highest in this study), and 136 isolates (14.5%) were resistant to 5 antimicrobials. Isolates from farms that used in-feed medication were significantly (P < 0.01) more likely to be resistant to > 2 antimicrobials than isolates from farms that did not. Likewise, isolates from weaner pigs were significantly (P < 0.01) more likely to be resistant to > 2 antimicrobials than isolates from finisher pigs.
Table III.
Resistance to two or more antimicrobials among fecal Escherichia coli from swine on 47 farrow-to-finish farms
| Prevalence of resistance (%)a |
Prevalence of resistance from farms with different in-feed medication practices (%)b |
||||
|---|---|---|---|---|---|
| Number of antimicrobials isolates resistant to | Weaners (n = 470 isolates) | Finishers (n = 470 isolates) | Farms that used in-feed antimicrobials (n = 680 isolates) | Farms with no in-feed antimicrobials (n = 260 isolates) | All farms (n = 940 isolates) |
| 2 | 7.9 | 18.5 | 13.5 | 12.3 | 13.2 |
| 3 | 12.6 | 14.5 | 15.3 | 8.8 | 13.5 |
| 4 | 18.5 | 12.8 | 17.9 | 9.6 | 15.6 |
| 5 | 21.7 | 7.2 | 16.5 | 9.2 | 14.5 |
| 6 | 13.8 | 6 | 10.3 | 8.8 | 9.9 |
| 7 | 2.6 | 1.1 | 2.1 | 1.2 | 1.8 |
| 8 | 2.6 | 1.9 | 2.8 | 0.8 | 2.2 |
| 9 | 0.6 | 0.6 | 0.9 | 0 | 0.6 |
| 10 | 1.5 | 0 | 0.6 | 1.2 | 0.7 |
| 11 | 0.2 | 0 | 0 | 0.4 | 0.1 |
| Total | 82 | 62.6 | 79.9 | 52.3 | 72.1 |
Overall, significantly different between weaners and finishers (P < 0.01; Fisher’s Exact Test).
Overall, significantly different between farms that used in-feed antimicrobials and those that did not (P < 0.01; Fisher’s Exact Test).
The patterns of resistance to ≥ 4 classes of antimicrobials are presented in Table IV. Resistance to the aminoglycosides and aminocyclitols, β-lactams, sulfonamides, and tetracyclines were most common among the observed patterns. Resistance was observed to up to 6 classes of antimicrobials; the most common resistance pattern (237 or 25.2% of isolates) was AMINO + BETA + SULF + TET.
Table IV.
Patterns of resistance to ≥ 4 antimicrobial classes among Escherichia coli from 47 farrow-to-finish farms
| Patterns of resistance to ≥ 4 antimicrobials | Number of isolates resistant | Percent of isolates resistant (n = 940) |
|---|---|---|
| AMINOa + BETAb + SULFc + TETd + NITe + QUINf | 1 | 0.1 |
| AMINO + BETA + SULF + TET + NIT + CARg | 2 | 0.2 |
| AMINO + BETA + SULF + TET + NIT + PHENh | 1 | 0.1 |
| AMINO + BETA + SULF + TET + PHEN + CAR | 6 | 0.6 |
| AMINO + BETA + SULF + TET + PHEN | 40 | 4.3 |
| AMINO + BETA + SULF + TET + CAR | 50 | 5.3 |
| AMINO + BETA + SULF + TET + NIT | 16 | 1.7 |
| AMINO + PHEN + SULF + TET + NIT | 4 | 0.4 |
| AMINO + BETA + CAR + TET + NIT | 4 | 0.4 |
| AMINO + BETA + SULF + TET + QUIN | 4 | 0.4 |
| AMINO + BETA + SULF + TET | 237 | 25.2 |
| AMINO + PHEN + SULF + TET | 88 | 9.4 |
| AMINO + CAR + SULF + TET | 58 | 6.2 |
| CAR + BETA + SULF + TET | 53 | 5.6 |
| AMINO + BETA + SULF + CAR | 52 | 5.5 |
| BETA + SULF + TET + NIT | 19 | 2 |
| AMINO + BETA + CAR + TET | 62 | 6.6 |
| AMINO + SULF + TET + NIT | 22 | 2.3 |
AMINO — Aminoglycosides and Aminocyclitols.
BETA —β-lactams (penicillins and cephalosporins).
SULF — Sulfonamides.
TET — Tetracyclines.
NIT — Nitrofurans.
QUIN — Quinolones (nalidixic acid and ciprofloxacin).
CAR — Quinoxalines (carbadox).
PHEN — Phenicols (chloramphenicol and florfenicol).
Discussion
This study aimed to determine the prevalence and patterns of antimicrobial resistance, including multiple antimicrobial resistance, among fecal E. coli from healthy weaner and finisher pigs on farrow-to-finish operations. Also compared, using simple statistical tests and without control for confounding or clustering, was the prevalence of resistance in isolates from farms that did or did not use in-feed antimicrobials. The results of multivariable analyses of these data, examining associations with various antimicrobial use and other factors, are presented separately (18).
Study farms were selected in part based on their in-feed medication practices in order to compare resistance prevalence (Figure 1). The results showed that resistance among E. coli was significantly (P < 0.01) higher on farms that used in-feed medication compared to those that did not (Table II). This finding is consistent with other evidence that nontherapeutic use of antimicrobials in-feed selects resistant organisms in the population of use (19–21).
The prevalence of resistance was significantly higher (P < 0.01) in weaner compared to finisher pigs (Table II), which might be due to the more extensive use of antimicrobials in weaners for growth promotion, treatment, and prophylaxis than in finisher pigs (22). Furthermore, weaners are younger and at risk of enteric infections, perhaps because of waning passive immunity, mixing with pigs from other litters or farms, and colonization by resistant organisms (23). Antimicrobials tend to be used less frequently in finishers, in part because they are close to marketing for human consumption and there is a need to avoid antimicrobial residues in pork.
Resistance to > 1 antimicrobial was common in this study (72.1% of isolates tested), most frequently to 2 to 6 antimicrobials. Potential selection pressures for multiple resistance were not explored, but one such factor may be co-selection, as this is consistent with phenotypic or genotypic evidence found in other studies (2,24–27). Therefore, finding multiple antimicrobial resistance in commensal E. coli from healthy pigs is worrisome, particularly if the genes encoding this resistance are available for transfer to pathogens. Also, the prevalence of resistance to > 2 antimicrobials was more common among E. coli from weaner pigs than from finisher pigs, which is consistent with the findings of other studies (23).
Resistance to ≥ 1 antimicrobials in this study was 90%, nevertheless, it should be noted that by declaring resistance at CLSI breakpoints, some strains may have been classified as susceptible that would have been classified as resistant using an MIC method. Such high prevalences of resistance increase in magnitude the potential reservoir of antimicrobial resistant bacteria and/or resistance genes that may be transferred to humans through the food chain, as suggested by other studies (5,26). Resistance was observed to some antimicrobials that are not specifically approved for use in pigs in Canada. For example, resistance to sulfamethoxazole is suggestive of cross-resistance with other sulfonamides that are approved for use in swine (22), and while neomycin was used by some study farms and is approved for therapeutic purposes in swine in Canada, kanamycin is not, and resistance to this antimicrobial could be due to cross-resistance. While the mechanisms of resistance in these study isolates were not characterized, it has been reported that resistance to kanamycin and neomycin may be encoded by the same genes (28).
Although chloramphenicol and nitrofurantoin were withdrawn from use in pigs in 1984 and 1994, respectively, resistance to these antimicrobials persisted in fecal E. coli of pigs (Table III). The persistence of resistance to chloramphenicol might be due to the process of co-selection by other antimicrobials that are still being used in pigs (29). This occurs when genes encoding for these withdrawn antimicrobials are linked with those encoding for other antimicrobials that are still being used (30). Another pathway for persistence of antimicrobial resistance is the linkage of chromosomally located antimicrobial resistance genes that are not readily lost by bacteria (31).
Eighty-one of the E. coli isolates were resistant to chloramphenicol at 32 μg/mL and 3 isolates were resistant to florfenicol at 16 μg/mL. In a recent study, chloramphenicol resistance among commensal porcine E. coli was in > 80% of the isolates encoded by the cmlA gene, whereas in < 15% of the isolates it was encoded by the catA1 gene. Chloramphenicol resistance due to expression of the floR gene was found in < 10% of enterotoxigenic E. coli (ETEC) and slightly > 20% of non-ETEC strains isolated from diarrheic pigs, but was not found in commensal E. coli (32). Since the E. coli in the present study were commensal isolates, most of the observed chloramphenicol resistance would likely also have been the result of expression of the cmlA gene (29). In the very few cases of resistance to both chloramphenicol and florfenicol, resistance may have been encoded by the floR gene that, via a multi-drug efflux pump mechanism, encodes resistance to both chloramphenicol and florfenicol (33).
An earlier study conducted in Ontario (11) assessed the prevalence of resistance in fecal E. coli of finisher pigs to ampicillin, carbadox, gentamicin, nitrofurantoin, spectinomycin, sulfamethoxazole, and tetracycline. In the present study, the prevalence of resistance among E. coli from finishers to antimicrobials that were members of classes still approved for use in pigs in Canada during the study period (ampicillin, carbadox, gentamicin, spectinomycin, sulfamethoxazole, and tetracycline) was higher in some cases [spectinomycin (44.5 vs. 27.3), sulfamethoxazole (46.6 vs. 38)] and similar in others [ampicillin (25.7 vs. 29), carbadox (4.04 vs. 3.5), gentamicin (0.4 vs. 0.6), tetracycline (73 vs. 71)]. In contrast, the prevalence of resistance to nitrofurantoin, which was banned from use in pigs in Canada in 1994, was considerably lower in this study (2.6%) than observed in the earlier study (27%). The differences in resistance proportions shown by the 2 analogous studies provide evidence of the effects of selection pressure due to continued use of these antimicrobials in pigs over time, and decrease in resistance resulting from withdrawal of certain antimicrobials.
Because of the public health importance of ciprofloxacin, resistance was tested at 3 different concentrations, including the breakpoint concentrations recommended by CLSI and 2 other lower concentrations. All E. coli isolates were susceptible at all 3 concentrations of ciprofloxacin, a finding consistent with other studies (13,34). This may reflect the fact that no quinolones or their derivatives are approved for use in pigs in Canada.
Like most observational studies, this study had some challenges. It was very difficult to find farms that did not use any in-feed medication and not all that were identified accepted the invitation to participate. Also, purposive sampling and exclusion of farms that had < 50 sows on-site might have introduced some selection bias. Farms were sampled in order to meet the stated objectives of the study and exclusion of smaller farms was based on the assumption that such farms proportionately contributed less to the food chain and antimicrobial resistance as a whole.
In conclusion, antimicrobial resistance among fecal E. coli of pigs from parts of Ontario and British Columbia varied widely amongst the antimicrobials tested. Resistance to some of the older antimicrobials that have been used in-feed for many years was comparatively high, while resistance to some of the newer antimicrobials that are either not approved at all in pigs, or used solely for therapy, was uncommon or absent. Antimicrobial resistance to ≥ 1 antimicrobials was more prevalent in E. coli from farms that used in-feed medication compared to those that did not, and also more prevalent in E. coli from weaner pigs compared to finisher pigs. There was evidence suggestive of cross-resistance and co-selection among the antimicrobials studied.
Acknowledgments
The authors thank Drs. X. Jia, H. Nicolidakis, and P. Pentney for assisting with data collection and Mrs. Kathleen Harris and Ms. Laura Martin for the isolation and identification of the E. coli isolates. Funding for the study was provided by Health Canada and the Canadian Commonwealth Scholarship Program.
References
- 1.Jensen BB. The impact of feed additives on the microbial ecology of the gut in young pigs. J Anim Feed Sci. 1998;7:45–64. [Google Scholar]
- 2.Harada K, Asai T, Kojima A, Sameshima T, Takahashi T. Contribution of multi-antimicrobial resistance to the population of antimicrobial resistant Escherichia coli isolated from apparently healthy pigs in Japan. Microbiol Immunol. 2007;51:493–499. doi: 10.1111/j.1348-0421.2007.tb03937.x. [DOI] [PubMed] [Google Scholar]
- 3.Stine OC, Johnson JA, Keefer-Norris A, et al. Widespread distribution of tetracycline resistance genes in a confined animal feeding facility. Int J Antimicrob Agents. 2007;29:348–352. doi: 10.1016/j.ijantimicag.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Akwar TH, Poppe C, Wilson J, et al. Risk factors for antimicrobial resistance among fecal Escherichia coli from residents on forty-three swine farms. Microb Drug Resist. 2007;13:69–76. doi: 10.1089/mdr.2006.9999. [DOI] [PubMed] [Google Scholar]
- 5.Lim SK, Lee HS, Nam HM, et al. Antimicrobial resistance observed in Escherichia coli strains isolated from fecal samples of cattle and pigs in Korea during 2003–2004. Int J Food Microbiol. 2007;116:283–286. doi: 10.1016/j.ijfoodmicro.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Hunter JE, Shelley JC, Walton JR, Hart CA, Bennett M. Apramycin resistance plasmids in Escherichia coli: Possible transfer to Salmonella typhimurium in calves. Epidemiol Infect. 1992;108:271–278. doi: 10.1017/s0950268800049748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kikuvi GM, Schwarz S, Ombui JN, Mitema ES, Kehrenberg C. Streptomycin and chloramphenicol resistance genes in Escherichia coli isolates from cattle, pigs, and chicken in Kenya. Microb Drug Resist. 2007;13:62–68. doi: 10.1089/mdr.2006.9998. [DOI] [PubMed] [Google Scholar]
- 8.van den Bogaard AE, Stobberingh EE. Epidemiology of resistance to antibiotics. Links between animals and humans. Int J Antimicrob Agents. 2000;14:327–335. doi: 10.1016/s0924-8579(00)00145-x. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien TF. Emergence, spread, and environmental effect of antimicrobial resistance: How use of an antimicrobial anywhere can increase resistance to any antimicrobial anywhere else. Clin Infect Dis. 2002;34:S78–84. doi: 10.1086/340244. [DOI] [PubMed] [Google Scholar]
- 10.Linton AH. Flow of resistance genes in the environment and from animals to man. J Antimicrob Chemother. 1986;18:189–197. doi: 10.1093/jac/18.supplement_c.189. [DOI] [PubMed] [Google Scholar]
- 11.Dunlop RH, McEwen SA, Meek AH, Black WD, Friendship RM, Clarke RC. Prevalences of resistance to seven antimicrobials among fecal Escherichia coli of swine on thirty-four farrow-to-finish farms in Ontario, Canada. Prev Vet Med. 1998;34:265–282. doi: 10.1016/s0167-5877(97)00094-9. [DOI] [PubMed] [Google Scholar]
- 12.Stege H, Jensen TK, Moller K, Baekbo P, Jorsal SE. Prevalence of intestinal pathogens in Danish finishing pig herds. Prev Vet Med. 2000;46:279–292. doi: 10.1016/s0167-5877(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 13.van den Bogaard AE, London N, Stobberingh EE. Antimicrobial resistance in pig faecal samples from the Netherlands (five abattoirs) and Sweden. J Antimicrob Chemother. 2000;45:663–671. doi: 10.1093/jac/45.5.663. [DOI] [PubMed] [Google Scholar]
- 14.Brown SD, Washington JA. Evaluation of the Repliscan system for identification of Enterobacteriaceae. J Clin Microbiol. 1978;8:695–699. doi: 10.1128/jcm.8.6.695-699.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NCCLS. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standards. National Committee for Clinical Laboratory Standards Document 1999; M31-A. NCCLS, Villanova, Pennsylvania.
- 16.NCCLS. Performance standards for antimicrobial susceptibility testing; eighth informational supplement. National Committee for Clinical Laboratory Standards Document 1998; M100-S8. NCCLS, Villanova, Pennsylvania.
- 17.FDA/USDA/CDC. Veterinary isolates. National Antimicrobial Resistance Monitoring Program Final Report; 1997. Enteric Bacteria. [Google Scholar]
- 18.Akwar HT, Poppe C, Wilson J, et al. Associations of antimicrobial uses and management factors with antimicrobial resistance of fecal Escherichia coli from pigs on 47 farrow-to-finish farms in Ontario and British Columbia. Can J Vet Res. 2008;72:202–210. [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. International Review Panel Evaluation of the Termination of the Use of Antimicrobial Growth Promoters in Denmark. World Health Organization; Geneva, Switzerland: 2003. [Google Scholar]
- 20.Emborg HD, Andersen JS, Seyfarth AM, Wegener HC. Relations between the consumption of antimicrobial growth promoters and the occurrence of resistance among Enterococcus faecium isolated from broilers. Epidemiol Infect. 2004;132:95–105. doi: 10.1017/s0950268803001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berge AC, Moore DA, Sischo WM. Field trial evaluating the influence of prophylactic and therapeutic antimicrobial administration on antimicrobial resistance of fecal Escherichia coli in dairy calves. Appl Environ Microbiol. 2006;72:3872–3878. doi: 10.1128/AEM.02239-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunlop RH, McEwen SA, Meek AH, Friendship RA, Clarke RC, Black WD. Antimicrobial drug use and related management practices among Ontario swine producers. Can Vet J. 1998;39:87–96. [PMC free article] [PubMed] [Google Scholar]
- 23.Langlois BE, Dawson KA, Leak I, Aaron DK. Effect of age and housing location on antibiotic resistance of fecal coliforms from pigs in a non-antibiotic-exposed herd. Appl Environ Microbiol. 1988;54:1341–1344. doi: 10.1128/aem.54.6.1341-1344.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nijsten R, London N, van den Bogaard A, Stobberingh E. Antibiotic resistance among Escherichia coli isolated from faecal samples of pig farmers and pigs. J Antimicrob Chemother. 1996;37:1131–1140. doi: 10.1093/jac/37.6.1131. [DOI] [PubMed] [Google Scholar]
- 25.Langlois BE, Dawson KA, Leak I, Aaron DK. Antimicrobial resistance of fecal coliforms from pigs in a herd not exposed to antimicrobial agents for 126 months. Vet Microbiol. 1988;18:147–153. doi: 10.1016/0378-1135(88)90060-0. [DOI] [PubMed] [Google Scholar]
- 26.Phongpaichit S, Liamthong S, Mathew AG, Chethanond U. Prevalence of class 1 integrons in commensal Escherichia coli from pigs and pig farmers in Thailand. J Food Prot. 2007;70:292–299. doi: 10.4315/0362-028x-70.2.292. [DOI] [PubMed] [Google Scholar]
- 27.Hsu SC, Chiu TH, Pang JC, Hsuan-Yuan CH, Chang GN, Tsen HY. Characterisation of antimicrobial resistance patterns and class 1 integrons among Escherichia coli and Salmonella enterica serovar Choleraesuis strains isolated from humans and swine in Taiwan. Int J Antimicrob Agents. 2006;27:383–391. doi: 10.1016/j.ijantimicag.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Tenover FC, Elvrum PM. Detection of two different kanamycin resistance genes in naturally occurring isolates of Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1988;32:1170–1173. doi: 10.1128/aac.32.8.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harada K, Asai T, Kojima A, Ishihara K, Takahashi T. Role of coresistance in the development of resistance to chloramphenicol in Escherichia coli isolated from sick cattle and pigs. Am J Vet Res. 2006;67:230–235. doi: 10.2460/ajvr.67.2.230. [DOI] [PubMed] [Google Scholar]
- 30.Summers AO. Generally overlooked fundamentals of bacterial genetics and ecology. Clin Infect Dis. 2002;34:S85–92. doi: 10.1086/340245. [DOI] [PubMed] [Google Scholar]
- 31.Sandvang D, Aarestrup FM. Characterization of aminoglycoside resistance genes and class 1 integrons in porcine and bovine gentamicin-resistant Escherichia coli. Microb Drug Resist. 2000;6:19–27. doi: 10.1089/mdr.2000.6.19. [DOI] [PubMed] [Google Scholar]
- 32.Travis RM, Gyles CL, Reid-Smith R, et al. Chloramphenicol and kanamycin resistance among porcine Escherichia coli in Ontario. J Antimicrob Chemother. 2006;58:173–177. doi: 10.1093/jac/dkl207. [DOI] [PubMed] [Google Scholar]
- 33.Singer RS, Patterson SK, Meier AE, Gibson JK, Lee HL, Maddox CW. Relationship between phenotypic and genotypic florfenicol resistance in Escherichia coli. Antimicrob Agents Chemother. 2004;48:4047–4049. doi: 10.1128/AAC.48.10.4047-4049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Threlfall EJ, Graham A, Cheasty T, Ward LR, Rowe B. Resistance to ciprofloxacin in pathogenic Enterobacteriaceae in England and Wales in 1996. Journal of Clinical Pathology. 1997;50:1027–1028. doi: 10.1136/jcp.50.12.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]