Abstract
The objective of this study was to describe antimicrobial resistance patterns in fecal generic Escherichia coli obtained from calves in western Canadian cow-calf herds. Susceptibility testing was completed on 1677 isolates obtained from 480 beef calves in 91 herds in the spring, and from 1187 isolates obtained from 394 calves in 45 herds in the fall of 2002. Resistance was rare to antimicrobials classified as being of very high importance to human health. Isolates were most commonly resistant to tetracycline, sulphamethoxazole, and streptomycin. Resistance to at least one antimicrobial was identified in 48.8% of the isolates collected in the spring, and 7.0% of those collected in the fall. Spring or fall calf resistance status was not associated with calf gender, breed, age of the dam, or if the calf was treated before sample collection. Calves ≤ 3 days of age were less likely to be resistance positive than calves > 10 days of age.
Résumé
La présente étude visait à décrire les patrons de résistance aux antimicrobiens d’isolats d’Escherichia coli génériques obtenus de veaux provenant de troupeaux vache-veau dans l’ouest canadien. Les épreuves de sensibilité ont été effectuées sur 1677 isolats obtenus de 480 veaux dans 91 troupeaux au printemps, et sur 1187 isolats provenant de 394 veaux dans 45 troupeaux à l’automne 2002. Une résistance envers des agents antimicrobiens classifiés comme très important à la santé humaine était rare. Les isolats étaient le plus fréquemment résistants à la tétracycline, au sulfaméthoxazole et à la streptomycine. De la résistance à au moins un antimicrobien a été identifiée chez 48,8 % des isolats obtenus au printemps et 7,0 % de ceux obtenus à l’automne. La résistance observée au printemps ou à l’automne n’était pas associée avec le sexe ou la race du veau, l’âge de la mère, ou si le veau avait été traité avant le prélèvement de l’échantillon. Les veaux âgés de 3 jours ou moins étaient moins susceptibles d’être porteur d’isolats résistants que les veaux âgés de plus de 10 jours.
(Traduit par Docteur Serge Messier)
Introduction
Although there is a growing amount of literature on antimicrobial resistance (AMR) there is no information currently available on AMR in cow-calf herds in western Canada. The cow-calf industry is a key component of the agricultural economy in all parts of Canada, but particularly in Saskatchewan and Alberta. These provinces account for more than 65% of the beef-cow, breeding heifer, and calf populations in Canada (1). A better understanding of AMR in this industry is essential to improve knowledge of the epidemiology of resistance in the Canadian beef industry, and to determine the need for future monitoring. This project was one component in a larger initiative examining the prevalence of and risk factors for AMR in cow-calf herds. The objective of this study was to describe AMR prevalence and patterns in Escherichia coli isolated from calves from western Canadian cow-calf herds in the spring and fall. Part II of this study describes AMR in cows sampled in the spring of 2002 and in cow-calf pairs sampled in the spring of 2003 (2).
Materials and methods
Herd and animal selection
Herds concurrently enrolled in a survey of cattle health and productivity (https://www.wissa.info) were used in this study. Criteria for herd inclusion were based on the objectives and economic constraints of the primary study. No information on antimicrobial use (AMU) or AMR was known at the time of herd enrollment. Private veterinary clinics across Alberta, Saskatchewan, and northeastern British Columbia were asked to participate. Within each practice, herds were enrolled based on the selection criteria that considered herd size, completeness of animal identification, existing calving records, presence of animal handling facilities, and a relationship with a local veterinary clinic. Herds of less than 50 animals were not included.
Targeted sampling was initiated in 2002 to investigate the point prevalence of AMR at different stages of production. The 6 veterinarians employed to collect data for the productivity survey (3) also collected data for the AMR study from a convenience subset of herds. Fecal samples were collected during a single herd visit between January and May 2002 (N = 91 herds; 44.8% of the 203 productivity survey herds) from calves readily accessible in the calving and nursery area. Calf samples came from the 1st individually identified animals successfully restrained in each herd. A 2nd group of calves were sampled during a single herd visit between September and December 2002 (N = 45 herds; 22.2% of the productivity survey herds). The fall samples were collected from randomly selected calves during routine processing procedures such as vaccination, castration, and sorting for sale. Due to logistical constraints, only 20 of the same herds and none of the same calves were sampled in both time periods. For the spring sampling, logistical constraints, including the number of accessible animals, determined the number of calves sampled per herd; for the fall sampling, 10 calves were sampled per farm. All fecal samples were obtained either per rectum or from the ground immediately after defecation. A separate disposable glove and container were used for each sample.
Laboratory methods
Escherichia coli culture
Fecal samples were packed on ice and sent by overnight courier to a commercial diagnostic laboratory (Prairie Diagnostic Services, Saskatoon, Saskatchewan). The samples were cultured onto MacConkey agar plates at 37°C for 18 h. At least 3 individual lactose fermenting colonies identified as E. coli using standard biochemical tests [including indole, triple sugar iron (TSI) slant, citrate, and urea] were saved from each sample. If both dry and mucoid colonies were detected within a sample, then 3 isolates from each colony type were tested. Individual E. coli isolates were stored in 50% glycerol and Luria-Bertani (LB) broth at −80°C.
Susceptibility testing methodology
The E. coli isolates were tested for susceptibility (Alberta Agriculture and Food) using broth microdilution (Sensititre; TREK Diagnostic Systems, Cleveland, Ohio, USA) and the standard 2002 National Antimicrobial Resistance Monitoring System (NARMS) CMV7CNCD gram-negative panel (4). Minimum inhibitory concentrations (MICs) were assessed for 16 antimicrobial agents (Tables I and II). Breakpoints for susceptibility were used as defined by the Clinical Laboratory Standards Institute (5,6). All isolates in the intermediate susceptibility range were classified as susceptible. Amikacin results > 4 μg/mL were not interpretable because the breakpoint is 4 dilutions beyond the range of the panel. The breakpoint used for streptomycin was 64 μg/mL (4).
Table I.
Minimum inhibitory concentrations (MICs) for fecal Escherichia coli isolates collected from calves in the spring of 2002 arranged by the Veterinary Drug Directorate, Health Canada, classification of drugs and presented as a percentage of the total number of isolates (n = 1677)
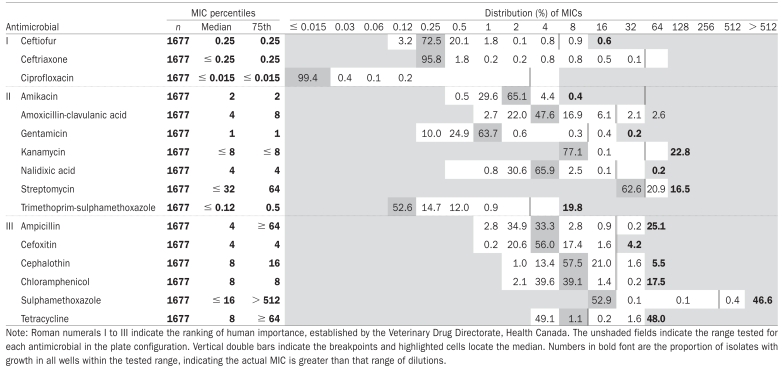 |
Table II.
Minimum inhibitory concentrations for fecal Escherichia coli isolates collected from calves in the fall of 2002 arranged by the Veterinary Drug Directorate, Health Canada, classification of drugs and presented as a percentage of the total number of isolates (n = 1186)
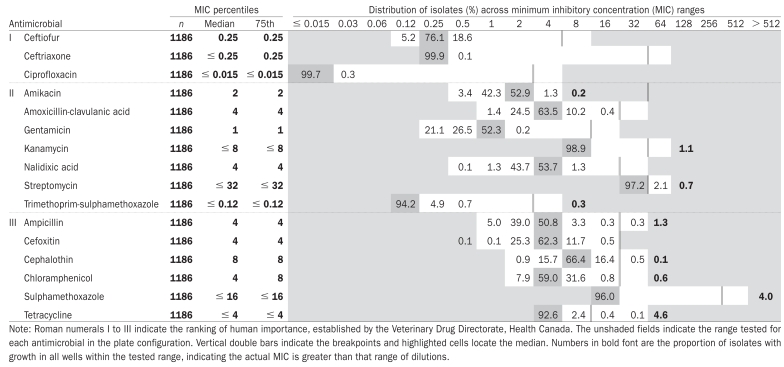 |
The significance of the results for each antimicrobial were classified according to the Veterinary Drug Directorate, Health Canada categorization as to the importance in human medicine (Tables I and II) (7). To facilitate comparisons with the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS), the same nomenclature for resistance patterns was used (7). Multiple resistance was defined as resistance to ≥ 2 antimicrobials.
Statistical analysis
Descriptive analyses were completed using SPSS (SPSS 11.0 for Windows; SPSS, Chicago, Illinois, USA). If any 1 isolate from a calf was resistant to a particular antimicrobial, that calf was considered positive for resistance to that antimicrobial. Additionally, if any calf from a herd was classified as positive, the herd was considered positive for resistance to that antimicrobial.
Population-average prevalence estimates and 95% confidence intervals (CI) for AMR in the spring and fall samples were determined using the intercept from null models. Models were developed using generalized estimating equations (GEE) to account for clustering within the herd [SAS v.8.2 for Windows (PROC GENMOD); SAS Institute, Cary, North Carolina, USA]. Model specifications included a binomial distribution, logit link function, repeated statement with subject equal to herd, and an exchangeable correlation structure. Where the proportion of isolates with resistance was equal to zero, Fleiss quadratic 95% CI formulas for a single proportion were calculated (8).
Using the same model specifications described previously, and in separate analyses for the calves sampled in the spring and those sampled in the fall, unconditional associations between resistance to any antimicrobial and calf age (categorized by quartiles), calf gender, calf breed (British, continental, or crossbred), health status, and whether the calf was ever treated prior to sampling were examined. Health status was determined by the veterinarian observing the calf during sample collection. The number of days since the last treatment was determined for calves where a treatment event had been recorded before sample collection. In addition, the age of the dam (2 y, 3 y, 4 to 10 y, and > 10 y) was considered in the spring calf analysis.
A model was developed to estimate the difference in the prevalence of AMR between the spring and fall calves where both sets of samples were available for the same herd (N = 20). The model used GEE, with the specifications outlined previously, to determine the effect of production phase (a fixed factor) on the total number of calves with any type of AMR (numerator) as a proportion of the total number of calves sampled (denominator).
Also, for the 20 herds sampled in both the spring and the fall, the proportion of isolates or calves with resistance to any antimicrobial in the spring was investigated to see if it was a predictor of the proportion [count of AMR positive isolates (or calves)/number of isolates (or calves) collected] with resistance to any antimicrobial in the fall using GEE and the aforementioned model specifications.
The extent of clustering of isolate resistance within individual calves and herds was described for the samples collected in the spring. The variance components for a 3-level model were estimated using penalized quasi-likelihood estimates (2nd order PQL) (MLwiN version 2.02; Centre for Multilevel Modeling, Institute of Education, London, UK), a binomial distribution, and logit link function. Data from this null model were used to estimate the proportion of variation explained at the isolate level (n = 1677) [π2/3/(σ2h + σ2c + π2/3)], calf level [σ2c/(σ2h + σ2c + π2/3)], and herd level [σ2h/(σ2h + σ2c + π2/3)] (9) where σ2h and σ2c are the estimates of total variance at the herd and individual animal level, respectively. The low prevalence of AMR in the fall samples allowed only a 2-level model; if a 3rd level for calf was included, the model would not converge. The proportion of variation between samples explained by the isolate (n = 1187) [π2/3/(σ2h + π2/3)] and the herd [σ2h/(σ2h + π2/3)] was reported (9).
Results
Spring calves
From the 480 calves sampled (212 female and 268 male), 1677 isolates were recovered for susceptibility testing. Healthy calves made up 92.5% (444/480) of the sample population. Calf age ranged from 0 to 151 days [median, 6; inter-quartile range (IQR), 4 to 10]. Median herd (N = 91) size was 177 (range, 89 to 411) breeding females. The median number of samples collected per herd was 5 [range, 1 to 11; interquartile range (IQR), 4 to 6]; 8.3% (40/480) of calves were reported as being treated prior to sampling with either oral or injectable antimicrobials or both. For treated calves, the number of days between last treatment and sample collection ranged from 0 to 117 d (median, 5; IQR, 1 to 10); 5% of all calves had been treated within a week of sample collection. Calf age at last treatment prior to sample collection ranged from 0 to 56 d (median, 2; IQR, 0 to 7).
Resistance to at least 1 antimicrobial was identified in 48.8% of spring calf isolates, 62.2% of calves, and in 91% of herds (Tables III to V). The resistance most commonly observed was to tetracycline and sulphamethoxazole. No resistance was identified to ceftriaxone or ciprofloxacin; resistance to ceftiofur and gentamicin was rare.
Table III.
Prevalence (%) of AMR for Escherichia coli isolates cultured from calves in the spring (n = 1677) and in the fall (n = 1186) of 2002 adjusted for clustering by herd
| Isolate prevalence for calves in the spring
|
Isolate prevalence for calves in the fall
|
|||||
|---|---|---|---|---|---|---|
| Antimicrobial | Prevalence (%) | Lower CI | Upper CI | Prevalence (%) | Lower CI | Upper CI |
| Amikacin | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.4 |
| Amoxicillin-clavulanic acid | 4.5 | 2.6 | 7.8 | 0.0 | 0.0 | 0.4 |
| Ampicillin | 22.7 | 18.0 | 28.2 | 1.6 | 0.7 | 3.3 |
| Cefoxtin | 4.1 | 2.3 | 7.2 | 0.0 | 0.0 | 0.4 |
| Ceftiofur | 1.7 | 0.7 | 3.8 | 0.0 | 0.0 | 0.4 |
| Ceftriaxone | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.4 |
| Cephalothin | 6.7 | 4.3 | 10.1 | 0.6 | 0.3 | 1.4 |
| Chloramphenicol | 14.8 | 10.8 | 19.9 | 0.6 | 0.2 | 1.8 |
| Ciprofloxacin | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.4 |
| Gentamicin | 0.5 | 0.2 | 1.3 | 0.0 | 0.0 | 0.4 |
| Kanamycin | 20.7 | 16.1 | 26.2 | 1.1 | 0.4 | 2.8 |
| Nalidixic acid | 0.2 | 0.02 | 1.2 | 0.0 | 0.0 | 0.4 |
| Streptomycin | 34.8 | 29.4 | 40.7 | 2.8 | 1.6 | 4.9 |
| Sulphamethoxazole | 42.8 | 36.9 | 48.9 | 4.0 | 2.7 | 6.1 |
| Tetracylcine | 46.4 | 40.2 | 52.7 | 5.0 | 3.4 | 7.5 |
| Trimethoprim-sulphamethoxazole | 16.3 | 12.2 | 21.4 | 0.3 | 0.1 | 1.0 |
| AMR (≥ 1 antimicrobial) | 48.8 | 42.6 | 55.1 | 7.0 | 4.8 | 9.9 |
| Multi AMR (≥ 2 antimicrobials) | 46.2 | 40.1 | 52.5 | 5.5 | 3.7 | 8.2 |
| A3Ca | 1.6 | 0.7 | 3.8 | 0.0 | 0.0 | 0.4 |
| ACSSuTb | 2.6 | 1.3 | 5.0 | 0.0 | 0.0 | 0.4 |
| AKSSuTc | 6.2 | 4.0 | 9.5 | 0.4 | 0.1 | 2.0 |
| ACKSSuTd | 5.0 | 2.9 | 8.5 | 0.0 | 0.0 | 0.4 |
CI — confidence interval.
A3C — ampicillin, cefoxitin, ceftiofur, cephalothin.
ACSSuT — ampicillin, chloramphenicol, streptomycin, sulphamethoxazole, and tetracycline.
AKSSuT — ampicillin, kanamycin, streptomycin, sulphamethoxazole, and tetracycline.
ACKSSuT — ampicillin, chloramphenicol, kanamycin, streptomycin, sulphamethoxazole, and tetracyclin.
The maximum number of antimicrobials to which an isolate demonstrated resistance was 10. Resistance to at least 6 antimicrobials was observed in 9.4% (157/1677) of isolates in 10.2% (49/480) of calves and 14% (13/91) of herds. The most common pattern was ampicillin, kanamycin-streptomycin-sulphamethoxazole-tetracycline-trimethoprim/sulphamethoxazole. Of the isolates with resistance to at least 6 antimicrobials, 75.8% (119/157) had a pattern including streptomycin, sulphamethoxazole, tetracycline, and trimethoprim/ sulphamethoxazole. The median minimum inhibitory concentration (MIC) for all antimicrobials were several dilutions away from the breakpoint, except for streptomycin and tetracycline. No resistance was detected to the Category I antimicrobials except for 1 isolate that had resistance to ceftiofur (Table I).
Calf AMR status was not significantly associated with calf gender (P = 0.54), breed (P = 0.40), dam age (P = 0.72), or calf treatment prior to sample collection (P = 0.65). Calves 0 to 3 d of age were 0.55 (95% CI, 0.30 to 1.0; P = 0.04) times as likely to be positive for any resistance as calves ≥ 10 d of age. The AMR status of calves 4 to 5 and 6 to 9 days of age were not significantly different from calves ≥ 10 d of age (P > 0.49). For the calves that had been treated, the number of days from last treatment was not associated with the presence of resistance (P = 0.92). Calves classified as unhealthy at the time of sample collection were 4.3 (95% CI, 1.16 to 2.04; P < 0.0001) times more likely to be positive for any resistance than calves that were healthy.
In the null model, the proportion of variance in AMR accounted for at the isolate, calf, and herd levels was 65.1%, 14.6%, and 20.3%, respectively.
Fall calves
Samples were collected from 394 healthy calves (242 female, 152 male) on 45 farms. Calf age ranged from 118 to 323 d (median, 219 d); 79% of the samples were collected from calves less than 250 d of age. The median number of samples collected per herd was 10 (range, 1 to 10; IQR, 10 to 10), and the median herd size was 125 (range, 52 to 265) breeding females. Before sample collection, 10.6% (39/367) of the calves had been treated with an oral and/or injectable antimicrobial. Calf age at last treatment ranged from 0 to 46 d of age (median, 14 d; IQR, 10 to 20). For treated calves, the median number of days between sample collection and the calf’s last treatment was 186 d (range, 140 to 284 d; IQR, 178 to 208).
Antimicrobial resistance was relatively less common in the 1186 isolates recovered from the fall samples than in those from the spring samples; 7.0% were resistant to at least 1 antimicrobial (Table III). At least 1 resistant isolate was identified in 12.7% of calves and 56% of herds (Tables IV and V). Most of the resistance detected was to tetracycline and sulphamethoxazole. No resistance was identified for 8/16 antimicrobials including: ceftiofur, ceftriaxone, cefoxitin, ciprofloxacin, and gentamicin.
Table IV.
Prevalence (%) of AMR in calves sampled in the spring (n = 480) and in the fall (n = 394) of 2002 accounting for clustering of AMR within herd
| Individual animal prevalence for calves in the spring
|
Individual animal prevalence for calves in the fall
|
|||||
|---|---|---|---|---|---|---|
| Antimicrobial | Prevalence (%) | Lower CI | Upper CI | Prevalence (%) | Lower CI | Upper CI |
| Amikacin | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 1.2 |
| Amoxicillin-clavulanic acid | 7.1 | 4.4 | 11.2 | 0.0 | 0.0 | 0.0 |
| Ampicillin | 31.1 | 25.2 | 37.7 | 3.0 | 1.4 | 6.4 |
| Cefoxtin | 6.4 | 4.1 | 10.0 | 0.0 | 0.0 | 1.2 |
| Ceftiofur | 2.9 | 1.2 | 6.6 | 0.0 | 0.0 | 1.2 |
| Ceftriaxone | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 1.2 |
| Cephalothin | 11.5 | 7.8 | 16.6 | 1.5 | 0.7 | 3.2 |
| Chloramphenicol | 22.3 | 16.6 | 29.2 | 0.8 | 0.3 | 2.3 |
| Ciprofloxacin | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 1.2 |
| Gentamicin | 1.0 | 0.4 | 2.4 | 0.0 | 0.0 | 1.2 |
| Kanamycin | 28.9 | 22.9 | 35.8 | 2.2 | 0.9 | 5.4 |
| Nalidixic acid | 0.2 | 0.03 | 1.5 | 0.0 | 0.0 | 1.2 |
| Streptomycin | 49.1 | 42.3 | 56.0 | 5.3 | 3.0 | 9.1 |
| Sulphamethoxazole | 56.3 | 49.6 | 62.7 | 7.3 | 4.8 | 11.0 |
| Tetracylcine | 60.0 | 53.3 | 66.4 | 9.9 | 6.2 | 15.5 |
| Trimethoprim-sulphamethoxazole | 24.3 | 18.7 | 31.0 | 0.5 | 0.1 | 1.9 |
| AMR (≥ 1 antimicrobial) | 62.2 | 55.4 | 68.5 | 12.7 | 8.5 | 18.4 |
| Multi AMR (≥ 2 antimicrobials) | 59.3 | 52.6 | 65.6 | 9.9 | 6.4 | 15.2 |
| A3Ca | 2.7 | 1.1 | 6.6 | 0.0 | 0.0 | 1.2 |
| ACSSuTb | 4.9 | 2.6 | 9.1 | 0.0 | 0.0 | 1.2 |
| AKSSuTc | 8.7 | 5.2 | 14.1 | 0.8 | 0.2 | 3.1 |
| ACKSSuTd | 10.0 | 6.7 | 14.6 | 0.0 | 0.0 | 1.2 |
CI — confidence interval.
A3C — ampicillin, cefoxitin, ceftiofur, cephalothin.
ACSSuT — ampicillin, chloramphenicol, streptomycin, sulphamethoxazole, and tetracycline.
AKSSuT — ampicillin, kanamycin, streptomycin, sulphamethoxazole, and tetracycline.
ACKSSuT — ampicillin, chloramphenicol, kanamycin, streptomycin, sulphamethoxazole, and tetracycline.
Table V.
Herd prevalence (%) of AMR as determined by calves sampled in the spring (N = 91 herds) and in the fall (N = 45 herds) of 2002
| Herd prevalence for calves in the spring
|
Herd prevalence for calves in the fall
|
|||||
|---|---|---|---|---|---|---|
| Antimicrobial | Prevalence (%) | Lower CI | Upper CI | Prevalence (%) | Lower CI | Upper CI |
| Amikacin | 0.0 | 0.0 | 5.0 | 0.0 | 0.0 | 9.8 |
| Amoxicillin-clavulanic acid | 22.0 | 14.6 | 31.6 | 0.0 | 0.0 | 9.8 |
| Ampicillin | 62.6 | 52.3 | 71.9 | 17.8 | 9.2 | 31.7 |
| Cefoxtin | 1.1 | 0.2 | 7.4 | 0.0 | 0.0 | 9.8 |
| Ceftiofur | 8.8 | 4.5 | 16.6 | 0.0 | 0.0 | 9.8 |
| Ceftriaxone | 0.0 | 0.0 | 5.0 | 0.0 | 0.0 | 9.8 |
| Cephalothin | 31.9 | 23.1 | 42.1 | 13.3 | 6.1 | 26.7 |
| Chloramphenicol | 41.8 | 32.1 | 52.1 | 6.7 | 2.2 | 18.7 |
| Ciprofloxacin | 0.0 | 0.0 | 5.0 | 0.0 | 0.0 | 9.8 |
| Gentamicin | 5.5 | 2.3 | 12.5 | 0.0 | 0.0 | 9.8 |
| Kanamycin | 57.1 | 46.8 | 66.9 | 11.1 | 4.7 | 24.1 |
| Nalidixic acid | 1.1 | 0.2 | 7.4 | 0.0 | 0.0 | 9.8 |
| Streptomycin | 80.2 | 70.8 | 87.2 | 26.7 | 15.8 | 41.3 |
| Sulphamethoxazole | 87.9 | 79.5 | 93.2 | 40.0 | 26.9 | 54.8 |
| Tetracylcine | 90.1 | 82.1 | 94.8 | 44.5 | 30.8 | 59.0 |
| Trimethoprim-sulphamethoxazole | 48.4 | 38.3 | 58.6 | 4.4 | 1.1 | 16.1 |
| AMR (≥ 1 antimicrobial) | 91.2 | 83.4 | 95.5 | 55.6 | 41.0 | 69.2 |
| Multi AMR (≥ 2 antimicrobials) | 90.1 | 82.1 | 94.8 | 46.7 | 32.8 | 61.1 |
| A3Ca | 7.7 | 3.7 | 15.3 | 0.0 | 0.0 | 9.8 |
| ACSSuTb | 13.2 | 7.6 | 21.8 | 0.0 | 0.0 | 9.8 |
| AKSSuTc | 26.4 | 18.4 | 36.4 | 4.4 | 1.1 | 16.1 |
| ACKSSuTd | 20.9 | 13.7 | 30.4 | 0.0 | 0.0 | 9.8 |
CI — confidence interval.
A3C — ampicillin, cefoxitin, ceftiofur, cephalothin.
ACSSuT — ampicillin, chloramphenicol, streptomycin, sulphamethoxazole, and tetracycline.
AKSSuT — ampicillin, kanamycin, streptomycin, sulphamethoxazole, and tetracycline.
ACKSSuT — ampicillin, chloramphenicol, kanamycin, streptomycin, sulphamethoxazole, and tetracycline.
The maximum number of antimicrobials to which an isolate demonstrated resistance was 5; 0.4% of isolates and 0.8% of calves demonstrated resistance to 5 antimicrobials. The most common multiresistance pattern was streptomycin-sulphamethoxazole-tetracycline.
The median MICs for all antimicrobials were several dilutions below the breakpoint with the exception of streptomycin, which was immediately below the breakpoint, and tetracycline and cephalothin, 2 dilutions below the breakpoint (Table II). No resistance was detected to the Category I antimicrobials.
Fall calf AMR status was not associated with calf age (P = 0.75), gender (P = 0.85), breed (P = 0.38), and treatment prior to sample collection (P = 0.13). For the calves previously treated, the number of days since last treatment (P = 0.74) was not associated with resistance. Based on a 2-level model, 84.9% of the total variation in AMR was accounted for between isolates within herds and 15.1% by variation between herds.
Comparison of AMR in spring and fall samples
Beef calves sampled in the spring were 9.6 (95% CI, 4.5 to 20.7) times more likely to have at least 1 resistant isolate than those sampled in the fall from the same herds (N = 20). In these 20 herds, neither the proportion of isolates or the proportion of calves positive for resistance in the spring were statistically significant predictors of the proportion of isolates (P = 0.82) or calves positive (P = 0.37) for resistance in the fall.
Discussion
Information on the extent and severity of AMR in the cow-calf industry is important, as these are the most common livestock operations in western Canada. Veterinary supervised herd health programs, however, are still relatively uncommon compared to those of other commodities. On-farm data from this study describing AMR in cow-calf herds show that resistance was rare to antimicrobials that are classified as highly important in human medicine. The E. coli isolates from both the spring and fall samples were most commonly resistant to tetracycline, sulphamethoxazole, and streptomycin. These findings are consistent with other reports of AMR in E. coli isolates collected from a variety of different animal species (10,11). The other key finding of this study was that young calves sampled in the spring had a higher prevalence of AMR than older calves sampled in the fall.
Resistance to drugs that were classified as being of very high importance to human medicine was detected in less than 1% of the isolates; for most drugs that are of interest to human medicine, the median MICs were well below the breakpoint for resistance. Median MICs several dilutions below the breakpoint indicate that the E. coli populations in these calves were highly susceptible to those drugs. Based on these findings, it appears that on-farm exposure to beef calves presents a low risk to human health. Hoyle et al (12) reported much higher levels of ampicillin (64%) and nalidixic acid (24%) resistance in calves on a Scottish beef farm. The discrepancy in prevalence may be the result of a variation in selection pressure due to different management systems.
Individual calf attributes such as age of the dam, calf gender, breed, and whether the calf had ever been treated prior to sampling were not associated with the occurrence of resistance in the beef calves. However, resistance was less common in calves less than 3 d of age than in calves of at least 10 d of age. A similar result was reported by Berge et al (13) who described a higher level of AMR in 2-wk-old dairy calves compared with day-old calves. Calves classified as unhealthy at the time of sample collection were also more likely to be AMR positive than healthy calves. Since AMR did not seem to be affected by treatment, this may be due to changes in the physiology of sick calves and subsequent changes in the gastrointestinal flora.
There was no association between the number of days from last treatment and the presence of AMR in treated calves. Berge et al (13) found that the effect of individual animal treatment was transitory, but was associated with AMR if the sample was collected less than 7 d post-treatment. In the present study, only 5% of all calves examined had been treated within 7 d of the spring sample collection, and none had been treated within 7 d of the fall sample collection. Therefore, the power to examine this risk factor was limited. The difference in findings between the two studies could also be the result of differences in selection pressure, host specific differences between beef and dairy calves, or differences in study design. For example, Berge et al (13) examined dairy calves longitudinally and the current project used a single sample collection for each animal.
While individual animal treatment history was not a significant predictor of calf AMR status, exposure to resistant organisms from the herd and the potential importance of exposure to antimicrobial use (AMU) in the herd cannot be ruled out by this analysis. The multi-level analysis suggested that most of the unexplained variation in AMR occurrence was at the isolate level. However, because interventions cannot be applied to the isolate, interventions to reduce AMR would be directed at the calf or herd level. No specific individual animal risk factors were identified, suggesting the need to look further at the herd environment. Any future studies of beef calves in the spring should consider a larger number of calf samples per herd to provide more reliable estimates of herd level variation and a better opportunity to assess herd level risk factors. Additional analyses of the herd level data showed that both the AMR status of the cow herd (2) and herd level AMU are important predictors of resistance in calves (14).
Spring beef calves were almost 10 times more likely to have resistant E. coli than fall calves. The reason for this difference is unknown; there was no association between individual calf treatment history and the occurrence of resistance. Other factors, not addressed directly in this analysis, might explain this finding, including increased intensity of herd AMU in the spring calving season compared to the summer pasture season, increased crowding and opportunity for AMR transmission in the spring calving season compared to the summer pasture season, and the intensity of shedding of resistant E. coli by the dam and the cow herd overall.
Age-related differences in calf physiology between the first few weeks of life and weaning also might explain the difference in AMR prevalence. Hoyle et al (12) demonstrated that beef calves preferentially lost resistant relative to susceptible bacteria as they aged. The presence of resistant E. coli in the absence of treatment and selection pressure could be due to fitness traits that make these bacteria better able to compete in the calf gut compared to susceptible strains. These traits could include non-scavenging mechanisms (siderophores), increased adhesion, and mechanisms that enhance colonization, reproduction, and spread (15–18).
This study was not designed to specifically examine the associations between calf age, season, and the difference in AMR between the spring and fall samples. To assess this association, individual calves could be followed longitudinally from birth through to weaning. Any changes in AMR prevalence with age could potentially be differentiated from the influence of herd level AMU and other management practices. Also, the study design precluded an assessment of the role of season in the observed variation.
Herd prevalence of AMR in spring calves was not predictive of AMR prevalence in the fall calves. This suggests that AMR profiles are not static, and may be affected by a number of factors potentially including calf physiology and environment. The potentially transitory nature of resistance has also been observed in feedlot cattle; in 1 study, feedlot resistance prevalence shifted towards a uniform population dictated by the feedlot environment, regardless of AMR prevalence at arrival (19).
Because herds were enrolled in the larger productivity study based on their ability to provide the required data, this sample probably represents some of the more progressive, commercially viable, and intensively managed herds in western Canada. In 2001, 65% of the beef farms in Canada had less than 47 cows per farm, but these producers managed less than 24% of the national herd (3). The study herds were larger than the national reported average of 53 cows. The Saskatchewan and Alberta herds included in the productivity study represented approximately 0.6% and 1.2% of the herds of similar size in their respective provinces (3).
This is the 1st study that describes AMR prevalence in calves in western Canadian beef herds during the calving season and at weaning. Baseline information is necessary to measure variation resulting from changing production practices and to develop strategies to control AMR emergence. Knowledge of production stage and timing of sample collection is critical to interpreting surveillance data from these herds. Additional research is needed to understand why AMR varies between spring and fall calves. Future studies should consider animal age, season, herd AMU, and management factors. Continued monitoring of AMR patterns in cow-calf herds will facilitate the detection of any emerging issues that could have a significant effect on human health.
Acknowledgments
Direct funding for this project was provided by the Canadian Adaptation and Rural Development (CARD) Fund, Saskatchewan Agriculture Development Fund, Horned Cattle Purchases Fund Advisory Committee, Cattle Marketing Deductions Fund Advisory Committee, and the Alberta Beef Producers (formerly Alberta Cattle Commission). The authors thank the Western Interprovincial Scientific Studies Association (WISSA) for support of the beef productivity study that provided much of animal and treatment data for this analysis. The producers and veterinarians who provided data and the project veterinarians who collected it are also thanked. The authors are grateful to the laboratory staff at PDS in Saskatoon and Agri-Food Laboratories Branch, Food Safety Division of Alberta Agriculture for their contribution to this project.
Footnotes
This work is part of a PhD thesis, University of Saskatchewan, Saskatoon, Saskatchewan.
References
- 1.Statistics Canada. [homepage on the Internet] [Last accessed 25 July 2006]; Available from www40.statcan.ca/l01/cst01/prim50a.htm.
- 2.Gow S, Waldner CL, Rajíc A, McFall M, Reid-Smith R. Prevalence of antimicrobial resistance in fecal generic Escherichia coli isolated in western Canadian cow-calf herds: Part II — Cows and cow-calf pairs. Can J Vet Res. 2008 in press. [PMC free article] [PubMed] [Google Scholar]
- 3.Western Interprovincial Scientific Studies Association (WISSA) Research Appendices: A study of 33,000 cattle. British Columbia: Alberta and Saskatchewan; 2006. Western Canada study of animal health effects associated with exposure to emissions from oil and natural gas field facilities. [Google Scholar]
- 4.National Antimicrobial Resistance Montioring System (NARMS) United States Food and Drug Administration. Department of Health and Human Services. [homepage on the Internet] [Last accessed 26 December 2007]; Available from http://www.fda.gov/cvm/narms_pg.html.
- 5.Clinical Laboratory Standards Institute (CLSI) formerly National Committee Clinical Laboratory Standards (NCCLS) NCCLS document M7-A5. 5. Wayne Pennsylvania, 19087–1898 USA: 2000 Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. [Google Scholar]
- 6.Clinical Laboratory Standards Institute (CLSI) formerly National Committee Clinical Laboratory Standards (NCCLS) NCCLS document M100-S14. Wayne, Pennsylvania, 19087–1898 USA: 2000 Performance standards for antimicrobial susceptibility testing; 12fth informational supplement. [Google Scholar]
- 7.Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2004 Annual Report, Government of Canada, 2006. [homepage on the Internet] [Last accessed 26 December 2007]; Available from http://www.phac-aspc.gc.ca/cipars-picra.
- 8.Fleiss JL, Levin B, Paik MC. Statistical inference for a single proportion. In: Balding DJ, Cressie NA, Fisher NI, et al., editors. Statistical Methods for Rates and Proportions. 3. Hooboken, New Jersey: John Wiley and Sons; 2003. [Google Scholar]
- 9.Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research. ACV Inc. University of Prince Edward Island; Charlottetown, Prince Edward Island: 2003. [Google Scholar]
- 10.Bywater R, Deluyker H, Deroover E, et al. A European survey of antimicrobial susceptibility among zoonotic and commensal bacteria isolated from food producing animals. J Antimicrob Chemother. 2004;54:744–754. doi: 10.1093/jac/dkh422. [DOI] [PubMed] [Google Scholar]
- 11.Khachatryan AR, Hancock DD, Besser TE, Call DR. Role of calf adapted Escherichia coli in maintenance of antimicrobial drug resistance in dairy calves. Appl Environ Microbiol. 2004;70:752–757. doi: 10.1128/AEM.70.2.752-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoyle DV, Knight HI, Shaw DJ, Hillman K, Pearce MC, Low JC, Gunn GJ, Woolhouse MEJ. Acquisition and epidemiology of antimicrobial resistant Escherichia coli in a cohort of newborn calves. J Antimicrob Chemother. 2004;53:867–871. doi: 10.1093/jac/dkh177. [DOI] [PubMed] [Google Scholar]
- 13.Berge ACB, Epperson WB, Prichard RH. Animal and farm influences on the dynamics of antimicrobial resistance in fecal Escherichia coli in young dairy calves. Prev Vet Med. 2005;69:25–38. doi: 10.1016/j.prevetmed.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Gow S. Factors associated with antimicrobial resistance in calves born on 89 western Canadian cow-calf herds. [PhD dissertation] Saskatoon, Saskatchewan: Univ. of Saskatchewan; 2007. [Google Scholar]
- 15.Allan BJ, van den Hurk JV, Potter AA. Characterization of Escherichia coli isolates from cases of avian colibacilosis. Can J Vet Res. 1993;57:146–151. [PMC free article] [PubMed] [Google Scholar]
- 16.Mandal P, Kapil A, Goswami K, Das B, Dwivedi SN. Uropathogenic Escherichia coli causing urinary tract infections. Indian J Med Res. 2001;114:207–211. [PubMed] [Google Scholar]
- 17.Simmons KW, Wooley RE, Brown J. Comparison of virulence factors and R-plasmids of Salmonella sp. isolated from healthy and ill swine. Appl Environ Microbiol. 1988;54:760–767. doi: 10.1128/aem.54.3.760-767.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visca JP, Filetici E, Anastoasio MP, Vetriani C, Fantasia M, Orsi N. Siderophore production by Salmonella species isolated from different sources. FEMS Microbiol Lett. 1991;63:225–231. doi: 10.1016/0378-1097(91)90090-w. [DOI] [PubMed] [Google Scholar]
- 19.Berge ACB, Epperson WB, Prichard RH. Assessing the effect of a single dose of florfenicol on the antimicrobial resistance patterns in faecal Escherichia coli. Vet Res. 2005;36:723–734. doi: 10.1051/vetres:2005027. [DOI] [PubMed] [Google Scholar]


