Abstract
Type 1 diabetes (T1D) is due to a loss of immune tolerance to islet antigens, such as glutamic acid decarboxylase 65 (GAD65), for which islet transplantation is a promising therapy. Therefore, the generation of tolerance aiming at both alloantigen and GAD65 will help therapeutic intervention greatly in T1D. In this study, we tested the effect of programmed death-1 ligands (PD-L1)-transfected dendritic cells (DC) loaded with GAD65 on the alloresponse and GAD65-reactive lymphocyte response. The DC2·4 cell line was transfected with PD-L1 and co-cultured with GAD65. BALB-c mice were primed, respectively, by intraperitoneal injection with GAD65, PD-L1-transfected- or non-transfected DC (PD-L1/DC or DC), and PD-L1-transfected- or non-transfected DC loaded with GAD65 (PD-L1/DC/GAD65 or DC/GAD65). Splenocytes of treated mice were isolated and restimulated in vitro with GAD65 or the various DC populations above being used as stimulators, respectively. In the mixed lymphocyte reaction, DC/GAD65 were able to stimulate both allogeneic and GAD65-reactive lymphocytes. However, PD-L1/DC/GAD65 were poorer than DC/GAD65 at activating the GAD65-reactive lymphocyte response. Further, although PD-L1/DC could inhibit the alloresponse, PD-L1/DC/GAD65 were more effective at down-regulating the GAD65-reactive lymphocyte response. More importantly, PD-L1/DC/GAD65-primed lymphocytes exhibited the weakest proliferation when again restimulated in vitro by PD-L1/DC/GAD65. Additionally, PD-L1/DC/GAD65 down-regulated interferon-γ and up-regulated interleukin-10 production by activated lymphocytes. Therefore, combined stimulation in vivo and in vitro by PD-L1/DC/GAD65 could inhibit both the alloresponse and the GAD65-reactive lymphocyte response, which may contribute to controlling diabetes and islet transplant rejection.
Keywords: dendritic cell, glutamic acid decarboxylase 65, lymphocyte proliferation, programmed death-1 ligands
Introduction
The ultimate goal of treatment for type 1 diabetes (T1D) is antigen- and/or site-specific suppression of pathology. Previous studies have shown that glutamic acid decarboxylase 65 (GAD65) is an important target of the immune response associated with β cell destruction [1–3]. Therefore, inhibition of the reactive T cell-mediated immune response in an antigen-specific manner without causing systemic immune suppression may prevent disease development [4,5]. Pancreatic-islet transplantation has long been considered a safer alternative and a potentially preferable alternative than conventional exogenous-insulin therapy for T1D [6]. Therefore, inhibiting the alloresponse to building allograft tolerance and controlling the aggressive response to generate GAD65-reactive immune tolerance simultaneously might be a promising strategy to prevent graft rejection and halt the progression of this disease.
Recent studies have revealed that tolerogenic donor dendritic cells (DC) can tolerize allogeneic T cells in vitro and can inhibit allograft rejection in vivo [7]. Negative co-stimulatory signals on DC delivered to T cells serve to limit alloantigen responses and to prevent inappropriate immune responses to build target-specific immune tolerance [8,9]. Some reports [10–12] have shown antigen-specific T regulatory (Treg) therapy which, working through secondary cell types such as DC, has the greatest potential to treat autoimmune diseases in which target antigens and epitopes have been identified, indicating further the potential use of antigen-specific DC in inducing target-specific immune tolerance. Therefore, DC are potentially useful candidates for inducing antigen-specific tolerance.
Termination of an immune response is achieved through a number of different mechanisms, including regulatory co-stimulatory molecules [13]. The inhibitory co-stimulatory molecules programmed death-1 ligands (PD-L1) have been shown to play an important role in regulating T cell activation and peripheral tolerance [8,14–16]. PD-L1 has been found to play a unique role in protecting the pancreas from T cell-mediated tissue damage [17]. Therefore, it suggests that these molecules play significant roles in maintaining immunological tolerance in physiological situations [18–20].
Thus, we propose a strategy of generating a modified DC line which can, on one hand, express high-level co-inhibitory molecules PD-L1 and, on the other hand, has the capability to present both target antigen of T1D, GAD65 and alloantigens. We presume that using these modified DC could induce allogeneic immunotolerance and prevent a GAD65-reactive response, also aiming directly at islet β cells. This study was performed to examine the role of these modified DC on activation and proliferation of both allogeneic and GAD65-reactive lymphocytes in a cell model seeking further to build the foundation for islet transplantation and prevention of T1D.
Materials and methods
Mice and cell culture
BALB/c (H-2d) male mice aged between 6 and 8 weeks, weighing approximately 20 g (Experimental Animal Center, Tongji Medical College, Wuhan, China) were maintained in standard environmental conditions with free access to food and water. They were allowed to adapt to their environment for 1 week before the experiments were initiated. The DC2·4 cell line (H-2b, kindly provided by Dr Cong-Yi Wang from the Georgia's Health Science University, GA, USA) originated from C57BL/6 mice, as described previously [21].
Expression and purification of GAD65 protein
Recombinant mGAD65 was produced from BL21(DE3)pLysS Escherichia coli containing the bacterial expression vector pET3ad10-mGAD65 (kindly provided by Dr Dai Yang from Torrey Pines Institute for Molecular Studies, San Diego, CA, USA). The expression procedure was conducted according to the pET system manual. Purification of the protein was carried out using HiTrapTM columns (Amersham Pharmacia Biotech, Uppsala, Sweden) according to the manufacturer's instructions. The presence and purity of GAD65 were confirmed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and enzyme-linked immunosorbent assay (ELISA) analysis. After removal of endotoxin with EndoTrap column (Profos AG, Regensburg, Germany), those fractions containing purified GAD65 were then pooled and dialysed 10 times against phosphate-buffered saline (PBS). The purified GAD65 was then lyophilized and resuspended to a concentration of 1 mg/ml. Endotoxin concentration was below the detection limit of 0·05 EU/ml using the Limulus amoebocyte lysate test (Profos AG).
Generation of a PD-L1-transfected dendrictic cell line
DC2·4 cells (DC) were plated in 24-well tissue culture plates (2 × 105 cells/well) in RPMI-1640 medium supplemented with 10% FCS (Gibco, Carlsbad, CA, USA) and 1% penicillin/streptomycin. The enhanced green fluorescence protein gene (pEGFP-N1) plasmid containing the gene for PD-L1 (PD-L1/pEGFP-N1, constructed in our laboratory) was transfected into DC2·4 cells by Lipofectamine™2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. After 72 h of transfection, the cells were selected with G418 (500 μg/ml; Sigma, St Louis, MO, USA) for 4 weeks for establishment of stable transfectants. An empty pEGFP-N1 vector was transfected into DC2·4 used as a control. To determine the expression of PD-L1 by transfected DC, the cells were stained with biotin-conjugated anti-mouse PD-L1 monoclonal antibody (mAb) and streptavidin-conjugated antigen presenting cells (APC) (BD Biosciences, San Jose, CA, USA), PD-L1-positive cells were gated, and its expression was evaluated using flow cytometry (FCM). Furthermore, the above DC were also stained with phycoerythrin (PE)-labelled mAb to CD40 (Biolegend, San Diego, CA, USA), CD80 (Biolegend), CD86 (Biolegend), major histocompatibility complex (MHC) class I or II (eBioscience, San Diego, CA, USA) molecules and analysed by flow cytometer (BD LSRII; BD Biosciences), respectively.
Antigen loading and endocytosis
PD-L1/DC or parental DC, respectively, were incubated with GAD65 at a final concentration of 100 μg/ml for 12 h prior to the following assay. For endocytosis experiments, fluorescein isothiocyanate (FITC)–dextran (molecular weight, 70 000, FD-70s; Sigma) uptake was performed as described previously [22]. The uptake of FITC–dextran was analysed by FCM and evaluated by fluorescence microscopy at 400× magnification.
Primed splenocytes purification and 5,6-carboxy-succinimidyl-fluorescein ester (CFSE) labelling
BALB/c mice were injected intraperitoneally (i.p.) with the following groups of DC (2 × 107/mouse): (i) parental DC2·4 cells (DC); (ii) DC loaded with GAD65 (DC/GAD65); (iii) PD-L1-transfected DC (PD-L1/DC); (iv) empty vector-transfected DC (GFP/DC); and (v) PD-L1-transfected DC loaded with GAD65 (PD-L1/DC/GAD-65), respectively. In addition, the sixth group of BALB/c mice was injected i.p. with a 0·5-ml immunofluorescence assay (IFA)/PBS solution containing 500 μg of GAD65 protein. After 14 days, the above six groups of mice were killed and their splenocytes were isolated. The above lymphocytes were labelled with CFSE tracking dye (Molecular Probes, Portland, OR, USA) at a concentration of 1 μM in PBS for 30 min at 37°C [23]. After washing, the labelled lymphocytes were used for further analyses.
Lymphocyte proliferation and cytokine assay
In all the experiments, after CFSE labelling, the above six groups of primed splenocytes (5 × 106/well as the responder) were restimulated in vitro with the same above five groups of DC (5 × 105/well as the stimulator), in 24-well, flat-bottomed plates for 5 days, respectively. GAD65 and ovalbumin (OVA) were also used as stimulators and their final working concentration was 100 μg/ml. Proliferation was analysed using FCM. Five days later, supernatants of the co-cultures were collected and the concentrations of interleukin (IL)-10 and interferon (IFN)-γ were measured by ELISA kits (eBioscience).
Statistical analysis
Statistical analysis was performed using theStatistical Package for the Social Sciences (SPSS version 12·0). Differences among groups were evaluated statistically by analysis of variance (anova) and comparison of means (Student's t-test). In both cases, P < 0·05 was considered significant.
Results
PD-L1/DC can express a high-level of PD-L1
First, we established a stable PD-L1/DC and GFP/DC line. FCM analyses indicated that the parental DC2·4 and GFP/DC expressed a low level of PD-L1 molecules, while PD-L1/DC showed a high level of PD-L1 (Fig. 1a). Fluorescent microscopy revealed that there was an obviously green fluorescence on the cell surface of PD-L1/DC (Fig. 1b).
Fig. 1.
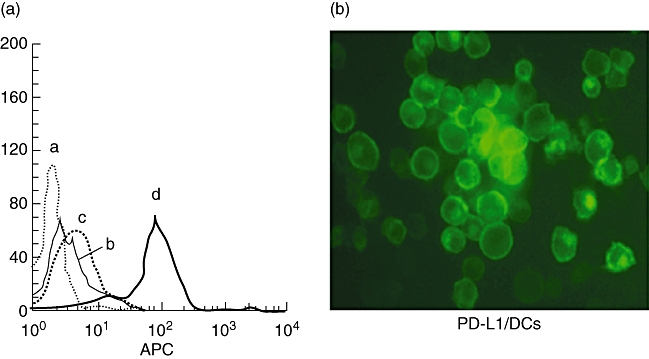
Expression of programmed death-1 ligands (PD-L1) by DC2·4 stable transfectants. (a) Cells were stained with biotin-labelled anti-PD-L1 monoclonal antibodies (mAb) and streptavidin-labelled antigen-presenting cells and analysed by flow cytometer. (a) Negative control; (b) parental DC; (c) green fluorescence protein gene (GFP/DC); (d) PD-L1/DC. The results are representative of at least three independent experiments. (b) Fluorescent microscopy of DC2·4 stable transfectants for PD-L1 (original magnification 400× ). PD-L1-GFP fusion protein was expressed on the cell surface of PD-L1/DC.
Transgene-derived PD-L1 molecules decreased the expression level of CD80 and CD86 on DC
In order to examine if high-level expression of PD-L1 molecules could impact the other phenotype on DC, we tested the expression of CD40, MHC-I, MHC-II molecules, CD80 and CD86 before and after DC transfected with PD-L1. No noticeable difference was found among PD-L1/DC, GFP/DC and parental DC for the expression level of CD40, MHC-I and MHC-II molecules (Fig. 2c). However, our results show that DC were 94·8% and 94·5% positive for CD80 and CD86, respectively, and there was no noticeable difference among the above three DC populations for the expression rate of CD80 and CD86. However, the mean fluorescence intensity (MFI) of both CD80 and CD86 by PD-L1/DC was lower than that of parental DC and GFP/DC (P < 0·01), but with no difference between parental DC and GFP/DC (Fig. 2b), showing that the enhanced PD-L1 molecules decreased the expression level, not the expression rate, of CD80 and CD86 on DC.
Fig. 2.
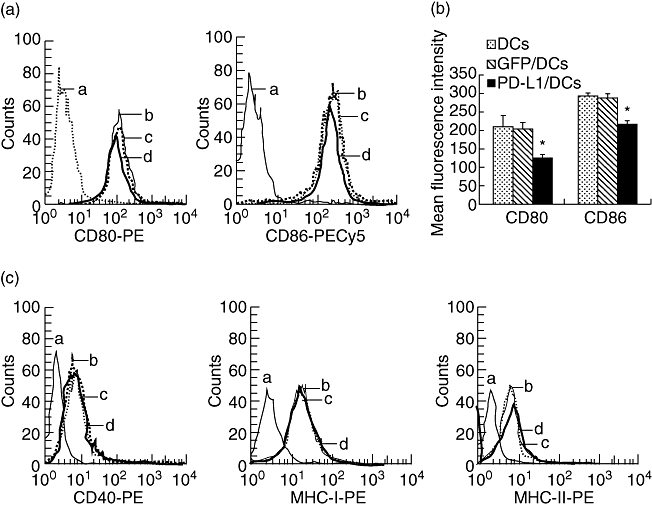
The high-level expression of programmed death-1 ligands (PD-L1) molecules resulted in a reduced level expression of CD80 and CD86 on dendritic cells (DC). (a) DC, GFP/DC and PD-L1/DC were stained with phycoerythrin (PE)-labelled anti-CD80 monoclonal antibodies (mAb) and PE-cy5-labelled anti-CD86 mAb and analysed by flow cytometer, respectively. (b) Results are expressed as the mean fluorescence intensity, which represents the average level of CD80 and CD86 surface expression on DC or PD-L1/DC. Bars represent standard deviation of the mean of three independent experiments. (c) The expression of CD40, major histocompatibility complex (MHC) class I and II antigens was not noticeably different between PDL1/DC and wild-type DC. DC, GFP/DC and PD-L1/DC were stained with PE-labelled mAb to CD40, MHC class I or II mAb and analysed by flow cytometer, respectively. (a) Negative control; (b) parental DC; (c) GFP/DC; (d) PD-L1/DC. The asterisks indicate that the difference in responses are statistically significant between three groups (*P < 0·01).
PD-L1/DC and parental DC both have high phagocytic capability
SDS-PAGE showed the presence of the putative GAD65 (Fig. 3a) and this was confirmed by ELISA analysis (data not shown). To analyse the DC-antigen capture, phagocytosis was assessed by the uptake of FITC–dextran. FCM analyses indicated that both PD-L1/DC and parental DC internalized large amounts of FITC–dextran (Fig. 3b), a marker of fluid macropinocytosis and mannose receptor-mediated endocytosis [24]. The phagocytosis rate of DC and PD-L1/DC at 37°C for 2 h was 78·6% and 79·1%, respectively. To confirm the FCM data, we next used FCM to visualize endocytosis directly by both PD-L1/DC and parental DC. After 2 h at 37°C, the fluid-phase marker FITC–dextran (green) was found clearly in intracellular vesicles in both PD-L1/DC and parental DC (Fig. 3c). The expression of PD-L1 molecules did not affect the endocytosis of DC.
Fig. 3.
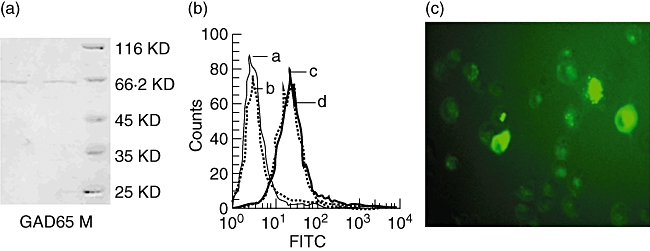
Programmed death-1 ligands/dendritic cells (PD-L1/DC) and parental DC both have high phagocytic capability. (a) The expression and purity of glutamic acid decarboxylase 65 (GAD65) protein. GAD65 protein and protein marker were loaded onto a sodium dodecyl sulphate-polyacrylamide gel electrophoresis gel and subsequently stained with Coomassie blue. M, protein marker. (b) Flow cytometry analyses of endocytosis by PD-L1/DC and parental DC. They were incubated in medium containing fluorescein isothiocyanate (FITC)–dextran for 2 h at 37°C or 4°C (as control). (a) Negative control; (b) parental DC (at 4°C); (c) parental DC (at 37°C); (d) PD-L1/DC (at 37°C). The results are representative of at least three independent experiments. (c) Fluorescent microscopy shows FD700-positive parental DC (magnified × 400) which were exposed to FITC–dextran (green) for 2 h at 37°C.
Stimulation of allogeneic and GAD65-reactive lymphocytes by DC/GAD65
In the following report, because GFP alone had no influence on the results in our study and the proliferation of GFP/DC-primed lymphocytes is similar to that of DC-primed lymphocytes, data for the proliferation of GFP/DC-primed lymphocytes are not shown.
First, we examined whether allogeneic and GAD65-reactive lymphocytes would be activated by injection with DC/GAD65. As shown in Fig. 4b, DC/GAD65-primed splenocytes showed a higher proliferative response to GAD65 than their response to OVA (P < 0·01), whereas DC-primed splenocytes showed a similar response to both GAD65 and OVA (data not shown), indicating GAD65-reactive stimulation by DC/GAD65. Furthermore, DC/GAD65-primed splenocytes also showed an obvious proliferative response to both DC/GAD65 and DC with, however, a higher response to DC/GAD65 than to DC (Fig. 4d, P < 0·01). In contrast, DC-primed splenocytes showed a similar response to both DC/GAD65 and DC (Fig. 4d). These results indicate that DC have high antigen-presenting capabilities and that combined stimulation in vivo and in vitro by DC/GAD65 was able to stimulate both allogeneic and GAD65-reactive lymphocytes.
Fig. 4.
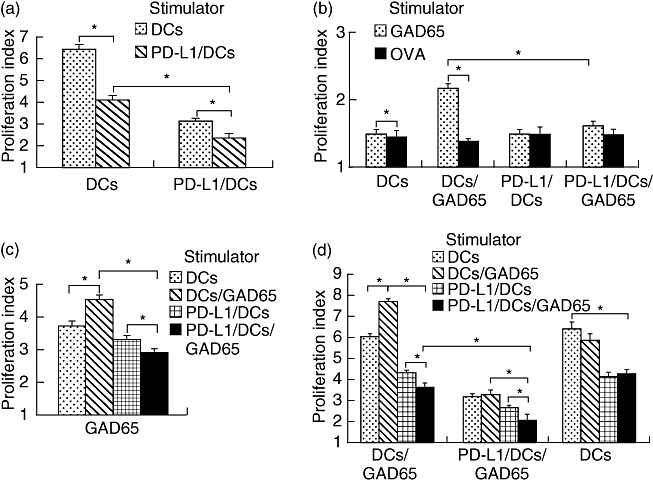
Programmed death-1 ligands/dendritic cells/glutamic acid decarboxylase 65 (PD-L1/DC/GAD65) inhibited both alloresponse and GAD65-reactive lymphocyte response. (a) PD-L1/DC inhibited alloresponse. Lymphocytes isolated from PD-L1/DC- or DC-pretreated mice were co-cultured with DC or PD-L1/DC. (b) PD-L1/DC/GAD65 were poorer than DC/GAD65 at activating GAD65-reactive response. Splenocytes isolated from DC/GAD65- or PD-L1/DC/GAD65-pretreated BALB/c mice were stimulated with GAD65 or ovalbumin, respectively. (c) PD-L1/DC/GAD65 had a reduced capability to stimulate GAD65-reactive response in vitro compared with DC/GAD65. Immunofluorescence assay (IFA)/GAD65-primed splenocytes were stimulated with DC, DC/GAD65, PD-L1/DC or PD-L1/DC/GAD65, respectively. (d) Combined stimulation in vivo and in vitro by PD-L1/DC/GAD65 inhibited both alloresponse and GAD65-reactive lymphocyte response. Splenocytes isolated from DC-, DC/GAD65- or PD-L1/DC/GAD65-pretreated BALB/c mice were stimulated with DC, DC/GAD65, PD-L1/DC or PD-L1/DC/GAD65, respectively. The proliferation of lymphocytes determined by 5,6-carboxy-succinimidyl-fluorescein ester labelling in mixed lymphocyte reaction for 5 days. The asterisks indicate that the difference in responses are statistically significant between two values indicated by lines (*P < 0·01). The data are each representative of three independent and reproducible experiments with similar results.
PD-L1/DC inhibited alloresponse
As shown in Fig. 4a, the proliferative response of lymphocytes isolated from DC-pretreated or PD-L1/DC-pretreated mice co-cultured with PD-L1/DC was significantly lower than that of those co-cultured with DC (P < 0·01). Furthermore, the proliferative response of PD-L1/DC-primed lymphocytes to the above stimulator DC was correspondingly lower than that of DC-primed lymphocytes (P < 0·01). These results indicate that combined stimulation in vivo and in vitro by PD-L1/DC inhibited the proliferation of allogeneic lymphocytes.
PD-L1/DC/GAD65 inhibited both alloresponse and GAD65-reactive lymphocyte response
First, compared with DC/GAD65-primed splenocytes, PD-L1/DC/GAD65-primed splenocytes displayed a lower response to GAD65 (Fig. 4b), which indicated that PD-L1/DC/GAD65 were poorer than DC/GAD65 at activating GAD65-reactive response. Secondly, IFA/GAD65-primed splenocytes, displaying a higher proliferative response to GAD65 than their response to OVA (data not shown), exhibited a lower response to PD-L1/DC/GAD65 compared with their response to DC/GAD65 (Fig. 4c), indicating that PD-L1/DC/GAD65 had a reduced capability to stimulate GAD65-reactive response in vitro compared with DC/GAD65. Thirdly, although PD-L1/DC/GAD65 and PD-L1/DC were both able to down-regulate the PD-L1/DC/GAD65-primed lymphocyte proliferation, the response of PD-L1/DC/GAD65-primed lymphocytes co-cultured with PD-L1/DC/GAD65 was even lower than those co-cultured with PD-L1/DC (Fig. 4d, P < 0·01). In contrast, for DC-primed lymphocytes, there was no significant difference between their response to PD-L1/DC/GAD65 and to PD-L1/DC (Fig. 4d). This observation indicated that PD-L1/DC/GAD65 were better at down-regulating a GAD65-reactive response than PD-L1/DC. Fourthly, we observed that when PD-L1/DC/GAD65 were used to pre-immunize BALB/c mice in vivo, the proliferation of primed lymphocytes would be reduced remarkably in vitro. As shown in Fig. 4d, all the proliferative responses of PD-L1/DC/GAD65-primed lymphocytes to the various stimulator DC were correspondingly lower than those of DC/GAD65-primed lymphocytes (P < 0·01). Additionally, our observations also showed that PD-L1/DC/GAD65-primed lymphocytes showed the weakest proliferation when they were again restimulated in vitro by PD-L1/DC/GAD65 (Fig. 4d). All these results indicate that combined stimulation in vivo and in vitro by PD-L1/DC/GAD65 inhibited the proliferation of allogeneic and GAD65-reactive lymphocytes.
PD-L1/DC/GAD65 altered the cytokine profiles of activated lymphocytes, with increases seen in IL-10 and a decrease in IFN-γ
In order to explore the mechanisms underlying the suppressive effect of PD-L1/DC/GAD65, IFN-γ and IL-10 levels in cultures were studied further. As shown in Fig. 5a and b, the highest levels of IFN-γ and undetectable levels of IL-10 were produced by activated lymphocytes isolated from DC/GAD65-pretreated mice after restimulation for 5 days with DC/GAD65, whereas IFN-γ production was greatly reduced and higher levels of IL-10 were detected when PD-L1/DC/GAD65 were used as stimulatory cells in those cultures. Furthermore, our results also demonstrate that lymphocytes isolated from PD-L1/DC/GAD65-pretreated mice secreted the highest IL-10 and the lowest IFN-γ after they were restimulated in vitro by PD-L1/DC/GAD65 (Fig. 5a and b). In addition, concentrations of IFN-γ and IL-10 of the other groups or cultures were either lower than the maximum or higher than the minimum (data not shown).
Fig. 5.
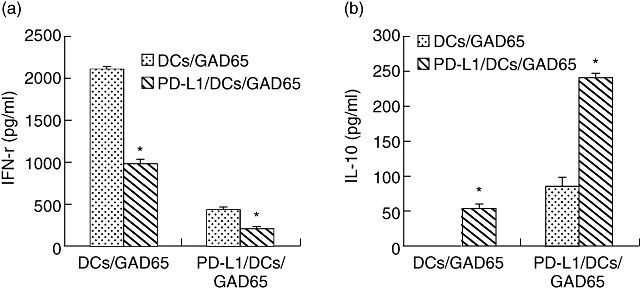
Programmed death-1 ligands/dendritic cells/glutamic acid decarboxylase 65 (PD-L1/DC/GAD65) altered the cytokine profiles of activated lymphocytes. (a) PD-L1/DC/GAD65 decreased interferon (IFN)-γ production by activated lymphocytes. (b) PD-L1/DC/GAD65 enhanced interleukin (IL)-10 production by activated lymphocytes. Lymphocytes isolated from PD-L1/DC/GAD65- or DC/GAD65-pretreated BALB/c mice were co-cultured with PD-L1/DC/GAD65 or DC/GAD65 (as stimulators), respectively. Cultures containing stimulators and primed-lymphocytes were incubated for 5 days before supernatants were removed for cytokine analysis. The levels of IL-10 and IFN-γ production were determined in duplicated by enzyme-linked immunosorbent assays (*P < 0·01). The data are representative of three independent and reproducible experiments with similar results.
Discussion
It has been reported that high-level expression of PD-L1 molecules by DC could inhibit T cell activation and suppress the function of activated T cells [25,26]. Therefore, by using DC with stably high expression of PD-L1, it is possible to prevent T cell activation or lead to inhibition/limitation of the activated T cell response. In this study, we exerted a negative co-stimulatory signal, PD-L1, to generate DC with a tolerogenic character. By this means we generated successfully a stable PD-L1-transfected DC2·4 cell line and examined the influence of enhanced PD-L1 on the function of those DC. On one hand, we have demonstrated that over-expression of PD-L1 decreased the expression of activation markers, CD80 and CD86, on DC (Fig. 2a and b). The reason for this phenomenon is not clear. Previous studies have reported that IL-10-pretreated DC expressed lower levels of CD80 and CD86 molecules than parental DC [27–28], so we presume that PD-L1 itself may be capable of decreasing CD80 and CD86 expression and finally contributed to these results. In addition, as we know, lower levels of these two molecules have been proposed to account for the poor capacity of DC to stimulate T cells. Therefore, this may be another potential mechanism contributing to the suppressive effect of PD-L1. On the other hand, by comparing the capacity to stimulate proliferative responses between PD-L1-expressing DC populations (including both PD-L1/DC and PD-L1/DC/GAD65) and wild-type DC (Fig. 4a and d), this indicates that over-expression of PD-L1 impaired the capacity of those DC to induce immune responses. Moreover, we also found that enhanced PD-L1 inhibited the proliferation and altered the cytokine profiles of activated lymphocytes, with increases seen in IL-10 and a decrease in IFN-γ (Fig. 5), which is consistent with the report by Dong et al. [29]. Taken together, these observations suggest that over-expression of PD-L1 impaired the ability of DC to induce an immune response and regulated the proliferation and cytokine production by activated lymphocytes.
Shen [21] has reported that DC2·4 cells express B7-1 and B7-2 and, as a result, can be used to present antigens on both MHC class I and class II molecules. Our data show that both DC2·4 and PD-L1/DC have a high phagocytic capability, which provides the opportunity for DC to present GAD65 to T cells. In the following experiments we found that PD-L1/DC/GAD65 were poorer stimulators compared with DC/GAD65. Further, both DC populations with hyperexpressing PD-L1 (including both PD-L1/DC and PD-L1/DC/GAD65) showed an inhibitory effect on activated lymphocyte response, indicating that PD-L1 make an important contribution to the poor stimulatory capacity of DC. In addition, we observed that although PD-L1/DC/GAD65-primed lymphocytes showed little proliferative response to GAD65, they still showed a further decreased proliferation when they were again restimulated by themselves. Therefore, it seems that combined stimulation in vivo and in vitro by PD-L1/DC/GAD65 may contribute to their suppressive effect. For these phenomena our interpretation is that, compared with DC/GAD65, PD-L1/DC/GAD65 might activate a lesser amount of lymphocytes or even render the activated lymphocytes anergic, which may have contributed finally to their down-regulative effect.
In summary, high-level expression of PD-L1 molecules can render DC immunosuppressive and impair their ability to induce immune response and regulate the proliferation and cytokine production by activated lymphocytes. Furthermore, PD-L1/DC/GAD65 can result in down-regulation of the alloresponse and the GAD65-reactive lymphocyte response. These findings are of special importance in application of DC-based immunotherapies for inhibition of graft rejection and prevention of T1D. Further islet-transplantation animal models will be built to validate this strategy.
Acknowledgments
This work was supported by the National Natural Science foundation of China (no. 30671954). We thank Zhihui Liang for the flow cytometric assays.
References
- 1.Ravanan R, Wong SF, Morgan NG, Mathieson PW, Smith RM. Inhalation of glutamic acid decarboxylase 65-derived peptides can protect against recurrent autoimmune but not alloimmune responses in the non-obese diabetic mouse. Clin Exp Immunol. 2007;148:368–72. doi: 10.1111/j.1365-2249.2007.03358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathis D, Vence L, Benoist C, et al. Beta-cell death during progression to diabetes. Nature. 2001;414:792–8. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 3.Gottlie PA, Eisenbarth GS. Insulin-specific tolerance in diabetes. Clin Immunol. 2002;102:2–11. doi: 10.1006/clim.2001.5142. [DOI] [PubMed] [Google Scholar]
- 4.Bach JF, Chatenoud L. Tolerance to islet autoantigens in type 1 diabetes. Annu Rev Immunol. 2001;19:131–61. doi: 10.1146/annurev.immunol.19.1.131. [DOI] [PubMed] [Google Scholar]
- 5.Cooke A, Phillips JM, Parish NM. Tolerance strategies to halt or prevent type 1 diabetes. Nat Immunol. 2001;2:810–15. doi: 10.1038/ni0901-810. [DOI] [PubMed] [Google Scholar]
- 6.Sebastien G, Blandine C, Michel E, Patrice D, Francois R, Benoit B. A new preservation solution increases islet yield and reduces graft immunogenicity in pancreatic islet transplantation. Transplantation. 2007;83:1397–400. doi: 10.1097/01.tp.0000261636.16197.45. [DOI] [PubMed] [Google Scholar]
- 7.Clarkson MR, Sayegh MH. T-cell costimulatory pathways in allograft rejection and tolerance. Transplantation. 2005;80:555–63. doi: 10.1097/01.tp.0000168432.60022.99. [DOI] [PubMed] [Google Scholar]
- 8.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 9.Khoury SJ, Sayegh MH. The roles of the new negative T cell costimulatory pathways in regulating autoimmunity. Immunity. 2004;20:529–38. doi: 10.1016/s1074-7613(04)00116-5. [DOI] [PubMed] [Google Scholar]
- 10.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–77. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serra P, Amrani A, Yamanouchi J, et al. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity. 2003;19:877–89. doi: 10.1016/s1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 12.Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2005;117:289–300. doi: 10.1111/j.1365-2567.2005.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carreno BM, Clollins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg MV, Maris MC, Hipkiss EL, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:86–92. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fife BT, Guleria I, Gubbels Bupp M, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;27:2737–47. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;17:883–95. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansari MJ, Salama AD, Chitnis T, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63–9. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang SC, Latchman YE, Buhlmann JE, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–16. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 20.Salama AD, Chitnis T, Imitola J, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003;198:71–8. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–30. [PubMed] [Google Scholar]
- 22.Steptoe RJ, Ritchie JM, Harrison LC. Increased generation of dendritic cells from myeloid progenitors in autoimmune-prone non-obese diabetic mice. J Immunol. 2002;168:5032–41. doi: 10.4049/jimmunol.168.10.5032. [DOI] [PubMed] [Google Scholar]
- 23.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. J Clin Invest. 1997;100:3173–83. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinya H, Satoru S, Hidetake M, Daiki F, Yasushi U, Yasuharu N. Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with TRAIL or programmed death-1 ligand1. J Immunol. 2005;174:1888–97. doi: 10.4049/jimmunol.174.4.1888. [DOI] [PubMed] [Google Scholar]
- 26.Kim HK, Guan H, Zu G, et al. High-level expression of B7-H1 molecules by dendritic cells suppresses the function of activated T cells and desensitizes allergen-primed animals. J Leukoc Biol. 2006;79:686–95. doi: 10.1189/jlb.0805436. [DOI] [PubMed] [Google Scholar]
- 27.Steinbrink K, Graulich E, Kubsch S, Knop J, Enk AH. CD4+ and CD8+ anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–76. doi: 10.1182/blood.v99.7.2468. [DOI] [PubMed] [Google Scholar]
- 28.Jonuleit H, Schmitt E, Steinbrink K, Enk AH. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol. 2001;22:394–400. doi: 10.1016/s1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- 29.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]


