Abstract
Inflammatory bowel disease (IBD) is characterized by detrimental immune reactivity in the gut, and the imbalance between proinflammatory and anti-inflammatory reactivity. The aims of this study were to determine whether oral administration of glabridin, a functional component of liquorice, could ameliorate dextran sulphate sodium (DSS)-induced colitis, as well as to understand the possible underlying mechanisms. Acute experimental colitis was induced in BALB/c mice by treatment with 5% DSS for 7 days. Glabridin (10 or 50 mg/kg/day) was given for 7 days. Treatment with glabridin significantly attenuated mortality, loss of body weight, shortening of the colon and severe clinical symptoms. This was associated with a remarkable amelioration of the disruption of the colonic architecture, a significant reduction in colonic myeloperoxidase (MPO) activity and the production of inflammatory mediators such as nitric oxide (NO), prostaglandin (PG) E2, and proinflammatory cytokines. These results suggest that glabridin-mediated anti-inflammatory action on colorectal sites may be a useful therapeutic approach to IBD.
Keywords: dextran sulphate sodium, glabridin, inflammation, inflammatory bowel disease, interleukin-6, tumour necrosis factor
Introduction
Inflammatory bowel diseases (IBD), represented mainly by ulcerative colitis (UC) and Crohn's disease (CD), are chronic, relapsing and remitting gastrointestinal diseases characterized by chronic inflammation of the intestine [1]. UC and CD are associated with intestinal and extra-intestinal clinical manifestations of disease, including weight loss, diarrhoea accompanied by blood and/or mucus, fever, gastric dysmotility and shortening of the colon [2,3]. UC is a condition that affects primarily the superficial layer of the colon mucosa, and histological analyses have shown ulceration of the mucosa, blunting and loss of crypts and inflammatory infiltrates [1]. The immune pathogenesis of IBD is associated with increased inflammatory mediators, including reactive oxygen species (ROS) such as nitric oxide (NO) [4,5], prostaglandins (PG) [6,7] and inflammatory cytokines such as tumour necrosis (TNF)-α, interferon (IFN)-γ and interleukin (IL)-6 [8–10].
Most of the current therapies for IBD involve treatment with glucocorticosteroids and 5-aminosalicylic acid; however, these display limited beneficial action [11,12]. Immunosuppressive drugs have also been used to control severe illness, regardless of their more serious complications and toxic side effects [13]. Although many types of treatments for IBD have been proposed and conducted clinically, additional therapeutic approaches are needed, as many patients either do not respond to the currently available options or demonstrate significant side effects, thereby precluding their continued use. Alternative medicine is becoming an increasingly attractive approach for the treatment of various inflammatory disorders among patients that are unresponsive to, or unwilling to take, standard medications. Among these alternative approaches is the use of food derivatives, which have the advantage of being relatively non-toxic. However, limited scientific evidence regarding the effectiveness of these natural derivatives, in conjunction with a lack of mechanistic understanding of their actions, has prevented their incorporation into the mainstream of medical care.
Glabridin is a naturally occurring substance found in liquorice that is used as a traditional medicinal plant and a non-sugar sweetener for humans in many countries. We have reported recently that the ethanolic extract of liquorice exerts potent anti-inflammatory effects on murine macrophages in vitro, and inhibits the production of inflammatory mediators such as NO, PGE2 and inflammatory cytokines [14]. Moreover, the ethanolic extract of liquorice was found to be effective at reducing the incidence of septic shock in endotoxin-treated mice. These previous observations led us to examine the effects of glabridin on intestinal inflammation. We were interested in whether glabridin has a therapeutic effect in inflammatory diseases and chose the dextran sulphate sodium (DSS)-induced mouse colitis model as the subject for this study. This model resembles human IBD, and is used for pharmacological analysis of potentially effective anti-inflammatory agents [15–17]. Furthermore, the DSS model faithfully reproduces many of the immunological disturbances observed in human IBD [15–19].
Materials and methods
Mice and experimental protocol
Six-week-old female BALB/c mice were purchased from SLC (Shizuoka, Japan) and maintained under specific pathogen-free conditions at the animal facility of Hallym University (Gangwon-do, Korea).
To induce experimental colitis, the mice were administered 5% DSS (40 000–50 000 MW; ICN Biomedicals, Aurora, OH, USA) dissolved in water that was filter-purified (Millipore Corp., Bedford, MA, USA) for 7 days. The control mice received the filtered water alone. Glabridin was dissolved in dimethylsulphoxide (DMSO) and freshly diluted in phosphate-buffered saline (PBS). Glabridin (10 or 50 mg/kg) or the vehicle (PBS with DMSO) was administrated orally for 7 days, commencing at the same time as the start of DSS exposure. In order to examine the therapeutic effect of glabridin, mice were given DSS for 6 days. After 1 day of applying DSS, glabridin (10 or 50 mg/kg) or the vehicle (PBS with DMSO) was administered orally for 7 days. We observed no differences in water consumption among groups (3·5–4·0 ml/day/mouse) throughout the experimental period. The study protocol was approved by the Animal Care and Use Committee of Hallym University.
Assessment of DSS-induced colitis
The mice were checked daily for colitis development by monitoring body weight, gross rectal bleeding, stool consistency and survival. The overall disease severity was assessed by a clinical scoring system on a scale of 0–4 [15]. In brief, scoring was as follows: 0, no weight loss, no occult blood in the stools and normal stool consistency; 1, weight loss of 1–5%, no occult blood and normal stool consistency; 2, 5–10% weight loss, positive for fecal occult blood and loose stools; 3, 10–20% weight loss, positive for fecal occult blood and loose stools; and 4, greater than 20% weight loss, gross rectal bleeding and diarrhoea.
Colon tissue culture
Colon tissues corresponding to the mid-colon were washed with RPMI-1640 (Hyclone, Logan, UT, USA) medium containing 2% fetal bovine serum (FBS; Hyclone) and penicillin and streptomycin (Hyclone) before being cut into smaller pieces. Then, approximately 0·5 cm of tissue was placed in 0·5 ml of 10% FBS containing RPMI-1460 medium in 48-well tissue culture plates and incubated for 24 h at 37°C in 5% CO2.
Measurement of cytokines, NO and PGE2
The TNF-α and IL-6 concentrations in the cell-free culture supernatants of the colon tissues were measured in triplicate using a Bio-Rad Multiplex bead array instrument and cytokine kits (Bio-Rad, Hercules, CA, USA) according to the manufacturer's protocol. The amount of nitrite and PGE2 produced was measured using the Griess reagent system (Promega, Madison, WI, USA) and an enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems (Minneapolis, MN, USA) according to the manufacturer's instructions, respectively.
Determination of colonic myeloperoxidase (MPO) activity in the colons
The mouse colons were rinsed with cold PBS, blotted dry and frozen immediately in liquid nitrogen. They were then stored at −80°C until assayed for MPO activity using the o-dianisidine method [18]. To perform the assay the tissue samples were thawed and weighed, and then suspended (10% wt/vol) in 50 mM potassium phosphate buffer (pH 6·0) containing 0·5% hexadecyltrimethylammonium bromide and homogenized. A sample of the homogenate (1 ml) was sonicated for 30 s, and then centrifuged at 200 g for 10 min at 4°C. The reaction was started by mixing and incubating the supernatant (100 μl) at 20°C for 10 min with a solution composed of 2810 μl of 50 mM potassium phosphate, 30 μl of 20 mg/ml o-dianisidine dihydrochloride and 30 μl of 20 mM hydrogen peroxide. After 10 min the reaction was terminated by the addition of 30 μl of 2% sodium azide. The change in absorbance was read at 460 nm using a SpectraMax M2 Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). MPO activity was expressed as the amount of enzyme necessary to produce a change in absorbance of 1·0 unit/min/g of tissue (wet weight).
Quantitative real-time reverse transcription–polymerase chain reaction (RT–PCR)
The total RNA from the colon homogenates was isolated with an RNAeasy mini kit (Quiagen, Hilden, Germany). After RNA preparation, the cDNA was transcribed using a single reverse transcriptase synthesis step with Superscript reagents (Invitrogen, Carlsbad, CA, USA). Quantitative PCR was performed with the incorporation of SYBR green into the double-stranded PCR products. The primers used in quantitative PCR were as follows: inducible NO synthase (iNOS), forward 5′-TGACGGCAAACATGACTTCAG-3′ and reverse 5′-GCCATCGGGCATCTGGTA-3′; cyclooxygenase-2 (COX-2), forward 5′-CATCCCAGGCCGACTAAATG-3′ and reverse 5′-TTTCAGAGCATTGGCCATAGAA-3′; TNF-α, forward 5′-TACCTTGTCTACTCCCAGGTTCTCT-3′ and reverse 5′-GTGTGGGTGAGGAGCACGTA-3′; IL-6, forward 5′-CTTCCTACCCCAATTTCCAATG-3′ and reverse 5′-ATTGGATGGTCTTGGTCCTTAGC-3′, intercellular adhesion molecule-1 (ICAM-1), forward 5′-CGCACAGAACTGGATCTCAGGC-3′ and reverse 5′-GCTTCAGAGGCAGGAAACAGGC-3′. The specificity of the reaction was tested via product separation by gel electrophoresis or by melting curve analysis when SYBR green was incorporated. The mean relative expressions of the cytokine genes were calculated, and differences were determined using the 2-ΔC(t) method.
Immunohistochemical study
Colonic tissues were fixed in 4% buffered paraformaldehyde, dehydrated through graded concentrations of ethanol, embedded in paraffin and sectioned. The sections (5 μm thick) were mounted on slides, cleared and hydrated; all were treated with a buffered blocking solution [3% bovine serum albumin (BSA)] for 15 min. Then, the sections were co-incubated with primary antibodies for iNOS (BD Biosciences PharMingen, San Diego, CA, USA) and COX-2 (BD Biosciences PharMingen) at a dilution of 1 : 500 at room temperature for 1 h. The sections were washed with PBS and then co-incubated with secondary antibody of peroxidase conjugated anti-sheep IgG (1 : 500 in PBS, v/v) at room temperature for 1 h. Thereafter, the sections were washed as before with 0·05 M Tris–HCl, pH 7·66, and then co-incubated with 3,3′-diaminobenzidine solution in the dark at room temperature for 10 min. Finally, the sections were washed with Tris–HCl and stained with haematoxylin according to standard protocols.
Statistical analysis
The data are presented as means ± standard error of the mean (s.e.m.) from at least three independent experiments. The values were evaluated by one-way analysis of variance (anova), followed by Duncan's multiple range tests using GraphPad Prism 4·0 software (GraphPad Software Inc., San Diego, CA, USA). Differences were considered significant at P < 0·05.
Results
Survival, body weight and clinical symptoms
We first observed symptomatic parameters such as the survival rate, body weight loss and disease activity index (DAI) caused by colitis 1 and 2 weeks after starting 5% DSS oral administration. As shown in Fig. 1a, the survival rate ofthe mice with colitis was enhanced in the glabridin-administered group. DSS exposure caused a 50% death rate at day 14. However, the survival rates of the 10 and 50 mg/kg glabridin-treated mice were 67% and 84%, respectively. The treatments of 10 or 50 mg/kg glabridin also reduced body weight loss in mice with colitis (Fig. 1b). Another common feature of the DSS-induced model of colitis is an increase in the DAI [4]. The DAI of the mice decreased significantly following glabridin administration (50 mg/kg) compared to the mice receiving vehicles from days 3–14 (Fig. 1c).
Fig. 1.
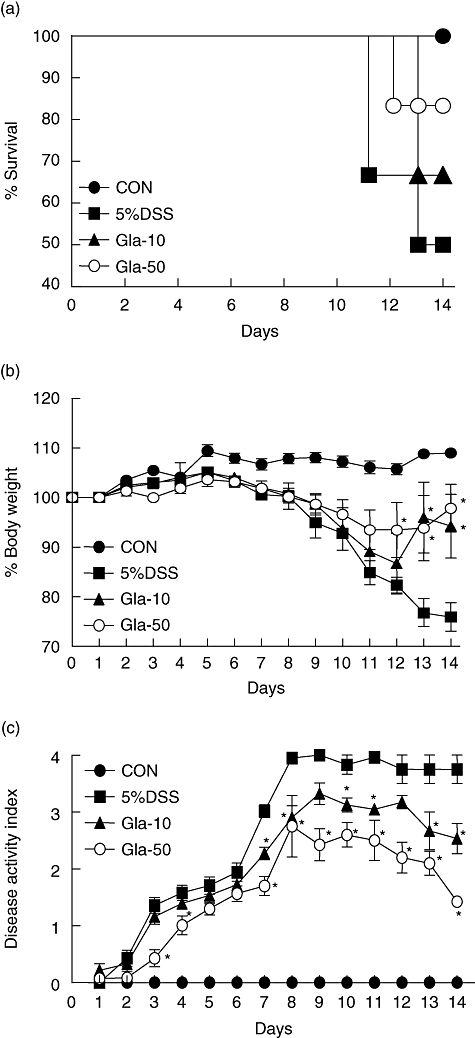
Time–course of survival rate, body weight and disease activity of dextran sulphate sodium (DSS)-induced colitis in glabridin-treated mice. (a) Survival rates, (b) body weight and (c) disease activity index of glabridin-treated mice and control mice administered 5% DSS were monitored every day. Data are the sum of results of three independent experiments (n = 10 per group). The values of body weight are expressed as the percentage of body weight on day 0. Each point represents the mean ± standard error of the mean. *Different from DSS group, P < 0·05.
Colon length and histopathological analysis
The DSS-induced model of colitis is associated with a significant decrease in colon length [2,3], and colon length measurement has often been used as a morphological parameter for the degree of inflammation in DSS colitis. To assess colon length in the present study, mice from each group were killed at days 7 and 14. At days 7 and 14 following DSS treatment, we found that colon length in the DSS-administered mice was significantly shorter than that in the 10 or 50 mg/kg glabridin-treated mice (Fig. 2a). Histological examination of the colonic sections assessed the intestinal inflammatory status. Microscopically, the samples from the DSS-induced colitic mice showed typical inflammatory changes in colonic architecture, such as ulceration, crypt dilation and goblet cell depletion, as well as mixed cell infiltration composed mainly of macrophages, lymphocytes, plasma cells and granulocytes (Fig. 2b). Conversely, histological analysis of the colons from the glabridin-treated mice showed greatly reduced numbers of infiltrating cells, degree of mucosal injury and oedema (Fig. 2b).
Fig. 2.
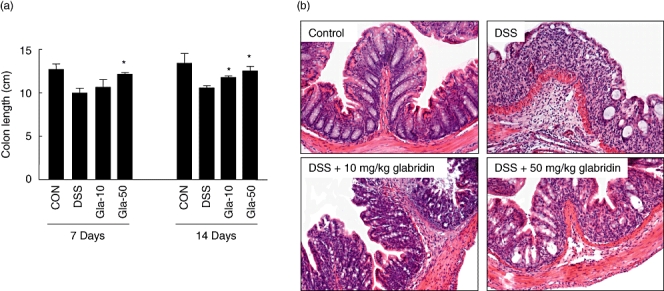
Evaluation of dextran sulphate sodium (DSS)-induced colitis in glabridin-treated mice. Colons were obtained 7 and 14 days after DSS administration. (a) Colon length was measured. Data are the sum of results of three independent experiments and are expressed as the mean ± standard error of the mean (n = 10 per group). *Different from DSS group, P < 0·05. (b) The colons were dissected for histological analysis with haematoxylin and eosin staining (original magnification × 100).
Production of NO, PGE2 and MPO activity in colon tissues
In recent years, NO has been considered an important inflammatory mediator playing a key role in the pathogenesis of IBD [5]. In addition, the dramatic increase in mucosal PG synthesis during inflammation was shown to correlate with the disease activity of human IBD and experimental colitis [6,7]. For this reason, we evaluated the effect of glabridin on colonic NO and PGE2 production in the DSS-induced experimental colitic colon. As shown in Fig. 3a and b, DSS administration produced increased levels of NO as well as PGE2. However, oral administration of glabridin reduced NO and PGE2 production in a concentration-dependent manner at days 7 and 14.
Fig. 3.
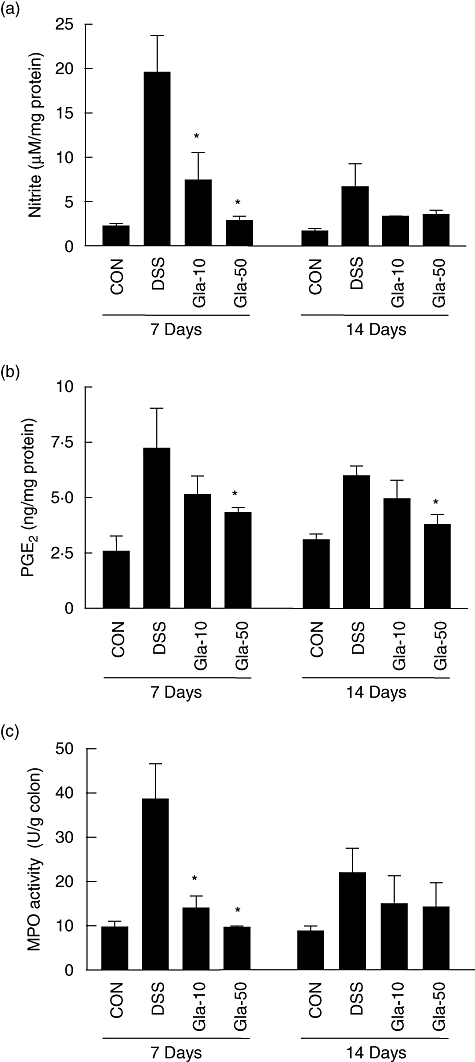
The production of (a) nitric oxide and (b) prostaglandin E2 in colon of dextran sulphate sodium (DSS)-induced colitis in glabridin-treated mice. A segment of colon in DSS-administered glabridin-treated mice and DSS-treated control mice was prepared on days 7 and 14 after DSS exposure and cultured without any stimulation for 24 h at 37°C. (c) Myeloperoxidase activity of colon was measured as described in Materials and methods. Values are mean ± standard error of the mean (n = 10). *Different from DSS group, P < 0·05.
MPO is an enzyme produced mainly by polymorphonuclear leucocytes and is associated with the degree of neutrophil infiltration in tissues. Following 7 days of DSS treatment via drinking water, MPO activity increased markedly to a level approximately four times higher than that in the control group (Fig. 3c). This increase in MPO activity was reduced significantly by glabridin administration at day 7, although the inhibitory effect disappeared at day 14. Because MPO activity is considered a biochemical maker of neutrophil infiltration [18], this result suggests that glabridin exerts anti-inflammatory effects by reducing neutrophil infiltration into the colonic mucosa.
iNOS and COX-2 immunohistochemistry of colons
Next, we examined the effects of glabridin on the expressions of iNOS and COX-2 in the colons, because iNOS and COX-2 are responsible for inducing NO and PGE2, respectively. In the control non-colitic animals, only weak iNOS and COX-2 immunoreactivities were detectable in the enterocyte cytoplasm located in the apical side of the ileal villi, as shown in Fig. 4. In animals that were selected 7 days after DSS administration, marked iNOS and COX-2 immunoreactivity was evident throughout the gut wall in both intestinal segments, but it was particularly noticeable in the cytoplasm of the epithelial cells. Administration of glabridin significantly decreased the iNOS- and COX-2-producing cells when compared with the DSS-treated group.
Fig. 4.
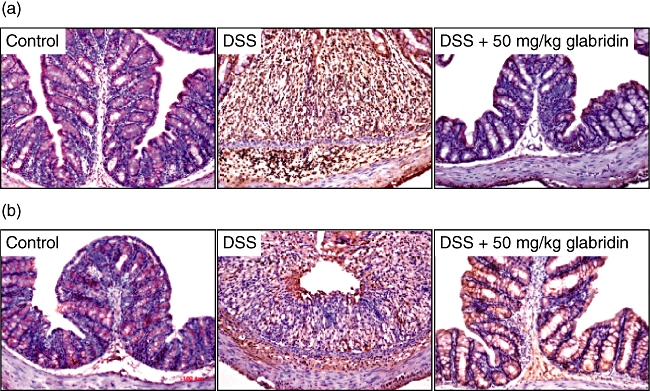
(a) Inducible NO synthase- and (b) cylooxygenase-2-producing cells in the colon of dextran sulphate sodium (DSS)-induced colitis in glabridin-treated mice. Colon in DSS-administered glabridin-treated mice (50 mg/kg) and DSS-treated control mice was prepared on day 7 after DSS exposure and immunostaining was performed as described in Materials and methods (original magnification × 100).
The production of inflammatory cytokines in colon tissues
To determine the effect of glabridin on major inflammatory cytokine expression, colonic TNF-α and IL-6 levels were determined (Fig. 5). After 7 days of DSS administration, TNF-α and IL-6 increased significantly to levels of 762 ± 217 and 5276 ± 1011 pg/mg protein, respectively, compared to 268 ± 49 and 946 ± 197 pg/mg protein in the control group, respectively. Glabridin administration (50 mg/kg) prevented significant increases in TNF-α and IL-6, and at day 7 levels were 355 ± 41 and 2302 ± 195 pg/mg protein, respectively. Similar effects of glabridin were shown at day 14.
Fig. 5.
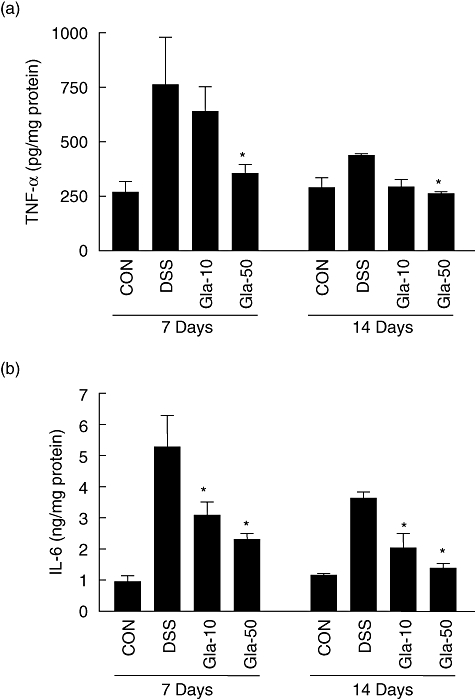
Cytokine production in colon of dextran sulphate sodium (DSS)-induced colitis in glabridin-treated mice. A segment of colon in DSS-administered glabridin-treated mice and DSS-treated control mice was prepared on days 7 and 14 after DSS exposure and cultured without any stimulation for 24 h at 37°C. (a) Tumour necrosis factor-α and (b) interleukin-6 secreted by colon in DSS-administered glabridin-treated and control mice were assessed by using a Bio-Rad Multiplex bead array instrument. Values are mean ± standard error of the mean (n = 10 per group). *Different from DSS group, P < 0·05.
mRNA expression of inflammatory mediators and cytokines in colon tissues
To provide further insight into the molecular mechanism underlying the suppression of colitis by glabridin, mRNA expression levels of inflammatory cytokines, iNOS and COX-2 in the colonic mucosa were measured by real-time RT–PCR. As shown in Fig. 6, mRNA levels for both TNF-α and IL-6 in the colons of DSS-treated mice were increased significantly 7 days after beginning DSS exposure. These increases were attenuated with glabridin administration (50 mg/kg) by 76% and 80%, respectively. The mRNA levels of iNOS and COX-2 in the DSS-treated mice also increased, while the glabridin-treated mice showed a 28% significant suppression of iNOS. On the other hand, the COX-2 mRNA level was not reduced significantly by glabridin treatment. As previous in vitro studies showed an inhibitory effect of glabridin on ICAM-1 expression in TNF-α-stimulated human umbilical vein endothelial cells [20], we also measured ICAM-1 mRNA expression. Markedly induced mRNA expression in the DSS-treated mice was suppressed significantly by glabridin treatment (Fig. 6e).
Fig. 6.
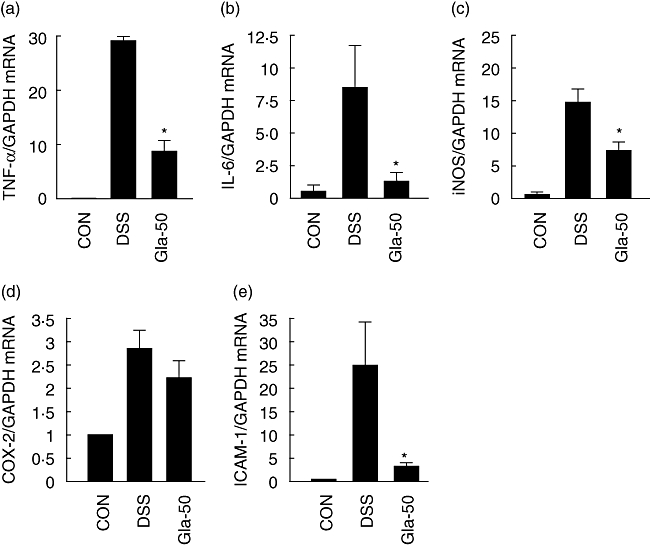
Colonic mRNA expression of (a) inducible NO synthase, (b) cyclooxygenase-2, (c) tumour necrosis factor-α, (d) interleukin-6 and (e) intercellular adhesion molecule-1 in colon of dextran sulphate sodium (DSS)-induced colitis in glabridin-treated mice. Colon in DSS-administered glabridin-treated mice and DSS-treated control mice was prepared on day 7 after DSS exposure and real-time reverse transcription–polymerase chain reaction was performed as described in Materials and methods. Values are mean ± standard error of the mean (n = 5). *Different from DSS group, P < 0·05.
Therapeutic effect of glabridin in DSS-induced colitis
The studies described above suggested that glabridin prevents colitis induced by DSS via controlled production of inflammatory mediators and enzymatic activities. Next, we examined the therapeutic effects of glabridin in the DSS-induced colitis model. In order to investigate the therapeutic effects, glabridin treatment was started at 7 days after DSS consumption. A summary of therapeutic effect in DSS-induced colitis is demonstrated in Fig. 7. In these experiments, untreated mice lost 20% of their original body weight on day 9 from colitis induction (Fig. 7a). In contrast, 10 and 50 mg/kg glabridin-treated mice suffered only 10% and 5% weight loss, respectively, in comparison to no treatment and, by day 12, regained their original weight (Fig. 7a). In addition, significantly decreased DAI and inhibited shortened colon length were shown in the glabridin-administered group (Fig. 7b,c). Finally, elevated MPO activity by DSS treatment was reduced significantly by glabridin administration at days 14 and 21 (Fig. 7d).
Fig. 7.
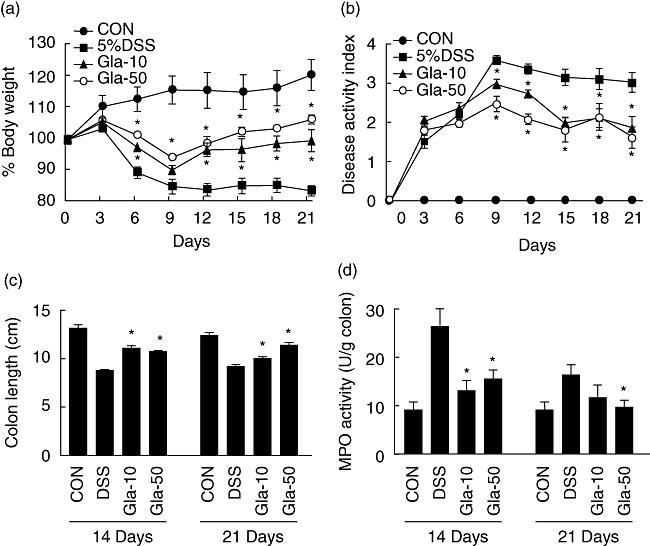
Therapeutic effect of glabridin in dextran sulphate sodium (DSS)-induced colitis. (a) body weight, (b) disease activity index, (c) colon length and (d) myeloperoxidase activity of glabridin-treated mice and control mice administered 5% DSS. Each point represents the mean ± standard error of the mean of three independent experiments. *Different from DSS group, P < 0·05.
Discussion
Glabridin is a polyphenolic flavonoid and a main constituent in the hydrophobic fraction of liquorice extract, and is known to have a variety of beneficial effects, including anti-microbial, anti-inflammatory, anti-atherosclerotic and anti-nephritic activities [20–25]. In terms of its anti-inflammatory effects, glabridin inhibits ICAM-1, which is involved liquorice in interactions with β2 integrins during inflammatory responses and inhibits PGE2 synthesis, NO production and iNOS gene expression in lipopolysaccharide (LPS)-stimulated macrophages [20,24,25]. In addition, in vivo studies using a septic shock model demonstrated that increases in plasma levels of NO and TNF-α by LPS were down-regulated by glabridin treatment [25]. Based on these results, and our previous study showing that the ethanolic extract of liquorice has strong anti-inflammatory effects [14], we hypothesized that glabridin has pharmacological activity to treat inflammatory responses in IBD.
In order to investigate the effect of glabridin on colonic inflammation, we selected a DSS-induced colitis model for the present study. This model exhibits many symptoms similar to those seen in human UC, such as diarrhoea, bloody faeces, body weight loss, mucosal ulceration and shortening of the colon [1,2,19]. In addition, the DSS-induced colitis animal model has a number of advantages over others, such as simple experimental methods, reproducibility of the time–course of development as well as colitis severity among individual mice, and relative uniformity of the induced lesions [15–19]. Therefore, this model is thought to be reliable for studying the pathogenesis of UC and for testing drugs or phytochemicals for treatment [1,4,15,26].
In this study, we have shown that administration of glabridin suppressed NO and PGE2 production, as well as iNOS and COX-2 protein expression in the DSS-induced colitis model. iNOS expression is regulated by several proinflammatory cytokines, and functions to generate high levels of NO via the oxidative metabolism of l-arginine. NO activates guanylate cyclases, enhancing cyclic GMP synthesis, and is involved in various functions that include vasodilatation, inhibition of platelet aggregation, skeletal muscle contractility and host defence [27–29]. In addition, NO is a highly reactive free radical, and can react rapidly with active oxygen species to generate peroxynitrite and cause severe detrimental effects [28]. Recent data demonstrate that local increases in iNOS expression correlate with increased severity of IBD [27,30]. Based on these results, the reduced iNOS expression in the colonic tissues of glabridin-administered mice represents a possible means for decreasing the severity of IBD.
Multiple lines of evidence suggest a significant role for PGE2 in IBD [31–33]. For example, increased levels of PGE2 are found at sites of intestinal inflammation and correlate with disease activity [31,32]. Non-steroidal anti-inflammatory drugs, which are prototypic inhibitors of COX activity and, therefore, PGE2 synthesis, can exacerbate or trigger IBD [6,26,33]. Experimental studies have also suggested an important role for PGE2. Both COX-1−/− and COX-2−/− mice showed increased susceptibility to DSS-induced colonic epithelial injury [34]. Recently, mice engineered to be deficient in EP4, a receptor for PGE2, were found to be sensitized to developing experimental colitis; treatment with an EP4 receptor agonist ameliorated colitis in wild-type mice [35]. In the present study, although COX-2 mRNA levels were not affected, expression of PGE2 and COX-2 was decreased in colon tissue by glabridin administration.
MPO is an enzyme found in neutrophils and its activity in the colon is related linearly to neutrophil infiltration. The assessment of MPO activity is well established for quantifying intestinal inflammation [18]. In inflammatory conditions such as IBD, the neutrophil levels of inflamed tissues, and consequently the MPO enzyme, increase. The activity of MPO, which is a major enzyme in the formation of ROS leading to tissue damage, increased in this colitis model. As oxidative stress is believed to play a key role in the pathogenesis of IBD-related intestinal damage [1–3,17,18], MPO is involved in the pathogenesis of IBD together with enhanced NO levels. The suppression of MPO activity by glabridin treatment in the DSS-induced colitic colon is effective for the inhibition of IBD progression.
Once the mucosal barrier is breached by DSS exposure, the submucosa is exposed to a vast pool of luminal antigens, including foods and bacteria, and innate immune responses are engaged to produce large amounts of cytokines. Analysis of inflammatory cytokine production by colon tissue culture and real-time RT–PCR revealed significant elevations of TNF-α and IL-6 in mice treated with DSS, compared to the glabridin-administered mice. Human and animal studies support the idea that TNF-α and IL-6 are important pathological mediators of IBD [8–12]. In humans with IBD, approximately two-thirds of patients respond to anti-TNF-α treatments [36], and in mice intestinal inflammation can be attenuated significantly by anti-IL-6 and/or anti-TNF-α monoclonal antibodies [37,38]. TNF-α and IL-6 production in the colons of DSS-exposed mice were substantially higher at 14 days post-DSS than in the glabridin-administered mice, which is consistent with the observed delay in recovery from inflammation in these mice. Reduced inflammatory cytokine expression corresponded with decreased susceptibility and prompted recovery for the glabridin- administered mice.
The exact molecular target(s) of glabridin in the DSS-induced colitic colon is still unknown. However, LPS-induced nuclear factor (NF)-kB/Rel DNA binding activity and NF-kB/Rel-dependent reporter gene activity were inhibited significantly by glabridin in murine macrophages, which was mediated through the inhibition of inhibitory factor-kB degradation and p65 nuclear translocation [25]. Moreover, glabridin treatment of various cell lines has revealed that the inhibitory effect of glabridin on NF-kB/Rel activation is not cell type-specific, and both inducible and constitutive NF-kB/Rel activation were suppressed by glabridin treatment. Further study demonstrated that the TNF-α-induced phosphorylation of Akt and extracellular signal-regulated kinase (ERK) were blocked by glabridin treatment in human umbilical vein endothelial cells [23]. These results indicate that the inhibitory effect of glabridin on DSS-induced colonic inflammation might be mediated, at least in part, by inhibiting the Akt, ERK and NF-kB/Rel signalling pathways.
In conclusion, this study demonstrated that the degree of colitis caused by DSS administration is attenuated significantly by glabridin. The anti-inflammatory effects of glabridin are associated with a reduction in the (i) production of inflammatory mediators, NO, PGE2 and inflammatory cytokines; (ii) attenuation of the recruitment of neutrophils; and (iii) ultimate tissue injury. As a relatively non-toxic natural product combined with excellent anti-inflammatory activity, glabridin could be useful in controlling inflammatory disorders such as IBD.
Acknowledgments
This work was supported by grants from the Korea Research Foundation funded by the Korean Government (MOEHRD) (2006-F00058).
References
- 1.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 2.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 3.Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15:79–94. doi: 10.1128/CMR.15.1.79-94.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araki Y, Sugihara H, Hattori T. The free radical scavengers edaravone and tempol suppress experimental dextran sulfate sodium-induced colitis in mice. Int J Mol Med. 2006;17:331–4. [PubMed] [Google Scholar]
- 5.Kolios G, Valatas V, Ward SG. Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle. Immunology. 2004;113:427–37. doi: 10.1111/j.1365-2567.2004.01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonner GF. Using COX-2 inhibitors in IBD: anti-inflammatories inflame a controversy. Am J Gastroenterol. 2002;97:783–5. doi: 10.1111/j.1572-0241.2002.05592.x. [DOI] [PubMed] [Google Scholar]
- 7.Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297–306. doi: 10.1016/s0016-5085(98)70196-9. [DOI] [PubMed] [Google Scholar]
- 8.Rojas-Cartagena C, Flores I, Appleyard CB. Role of tumor necrosis factor receptors in an animal model of acute colitis. Cytokine. 2005;32:85–93. doi: 10.1016/j.cyto.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Ito R, Shin-Ya M, Kishida T, et al. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin Exp Immunol. 2006;146:330–8. doi: 10.1111/j.1365-2249.2006.03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atreya R, Neurath MF. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol. 2005;28:187–96. doi: 10.1385/CRIAI:28:3:187. [DOI] [PubMed] [Google Scholar]
- 11.Podolsky DK. Inflammatory bowel disease (1) N Engl J Med. 1991;325:928–37. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- 12.Podolsky DK. Inflammatory bowel disease (2) N Engl J Med. 1991;325:1008–16. doi: 10.1056/NEJM199110033251406. [DOI] [PubMed] [Google Scholar]
- 13.Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology. 2001;20:622–35. doi: 10.1053/gast.2001.22122. [DOI] [PubMed] [Google Scholar]
- 14.Kim JK, Oh SM, Kwon HS, Oh YS, Lim SS, Shin HK. Anti-inflammatory effect of roasted licorice extracts on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biochem Biophys Res Commun. 2006;345:1215–23. doi: 10.1016/j.bbrc.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 15.Copper HS, Murthy SNS, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;66:635–9. [PubMed] [Google Scholar]
- 16.Camuesco D, Galvez J, Nieto A, et al. Dietary olive oil supplemented with fish oil, rich in EPA and DHA (n-3) polyunsaturated fatty acids, attenuates colonic inflammation in rats with DSS-induced colitis. J Nutr. 2005;135:687–94. doi: 10.1093/jn/135.4.687. [DOI] [PubMed] [Google Scholar]
- 17.Ramakers JD, Verstege MI, Thuijls G, Te Velde AA, Mensink RP, Plat J. The PPARgamma agonist rosiglitazone impairs colonic inflammation in mice with experimental colitis. J Clin Immunol. 2007;27:275–83. doi: 10.1007/s10875-007-9074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity: assessment of inflammation in rat and hamster models. Gastroenterology. 1984;84:1344–50. [PubMed] [Google Scholar]
- 19.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 20.Kang JS, Yoon YD, Han MH, et al. Glabridin suppresses intercellular adhesion molecule-1 expression in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells by blocking sphingosine kinase pathway: implications of Akt, extracellular signal-regulated kinase, and nuclear factor-kappaB/Rel signaling pathways. Mol Pharmacol. 2006;69:941–9. doi: 10.1124/mol.105.017442. [DOI] [PubMed] [Google Scholar]
- 21.Yokota T, Nishio H, Kubota Y, Mizoguchi M. The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Res. 1998;11:355–61. doi: 10.1111/j.1600-0749.1998.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 22.Fukai T, Satoh K, Nomura T, Sakagami H. Preliminary evaluation of antinephritis and radical scavenging activities of glabridin from Glycyrrhiza glabra. Fitoterapia. 2003;74:624–9. doi: 10.1016/s0367-326x(03)00164-3. [DOI] [PubMed] [Google Scholar]
- 23.Fuhrman B, Buch S, Vaya J, et al. Licorice extract and its major polyphenol glabridin protect low-density lipoprotein against lipid peroxidation: in vitro and ex vivo studies in humans and in athero-sclerotic apolipoprotein E-deficient mice. Am J Clin Nutr. 1997;66:267–75. doi: 10.1093/ajcn/66.2.267. [DOI] [PubMed] [Google Scholar]
- 24.Choi EM. The licorice root derived isoflavan glabridin increases the function of osteoblastic MC3T3–E1 cells. Biochem Pharmacol. 2005;70:363–8. doi: 10.1016/j.bcp.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Kang JS, Yoon YD, Cho IJ, et al. Glabridin, an isoflavan from licorice root, inhibits inducible nitric oxide synthase expression and improves survival of mice in experimental model of septic shock. J Pharmacol Exp Ther. 2004;321:1187–94. doi: 10.1124/jpet.104.077107. [DOI] [PubMed] [Google Scholar]
- 26.Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporine. Dig Dis Sci. 1993;37:1722–34. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 27.Cross RK, Wilson KT. Nitric oxide in inflammatory bowel disease. Inflamm Bowel Dis. 2003;9:179–89. doi: 10.1097/00054725-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Pryor WA, Squadrito GL. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol. 1995;268:L699–722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 29.Shah YM, Morimura K, Gonzalez FJ. Expression of peroxisome proliferator-activated receptor-gamma in macrophage suppresses experimentally induced colitis. Am J Physiol Gastrointest Liver Physiol. 2007;29:G657–66. doi: 10.1152/ajpgi.00381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Logan RF. Inflammatory bowel disease incidence: up, down or unchanged? Gut. 1998;42:309–11. doi: 10.1136/gut.42.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carty E, De Brabander M, Feakins RM, Rampton DS. Measurement of in vivo rectal mucosal cytokine and eicosanoid production in ulcerative colitis using filter paper. Gut. 2000;46:487–92. doi: 10.1136/gut.46.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiercinska-Drapalo A, Flisiak R, Prokopowicz D. Effects of ulcerative colitis activity on plasma and mucosal prostaglandin E2 concentration. Prostaglandins Other Lipid Med. 1999;58:159–65. doi: 10.1016/s0090-6980(99)00032-5. [DOI] [PubMed] [Google Scholar]
- 33.Cipolla G, Crema F, Sacco S, Moro E, de Ponti F, Frigo G. Nonsteroidal anti-inflammatory drugs and inflammatory bowel disease: current perspectives. Pharmacol Res. 2002;46:1–6. doi: 10.1016/s1043-6618(02)00033-6. [DOI] [PubMed] [Google Scholar]
- 34.Morteau O, Morham SG, Sellon R, et al. Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J Clin Invest. 2000;105:469–78. doi: 10.1172/JCI6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabashima K, Saji T, Murata T, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002;109:883–93. doi: 10.1172/JCI14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289–98. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- 37.Ogata H, Hibi T. Cytokine and anti-cytokine therapies for inflammatory bowel disease. Curr Pharm Des. 2003;9:1107–13. doi: 10.2174/1381612033455035. [DOI] [PubMed] [Google Scholar]
- 38.Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]


