Abstract
The anti-inflammatory cytokine interleukin (IL)-10 plays an important role in the regulation of host-immune responses. Here we studied the role IL-10 plays in host responses to cytomegalovirus (CMV) infection. We demonstrate that manifestations of murine CMV (MCMV) disease are more severe in IL-10 knock-out mice, despite significantly reduced levels of viral replication. Cytokine analysis of serum revealed increased levels of interferon (IFN)-γ, monocyte chemotactic protein 1 (MCP-1) and IL-6, all of which are potent stimulators of inflammatory responses. Depletion of IFN-γ by monoclonal antibodies in IL-10 knock-out mice failed to improve the physical condition of the mice, while increasing viral replication. In contrast, serum levels of IL-6 in the knock-out animals were unaffected by IFN-γ depletion and remained significantly elevated early in the course of infection. These data suggest that increased weight loss observed in IL-10 knock-out mice may be attributed to the uncontrolled production of proinflammatory cytokines, including IL-6.
Keywords: cytokines, cytomegalovirus, interferon, interleukin-10, knock-out
Introduction
Human cytomegalovirus (CMV) is a major cause of morbidity and mortality, especially in immunocompromised patients, and new treatment options are needed urgently. Observational studies of transplant patients have suggested that interleukin (IL)-10 may play a role in protecting against the development of human CMV infection and/or curtailing the severity of disease [1], and IL-10 has been advocated as a possible therapeutic modality [2]. However, there is a growing awareness that detrimental host damage can emanate from the same immune responses necessary to combat invading microbes [3–6], suggesting that it is critical to understand the control of both the beneficial antiviral and detrimental host effects of proinflammatory cytokines and their modulation by IL-10 during CMV disease. To understand more clearly the role that IL-10 plays in host response to CMV infection, we studied cytokine responses in a murine model of CMV (MCMV), including the immune responses in mice deficient in IL-10.
IL-10 is a pleiotropic, immunomodulatory cytokine that may act as an anti-inflammatory agent with potent immunosuppressive actions and function to block proinflammatory cytokine synthesis and inhibit antigen presentation. The T helper 1 (Th1)–Th2 paradigm posits that host response to a variety of pathogens depends upon a balance between the cytokines characteristically produced by the two subsets of CD4+ cells. An important function of the Th2-type cytokine IL-10 is to down-regulate production of the Th1-type cytokine interferon (IFN)-γ, suggesting that IL-10 has a significant role in curtailing inflammatory host responses.
Previous studies using mouse models suggest that IL-10 functions to limit inflammation resulting from the host response to a variety of bacterial, fungal and parasitic diseases [5,7,8]. Because IL-10 is required for inhibition of the Th1 response, IL-10 knock-out (KO) mice may respond to antigenic challenge with increased production of proinflammatory Th1 cytokines. In some cases this will lead to increased disease resistance, but in other cases the exaggerated inflammatory response can damage the host and exacerbate immunopathology [9]. While the role of IL-10 has been well studied in a variety of bacterial and parasitic infections [2,5,10–20], the cytokine response to viral infection has been investigated less extensively [5]. We hypothesized that IL-10 might function to down-regulate IFN-γ or other proinflammatory cytokines in order to attenuate damage to the host during the inflammatory response and facilitate survival during MCMV infection.
Materials and methods
Mice
Five to 6-week-old female C57BL/6 mice were obtained from Harlan Sprague Dawley (Indianapolis, IN, USA). Five to 6-week old-female IL-10-deficient (IL-10 KO) animals were obtained from Jackson Laboratories (Bar Harbor, ME, USA). All animals were housed under specific pathogen-free conditions in barrier-filtered cages in our Association for Assessment and Accreditation of Laboratory Animal Care (AALAC)-approved facilities. Animal studies were approved by the Institutional Animal Care and Use Committee of the Lexington Veterans Affairs Medical Center.
Cytomegalovirus
The Smith strain of MCMV (originally obtained as a gift from M. C. Jordan MD) was maintained by salivary gland passage in BALB/c mice. Salivary gland homogenates (10% w/v) were prepared as described previously [21] and stored in RPMI-1640 (Gibco laboratories, Grand Island, NY, USA) with 10% fetal calf serum and 10% dimethylsulphoxide at −70°C. To establish MCMV infection, mice were injected intraperitoneally with 104 plaque-forming units (pfu) of virus, as determined by viral plaque assays for each batch of virus. Plaque assays were performed as described previously [21]. Briefly, 10% w/v homogenates of spleens were made in RPMI-1640. Serial dilutions of the homogenate were adsorbed onto semiconfluent layers of 3T3 fibroblasts. Primary plaques were counted following a 5–6-day incubation at 37°C.
In vivo depletion of IFN-γ
Rat anti-mouse IFN-γ monoclonal antibody (mAb) (IgG1) from clone R4–6A2 [22] was purified partially from culture supernatants generated using a hollow filter cartridge system (Cellmax; Spectrum Laboratories Inc., Rancho Dominguez, CA, USA) and tested for its neutralizing ability in vivo. For in vivo depletion of IFN-γ, mice were administered 200 μg three times per week by intraperitoneal injection, as described previously [22]
Cytokine assays
Mice were killed by CO2 narcosis and blood collected immediately by cardiac puncture, allowed to clot, and spun to collect serum that was stored at −70°C. Serum levels of tumour necrosis factor (TNF)-α, IFN-γ, IL-1α and -1β, IL-2, IL-4, IL-6 and IL-10 were assayed by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (R&D Systems, Minneapolis, MN, USA). Assays were performed in duplicate on serum samples from a minimum of five mice per time-point. Also, serum levels of IL-6, monocyte chemotactic protein 1 (MCP1), IFN-γ, TNF-α, IL-12p70 and IL-10 cytokine levels were determined using mouse inflammation cytokine cytometric bead array (CBA) kits (BD Pharmingen, San Diego, CA, USA) in accordance with the manufacturer's instructions. Samples were examined on a fluorescence activated cell sorter (FACSCalibur) cytofluorometer (BD Pharmingen).
Detection of cytokine mRNA
RNase protection assays were performed using the Riboquant multi-probe RNase protection assay system (BD Pharmingen). Synthesis of radiolabelled anti-sense probe was carried out in a 20 μl reaction containing 100 μCi [α-32P] uridine triphosphate (UTP) (3000 Ci/mmol, 10 mCi/ml, Amersham, Frieburg, Germany), dithiothreitol (DTT) (10 mM), RNase inhibitor (40 U), T7 RNA polymerase (20 U), guanine, adenosine, cytidine and uridine (GACU) pool [(UTP): 3·0 μM; guanosine triphosphate (GTP), adenosine triphosphate (ATP) and cytidine triphosphate (CTP): 140 mM each], transcription buffer (1× ) and 50 mg of template (mCK-2). Hybridization and digestion were carried out according to the manufacturer's instructions. The samples were purified by phenol/chloroform extraction and ethanol precipitation and resolved on a 5% acrylamide sequencing gel containing 8 M urea in 0·5× Tris–borate–ethylenediaminne tetraacetic acid (TBE) buffer. For quantification of cytokine mRNA, dried gels were exposed to a phosphorous imaging screen and analysed with a phosphor-Imager using ImageQuant software (Molecular Dynamics, Sunnyvale, CA, USA). Cytokine mRNA values are expressed as a ratio of the housekeeping genes L32 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to normalize the results.
Detection of intracellular cytokine production
Spleens were harvested and single cell suspensions of splenocytes prepared and counted. For FACS analysis, cells were cultured for 4 h with 50 ng/ml phorbol myristate acetate (PMA) and 500 ng/ml ionomycin, and in the presence of 10 μg/ml brefeldin A for the final 2 h 106 cells were incubated with anti-CD4-Cy-chrome (BD Pharmingen) and anti-CD8a-APC (BD Pharmingen) for 20 min on ice. Cells were then washed, fixed in 2% formaldehyde, permeabilized in 0·5% saponin and incubated with anti-IFN-γ-fluorescein isothiocyanate (FITC) (BD Pharmingen) and anti-IL-10-phycoerythrin (PE) (BD Pharmingen) for 20 min at room temperature. After washing and resuspension in phosphate-buffered saline (PBS), cells were analysed using a FACSCalibur (Becton-Dickinson, Mountain View, CA, USA) equipped with a red diode laser for four-colour analysis of at least 10 000 events.
Statistical analysis
Differences between experimental groups were determined using Student's t-test or analysis of variance (anova) as appropriate and were considered statistically significant when P < 0·05. SigmaStat statistical software (SPSS, Inc., Chicago, IL, USA) was used for all analyses.
Results
Serum IL-10 concentrations in immunocompetent mice during MCMV infection
To understand the role of IL-10 in response to MCMV infection, we first studied serum IL-10 levels in immunocompetent C57BL/6 mice during viral infection. Serum concentrations of IL-10 began to increase 2–3 days after viral infection and persisted at elevated levels to at least day 7, returning towards baseline over the following 7 days (Fig. 1). Lower serum levels were observed in mice receiving a lower dose of MCMV, but the kinetics of cytokine production were comparable (data not shown). In both scenarios, detectable serum levels of IL-10 first appeared as mice began to develop clinical symptoms (day 3), and persisted after mice had recovered from overt clinical illness (days 10–12).
Fig. 1.

Serum concentrations of interleukin (IL)-10 (pg/ml ± standard deviation) in C57BL/6 mice after murine cytomegalovirus (MCMV) infection. Each data point represents the results of enzyme-linked immunosorbent assays (ELISAs) performed in duplicate using serum from five to seven mice. Serum levels of IL-10 were significantly greater in MCMV-infected mice at days 1, 2, 3, 4 and 7 after infection compared to uninfected animals (*P < 0·01).
MCMV infection in IL-10 KO versus immunocompetent mice
We next studied the course of viral illness in IL-10 KO mice compared to immunocompetent mice. In response to MCMV infection, IL-10 KO mice lost more weight (Fig. 2). By day 3, IL-10 KO mice had lost a greater percentage of body weight than had their immunocompetent counterparts. The differential weight loss persisted through days 8–10 after MCMV infection. In concert with the loss of body weight, IL-10 KO mice also suffered more severe manifestations of clinical disease, including hunched behaviour, ruffled fur and inactivity (data not shown).
Fig. 2.

Percentage change in body weights of C57BL/6 immunocompetent (solid symbols) and interleukin (IL)-10 knock-out (KO) (open symbols) mice after murine cytomegalovirus (MCMV) infection (triangles) and in uninfected controls (circles). Each data point represents the change in body weight from baseline measured in five to 18 mice. Loss of body weight was significantly greater in IL-10 KO mice compared to immunocompetent mice infected with MCMV (*P < 0·05, immunocompetent versus IL-10 KO mice with MCMV infection).
Cytokine production in IL-10 KO versus immunocompetent mice
We hypothesized that aberrant cytokine responses might explain the increased weight loss in the IL-10 KO animals. Because a role of IL-10 in other disease models is to down-regulate Th1 cytokine responses including IFN-γ production and because our previous work suggested that IFN-γ can contribute to end-organ inflammation and damage during MCMV infection [21], we went on to determine cytokine responses to MCMV infection in the two groups of animals. We first studied serum concentrations of IL-10, IFN-γ and other proinflammatory cytokines (Fig. 3). As expected, immunocompetent animals produced IL-10 in response to MCMV infection [21], while IL-10 KO animals did not (Fig. 3a). Both immunocompetent and IL-10 KO mice demonstrated elevated serum levels of IFN-γ TNF-α and IL-6 (Fig. 3b–d), but not IL-2 or IL-4 (data not shown).
Fig. 3.

Serum cytokine concentrations (pg/ml ± standard deviation) in immunocompetent C57BL/6 mice (black bars) and interleukin (IL)-10 knock-out (KO) mice (grey bars) after murine cytomegalovirus (MCMV) infection. Cytokines studied were IL-10 (a), interferon (IFN)-γ (b), IL-6 (c) and tumour necrosis factor (TNF)-α (d). Results shown are from one of three sets of experimental animals; similar results were obtained in two additional experiments. Data represent the results of assays performed in duplicate using serum from five to 10 mice at each time-point. Cytokine levels of IL-10, IFN-γ and IL-6 were significantly different in the two groups (*P < 0·05); n.d.: time-points at where data were not available.
However, for several of the proinflammatory cytokines, serum concentrations were significantly different in the IL-10 KO mice compared with the control animals. IL-10 KO mice had slightly higher constitutive serum levels of IFN-γ than did immunocompetent mice (Fig. 3b, day 0). IL-10 KO mice had significantly greater serum concentrations of IFN-γ than control mice at 3, 5, 7 and 12 days after MCMV infection (Fig. 3b). By day 7 after infection, IFN-γ levels in control mice had returned to levels comparable to those observed at baseline in control mice. In contrast, serum IFN-γ levels in IL-10 KO mice continued to be elevated as late as 12 days after infection (Fig. 3b).
IL-6 serum levels were elevated in IL-10 KO mice following infection and were significantly greater in IL-10 KO mice at 12 days after MCMV infection (Fig. 3c). TNF-α serum levels were not consistently significantly different in the two groups of animals (Fig. 3d).
To characterize cytokine responses further during MCMV infection, we went on to determine splenic cytokine mRNA levels in immunocompetent and IL-10 KO mice (Fig. 4). As expected, IL-10 mRNA production was detected in response to MCMV infection in immunocompetent but not IL-10 KO mice (Fig. 4a). Consistent with the serum protein concentrations, IFN-γ mRNA was significantly greater in the IL-10 KO animals compared to the controls (Fig. 4b). IL-6 mRNA levels were greater in IL-10 KO mice 7 days after infection (Fig. 4c), and in other experiments at 5 and 7 days after infection (data not shown). Analogous to the findings for serum protein concentrations, TNF-α mRNA levels were not consistently different in the two groups of animals (Fig. 4d); however, IL-10 KO animals showed reduced message for TNF-α at 12 days post-infection. Thus, MCMV infection in IL-10 KO mice was characterized by exaggerated and prolonged production of IFN-γ and IL-6.
Fig. 4.

Cytokine mRNA detection (relative mRNA concentration ± standard deviation) in immunocompetent C57BL/6 mice (black bars) and interleukin (IL)-10 knock-out (KO) mice (grey bars) after murine cytomegalovirus (MCMV) infection. Cytokines studied were IL-10 (a), interferon (IFN)-γ (b), IL-6 (c) and tumour necrosis factor (TNF)-α (d). Results shown are from one of three sets of experimental animals; similar results were obtained in two additional experiments. Data represents the results of assays performed in duplicate using spleens from five to 10 mice at each time-point. Cytokine mRNA levels of IL-10, IFN-γ and IL-6 were significantly different in the two groups (*P < 0·05).
Cell types responsible for aberrant IFN-γ production
To identify the cells responsible for the aberrant IFN-γ production observed in MCMV-infected IL-10 KO mice, we assessed intracellular cytokines in CD4+ and CD8+ splenocytes. Although numbers of CD4+ cells producing IFN-γ increased in both groups of animals at day 7 after MCMV infection, the number of CD4+ cells producing IFN-γ was significantly greater in IL-10 KO mice than in the immunocompetent animals (Fig. 5a). At day 7 after MCMV infection, the numbers of CD4+, IFN-γ-producing splenic cells was fourfold greater than at baseline in control (infected) animals, but more than 10-fold greater in IL-10 KO animals. Similar results were obtained in experiments using two additional sets of animals. The number of CD8+ cells producing IFN-γ in response to MCMV infection increased at days 7 and 14 in both groups of animals, but significantly disproportionately increased numbers of CD8+ IFN-γ cells in IL-10 KO animals were detected only at day 14 (Fig. 5b).
Fig. 5.

Detection of intracellular interferon (IFN)-γ in CD4+ (a) and CD8+ (b) splenocytes (number of cells ± standard deviation) from C57BL/6 immunocompetent (black bars) and interleukin (IL)-10 knock-out (KO) (grey bars) mice after murine cytomegalovirus (MCMV) infection. On day 7 after MCMV infection, the number of CD4+/IFN-γ+ cells was significantly greater in the IL-10 KO mice compared to immunocompetent animals (*P < 0·05); and on day 14 after MCMV infection, the number of CD8+/IFN-γ+ cells was significantly greater in the IL-10 KO mice compared to immunocompetent animals (*P < 0·05).
Given the detection of aberrant production of IFN-γ as early as 3 days after infection, we postulated that natural killer (NK) cells might also be a source of the increased IFN-γ production in IL-10 KO mice. However, FACS analysis revealed that the number of CD4–, CD8– cells producing IFN-γ was <1% at days 0, 3, 7 and 14 after infection and that there was no difference in the number of these cells in the two groups of animals at any time-point (data not shown).
Depletion of IFN-γ in IL-10 KO animals
We hypothesized that the increased morbidity seen in the IL-10 KO mice following primary infection with MCMV was attributed to the unopposed production of IFN-γ in these mice. To define further the role of IFN-γ in the development of disease in IL-10 KO mice, we neutralized circulating IFN-γ with specific antibodies. Following treatment with the antibodies, serum levels of IFN-γ were depleted significantly within 3 days (data not shown), and administration of antibody every third day was sufficient to keep levels of IFN-γ suppressed.
We then characterized disease progression in IL-10 KO mice treated with and without anti-IFN-γ antibody. Despite significantly reduced levels of serum IFN-γ in treated IL-10 KO mice, we did not observe any attenuation of morbidity, as assessed by weight loss. Serum cytokine analyses revealed that levels of serum MCP-1 were increased significantly in IL-10 KO animals compared to wild-type controls, and that following treatment with IFN-γ antibodies, serum levels of MCP-1 were reduced to undetectable levels (Fig. 6a). Please note that wild-type animals were matched to the availability of IL-10 KO animals. In contrast, however, elevated serum IL-6 concentrations observed from day 3 in IL-10 KO mice in this experiment remained elevated following IFN-γ depletion (Fig. 6b). These data demonstrate that elevated serum IL-6 levels correlate with increased weight loss observed in animals deficient in IL-10. Weight loss in IL-10 KO mice was significantly greater than in wild-type controls whether treated or not with neutralizing IFN-γ antibodies (Fig. 7a).
Fig. 6.
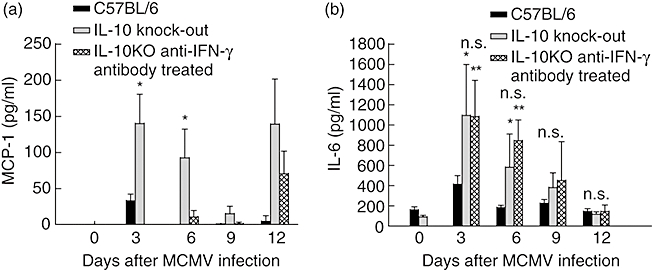
Detection of serum interleukin (IL)-6 (a) and monocyte chemotactic protein 1 (MCP-1) (b) concentrations (pg/ml ± standard deviation) in immunocompetent C57BL/6 mice (black filled), IL-10 knock-out (KO) mice (grey filled) and IL-10 KO mice treated with interferon (IFN)-γ neutralizing antibody every third day starting at day 3 (checkered filled). Results shown are means of four to five animals per group. Levels of IL-6 are significantly greater in IL-10 KO mice at days 3 and 5 post-infection compared to normal controls (*P < 0·05). By days 7 and 12 post-infection there was no statistical difference (n.s.) between any of the groups. Treatment of IL-10 KO animals with IFN-γ neutralizing antibody had no effect on IL-6 levels throughout the course of these experiments. Serum MCP-1 levels were increased significantly in IL-10 KO animals; treatment with IFN-γ neutralizing antibody reduced levels to that below detectable levels.
Fig. 7.

(a) Percentage change in body weights of C57BL/6 immunocompetent (solid circles), interleukin (IL)-10 knock-out (KO) (open circles) and anti-interferon (IFN)-γ-treated IL-10 KO (solid triangles) mice after murine cytomegalovirus (MCMV) infection. Each data point represents the change in body weight from baseline measured in five to 18 mice. Loss of body weight was significantly greater in IL-10 KO mice at days 4, 5 and 7 post-infection compared to immunocompetent C57BL/6 mice infected with MCMV (*P < 0·05). No differences were noted in anti-IFN-γ-treated mice compared to untreated IL-10 KO mice. (b) Viral titres of spleens from adult C57BL/6 (black filled), IL-10 KO (grey filled) and IL-10 KO mice treated with anti-IFN-γ monoclonal antibody (200 mg) (checkered filled). Mice were injected intraperitoneally with 5 × 104 plaque-forming units of MCMV; 10% w/v spleen suspensions were quantified by the plaque assay method using CCL163 fibroblasts. Each data point represents the mean ± standard deviation of three animals. At all time-points, IL-10 KO mice showed significantly decreased levels of viral replication compared to C57BL/6 mice (*P < 0·05). IL-10 KO mice that received anti-IFN-γ antibody showed significantly increased levels of viral replication compared to non-treated IL-10 KO mice (**P < 0·05) as early as 3 days post-treatment (6 days post-infection). Continued treatment of IL-10 KO mice with anti-IFN-γ antibody led to significantly increased levels of viral replication at days 9 and 12 post-infection.
Viral replication in IL-10 KO versus immunocompetent mice
To determine if the increased severity of MCMV disease in IL-10 KO mice could be attributed to differences in viral replication, we assessed viral replication in the spleens of C57BL/6- and IL-10 KO-infected animals (Fig. 7b). Viral replication in C57BL/6 mice peaked at day 9 post-infection, while viral replication in IL-10 KO mice was significantly lower than that of control mice.
Levels of viral replication in IL-10 KO animals were at the limit of detection for the assay (1 × 102 pfu/g). The importance of IFN-γ-secreting cells on the control of initial infection has been reported previously [23]. IL-10 KO animals demonstrate increased IFN-γ secretion and ultimately reduced viral titres in tissues following initial infection. Treatment of IL-10 KO mice with neutralizing antibodies for IFN-γ inhibited the enhanced ability of the IL-10 KO mice to clear the virus, resulting in increased levels of viral replication compared to that of untreated IL-10 KO mice. After only 3 days of treatment with anti-IFN-γ antibody, mice already exhibited increased viral replication. In these animals, viral replication peaked at 12 days post-infection, 9 days post-anti-IFN-γ antibody treatment.
Discussion
Our studies demonstrate that the normal host response to MCMV infection is characterized by production of proinflammatory cytokines such as IFN-γ, IL-1α, IL-1β, TNF-α and IL-6. These results are consistent with previous studies demonstrating that IFN-γ, TNF-α, IL-12 and IFN-α/β are produced in response to MCMV infection and are critical to effective anti-viral defences [24–33]. Production of proinflammatory cytokines, especially IFN-γ, in response to MCMV and their importance in control of MCMV replication is demonstrated here and is also supported by previous in vitro and in vivo studies [21,34,35]. However, little is known about the mechanisms by which the production of proinflammatory cytokines is curtailed after effective host response to MCMV infection; our studies were designed to address this question.
Our studies show that IL-10 is produced quickly in response to MCMV infection in the normal host. We found that serum IL-10 levels and mRNA production were increased in response to the viral infection. These findings are consistent with previous reports of production of IL-10 in MCMV-infected mice [36,37] and accelerated in vitro production of IL-10 by macrophages exposed to MCMV [38]. Our current studies demonstrate that increased IL-10 levels persist throughout the time of recovery from clinically evident illness.
The established ability of IL-10 to down-regulate IFN-γ suggested to us that IL-10 might contribute to recovery from MCMV disease by modulating production of IFN-γ and other proinflammatory cytokines.
To define more clearly the role of IL-10 in MCMV infection, we studied the course of the viral disease in mice deficient in IL-10. We found that IL-10 KO mice had more severe disease due to MCMV. Importantly, these differences were not attributable to increases in viral replication, as IL-10 KO mice had significantly reduced viral loads as measured by plaque assay of spleens. Instead, the increased morbidity occurred in concert with exuberant and prolonged production of proinflammatory cytokines, especially IFN-γ, IL-1 and IL-6. Our findings are consistent with previous observations that proinflammatory cytokines can contribute to host damage occurring during MCMV infection. Previously, we have reported the contribution of IFN-γ in the exacerbation of symptomatic manifestations in MCMV disease [21]. In addition, IL-1 and/or TNF-α contribute to acinar necrosis in a model of MCMV-induced pancreatitis [39]. Also, recent studies have indicated a role for IL-6 in CMV-associated inflammatory bowel disease [6]. The results of the current studies demonstrate increased and prolonged production of proinflammatory cytokines, including IFN-γ, IL-6 and IL-1α and 1β, during MCMV infection in the absence of IL-10. Therefore, we conclude that aberrant cytokine responses in MCMV-infected IL-10 KO mice mediate increased host pathology via unopposed production of proinflammatory cytokines.
To define further the role of IFN-γ in the exacerbated disease seen in IL-10 KO mice, we investigated the disease progression in IL-10 KO mice treated with neutralizing monoclonal antibodies for IFN-γ. Treatment with anti-IFN-γ antibodies reduced serum levels of IFN-γ and MCP-1 successfully to below detectable levels. However, neutralization of IFN-γ did not attenuate the severity of MCMV disease. We observed no differences in clinical disease progression in IL-10 mice whether or not they were treated treated with neutralizing antibody for IFN-γ. Interestingly, IL-10 KO mice treated with neutralizing antibody showed increased viral load shortly after the first treatment. More important was that the viral load remained elevated as late as 12 days post-infection. This is consistent with the crucial role for IFN-γ in controlling CMV viral replication [40]. However, we did not observe exacerbated disease in these IL-10 KO animals treated with anti-IFN-γ antibody. More recent work [41] has shown that blockade of the IL-10 receptor led to an increase in IFN-γ-secreting cells and a subsequent decrease in viral load, similar to our findings (Fig. 5a). Interestingly, levels of IL-6 were not reduced in anti-IFN-γ treated mice, and that these mice lost weight comparable to infected IL-10 KO animals suggesting a role for IL-6, not IFN-γ or MCP-1, in the weight loss associated with MCMV disease.
Taken together, our studies suggest that host response to MCMV infection reflects a balance between the protective antiviral defences mediated by proinflammatory cytokines and the modulating effect of anti-inflammatory cytokines such as IL-10 to protect the host from collateral damage caused by anti-microbial cytokines [3,5]. This balance would determine the effectiveness of the anti-viral activity and the extent of the detrimental impact of the inflammatory response on the host.
Previous work in other models of infections has demonstrated that in some cases attenuation of the inflammatory response by IL-10 is important to protect the host [11,13,14,16,17,42], but in other scenarios can impair host anti-microbial defences [43–45]. The situation is probably the same in host defence against some viruses. In recent studies of MCMV infection, susceptible strains of mice that had greater levels of viral DNA in the lungs also had greater lung IL-10 mRNA levels than did resistant strains of mice [37], suggesting that IL-10 may interfere with the initial host response and control of viral replication. Treatment with IL-10 in a mouse model of viral myocarditis attenuates disease severity [46] and suppresses stromal keratitis due to herpes simplex virus [47]. IL-10 KO mice infected with mouse hepatitis virus experience increased central nervous system damage, due presumably to unopposed proinflammatory cytokines [48]. We now present data demonstrating that in MCMV, the protective role of IL-10 is predominant and critical to limiting host inflammation. Our finding of significantly reduced MCMV replication but increased morbidity and proinflammatory cytokine production in IL-10 KO mice suggests that, in this infection, IL-10 mediates collateral damage successfully by proinflammatory cytokines, even though it reduces the effectiveness of the host immune response in controlling viral replication. Thus, we demonstrate that interfering with the delicate cytokine balance (by deleting IL-10 responses) results in detrimental effects to the host.
It is clear that host responses to microbial insults are not controlled simply by individual processes, and that interference in a specific cytokine response has a significant effect on the overall immune responses. These experiments demonstrate that levels of IFN-γ and other proinflammatory cytokines are increased dramatically in IL-10 KO mice, and that these levels correlate with disease severity. However, the failure to reduce morbidity by neutralizing IFN-γ suggests that it is not IFN-γ, and is most probably a combination of the other cytokines identified in these studies that contribute to the development of disease morbidity.
Observations of cytokine responses in immunocompromised patients with CMV infections have raised intriguing questions about the role of cytokines in host response to human CMV. In renal transplant recipients, patients with CMV infection have elevated TNF-α and IL-10 levels [1]. Importantly, patients with active CMV disease had a more moderate IL-10 increase than did those with asymptomatic infection, suggesting that IL-10 might have beneficial effects in CMV-infected patients. The authors hypothesized that this benefit reflected a down-regulatory effect of IL-10 on pathogenic effects of proinflammatory cytokines. In a study of bone marrow transplant patients, those with CMV infection had higher serum levels of TNF-α and IL-6, with the highest levels of IL-6 observed in patients with active disease [49], leading the investigators to speculate that up-regulation of proinflammatory cytokines might play a role in the dissemination of CMV. Recent reports showing that CMV produces IL-10 homologues actively [50,51] would indicate some advantages to the virus for up-regulation of proinflammatory cytokines. Analogous observations in liver transplant patients have demonstrated a relationship between elevated TNF-α and IL-6 levels and risk of CMV disease [52]. Unfortunately, IL-10 production was not assessed in these two later studies. Nevertheless, these human studies suggest that a balance of the activity of both proinflammatory and anti-inflammatory cytokines are critical to effective host response to the virus [53], and that IL-10 may play a protective role by down-regulating proinflammatory cytokines, analogous to the findings in our mouse model of CMV disease. Our results highlight the importance of understanding completely the impact of cytokines such as IL-10 on both beneficial host defence and detrimental host damage when embarking on attempts to design immunomodulatory therapies.
Acknowledgments
This work was supported in part by funds from the Merit Review Program, Department of Veterans Affairs. The authors thank Kevin Schuer, Wayne Young and Whitney Durham for technical assistance. This work was supported by Public Health Service grants from the National Institutes of Health (HL-62053 and HL-64524) to B. A. G. and a VA Merit Review Grant to C. P.
References
- 1.Nordoy I, Muller F, Nordal KP, et al. The role of the tumor necrosis factor system and interleukin-10 during cytomegalovirus infection in renal transplant recipients. J Infect Dis. 2000;181:51–7. doi: 10.1086/315184. [DOI] [PubMed] [Google Scholar]
- 2.Opal SM, Wherry JC, Grint P. Interleukin-10: potential benefits and possible risks in clinical infectious diseases. Clin Infect Dis. 1998;27:1497–507. doi: 10.1086/515032. [DOI] [PubMed] [Google Scholar]
- 3.Casadevall A, Pirofski LA. Host–pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun. 1999;67:3703–13. doi: 10.1128/iai.67.8.3703-3713.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang HR, Bistrian B. The role of cytokines in the catabolic consequences of infection and injury. J Parenter Enteral Nutr. 1998;22:156–66. doi: 10.1177/0148607198022003156. [DOI] [PubMed] [Google Scholar]
- 5.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 6.Rahbar A, Bostrom L, Lagerstedt U, Magnusson I, Soderberg-Naucler C, Sundqvist VA. Evidence of active cytomegalovirus infection and increased production of IL-6 in tissue specimens obtained from patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2003;9:154–61. doi: 10.1097/00054725-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 8.Mosmann TR. Properties and functions of interleukin-10. Adv Immunol. 1994;56:1–26. [PubMed] [Google Scholar]
- 9.Rennick D, Davidson N, Berg D. Interleukin-10 gene knock-out mice: a model of chronic inflammation. Clin Immunol Immunopathol. 1995;76:S174–8. doi: 10.1016/s0090-1229(95)90144-2. [DOI] [PubMed] [Google Scholar]
- 10.Standiford TJ, Tsai WC, Mehrad B, Moore TA. Cytokines as targets of immunotherapy in bacterial pneumonia. J Lab Clin Med. 2000;135:129–38. doi: 10.1067/mlc.2000.103196. [DOI] [PubMed] [Google Scholar]
- 11.Howard M, Muchamuel T, Andrade S, Menon S. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993;177:1205–8. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchant A, Bruyns C, Vandenabeele P, et al. Interleukin-10 controls interferon-gamma and tumor necrosis factor production during experimental endotoxemia. Eur J Immunol. 1994;24:1167–71. doi: 10.1002/eji.1830240524. [DOI] [PubMed] [Google Scholar]
- 13.Standiford TJ, Strieter RM, Lukacs NW, Kunkel SL. Neutralization of IL-10 increases lethality in endotoxemia. Cooperative effects of macrophage inflammatory protein-2 and tumor necrosis factor. J Immunol. 1995;155:2222–9. [PubMed] [Google Scholar]
- 14.Chmiel JF, Konstan MW, Knesebeck JE, et al. IL-10 attenuates excessive inflammation in chronic Pseudomonas infection in mice. Am J Respir Crit Care Med. 1999;160:2040–7. doi: 10.1164/ajrccm.160.6.9901043. [DOI] [PubMed] [Google Scholar]
- 15.Cusumano V, Genovese F, Mancuso G, Carbone M, Fera MT, Teti G. Interleukin-10 protects neonatal mice from lethal group B streptococcal infection. Infect Immun. 1996;64:2850–2. doi: 10.1128/iai.64.7.2850-2852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawa T, Corry DB, Gropper MA, Ohara M, Kurahashi K, Wiener-Kronish JP. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J Immunol. 1997;159:2858–66. [PubMed] [Google Scholar]
- 17.Gazzinelli RT, Wysocka M, Hieny S, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 18.Suzuki Y, Sher A, Yap G, et al. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J Immunol. 2000;164:5375–82. doi: 10.4049/jimmunol.164.10.5375. [DOI] [PubMed] [Google Scholar]
- 19.Holscher C, Mohrs M, Dai WJ, et al. Tumor necrosis factor alpha-mediated toxic shock in Trypanosoma cruzi-infected interleukin 10-deficient mice. Infect Immun. 2000;68:4075–83. doi: 10.1128/iai.68.7.4075-4083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter CA, Ellis-Neyes LA, Slifer T, et al. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol. 1997;158:3311–16. [PubMed] [Google Scholar]
- 21.Pomeroy C, Delong D, Clabots C, Riciputi P, Filice GA. Role of interferon-gamma in murine cytomegalovirus infection. J Lab Clin Med. 1998;132:124–33. doi: 10.1016/s0022-2143(98)90007-5. [DOI] [PubMed] [Google Scholar]
- 22.Havell EA. Purification and further characterization of an anti-murine interferon-gamma monoclonal neutralizing antibody. J Interferon Res. 1986;6:489–97. doi: 10.1089/jir.1986.6.489. [DOI] [PubMed] [Google Scholar]
- 23.Daniels KA, Devora G, Lai WC, O'Donnell CL, Bennett M, Welsh RM. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J Exp Med. 2001;194:29–44. doi: 10.1084/jem.194.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carr JA, Rogerson JA, Mulqueen MJ, Roberts NA, Nash AA. The role of endogenous interleukin-12 in resistance to murine cytomegalovirus (MCMV) infection and a novel action for endogenous IL-12 p40. J Interferon Cytokine Res. 1999;19:1145–52. doi: 10.1089/107999099313082. [DOI] [PubMed] [Google Scholar]
- 25.Heise MT, Virgin HW. The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J Virol. 1995;69:904–9. doi: 10.1128/jvi.69.2.904-909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucin P, Pavic I, Polic B, Jonjic S, Koszinowski UH. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J Virol. 1992;66:1977–84. doi: 10.1128/jvi.66.4.1977-1984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orange JS, Biron CA. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–42. [PubMed] [Google Scholar]
- 28.Orange JS, Biron CA. Characterization of early IL-12, IFN-alphabeta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol. 1996;156:4746–56. [PubMed] [Google Scholar]
- 29.Orange JS, Wang B, Terhorst C, Biron CA. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med. 1995;182:1045–56. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polic B, Hengel H, Krmpotic A, et al. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J Exp Med. 1998;188:1047–54. doi: 10.1084/jem.188.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruzek MC, Miller AH, Opal SM, Pearce BD, Biron CA. Characterization of early cytokine responses and an interleukin (IL)-6-dependent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J Exp Med. 1997;185:1185–92. doi: 10.1084/jem.185.7.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shanley JD, Goff E, Debs RJ, Forman SJ. The role of tumor necrosis factor-alpha in acute murine cytomegalovirus infection in BALB/c mice. J Infect Dis. 1994;169:1088–91. doi: 10.1093/infdis/169.5.1088. [DOI] [PubMed] [Google Scholar]
- 33.Yerkovich ST, Olver SD, Lenzo JC, Peacock CD, Price P. The roles of tumour necrosis factor-alpha, interleukin-1 and interleukin-12 in murine cytomegalovirus infection. Immunology. 1997;91:45–52. doi: 10.1046/j.1365-2567.1997.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schut RL, Gekker G, Hu S, et al. Cytomegalovirus replication in murine microglial cell cultures: suppression of permissive infection by interferon-gamma. J Infect Dis. 1994;169:1092–6. doi: 10.1093/infdis/169.5.1092. [DOI] [PubMed] [Google Scholar]
- 35.Shanley JD, Shanley JA, Albert G, Biegel D. Characterization of virus-induced interferon-gamma responses in mice previously infected with murine cytomegalovirus. J Infect Dis. 2001;183:697–706. doi: 10.1086/318830. [DOI] [PubMed] [Google Scholar]
- 36.Ruzek MC, Pearce BD, Miller AH, Biron CA. Endogenous glucocorticoids protect against cytokine-mediated lethality during viral infection. J Immunol. 1999;162:3527–33. [PubMed] [Google Scholar]
- 37.Geist LJ, Hinde SL. Susceptibility to cytomegalovirus infection may be dependent on the cytokine response to the virus. J Invest Med. 2001;49:434–41. doi: 10.2310/6650.2001.33788. [DOI] [PubMed] [Google Scholar]
- 38.Redpath S, Angulo A, Gascoigne NR, Ghazal P. Murine cytomegalovirus infection down-regulates MHC class II expression on macrophages by induction of IL-10. J Immunol. 1999;162:6701–7. [PubMed] [Google Scholar]
- 39.Price P, Baxter AG, Allcock RN, Papadimitriou JM. Factors influencing the effects of murine cytomegalovirus on the pancreas. Eur J Clin Invest. 1998;28:546–53. doi: 10.1046/j.1365-2362.1998.00314.x. [DOI] [PubMed] [Google Scholar]
- 40.Gamadia LE, Remmerswaal EB, Weel JF, Bemelman F, van Lier RA, Ten Berge IJ. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood. 2003;101:2686–92. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- 41.Humphreys IR, de Trez C, Kinkade A, Benedict CA, Croft M, Ware CF. Cytomegalovirus exploits IL-10-mediated immune regulation in the salivary glands. J Exp Med. 2007;204:1217–25. doi: 10.1084/jem.20062424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sasaki S, Nishikawa S, Miura T, et al. Interleukin-4 and interleukin-10 are involved in host resistance to Staphylococcus aureus infection through regulation of gamma interferon. Infect Immun. 2000;68:2424–30. doi: 10.1128/iai.68.5.2424-2430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai WJ, Kohler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–67. [PubMed] [Google Scholar]
- 44.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995;155:722–9. [PubMed] [Google Scholar]
- 45.van der Poll T, Marchant A, Keogh CV, Goldman M, Lowry SF. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J Infect Dis. 1996;174:994–1000. doi: 10.1093/infdis/174.5.994. [DOI] [PubMed] [Google Scholar]
- 46.Nishio R, Matsumori A, Shioi T, Ishida H, Sasayama S. Treatment of experimental viral myocarditis with interleukin-10. Circulation. 1999;100:1102–8. doi: 10.1161/01.cir.100.10.1102. [DOI] [PubMed] [Google Scholar]
- 47.Tumpey TM, Elner VM, Chen SH, Oakes JE, Lausch RN. Interleukin-10 treatment can suppress stromal keratitis induced by herpes simplex virus type 1. J Immunol. 1994;153:2258–65. [PubMed] [Google Scholar]
- 48.Lin MT, Hinton DR, Parra B, Stohlman SA, van der Veen RC. The role of IL-10 in mouse hepatitis virus-induced demyelinating encephalomyelitis. Virology. 1998;245:270–80. doi: 10.1006/viro.1998.9170. [DOI] [PubMed] [Google Scholar]
- 49.Humar A, St Louis P, Mazzulli T, et al. Elevated serum cytokines are associated with cytomegalovirus infection and disease in bone marrow transplant recipients. J Infect Dis. 1999;179:484–8. doi: 10.1086/314602. [DOI] [PubMed] [Google Scholar]
- 50.Spencer JV, Lockridge KM, Barry PA, et al. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J Virol. 2002;76:1285–92. doi: 10.1128/JVI.76.3.1285-1292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotenko SV, Saccani S, Izotova LS, Mirochnitchenko OV, Pestka S. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10) Proc Natl Acad Sci USA. 2000;97:1695–700. doi: 10.1073/pnas.97.4.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fietze E, Prosch S, Reinke P, et al. Cytomegalovirus infection in transplant recipients. The role of tumor necrosis factor. Transplantation. 1994;58:675–80. [PubMed] [Google Scholar]
- 53.Dinarello CA. Role of pro- and anti-inflammatory cytokines during inflammation: experimental and clinical findings. J Biol Regul Homeost Agents. 1997;11:91–103. [PubMed] [Google Scholar]


