Abstract
Probiotics, defined as live or attenuated bacteria or bacterial products, confer a significant health benefit to the host. Recently, they have been shown to be useful in the treatment of chronic inflammatory bowel disease and infectious colitis. In this study, we investigated the effect of probiotics on the development of experimental colitis using Toll-like receptor 4 (TLR-4) mutant (lps–/lps–) mice. TLR-4lps–/lps– and wild-type (WT) mice were given 2·5% dextran sulphate sodium (DSS) in drinking water to induce colitis with or without Lactobacillus casei pretreatment. Clinical and histological activity of DSS-colitis was attenuated markedly both in TLR-4lps–/lps– and WT mice pretreated with L. casei. Interestingly, histological activity was less severe in TLR-4lps–/lps– mice than in WT mice. The levels of myeloperoxidase activity and interleukin (IL)-12p40 were attenuated in pretreated TLR-4lps–/lps– mice after DSS administration. By contrast, transforming growth factor (TGF)-β and IL-10 mRNA and protein expressions were increased markedly in pretreated TLR-4lps–/lps– mice. The current results suggest that L. casei has a preventive effect in the development of acute DSS-induced colitis and its action depends largely upon TLR-4 status. L. casei modulates the expression of inflammatory cytokines and down-regulates neutrophilic infiltration in the case of incomplete TLR-4 complex signalling.
Keywords: dextran sulphate sodium-induced colitis, Lactobacillus casei, probiotics, Toll-like receptor 4
Introduction
Inflammatory bowel diseases (IBD), including Crohn's disease and ulcerative colitis, are characterized by chronic and relapsed inflammation of the gut, but their aetiology remains unknown [1]. Recently, there has been growing evidence that IBD results from abnormal immune responses to normal gut bacterial flora. The human intestinal tract contains hundreds of different bacterial species that are largely tolerated by the host. It is hypothesized that this tolerance to enteric bacteria exists because of the effectiveness of the epithelial barrier in separating the mucosal immune system and enteric antigens and/or because of active or indirect suppression of mucosal immune responses to such antigens [2].
Although no single bacterial species has been associated with the development of either Crohn's disease or ulcerative colitis, probiotic therapies, which aim to decrease disease severity by changing the flora of the gut, are currently being tested in various clinical trials and have shown some efficacy [3]. Lactobacilli and Bifidobacteria are natural components of the colonic microbiota, and as probiotic agents they have been tested in the prevention and treatment of IBD [4,5]. A strain of Lactobacillus casei, DN-114 001, has been shown to decrease the secretion of tumour necrosis factor (TNF)-α from the inflamed ileum of Crohn's disease patients [6,7]. It was also shown to have an anti-inflammatory effect on Shigella-infected human intestinal epithelial cells through a process that involves manipulation of the ubiquitin/proteasome pathway upstream of I-κBα [8]. The beneficial effect of probiotics is proposed to be due, at least in part, to interference with the innate immune system and possibly the orientation of adaptive immunity. Indeed, the probiotic preparation VSL#3® (a mixture of eight different Gram-positive bacteria) as well as different lactobacilli strains were shown to increase production of the anti-inflammatory cytokine interleukin (IL)-10 by dendritic cells [9,10].
The innate immune system recognizes the presence of specific bacterial antigens through pattern recognition receptors. Toll-like receptor 4 (TLR-4) is one of an extensive family of pattern recognition receptors that have been found in Drosophila melanogaster and mammals, and compelling research has shown that lipopolysaccharide (LPS), which is the major component of the outer membrane of Gram-negative bacteria, binds to TLR-4 [11–13]. The triggering of TLR-4 complex signalling by LPS results in a cascade of events that leads to the secretion of proinflammatory mediators from monocytes and dendritic cells, which leads ultimately to activation of the acquired immune response [14,15]. Thus, signalling through the TLR-4 complex contributes actively to the development of inflammation and may help to maintain an ongoing inflammatory response. In the gastrointestinal tract, it has been reported that intestinal epithelial cells express some pattern recognition receptors in vivo [16] and that expression of TLR-4, but not TLR3, was observed in epithelial cells from the colons of patients with IBD [17]. In the mouse colon, it has been reported that TLR-4 protein and mRNA expression were up-regulated significantly during dextran sulphate sodium (DSS)-induced colitis [18].
Because probiotics such as L. casei represents a subclass of commensals that modulate mucosal innate responses and possibly exhibit an anti-inflammatory function [6], we aimed to analyse the effect of L. casei on the development of experimental colitis induced by DSS and clarify the mechanism of action with regard to the cytokine network and TLR signalling using mutant mice functionally defective in TLR-4 signalling (TLR-4lps–/lps–). We found that L. casei had a preventive effect on the development of acute DSS-induced colitis and its action depended largely upon TLR-4 status. L. casei demonstrated up- or down-regulation of multiple inflammatory cytokines expression and down-regulation of neutrophilic infiltration in TLR-4lps–/lps– mice.
Materials and methods
Animals
BALB/cJ and BALB/c congenic C.C3 female TLR-4lps–/lps– mice (n = 29, 4–6 weeks old) and female wild-type (WT) BALB/c mice (n = 38, 5–7 weeks old) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were inbred under a light : dark cycle every 12 h at room temperature and fed food and water ad libitum. All animal experiments were approved by the Animal Experiment Ethnics Committee of the Hanyang University Medical Center.
Bacterial strain
The probiotic strain, L. casei DN-114 001 (Hanwha Pharmaceutics, Seoul, Korea), was cultured in deMane–Rogosa–Shape broths and agar (Difco Laboratories, Detroit, MI, USA) and incubated overnight at 37°C in anaerobic chamber.
Study design
Eight TLR-4lps–/lps– and 10 WT mice were used as control. To induce colitis, 21 TLR-4lps–/lps– and 28 WT mice were given 2·5% DSS in their drinking water for 9 days. Of these mice, 10 TLR-4lps–/lps and 14 WT mice were pretreated daily by intragastric administration of 1 × 106 colony-forming units (CFU)/ml of L. casei for 3 days prior to the induction of colitis. Animals were killed at the end of 9 days. All studies were performed in a blind fashion.
Assessment of disease activity
Body weight was assessed at baseline and every day for the duration of the experiment. Weight change was calculated as percentage change in weight compared with baseline. Animals were monitored clinically for rectal bleeding, diarrhoea and general signs of morbidity, including hunched posture and failure to groom.
Histological scoring
Colon tissue was fixed with 10% neutral formalin, paraffin-embedded, sectioned at 3–6 μm and stained with haematoxylin and eosin. For histological evaluation of colitis, specimens were quantified microscopically as described previously (Table 1) [19]. The colocaecal damage was categorized into six segments: severity and extent of inflammation, degree of regeneration, degree of crypt damage and percentage involvement. Total scores are the sum of the scores of each individual segment. To exclude bias, the histological score was determined in a blind manner by a trained pathologist.
Table 1.
Histological grading system of colitis.
| Segment | Grade | Description |
|---|---|---|
| Severity | 0 | None |
| 1 | Slight | |
| 2 | Moderate | |
| 3 | Severe | |
| Extent | 0 | None |
| 1 | Mucosa | |
| 2 | Mucosa and submucosa | |
| 3 | Transmural | |
| Regeneration | 4 | No tissue repair |
| 3 | Surface epithelium not intact | |
| 2 | Regeneration with crypt depletion | |
| 1 | Almost complete regeneration | |
| 0 | Complete regeneration or normal tissue | |
| Crypt damage | 0 | None |
| 1 | Basal 1/3 damaged | |
| 2 | Basal 2/3 damaged | |
| 3 | Only surface epithelium intact | |
| 4 | Entire crypt and epithelium lost | |
| Percentage involvement | 1 | 1–25% |
| 2 | 26–50% | |
| 3 | 51–75% | |
| 4 | 76–100% |
Enzyme-linked immunosorbent assay (ELISA)
IL-6, IL-12p40, IL-10 and transforming growth factor (TGF)-β concentrations were determined by commercially available specific ELISA using duo-paired murine cytokines as per the manufacturer's recommendations (eBioscience and BD Pharmingen, San Diego, CA, USA). Optical densities were measured on a Bio-Rad Novapath ELISA reader at a wavelength of 450 nm (Hercules, CA, USA). Data were analysed against the linear portion of the generated standard curve.
Semi-quantitative reverse transcription–polymerase chain reaction (RT–PCR) analysis
Total cellular RNA was extracted from cells with Trizol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcription was performed as described previously [20]. Oligonucleotide primers used for PCR amplification and the size of the PCR products obtained from target cellular RNA are shown in Table 2. PCR amplification consisted of 35 cycles of 1 min of denaturation at 95°C, 2·5 min of annealing at 60°C, and 1 min of extension at 72°C. The amplified products were separated by electrophoresis on a 1·5% agarose gel and visualized by ultraviolet (UV) light illumination using ethidium bromide staining. The band intensities of RT–PCR products for TGF-β, IL-10 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were evaluated using a fluorescence densitometer (Molecular Dynamics, Sunnyvale, CA, USA). The relative fluorescence intensity was determined by dividing the intensity of TGF-β and IL-10 mRNA band by density of GAPDH band and expressed as ratio of relative fluorescence units.
Table 2.
Polymerase chain reaction (PCR) primers used for PCRs.
| Gene name | Forward | Reverse | Length |
|---|---|---|---|
| GAPDH | AACGGATTTGGCCGTATT | ACTGTGGTCATGAGCCCTT | 520 |
| TGF-β | TGACGTCACTGGAGTTGTACGG | GGTTCATGTCATGGATGGTGC | 340 |
| IL-10 | GGTTGCCAAGCCTTATCGGA | ACCTGCTCCACTGCCTTGCT | 280 |
GAPDH: glyceraldehyde-3-phosphate dehydrogenase; TGF: transforming growth factor; IL: interleukin.
Myeloperoxidase (MPO) activity
Colonic myeloperoxidase (MPO) activity was determined as described previously [21]. Briefly, colonic tissue was homogenized in 1 ml of 50 mmol/l potassium phosphate buffer (pH 6·0) containing 0·5% hexadecyltrimethylammonium hydroxide and centrifuged at 120 g at 4°C for 20 min; 10 μl of the supernatant was transferred into phosphate buffer (pH 6·0) containing 0·17 mg/ml 3,3′-dimethoxybenzidine and 0·0005% H2O2. MPO activity of the supernatant was determined by measuring the H2O2-dependent oxidation of 3,3′-dimethoxybenzidine and expressed as units per gram of total protein. Total protein content of the samples was analysed using a bicinchoninic acid protein assay kit (BioSource, Camarillo, CA, USA).
Statistical analysis
Data are presented as the means ± standard deviations. The results were analysed statistically using the unpaired Student's t-test. P-values < 0·05 were considered to be statistically significant.
Results
L. casei-pretreated mice manifest less severe DSS-induced colitis
There was no lethality associated with DSS administration. As shown in Fig. 1, significant weight loss was developed after induction of DSS colitis both in TLR-4lps–/lps– and WT mice. By contrast, L. casei administration for 3 days prior to the induction of colitis led to a significant reduction in weight loss in both TLR-4lps–/lps– and WT mice and the difference was statistically significant on day 5 after DSS-colitis induction. Other clinical features, including rectal bleeding, diarrhoea and general signs of morbidity, however, were not significantly different between pretreated and non-pretreated mice.
Fig. 1.
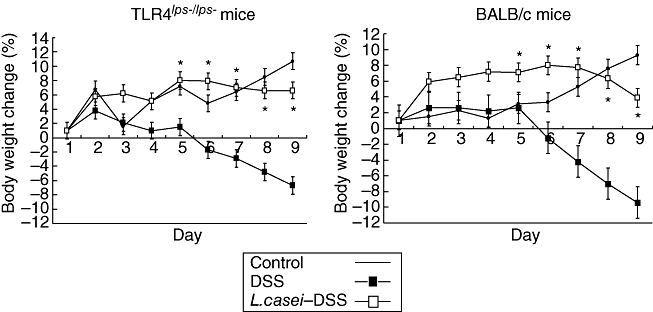
Weight changes of mice after induction of dextran sulphate sodium (DSS)-induced colitis with or without Lactobacillus casei-pretreatment according to the protocol depicted in Fig. 1. Each point represents the cumulative mean weight data from three different groups. Bars represent standard deviations. Difference between L. casei-pretreated mice and non-pretreated mice is significant at P < 0·05 (*).
In addition, L. casei-pretreated mice tissue showed a less severe grade of colonic inflammation compared with non-pretreated mice tissue both in TLR-4lps–/lps– and WT mice. The extensive superficial ulceration with severe acute inflammation both in TLR-4lps–/lps– and WT mice (Fig. 2b, e, respectively) was markedly abolished in mice pretreated with L. casei (Fig. 2c, f, respectively). Histological scores for tissue damage and extent of lesion were also lower in L. casei-pretreated mice compared with non-pretreated mice (6·7 ± 2·6 and 9·2 ± 3·2 in TLR-4lps–/lps– mice, 8·6 ± 4·5 and 10·0 ± 3·9 in WT mice, respectively, P< 0·05) (Fig. 3). Interestingly, histological scores were less severe in TLR-4lps–/lps– mice than in WT mice regardless of L. casei pretreatment. Histological score was significantly lower in L. casei-pretreated TLR-4lps–/lps– mice than in L. casei-pretreated WT mice.
Fig. 2.
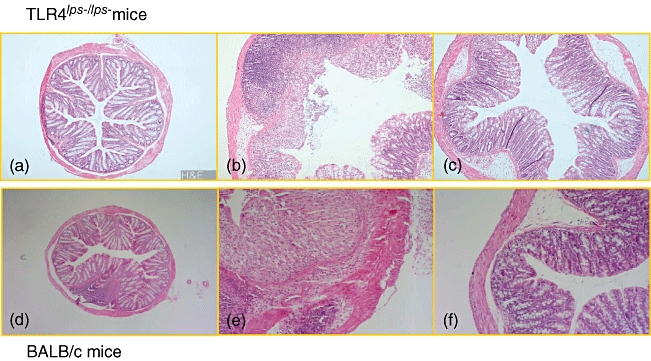
Microscopic evaluation of dextran sulphate sodium (DSS)-induced colitis in mice with or without Lactobacillus casei-pretreatment. (a, d) Photomicrograph (× 100) of a haematoxylin and eosin-stained (H&E) paraffin section of a representative colon from Toll-like receptor (TLR)-4lps–/lps– and wild-type (WT) mice without DSS-colitis, respectively. (b, e) Photomicrograph (× 100) of an H&E-stained paraffin section of a representative colon from non-pretreated TLR-4lps–/lps– and WT mice after induction of DSS-colitis, respectively. Severe neutrophil and mononuclear cell infiltrate and disruption of the normal crypt architecture with epithelial crypt ulceration and loss of goblet cells is evident. (c, f) Photomicrograph (× 100) of an H&E-stained paraffin section of a representative colon from L. casei-pretreated TLR-4lps–/lps– and WT mice after induction of DSS-colitis, respectively. Only small scattered areas of cellular infiltration are present.
Fig. 3.
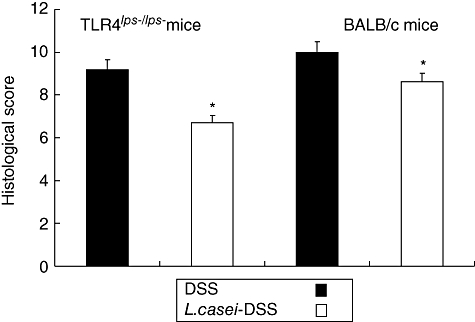
Histological scores of dextran sulphate sodium (DSS)-induced colitis in mice with or without Lactobacillus casei-pretreatment. Each point represents the cumulative mean histological score from two different groups. Bars represent standard deviations. The difference between L. casei-pretreated mice and non-pretreated mice is significant at P < 0·05 (*). Interestingly, histological scores in Toll-like receptor 4lps–/lps– mice is lower than in wild-type mice.
IL-12p40 level, but not IL-6 level, was attenuated in the colons of L. casei-pretreated TLR-4lps–/lps– mice
ELISA was used to determine the levels of IL-6 and IL-12p40 both in the colons of TLR-4lps–/lps– and WT mice with or without L. casei pretreatment. IL-6 levels were not significantly different between control, L. casei-pretreated and non-pretreated mice both in TLR-4lps–/lps– and WT mice [0·071 ± 0·02, 0·066 ± 0·02 and 0·081 ± 0·02 in TLR-4lps–/lps– mice, 0·087 ± 0·03, 0·104 ± 0·04 and 0·105 ± 0·05 in WT mice, respectively, P = not significant (n.s.)] (Fig. 4). By contrast, IL-12p40 levels were attenuated significantly in L. casei-pretreated TLR-4lps–/lps– mice compared to non-pretreated TLR-4lps–/lps– mice. Interestingly, these effects were not observed in WT mice (84·3 ± 22·6, 93·9 ± 12·4 and 61·3 ± 18·8 in TLR-4lps–/lps– mice, 68·9 ± 13·9, 84·4 ± 26·4 and 82·2 ± 27·0 in WT mice, P = 0·005) (Fig. 5).
Fig. 4.
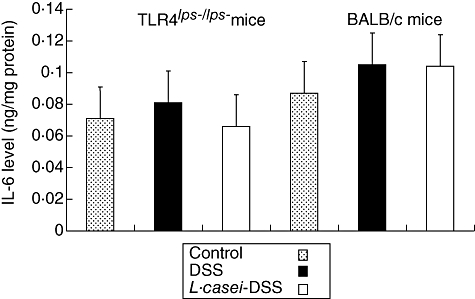
Interleukin (IL)-6 contents in the colons of dextran sulphate sodium (DSS)-induced colitis in mice with or without Lactobacillus casei-pretreatment. Each point represents the cumulative mean levels of IL-6 from three different groups. Bars represent standard deviations. There is no significant difference between control, L. casei-pretreated mice and non-pretreated mice.
Fig. 5.
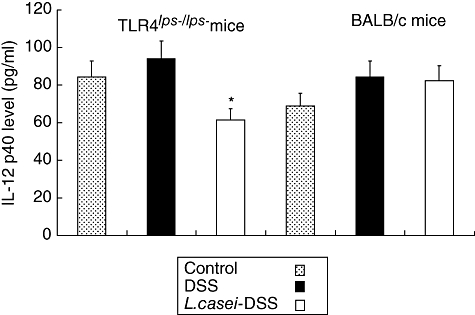
Interleukin (IL)-12p40 contents in the colons of dextran sulphate sodium (DSS)-induced colitis in mice with or without Lactobacillus casei-pretreatment. Each point represents the cumulative mean levels of IL-12p40 from three different groups. Bars represent standard deviations. Difference between pretreated and non-pretreated Toll-like receptor 4lps–/lps– mice reaches statistical significance at P < 0·01 (*). However, there is no significant difference in wild-type mice.
MPO activity was attenuated in the colons of L. casei-pretreated TLR-4lps–/lps– mice
MPO activity is a marker of neutrophil contents and is up-regulated in the tissue under inflammatory conditions. The level of MPO activity was increased markedly after DSS administration in TLR-4lps–/lps– mice, but attenuated significantly in L. casei-pretreated mice (21·8 ± 0·3, 45·2 ± 0·7 and 17·7 ± 0·2 in TLR-4lps–/lps– mice, P< 0·05, respectively). These effects were not observed in WT mice (Fig. 6).
Fig. 6.
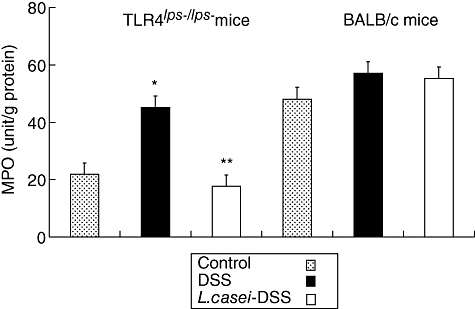
Myeloperoxidase (MPO) activity in the colons of dextran sulphate sodium (DSS)-induced colitis in mice with or without Lactobacillus casei pretreatment. Each point represents the cumulative mean levels of MPO activity from three different groups. Bars represent standard deviations. Difference between control and non-pretreated Toll-like receptor (TLR)-4lps–/lps– mice is significant at P < 0·05 (*). Difference between pretreated and non-pretreated TLR-4lps–/lps– mice also reaches statistical significance at P < 0·05 (**). However, there is no significant difference in wild-type mice.
TGF-β and IL-10 mRNA and protein expressions were increased in colons of L. casei-pretreated TLR-4lps–/lps– mice
To clarify the reason why DSS-induced colitis manifested less severely in L. casei-pretreated mice, semiquantitative RT–PCR analysis for TGF-β and IL-10 mRNA was performed. The relative fluorescence intensity of TGF-β and IL-10 mRNA bands showed fourfold increases in L. casei-pretreated TLR-4lps–/lps– mice compared to non-pretreated TLR-4lps–/lps– mice. No significant difference was found in WT mice (Figs 7 and 8). Next, we tried to confirm these results by protein analysis using ELISA. TGF-β levels were moderately increased in L. casei-pretreated TLR-4lps–/lps– mice compared to non-pretreated TLR-4lps–/lps– mice (289·4 ± 91·9, 285·5 ± 154·2 and 411·0 ± 129·7 in TLR-4lps–/lps– mice, P = 0·08) (Fig. 9). Moreover, IL-10 levels were increased significantly in L. casei-pretreated TLR-4lps–/lps– mice compared to non-pretreated TLR-4lps–/lps– mice (52·3 ± 9·2, 54·1 ± 5·2 and 75·5 ± 6·2 in TLR-4lps–/lps– mice, P = 0·02) (Fig. 10). No significant difference was found in WT mice.
Fig. 7.
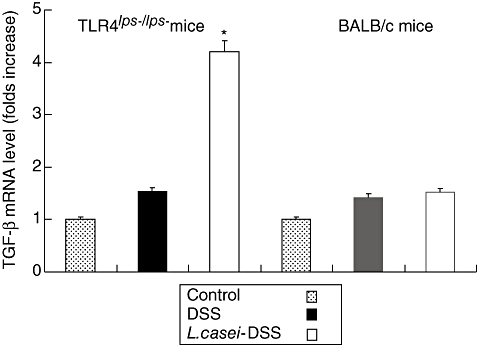
Semi-quantitative reverse transcription–polymerase chain reaction analysis for transforming growth factor (TGF)-β mRNA in the colons of dextran sulphate sodium (DSS)-induced colitis in mice with or without Lactobacillus casei pretreatment. Each point represents the cumulative mean levels of TGF-β mRNA from three different groups. Bars represent standard deviations. Difference between pretreated and non-pretreated Toll-like receptor (TLR)-4lps–/lps– mice is significant at P < 0·05 (*). However, there is no significant difference in wild-type mice.
Fig. 8.
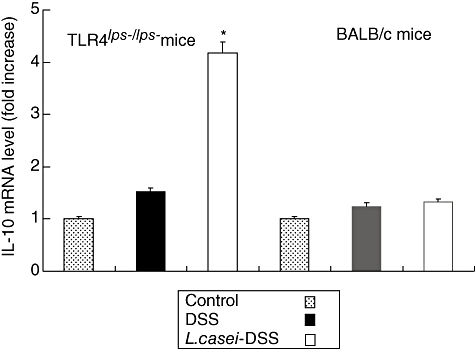
Semi-quantitative reverse transcription–polymerase chain reaction analysis for interleukin (IL)-10 mRNA in the colons of dextran sulphate sodium (DSS)-induced colitis in mice with or without Lactobacillus casei pretreatment. Each point represents the cumulative mean levels of IL-10 mRNA from three different groups. Bars represent standard deviations. Difference between pretreated and non-pretreated Toll-like receptor 4lps–/lps– mice is significant at P < 0·05 (*). However, there is no significant difference in wild-type mice.
Fig. 9.
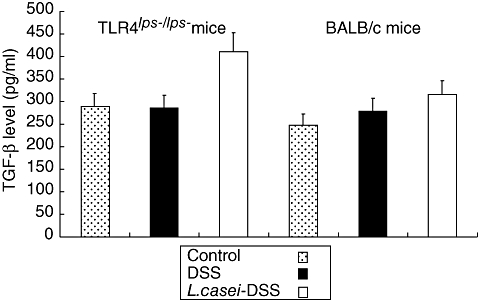
Transforming growth factor (TGF)-β contents in the colons of dextran sulphate sodium (DSS)-induced colitis in mice with or without Lactobacillus casei pretreatment. Each point represents the cumulative mean levels of TGF-β from three different groups. Bars represent standard deviations. Difference between pretreated and non-pretreated Toll-like receptor 4lps–/lps– mice shows borderline significance (P= 0·08). However, there is no significant difference in wild-type mice.
Fig. 10.
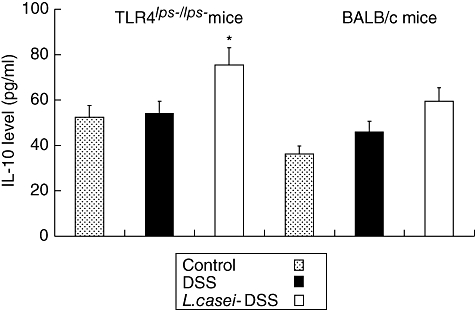
Interleukin (IL)-10 contents in the colons of dextran sulphate sodium (DSS)-induced colitis in mice with or without Lactobacillus casei pretreatment. Each point represents the cumulative mean levels of IL-10 from three different groups. Bars represent standard deviations. Difference between pretreated and non-pretreated Toll-like receptor 4lps–/lps– mice is significant at P < 0·05 (*). However, there is no significant difference in wild-type mice.
Discussion
Persuasive evidence indicates that intestinal microflora play an important role in the initiation and the perpetuation of murine experimental colitis and human IBD [1,22,23]. This fact initially provided the rationale for the use of probiotics to manipulate the intestinal microenvironment. In fact, recent studies have demonstrated the therapeutic effects of probiotics in various models of experimental colitis and in several clinical trials [3–10]. However, the molecular mechanisms by which probiotics exert their therapeutic effects have not yet been identified. In the present study, we demonstrated that acute DSS-induced colitis occurred in TLR-4lps–/lps– mice as well as in WT mice. Interestingly, TLR-4lps–/lps– mice showed less devastating colonic inflammation than WT mice. Daily administration of L. casei before induction of DSS-colitis resulted in a milder form of DSS-colitis. This protective activity was attributable to reduced neutrophilic infiltration, emerging from histology and MPO activity, increased expression of anti-inflammatory cytokines such as TGF-β and IL-10 and attenuated expression of proinflammatory cytokine, IL-12p40. Moreover, these effects were demonstrated only in TLR-4lps–/lps– mice, not in WT mice, which may suggest that this protective activity of L. casei depends mainly on the TLR-4 status of the host.
Our study confirms and extends the hypothesis that certain commensal microorganisms have the potential to influence actively the homeostatic control of intestinal inflammation [24]. Indeed, co-culture of human tissues with L. casei significantly reduced TNF-α release from Crohn's disease patients' inflamed mucosa [6], even in the presence of a non-pathogenic Escherichia coli that alone enhanced TNF-α release from inflamed tissues [7]. Recently, another anti-inflammatory mechanism induced by a commensal bacteria strain has been described. Bacteroides thetaiotaomicron attenuates proinflammatory cytokine expression by inducing the nuclear export of complexes formed by nuclear factor (NF)-κB and peroxisome proliferator-activated receptor-γ (PPAR-γ) [25]. In our study, effect of L. casei on the NF-κB pathway and PPAR-γ could not be obtained and should be clarified in future study.
Little is known about the role of TLR-4 in most of the mouse models of colitis. In the DSS model, evidence suggests that TLR-4 may play a partial role in the development of severe disease, at least in certain mouse strains [26]. Our results confirm these findings in this DSS model. Moreover, genetic variants of the TLR-4 mutant mice, TLR-4lps–/lps– mice, were less susceptible to the development of DSS-colitis, suggesting that genetic factors can influence the relative importance of TLR-4 in the development of colitis.
The exact role for TLR-4 in intestinal inflammation remains controversial. Although most studies showed a role for TLR-4 in inflammatory responses, Rakoff-Nahoum et al. [27] have demonstrated recently an important role for TLR-4 in the maintenance of intestinal epithelial homeostasis and protection from direct epithelial injury. Their results show that mice deficient in TLR signalling, due to a deficiency in MyD88, are more susceptible than WT mice to intestinal epithelial damage. They argued that constitutive signalling through TLR in intestinal epithelial cells results in the production of tissue protective factors, such as IL-6, keratinocyte-derived chemokine (KC)-1 and TNF. Araki et al. [28] also reported that they observed severe colitis unexpectedly in MyD88-deficient mice fed DSS. Both macrophages and CD4+ T cells were increased significantly in the inflamed mucosa of DSS-fed MyD88-deficient mice compared with WT mice. They concluded that innate immune responses through MyD88 in the gut might have a protective role in some phase of the development of colitis. At this point, one important matter should be noted. These two studies commonly used TLR-4–/– or Myd88–/– mice provided by Dr Akira (Osaka University, Osaka, Japan), in which all TLR signalling is completely inhibited. In contrast to those mice, our study used a variant of TLR-4 mutant mice, TLR-4lps–/lps– mice, in which TLR signalling is blocked only partially. Indeed, we confirmed a missense mutation in the third exon of the TLR-4 gene (TLR-4) in gene sequence analysis, which predicted replacing proline with histidine at position 712 of the polypeptide chain. Putting these results together, it may be possible that partial inhibition of TLR-4 signalling may be beneficial in some cases of acute epithelial injury, although complete inhibition of all TLR signalling may be more injurious than beneficial. The result of Fort et al. [2], that treatment with a synthetic TLR-4 antagonist, CRX-526, inhibited successfully the development of moderate to severe disease in DSS-colitis model, also supports our hypothesis. However, the specific elements of the signalling pathway in TLR-4lps–/lps– mice remain to be characterized. Further studies comparing TLR-4 mutant to knock-out mice will be extremely informative.
In the present study, how L. casei showed protective effects on WT mice has not been identified. Recently, Dubuquoy et al. [29] reported that the commensal intestinal flora affected the expression and activation of PPAR-γ through the TLR-4 signalling pathway and was, in part, I-kappa-B kinase β (IKKβ)-dependent. They postulated that bacteria up-regulate PPAR-γ in epithelial cells where PPAR-γ is acting in a negative feedback loop, uncoupling NF-κB-dependent target genes that are important for inflammatory responses. They also found that PPAR-γ expression was considerably impaired in patients with ulcerative colitis. From this viewpoint, different mechanisms of the protective effects of L. casei between TLR-4lps–/lps– mice and WT mice can, to some extent, be explained. A difference in the degree of TLR-4 signalling might result in different mechanisms of the protective effects of L. casei, and PPAR-γ is possibly considered to play a role in WT mice. Further experiments are highly warranted.
In conclusion, L. casei has a preventive effect in the development of acute DSS-induced colitis and its action depends largely upon TLR-4 status. L. casei up- and down-regulates multiple inflammatory cytokine expression and down-regulates neutrophilic infiltration in circumstances of incomplete TLR-4 signalling. Further studies are necessary to clarify the precise mechanism of L. casei under normal TLR-4 signalling, especially with regard with PPAR-γ. Moreover, as we have demonstrated only the preventive effect of probiotics in acute colitis, further studies are necessary to clarify whether probiotics treatment is also effective in acute colitis.
Acknowledgments
This work was supported by a Hanyang University Research Foundation grant (2007-000-4655).
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Fort MM, Mozaffarian A, Stover AG, et al. A synthetic TLR-4 antagonist has anti-inflammatory effects in two murine models of inflammatory bowel disease. J Immunol. 2005;174:6416–23. doi: 10.4049/jimmunol.174.10.6416. [DOI] [PubMed] [Google Scholar]
- 3.Shanahan F. Probiotics in inflammatory bowel disease. Gut. 2001;48:609. doi: 10.1136/gut.48.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–9. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 5.Cui HH, Chen CL, Wang JD, et al. Effects of probiotic on intestinal mucosa of patients with ulcerative colitis. World J Gastroenterol. 2004;10:1521–5. doi: 10.3748/wjg.v10.i10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borruel N, Carol M, Casellas F, et al. Increased mucosal tumour necrosis factor alpha production in Crohn's disease can be downregulated ex vivo by probiotic bacteria. Gut. 2002;51:659–64. doi: 10.1136/gut.51.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borruel N, Casellas F, Antolin M, et al. Effects of nonpathogenic bacteria on cytokine secretion by human intestinal mucosa. Am J Gastroenterol. 2003;98:865–70. doi: 10.1111/j.1572-0241.2003.07384.x. [DOI] [PubMed] [Google Scholar]
- 8.Tien MT, Girardin SE, Regnault B, et al. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J Immunol. 2006;176:1228–37. doi: 10.4049/jimmunol.176.2.1228. [DOI] [PubMed] [Google Scholar]
- 9.Drakes M, Blanchard T, Czinn S. Bacterial probiotic modulation of dendritic cells. Infect Immun. 2004;72:3299–309. doi: 10.1128/IAI.72.6.3299-3309.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168:171–8. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 11.Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 12.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 13.Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Curr Opin Immunol. 2003;15:396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 14.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 15.Barton GM, Medzhitov R. Control of adaptive immune responses by Toll-like receptors. Curr Opin Immunol. 2002;14:380–3. doi: 10.1016/s0952-7915(02)00343-6. [DOI] [PubMed] [Google Scholar]
- 16.Hornef MW, Frisan T, Vandewalle A, et al. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195:559–70. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR-4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–17. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortega-Cava CF, Ishihara S, Rumi MA, et al. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol. 2003;170:3977–85. doi: 10.4049/jimmunol.170.8.3977. [DOI] [PubMed] [Google Scholar]
- 19.Dieleman LA, Palmen MJ, Akol H, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–91. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung HC, Eckmann L, Yang SK, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herfarth H, Brand K, Rath HC, et al. Nuclear factor-kappa B activity and intestinal inflammation in dextran sulphate sodium (DSS)-induced colitis in mice is suppressed by gliotoxin. Clin Exp Immunol. 2000;120:59–65. doi: 10.1046/j.1365-2249.2000.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 23.Rachmilewitz D, Katakura K, Karmeli F, et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–8. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953–64. doi: 10.1038/nri1499. [DOI] [PubMed] [Google Scholar]
- 25.Kelly D, Campbell JI, King TP, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004;5:104–12. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 26.Lange S, Delbro DS, Jennische E, et al. The role of the Lps gene in experimental ulcerative colitis in mice. APMIS. 1996;104:823–33. doi: 10.1111/j.1699-0463.1996.tb04948.x. [DOI] [PubMed] [Google Scholar]
- 27.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Araki A, Kanai T, Ishikura T, et al. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J Gastroenterol. 2005;40:16–23. doi: 10.1007/s00535-004-1492-9. [DOI] [PubMed] [Google Scholar]
- 29.Dubuquoy L, Jansson EA, Deeb S, et al. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology. 2003;124:1265–76. doi: 10.1016/s0016-5085(03)00271-3. [DOI] [PubMed] [Google Scholar]


