Abstract
Persistence of hepatitis B virus (HBV) infection is associated with reduced anti-viral T cell responses. Impaired dendritic cell (DC) function was suggested as the cause of reduced T cell stimulation in chronic HBV carriers. Thus, we compared myeloid (mDC) and plasmacytoid DC (pDC) from chronic HBV carriers and controls. Frequency and phenotype of isolated DC were analysed by fluorescence activated cell sorter staining, DC function by mixed lymphocyte reaction, cytokine bead array, intracellular cytokine staining, enzyme-linked immunosorbent assay and enzyme-linked immunospot. Expression of HBV DNA and mRNA was studied by polymerase chain reaction (PCR). Circulating total DC, mDC or pDC were not reduced in chronic HBV carriers. Isolated mDC and pDC from chronic HBV carriers exhibited similar expression of co-stimulatory molecules and alloreactive T helper cell stimulation as control DC, whether tested directly ex vivo or after in vitro maturation. Secretion of pro- and anti-inflammatory cytokines by CD40 or Toll-like receptor ligand-stimulated patient DC was intact, as was human leucocyte antigen A2-restricted HBV-specific cytotoxic lymphocyte stimulation. Although both DC populations contained viral DNA, viral mRNA was undetectable by reverse transcription–PCR, arguing against viral replication in DC. We found no quantitative, phenotypic or functional impairment of mDC or pDC in chronic hepatitis B, whether studied ex vivo or after in vitro maturation.
Keywords: cytotoxic T lymphocytes, dendritic cells, hepatitis B virus, T helper cells
Introduction
Approximately 350 000 000 chronic hepatitis B virus (HBV) carriers worldwide are at risk of developing liver cirrhosis and cancer. The outcome from acute infection seems to be determined by the host immune response: the close correlation of viral clearance with strong anti-viral T helper (Th) cell and cytotoxic lymphocyte (CTL) activities and chronic infections with weak anti-viral responses suggests that this HBV-specific T cell failure might cause viral persistence [1,2]. Dendritic cells (DC) are the most important antigen-presenting cells (APC) for T cell stimulation [3]. A defect of these cells could therefore account for impaired T cell stimulation in chronic HBV carriers. Several studies have reported phenotypical and functional impairment of peripheral blood DC from chronic HBV carriers [4–7]. These findings were challenged by our recent studies demonstrating intact alloreactive and antigen-specific T cell stimulation [8]. Thus, the experimental findings are controversial, and a general DC failure would contrast with the clinical immunocompetence of HBV carriers [6–8].
The major drawback of these previous studies is the unknown physiological relevance of in vitro-generated monocyte-derived DC. In peripheral blood, two major DC populations can be identified directly: CD11c+ myeloid DC (mDC) and CD123+ plasmacytoid DC (pDC) [9]. Both are immature precursor DC derived from bone marrow stem cells that are on migration to their target sites. Myeloid DC home preferentially to non-lymphoid tissues, where they are specialized in uptake of invading pathogens. After engagement of Toll-like receptors (TLR)-2 and -4, they rapidly secrete proinflammatory cytokines [10]. In contrast, pDC express TLR-7 and -9 and are located preferentially in the T cell areas of lymphoid tissues. They produce large amounts of interferon (IFN)-α after TLR ligation. Thus, mDC are believed to evoke preferentially T cell responses against invading pathogens, while pDC might serve as a link to innate immunity and to maintain tolerance against self-antigens [10].
Thus, we performed comprehensive phenotypic and functional analyses of mDC and pDC from chronic HBV carriers and controls either directly ex vivo or after in vitro maturation.
Materials and methods
Patients and donors
Seventy-six patients with chronic HBV infection were studied (Table 1): 38 were highly viraemic [alanine aminotransferase (ALT) > 2 × upper limit of normal (ULN), HBV-DNA > 105 copies (cps)/ml]; 38 were low viraemic [ALT ≤ 2 × ULN, seropositive for anti-hepatitis B envelope (HBe), HBV DNA < 105 cps/ml]. Moreover, eight healthy donors with spontaneously resolved hepatitis B and 38 healthy HBV-naive blood donors served as controls. All patients were treatment-naive and had no serious concomitant diseases. Cirrhosis was excluded in all patients by histology or ultrasound plus biochemistry. All patients and controls were tested seronegative for anti-HIV-1/-2, anti-HCV and anti-HDV antibodies and gave informed consent according to the ethical guidelines from Helsinki. The study was approved by the institutional ethical committee.
Table 1.
Patient characteristics.
| High viraemic (n = 38) | Low viraemic (n = 38) | Resolved (n = 10) | Controls (n = 38) | |
|---|---|---|---|---|
| Age (years) | 42 | 41 | 41 | 31 |
| Male/female | 25/13 | 26/12 | 7/3 | 21/17* |
| ALT (U/l)†‡ | 131 ± 109 | 31 ± 15 | 25 ± 14 | n.t. |
| HBV viraemia (cps/ml)‡§ | 3 ± 4·9 × 107 | 2·2 ± 2 × 104 | 0·9 ± 3·9 × 103 | n.t. |
| HBe+/aHBe+‡ | 20/18 | 0/38 | 0/10 | 0/0 |
P < 0·05 for highly viraemic against three other groups.
Mean ± standard deviation (s.d.); n.t., not tested.
P < 0·01 for highly viraemic against both other groups.
Mean ± s.d.; quantitative hepatitis B virus (HBV) Amplicor Assay in copies/ml (cps/ml; Roche Diagnostics; lower limit of detection 103 cps/ml). HBe: hepatitis B envelope.
Peripheral blood mononuclear cells (PBMC) and hepatitis B core (HBc)18−27-specific CTL clone
PBMC for DC separation were isolated from heparinized blood by Ficoll centrifugation. CD4-positive Th cells used as responder cells in allogeneic mixed lymphocyte reaction (MLR) were obtained from healthy buffy coat donors from our institutional Blood Transfusion Center after Ficoll centrifugation by immunomagnetic cell sorting using anti-CD4 beads (Miltenyi Biotech, Gladbach, Germany). The CD8+ HBc18−27-specific and HLA A2·1-restricted CTL clone was generated from a patient during the acute phase of self-limited HBV infection, as described previously [11]. After thawing, the clone was restimulated with allogeneic irradiated (3000 rad) PBMC and expanded in RPMI-1640 + 5% human antibody serum serum (HUS), supplemented with interleukin (IL)-7, IL-15 (both 10 ng/ml; R&D Systems, Heidelberg, Germany) and IL-2 (60 U/ml; R&D Systems) for a further 2 weeks. Supplementation with cytokines was stopped 6 days before co-incubation with peptide-pulsed allogeneic HLA A2·1-matched mDC and pDC.
Dendritic cell isolation and culture
DC were isolated from PBMC by the use of immunomagnetic anti-BDCA-1 and -BDCA-4 beads according to the manufacturer's instructions with slight modifications (Miltenyi Biotech) [12]. At first, CD3+ and CD19+ cells were removed from PBMC by immunomagnetic cell sorting (MACS; Miltenyi Biotech), before the negative fraction was labelled with blood dendritic cell antigen (BDCA)-1 beads. This cell suspension was run over two consecutive columns. The BDCA-1-positive fraction was washed and further used as CD1c-positive mDC. The BDCA-1-negative fraction was incubated with BDCA-4 beads and run over two consecutive columns. The positive fraction was recovered, washed and studied further as pDC. The purity of recovered DC was routinely > 95%; cell preparations with lower purity were discarded. The DC yield averaged 3–8 × 105 mDC and pDC per donor.
For MLR, CTL stimulation and cytokine secretion assays, mDC were kept in RPMI-1640 medium + 5% HUS supplemented with 800 U/ml granulocyte–macrophage colony-stimulating factor (GM-CSF) (Leukomax, Sandoz, Basel, Switzerland) and 1000 U/ml IL-4 (Strathmann Biotech, Hannover, Germany); pDC in RPMI-1640 + HUS containing IL-3 (10 ng/ml; CellGenix, Freiburg, Germany). Where indicated in the text, DC maturation was induced for 24 h by supplementation with IL-1β, tumour necrosis factor (TNF)-α (both: 10 ng/ml; R&D Systems), IL-6 (1000 U/ml; Strathmann Biotech) and prostaglandin E2 (1 mg/ml; Sigma, Munich, Germany) [8,13].
Flow cytometry of surface markers
DC were analysed in whole blood after staining with anti-lin-1-fluorescein isothiocyanate (FITC) (i.e. anti-CD3, -CD14, -CD16, -CD19, -CD20, -CD56), anti-CD11c-PE, anti-CD123-phycoerythrin (PE) and anti-human leucocyte antigen D-related (HLA-DR)-cychrome (all BD Biosciences). Erythrocytes were lysed using lysis buffer (Optilyse B; Immunotech, Bad Soden, Germany). A total of 120 000 events were analysed on a fluorescence activated cell sorter (FACScan) utilizing CellQuest (Becton Dickinson, San Jose, CA, USA). Gate R1 was set according to light-scatter properties on monocytes and lymphocytes, region R2 marked HLA-DR+ lin-1– cells within gate R1, and cychrome-counterstaining for CD11c or CD123 uncovered myeloid (mDC) and plasmacytoid DC (pDC) within region R2.
Expression of surface markers was analysed using mouse anti-human antibodies: FITC-anti-CD1c, FITC-BDCA-2, FITC-BDCA-4 (all Miltenyi Biotech); PE-anti-CD11c, FITC-anti-CD40, PE-anti-CD80, FITC-anti-CD83, PE-anti-CD86 and FITC-anti-HLA-DR (all BD Biosciences). Analysis of 5000 acquired events within the gate was performed on a FACScan. Quadrants were set according to staining with the respective IgG1 and IgG2a isotype controls.
Cytokine secretion by ex vivo DC
DC (3 × 104/well) were stimulated in mDC- or pDC-medium for 48 h with 1 × 103 or 5 × 103/well mouse fibroblasts stably transfected with human CD40L (CD40L cells [14]) or TLR ligands: SAC (protein A from Staphylococcus aureus Cowan I, 1 : 1000; Calbiochem, Darmstadt, Germany), unmethylated oligonucleotide CpG2006 (Hermann GbR, Freiburg, Germany; 10 μg/ml) or poly(I:C) (20 or 30 μg/ml; Amersham, Piscataway, NJ, USA). Culture supernatants were collected after 48 h to assess the secretion of TNF-α, IL-1β, IL-6, IL-10 and IL-12p70 by the flow cytometry-based cytokine bead array (CBA; BD Biosciences). IFN-α secretion was assessed by enzyme-linked immunosorbent assay (ELISA) (Bender Medsystems, Vienna, Austria).
Cytokine secretion by contaminating cell populations was assessed by intracellular FACS staining for IL-1β, IL-6 and TNF-α of highly purified mDC and pDC from 10 healthy buffy coat donors, stimulated with SAC (mDC) or CpG2006 (pDC) for 48 h, or phorbol myristate acetate (PMA) (10 ng/ml) plus ionomycin (250 ng/ml) for 5 h. Cytokine staining was performed using reagents from BD Biosciences, according to the manufacturer's instructions. In brief, stimulated cells were incubated with GolgiStop (for IL-6 and IL-1β) or GolgiPlug (TNF-α) reagent (5 h), 10% human antibody serum and stained with FITC-CD11c (mDC) or FITC-BDCA-2 (pDC). After 30 min, cells were fixed and permeabilized with Cytofix/Cytoperm solution (20 min) and stained for the respective cytokine. Two-colour analysis was performed on all living cells on a FACScan (Becton Dickinson).
T cell stimulation
Mixed lymphocyte reactions (MLR) were performed by co-incubation of mDC/pDC isolated from eight HBV patients and healthy donors each with 105/well (triplicates) isolated CD4 cells from three to four allogeneic healthy buffy coat donors. After 4 days of culture in mDC- or pDC-medium, the plates were pulsed with 0·25 μCi/well [3H]-thymidine for 18 h before the incorporated radioactivity was measured by liquid scintillation technique.
The HBc18−27-specific HLA A2·1-restricted CTL clone was used as a responder cell in co-culture assays with isolated DC pulsed with 10 μg/ml of the synthetic HBc18−27 peptide or an unrelated A2·1-restricted Epstein–Barr virus (EBV)280−288 peptide. Therefore, DC from five HLA A2·1-positive chronic HBV patients or controls were each peptide-pulsed for 2 h and washed in phosphate-buffered saline (PBS) before co-incubation at 104/well with 12 × 103, 4 × 103 or 1·3 × 103 cells of the CTL clone in 96-well plates. After 4 days, supernatants were harvested for IFN-γ detection by CBA (BD Biosciences). In order to avoid artefacts due to different activation states of the responder clone, the full set of experiments was performed simultaneously with DC from all donors, kept frozen and thawed immediately before use in co-culture.
HBV DNA, replicative intermediates and HBs antigen expression
DNA was isolated from 8·0 × 105 DC by the Qiagen® method (Qiagen, Hilden, Germany). Cellular and serum HBV DNA were detected using the HBV-Amplicor® Assay (Roche Diagnostics, Mannheim, Germany).
Highly sensitive analyses of viral mRNA were performed from cellular RNA extracted from 105 purified DC by specific reverse transcription–polymerase chain reaction (RT–PCR) of the X-gene region [15], recognizing all HBV mRNA species from all currently known HBV genotypes. The lower detection limit as determined by serial dilutions is 104 cps per culture (i.e. per 105 purified DC).
Statistics
The Mann–Whitney test for non-parametric data was calculated utilizing the StatView software (Abacus Concepts Inc., Berkeley, CA, USA). Two-sided Student's t-tests for impaired data were performed utilizing Excel software (Microsoft, Munich, Germany).
Results
Frequencies of mDC and pDC in peripheral blood
When frequencies of HLA-DR-positive, lineage marker-negative DC were analysed in whole blood, FACS staining for CD11c and CD123 revealed similar frequencies of total DC and myeloid or plasmacytoid DC subpopulations in low and highly viraemic HBV carriers and controls. The mean frequencies of these cells ranged between 0·2 and 0·8% in chronic or recovered HBV patients and healthy controls (Table 2). Due to high interindividual variations, none of these differences reached statistical significance. Serial FACS analyses in individual healthy donors and chronic HBV carriers revealed large intra-individual frequency variations of all peripheral blood DC populations over time (Table 3).
Table 2.
Frequencies of total, myeloid and plasmacytoid dendritic cells (DC) in peripheral blood of patients and controls.
| HLA-DR+/lin– | |||
|---|---|---|---|
| Total* | CD11c+* | CD123+* | |
| High viraemic (n = 14) | 0·6 ± 0·3%† | 0·2 ± 0·2%† | 0·2 ± 0·1%† |
| Low viraemic (n = 7) | 0·8 ± 0·4%† | 0·3 ± 0·2%† | 0·3 ± 0·2%† |
| Resolved (n = 4) | 0·5 ± 0·3%† | 0·2 ± 0·1%† | 0·2 ± 0·2%† |
| Controls (n = 9) | 0·8 ± 0·2%† | 0·4 ± 0·1%† | 0·4 ± 0·1%† |
Total DC (DR+ lin–), myeloid DC (CD11c+) and plasmacytoid DC (CD123+) as percentage of total blood cells; per sample 120 000 cells were analysed and results presented as means ± standard deviation.
Not signficant versus three other patient groups. HLA-DR: human leucocyte antigen D-related.
Table 3.
Frequencies of total, myeloid and plasmacytoid dendritic cells (DC) in peripheral blood of three untreated low viraemic chronic hepatitis B virus (HBV) carriers and healthy controls (CTR) during follow-up.
| CTR 1 | CTR 2 | CTR 3 | HBV 1 | HBV 2 | HBV 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d0* | w46* | d0 | w53 | d0 | w32 | d0 | w24 | d0 | w49 | d0 | w52 | |
| Total DC | 1·0 | 1·32 | 0·93 | 0·23 | 0·75 | 0·44 | 0·67 | 0·93 | 0·25 | 0·39 | 0·55 | 0·79 |
| CD11c + mDC | 0·37 | 0·65 | 0·33 | 0·18 | 0·23 | 0·1 | 0·46 | 0·12 | 0·03 | 0·19 | 0·21 | 0·25 |
| CD123 + pDC | 0·51 | 0·4 | 0·52 | 0·05 | 0·42 | 0·31 | 0·12 | 0·2 | 0·08 | 0·1 | 0·1 | 0·05 |
d, day; w, week. HBV: hepatitis B virus.
Phenotype of DC ex vivo and after in vitro maturation
Highly purified mDC showed slight to moderate expression of CD40 and CD86, strong expression of CD11c and HLA-DR, but were almost negative for CD80 and the maturation marker CD83 (Fig. 1). Plasmacytoid DC stained moderately positively for CD40, CD86 and HLA-DR, were strongly positive for the IL-3 receptor CD123 and the antigen uptake receptor BDCA-2 [16], but almost negative for CD80 and CD83 (Fig. 1). After in vitro maturation, both DC subsets strongly up-regulated CD83, CD86 and HLA-DR, and to a lesser extent CD40 and CD80 (Fig. 1). There were no statistically significant differences between ex vivo or maturated mDC or pDC from healthy donors and chronic HBV carriers in the expression of any of these molecules, whether analysed as percentage of positive cells (not shown) or mean fluorescence intensity (MFI) values (Fig. 1). Moreover, the DC phenotype of low viraemic HBV carriers did not differ from that of carriers with high viraemia (not shown). When purified mDC and pDC from four healthy donors or chronic HBV carriers were assessed serially during spontaneous follow-up, large and inconsistent intra-individual variations were found in the expression of all DC markers assessed (Fig. 2).
Fig. 1.
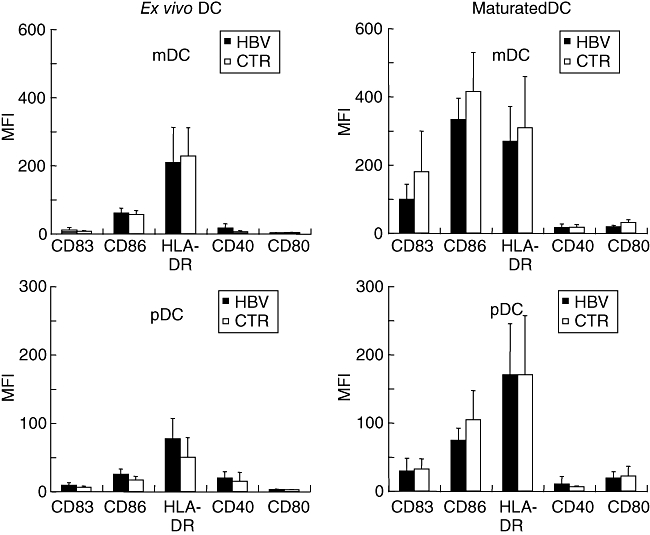
Phenotype of isolated myeloid or plasmacytoid dendritic cells (DC) analysed directly ex vivo or after 24 h of in vitro maturation with interleukin (IL)-1β, tumour necrosis factor-α, IL-6 and prostaglandin E2. Mean fluorescence intensities and standard deviations after staining for CD83, CD86, human leucocyte antigen D-related, CD40 and CD80 are shown of DC from 17 chronic hepatitis B virus carriers and controls each; none of the differences reached statistical significance.
Fig. 2.
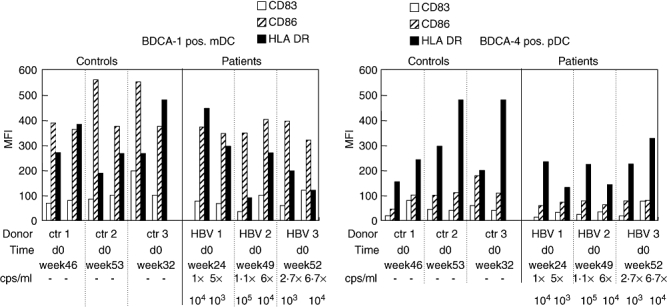
Phenotype of isolated myeloid or plasmacytoid dendritic cells (DC) separated at two different time-points from three untreated low viraemic hepatitis B virus (HBV) carriers and controls each and analysed ex vivo. Mean fluorescence intensities after staining for CD83, CD86 and human leucocyte antigen D-related are shown of DC from three representative chronic HBV carriers and controls each.
Cytokine secretion of ex vivo DC
Next, we analysed isolated DC populations for secretion of pro- and anti-inflammatory cytokines either spontaneously or after stimulation via CD40 ligation [14] or the TLR ligands SAC [17], poly(I:C) [18] or CpG2006 [19]. However, no significant differences were found in the secretion of any tested cytokine by both DC subsets from chronic HBV patients or controls, irrespective of the stimulation mode (Fig. 3). Secretion of IL-12 was low (< 50 pg/ml, not shown) in both DC subsets, whatever stimulus was used (not shown).
Fig. 3.
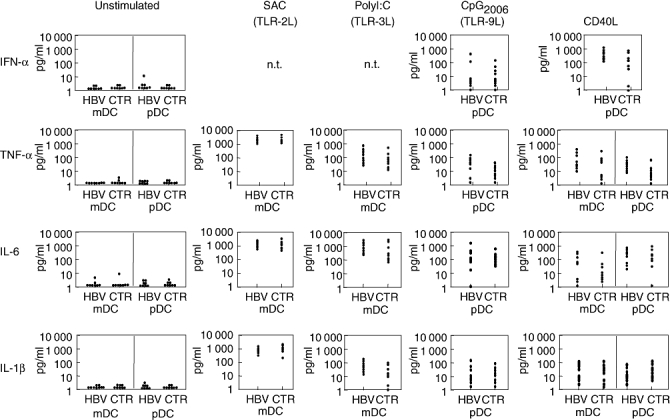
Cytokine secretion by separated ex vivo dendritic cells (DC) either unstimulated or stimulated via CD40 or Toll-like receptor ligation. Separated myeloid DC (mDC) and plasmacytoid DC (pDC) from eight patients and controls each were stimulated in vitro with the indicated stimuli; after 48 h the indicated cytokines were determined in culture supernatants by enzyme-linked immunosorbent assay (interferon-α) or cytokine bead array.
Cytokine secretion by contaminating cells was assessed by intracellular cytokine staining of mDC and pDC isolated from five healthy donors and stimulated for 48 h by SAC (mDC) or CpG2006 (pDC). As shown in Fig. 4a, CD11c+ mDC produced IL-6, TNF-α and IL-1β in response to SAC, and BDCA-4+ pDC showed production of IL-6, TNF-α and IL-1β in response to CpG2006. Frequencies of SAC-stimulated cytokines producing mDC were significantly higher than those of CpG-stimulated pDC (Fig. 4), reflecting the generally higher amounts of cytokines secreted by mDC (compare Fig. 3). Importantly, no cytokine secretion was detected in the minute non-DC population (Fig. 4).
Fig. 4.
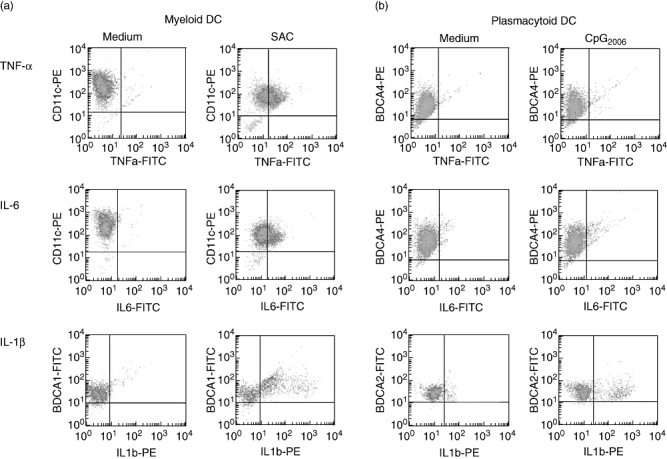
Cytokine secretion by separated dendritic cells (DC) after stimulation with Staphylococcus aureus Cowan I (SAC) or CpG2006 by intracellular cytokine staining. Separated myeloid DC (mDC) and plasmacytoid DC (pDC) from six healthy controls stimulated in vitro with SAC or CpG were stained for the indicated cytokines. Plots of one experiment representative of six independent experiments are shown.
T cell stimulatory capacity of ex vivo DC
To assess the capacity of DC to stimulate primary Th cells, a panel of CD4 cells isolated from healthy buffy coat donors were stimulated by mDC and pDC from eight chronic HBV carriers and controls each either directly after isolation or after in vitro maturation. As shown in Fig. 5, the curves of immature mDC and pDC from HBV patients and controls were almost overlapping over a wide range of DC : CD4 cell ratios, while for mature mDC and pDC minor differences between patients and controls occurred that were not significant and inconsistent between the four different responder CD4 cell populations. However, isolated CD4 cells incubated without DC did not show significant proliferation, as indicated by [3H]-thymidine incorporation below 150 counts per minute (cpm) (not shown).
Fig. 5.
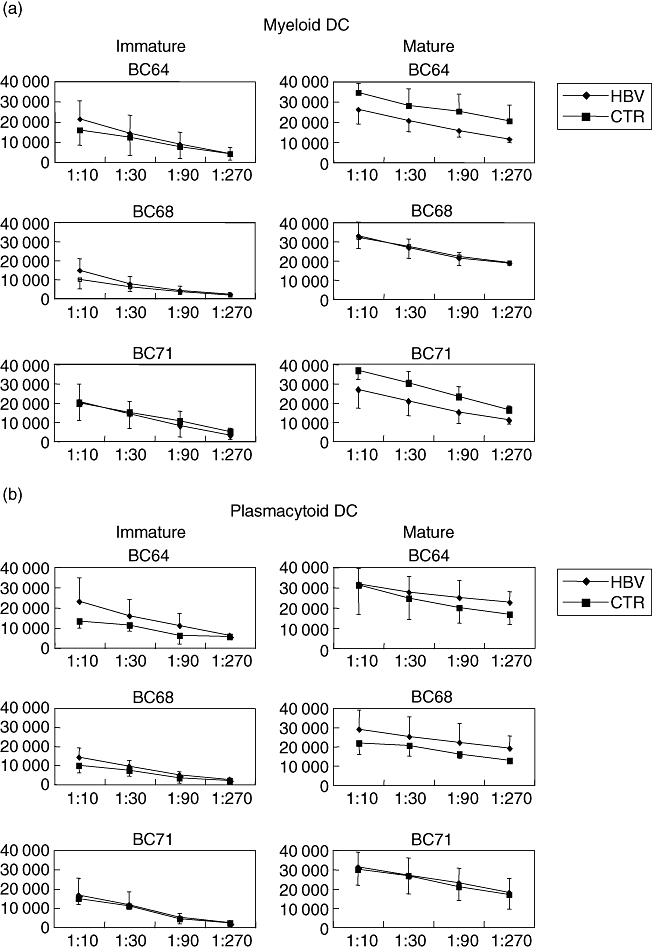
Allogeneic T cell stimulation by ex vivo dendritic cells (DC) or maturated DC in mixed lymphocyte reactions. Separated myeloid DC (a) and plasmacytoid DC (b) from eight chronic hepatitis B virus carriers and healthy controls (CTR) were each co-incubated either directly ex vivo or after 24 h of in vitro maturation with a panel of allogeneic responder CD4 cells from four healthy buffy coat (BC) donors, before the cultures were pulsed with [3H]-thymidine and uptake was detected as counts per minute. Means and standard deviations of three representative BC donors (BC 64, 68, 71) are shown.
To study the DC function to stimulate HBV-specific HLA class I-restricted CTL, isolated mDC and pDC from six HLA A2·1-positive chronic HBV patients (three low, three highly viraemic) and controls were each pulsed with the HBV- or EBV-derived CTL epitopes HBc18−27 or EBV280−288. In order to avoid potential peptide autopresentation, pulsed DC were washed extensively before co-incubation with a HBc18−27-specific HLA A2·1-restricted CTL clone. Both DC subsets were highly effective in peptide-specific CTL stimulation, as indicated by IFN-γ levels up to 8000 pg/ml when pulsed with the respective epitope, although pDC were slightly weaker CTL stimulators (Fig. 6). No relevant IFN-γ levels were found when DC loaded with EBV control peptide, HLA A2·1-negative DC loaded with HBc18−27 peptide, or the peptide without DC were used as stimulators, demonstrating the specificity and HLA restriction of CTL stimulation. Importantly, there were no differences in HBV-specific CTL stimulation between patient or control DC, excluding a T cell stimulation failure of mDC or pDC in chronic HBV infection.
Fig. 6.
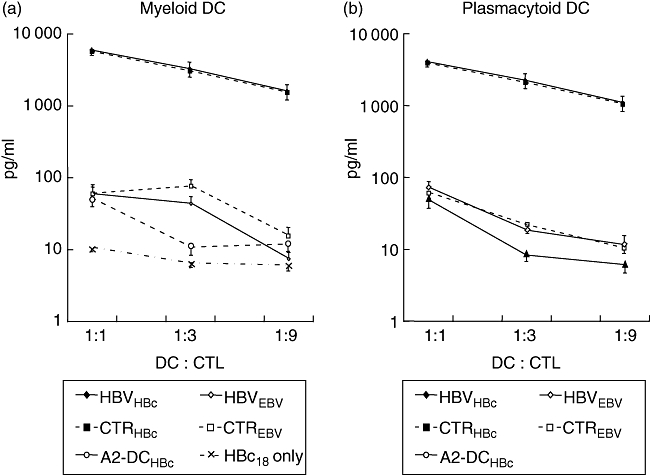
Hepatitis B core (HBc)18−27 peptide-specific cytotoxic lymphocyte (CTL) stimulation by ex vivo dendritic cells (DC). Separated myeloid DC (a) and plasmacytoid DC (bB) from five human leucocyte antigen (HLA) A2·1-positive patients (HBV) and controls (CTR) were each loaded with synthetic HLA A2·1-restricted peptide epitopes HBc18−27 or control peptide EBV280−288, and co-incubated with an HLA A2·1-restricted HBc18−27-specific CTL clone derived from a patient with acute self-limited hepatitis B. Myeloid DC and plasmacytoid DC from five HLA A2-negative donors pulsed with HBc18−27 or the peptide HBc18−27 without DC served as negative controls. CTL-derived interferon-γ secretion was quantified in 48 h supernatants by cytokine bead array. Means and standard deviation of five simultaneously analysed DC donors per group are presented.
HBV infection of mDC and pDC
As viral replication in DC could interact with their APC function, isolated DC preparations were assessed for HBV infection using quantitative PCR for HBV DNA and real-time PCR for viral mRNA. PCR analyses of DC lysates indeed revealed HBV DNA in mDC and pDC, as shown in Table 4. The highest amount of HBV DNA was detected in highly viraemic patients, while DC from only one carrier with low viraemia but none of the controls were positive. Viral replication was investigated by a well-established RT–PCR assay with high sensitivity for the replicative intermediate HBV mRNA [15]. However, results for HBV mRNA in mDC and pDC from high and low viraemic donors were uniformly negative (Table 4).
Table 4.
Patient characteristics and expression of the hepatitis B virus (HBV) replicative intermediate mRNA in myeloid dendritic cells (mDC) and plasmacytoid DC (pDC) (by reverse transcription–polymerase chain reaction).
| HBe | ALT | DNA* | DNA† | mRNA | ||||
|---|---|---|---|---|---|---|---|---|
| Donor | Diagnosis | (serum) | (U/l) | (plasma) | mDC | pDC | mDC | pDC |
| C-11 | Chronic | Pos. | 91 | 1·1 × 108 | 5200 | 9390 | – | – |
| C-12 | Chronic | Pos. | 80 | 1·7 × 107 | 630 | 1800 | – | – |
| C-13 | Chronic | Pos. | 69 | 4 × 107 | 910 | 650 | – | – |
| C-14 | Chronic | Pos. | 107 | 4 × 107 | 350 | 731 | – | – |
| C-15 | Chronic | Pos. | 49 | 7·1 × 106 | 124 | 110 | – | – |
| C-16 | Chronic | Pos. | 324 | 4 × 107 | 140 | 91 | – | – |
| C-17 | Chronic | Neg. | 130 | 3·7 × 106 | 130 | 170 | – | – |
| C-18 | Chronic | Neg. | 96 | 1·7 × 106 | 58 | 0 | – | – |
| C-19 | Chronic | Neg. | 42 | 8·3 × 104 | 22 | 11 | – | – |
| C-20 | Chronic | Neg. | 30 | 2·9 × 103 | 21 | 51 | – | – |
| C-21 | Chronic | Neg. | 22 | 1·5 × 103 | 0 | 0 | – | – |
| C-22 | Chronic | Neg. | 31 | < 103 | 0 | 0 | – | – |
| H-11 | Resolved | Neg. | 23 | < 103 | 0 | 0 | – | – |
| H-12 | Resolved | Neg. | n.t. | < 103 | 0 | 0 | – | – |
| H-13 | Resolved | Neg. | n.t. | < 103 | 0 | 0 | – | – |
| H-14 | Resolved | Neg. | n.t. | < 103 | 0 | 0 | – | – |
HBV DNA in copies/ml by Roche Amplicor (cps/ml).
HBV DNA in copies/DC by Roche Amplicor. n.t., not tested. HBe: hepatitis B envelope; ALT: alanine aminotransferase.
Discussion
Most previous reports studied DC generated in vitro from peripheral blood monocytes demonstrating reduced expression of co-stimulatory molecules, impaired cytokine secretion and lower allostimulatory capacity by DC from chronic HBV carriers [4–6,20,21]. In contrast, we found no evidence of reduced allogeneic or HBc antigen-specific T cell stimulation by monocyte-derived DC from chronic HBV carriers [8]. These studies were limited widely by prolonged in vitro culture, different maturation protocols and use of monocytic precursor cells. Moreover, the relevance of these approaches for the situation in situ has never been demonstrated. In contrast, a recent study reported comparable frequencies but impaired function and phenotype of mDC and pDC from chronic HBV carriers after isolation and short in vitro culture [7]. However, as DC are not susceptible to HBV infection or replication [22], it is unclear how the HBV should cause an HBV-specific DC failure. Thus, the aim of this study was to reassess these findings in myeloid and plasmacytoid DC, the two major DC populations in peripheral blood [23,24].
A reduction of peripheral blood DC could lead to reduced numbers of organ resident DC resulting in a T cell stimulation failure [10]. In our analyses, no reduction of any DC subset was found in blood of patients with chronic hepatitis B compared to healthy donors or donors with resolved hepatitis B. This is in accordance with recent data [7], although other authors found reduced mDC or pDC frequencies in peripheral blood of chronic HBV carriers, due probably to slight differences in technique or patient selection [6,21,25]. However, DC frequencies in blood are very low, depend on exact gating, and vary considerably in the same individual tested at different time-points, questioning the physiological relevance of minor differences.
In this present study, isolated mDC or pDC showed an immature expression pattern of surface markers without differences between DC from patients and controls, thus ruling out an impaired expression of co-stimulatory molecules in vivo. This is in concordance with the findings of a recent report on ex vivo DC utilizing a similar protocol [7]. In contrast, after in vitro maturation the previous study found a reduced up-regulation of CD80 and CD86 on mDC of chronic HBV carriers that was not confirmed by our studies. However, our longitudinal analyses of DC phenotype in HBV carriers and healthy controls revealed substantial fluctuations in the surface expression of DC markers within the same individual over time, limiting the relevance of minor differences. Finally, studies of in vitro-cultured DC are hampered further by the low viability of such cells and are therefore prone to artefacts due to contamination with dying cells [26]. In addition, the different maturation protocols might account for different results.
Cytokine secretion by DC results from activation of TLR by bacterial or viral stimuli or from CD40 ligation by T cells [10,27]. In our studies, the secretion of IFN-α and other cytokines by mDC or pDC in response to CD40 ligation and different TLR agonists [19,28] varied considerably from donor to donor but revealed no differences between chronic HBV patients and controls. The detected cytokines were indeed derived from DC, because intracellular cytokine staining failed to demonstrate production of cytokines after TLR-stimulation by the minute contaminating non-DC population. This uncompromised cytokine secretion by mDC and pDC from chronic HBV carriers utilizing different stimuli contrasts with the findings of reduced mDC-derived TNF-α production and pDC-derived IFN-α production shown previously [7]. This might be due to different patient selection (in our study: all patients treatment-naive, Caucasian, lower viraemia and higher ALT), stimulation modes and cytokine detection assays [7]. However, our extensive MLR studies involving a large and fixed panel of responder CD4 cells ruled out an allogeneic Th cell stimulation defect of ex vivo DC or in vitro maturated DC. Moreover, HBV core peptide-specific CTL stimulation was uncompromised in mDC and pDC from chronic HBV carriers. Thus, a relevant defect of co-stimulatory molecules or cytokine secretion was excluded further by the finding of successful alloreactive and HBV-specific Th cell and CTL stimulation by patient-derived DC.
Finally, purified mDC and pDC were assessed for viral DNA and RNA. The negative proof of the replicative mRNA intermediates in ex vivo DC by highly sensitive and specific PCR recognizing all HBV mRNA species [15] excluded relevant HBV replication in DC and is in accordance with a recent report [22]. Moreover, detection of the highest viral DNA contents in DC from HBV carriers with high viraemia suggests contamination by serum DNA. Thus, we found no evidence for HBV infection of DC, which is in line with the intact phenotype and function of these cells.
The major limitation of our study is the necessity to include different subjects into different experiments, as the number of purified DC available from an individual donor sample is very limited and patients cannot be studied serially due to the initiation of anti-viral treatment and the high spontaneous fluctuations over time that would interfere with the results.
In conclusion, our studies indicate the quantitative and qualitative integrity of peripheral blood-derived myeloid and plasmacytoid DC from chronic HBV carriers, when studied directly ex vivo or after in vitro maturation. Controversial previous findings of mDC or pDC alterations were disproved by the finding of uncompromised Th cell and CTL stimulation. This seems reasonable, as a general DC defect would not be compatible with the general immunocompetence of chronic HBV carriers. Thus, autologous DC might represent fully functional and useful tools for future immunotherapies.
Acknowledgments
We thank Dr Pierre Garrone (Schering Plough, Dardilly, France) for kindly providing us with CD40L cells and Dr Antonio Bertoletti (University College of London) for his helpful discussions. We thank Sandra Weyer and Silke Schmitt for excellent technical assistance. The work was supported by grants to W. O. B. from Deutsche Forschungsgemeinschaft (SFB490/A8) and Kompetenznetz Hepatitis.
References
- 1.Bertoletti A, Ferrari C. Kinetics of the immune response during HBV and HCV infection. Hepatology. 2003;38:4–13. doi: 10.1053/jhep.2003.50310. [DOI] [PubMed] [Google Scholar]
- 2.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–29. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Wang FS, Xing LH, Liu MX, et al. Dysfunction of peripheral blood dendritic cells from patients with chronic hepatitis B virus infection. World J Gastroenterol. 2001;7:537–41. doi: 10.3748/wjg.v7.i4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arima S, Akbar SM, Michitaka K, et al. Impaired function of antigen-presenting dendritic cells in patients with chronic hepatitis B: localization of HBV DNA and HBV RNA in blood DC by in situ hybridization. Int J Mol Med. 2003;11:169–74. [PubMed] [Google Scholar]
- 6.Beckebaum S, Cicinnati VR, Dworacki G, et al. Reduction in the circulating pDC1/pDC2 ratio and impaired function of ex vivo-generated DC1 in chronic hepatitis B infection. Clin Immunol. 2002;104:138–50. doi: 10.1006/clim.2002.5245. [DOI] [PubMed] [Google Scholar]
- 7.Van der Molen RG, Sprengers D, Binda RS, et al. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40:738–46. doi: 10.1002/hep.20366. [DOI] [PubMed] [Google Scholar]
- 8.Tavakoli S, Schwerin W, Rohwer A, et al. Phenotype and function of monocyte derived dendritic cells in chronic hepatitis B virus infection. J Gen Virol. 2004;85:2829–36. doi: 10.1099/vir.0.80143-0. [DOI] [PubMed] [Google Scholar]
- 9.Robinson SP, Patterson S, English N, Davies D, Knight SC, Reid CD. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769–78. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–62. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 11.Bertoletti A, Southwood S, Chesnut R, et al. Molecular features of the hepatitis B virus nucleocapsid T-cell epitope 18–27: interaction with HLA and T-cell receptor. Hepatology. 1997;26:1027–34. doi: 10.1002/hep.510260435. [DOI] [PubMed] [Google Scholar]
- 12.Dzionek A, Fuchs A, Schmidt P, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–46. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 13.Jonuleit H, Kuhn U, Muller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–42. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 14.Garrone P, Neidhardt EM, Garcia E, Galibert L, van Kooten C, Banchereau J. Fas ligation induces apoptosis of CD40-activated human B lymphocytes. J Exp Med. 1995;182:1265–73. doi: 10.1084/jem.182.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glebe D, Aliakbari M, Krass P, Knoop EV, Valerius KP, Gerlich WH. Pre-s1 antigen-dependent infection of Tupaia hepatocyte cultures with human hepatitis B virus. J Virol. 2003;77:9511–21. doi: 10.1128/JVI.77.17.9511-9521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dzionek A, Sohma Y, Nagafune J, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–34. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akira S. Mammalian Toll-like receptors. Curr Opin Immunol. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 18.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 19.Krug A, Towarowski A, Britsch S, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–37. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Zheng BJ, Zhou J, Qu D, et al. Selective functional deficit in dendritic cell–T cell interaction is a crucial mechanism in chronic hepatitis B virus infection. J Viral Hepat. 2004;11:217–24. doi: 10.1111/j.1365-2893.2004.00497.x. [DOI] [PubMed] [Google Scholar]
- 21.Duan XZ, Zhuang H, Wang M, Li HW, Liu JC, Wang FS. Decreased numbers and impaired function of circulating dendritic cell subsets in patients with chronic hepatitis B infection (R2) J Gastroenterol Hepatol. 2005;20:234–42. doi: 10.1111/j.1440-1746.2004.03529.x. [DOI] [PubMed] [Google Scholar]
- 22.Untergasser A, Zedler U, Langenkamp A, et al. Dendritic cells take up viral antigens but do not support the early steps of hepatitis B virus infection. Hepatology. 2006;43:539–47. doi: 10.1002/hep.21048. [DOI] [PubMed] [Google Scholar]
- 23.Liu YJ, Kanzler H, Soumelis V, Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol. 2001;2:585–9. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 24.Grabbe S, Kampgen E, Schuler G. Dendritic cells: multi-lineal and multi-functional. Immunol Today. 2000;21:431–3. doi: 10.1016/s0167-5699(00)01694-7. [DOI] [PubMed] [Google Scholar]
- 25.Duan XZ, Wang M, Li HW, Zhuang H, Xu D, Wang FS. Decreased frequency and function of circulating plasmocytoid dendritic cells (pDC) in hepatitis B virus infected humans. J Clin Immunol. 2004;24:637–46. doi: 10.1007/s10875-004-6249-y. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–20. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 27.Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–9. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]


