Abstract
Enterovirus infections have been diagnosed more frequently in type 1 diabetic patients than in the healthy population, and enteroviruses have also been found in the pancreas of diabetic patients. Primary replication of the virus occurs in the gut, but there are no previous studies evaluating possible presence of virus in the intestine of diabetic patients. The purpose of this study was to investigate if enteroviruses can be found in small intestinal tissue of type 1 diabetic patients. Formalin-fixed, paraffin-embedded upper intestinal biopsy samples were analysed for the presence of enterovirus using in situ hybridization and immunohistochemistry. Enterovirus was detected by in situ hybridization in six (50%) of the type 1 diabetic patients (n = 12) but in none of the control subjects (n = 10, P = 0·015). Immunohistochemistry identified enterovirus in nine (75%) of the patients and one (10%) control subject (P = 0·004). The presence of the virus was confirmed by reverse transcription–polymerase chain reaction in one of the four patients from whom a frozen and unfixed sample was available. Intestinal morphology was normal in all study subjects. The results suggest that a substantial proportion of type 1 diabetic patients have an ongoing enterovirus infection in gut mucosa, possibly reflecting persistent enterovirus infection. This observation opens new avenues for further studies on the possible role of enteroviruses in human type 1 diabetes.
Keywords: enteroviruses, immunohistochemistry, in situ hybridization, small intestine, type 1 diabetes
Introduction
Previous studies have found that enterovirus infections are more common in type 1 diabetic and prediabetic subjects than in the healthy population [1]. Enteroviruses were also found in the pancreas of a few diabetic cases [2–7]. However, possible mechanisms that explain how enterovirus infections could cause type 1 diabetes still remain uncertain. The primary replication site of enteroviruses is in the gut mucosa. In light of this well-known fact it is surprising that no studies have been conducted so far to search for enteroviruses in the intestinal tissue of diabetic patients. In addition, previous studies have provided evidence that the small intestine of type 1 diabetic patients shows enhanced immune activation [8,9]. This type of activation of the gut immune system could be explained by a local virus infection in intestinal mucosa. The purpose of this study was to analyse if enteroviruses can be found in small intestinal mucosa of type 1 diabetic patients. We analysed small intestine biopsies of type 1 diabetic patients and healthy subjects for the presence of enterovirus using different virological methods.
Subjects and methods
Study series
Small intestine biopsy samples were taken from 12 type 1 diabetic patients and 10 control subjects during the years 1995–2000 at the Department of Gastroenterology, Tampere University Hospital. Type 1 diabetes had been diagnosed in all patients and all of them were on insulin treatment (Table 1). Their ages varied from 18 to 53 years (median 30 years) and duration of diabetes from 0 to 51 years (median 13 years). Two of the patients were male. All control subjects were non-diabetic and their age varied from 23 to 71 years (median 54 years). Three subjects were male. All study subjects underwent gastroscopy due to unspecific gastrointestinal symptoms and small bowel mucosal biopsies were taken for morphological analyses and for basic research. Morphological analyses indicated normal gut mucosa in all study subjects. Coeliac disease was excluded from all study subjects by negative endomysial antibody result and normal villous morphology. For in situ hybridization and immunohistochemistry, biopsy samples were formalin-fixed and embedded in paraffin, after which they were cut into 5 µm sections onto microscopic slides. For reverse transcription–polymerase chain reaction (RT–PCR), unfixed samples were stored frozen in optimal cutting temperature (OCT) medium at −70°C. Formalin-fixed samples were available from all study subjects, while frozen samples were available from four patients. Study protocol was approved by the ethical committee of Tampere University Hospital, and all subjects gave their informed consent.
Table 1.
Clinical characteristics and enterovirus analysis of type 1 diabetic patients.
| Patient | Sex | Age at biopsy | Age at DM dg | In situ hybridization | Immunohistochemistry | RT–PCR* |
|---|---|---|---|---|---|---|
| 1 | F | 18 | 13 | – | + | n.a. |
| 2 | F | 19 | Under age 10 | – | – | n.a. |
| 3 | F | 23 | 23 | + | + | n.a. |
| 4 | F | 29 | Under age 10 | + | – | n.a. |
| 5 | M | 29 | 10 | + | + | – |
| 6 | F | 30 | 18 | + | + | – |
| 7 | M | 30 | 26 | – | – | n.a. |
| 8 | F | 31 | 6 | + | + | + |
| 9 | F | 37 | 5 | – | + | n.a. |
| 10 | F | 43 | 15 | – | + | n.a. |
| 11 | F | 50 | 41 | – | + | n.a. |
| 12 | F | 53 | 51 | + | + | – |
A frozen sample for reverse transcription–polymerase chain reaction (RT–PCR) was available from only four patients who were positive in both in situ hybridization and immunohistochemistry; n.a.: not analysed (there was no frozen biopsy sample available from these patients).
In situ hybridization
An enterovirus-specific oligonucleotide probe (sequence from 5′ to 3′GAA ACA CGG ACA CCC AAA GTA GTC GGT TCC GCT GCR GAG TTR CCC RTT ACG ACA) was designed to hybridize with the conserved, group-common sequence in the 5′ non-coding region of the enteroviral genome to detect all known enterovirus types. The probe was 3′ end-labelled with digoxigenin (DIG) using the DIG oligonucleotide tailing kit (Roche Diagnostics Ltd, Welwyn Garden City, UK). Ten pmol of the probe was used for one labelling reaction. The hybridization was performed using earlier published instructions [10]. The amount of probe in the hybridization cocktail was 250 ng, and the used hybridization time was 3 h. Binding of the probes was documented by anti-DIG antibody, which was conjugated with alkaline phosphatase. This enzyme, together with its substrate nitroblue tetrazolium/bromo-chloro-3-indolyl-phosphate (NBT/BCIP), yields an insoluble purple precipitate, which can be visualized using a light microscope. Enterovirus-infected and mock-infected green monkey kidney (GMK) cells were used as controls.
Immunohistochemical staining
A monoclonal mouse enterovirus-specific antibody (DakoCytomation Denmark A/S, Glostrup, Denmark; clone 5-D8/1) (1 : 1000) was used to detect enteroviral VP1 protein in biopsy samples using the EnVision+ polymer technique (DakoCytomation Denmark A/S). Antigen retrieval was performed on rehydrated sections in a microwave oven at 850 W for two 7-min cycles using Tris-ethylenediamine tetraacetic acid (EDTA) buffer (pH 9·0) as the retrieval solution. Immunostaining was carried out in a TechMate™ 500 Immunostainer (DakoCytomation Denmark A/S). Diaminobenzidine (DAB) was used as a chromogen and haematoxylin as a nuclear stain. The specificity of immunohistochemistry was controlled by omitting the primary antibodies or replacing them with irrelevant anti-sera. Known positive tissue samples from enterovirus-infected mice were used to confirm the staining reliability of all separate staining batches. Enterovirus-infected and mock-infected GMK cells were used as controls.
RT–PCR
The biopsy samples were first cut out from the OCT medium, after which they were homogenized using a SilentCrusher S homogenizer (Heidolph, Schwabach, Germany). RNA extraction was carried out using the RNeasy Mini Kit (Qiagen, Hilden, Germany). We used a highly sensitive RT–PCR method which amplifies a sequence common for all known enterovirus serotypes. The details of this method have been described in our earlier studies [11].
Statistical methods
Statistical analyses were performed using spss 15·0 for Windows. Frequency comparison was performed using the Pearson χ2 test.
Results
In total, 12 type 1 diabetic patients and 10 control subjects were analysed for the presence of enterovirus in small intestinal biopsies using in situ hybridization and immunohistochemistry. Six of the patients were clearly positive in the hybridization assay, whereas all control subjects remained negative (P = 0·015; Table 2 and Fig. 1). Four of the enterovirus-positive cases had the virus in the epithelial cells of villi and crypts as well as in the cells of lamina propria; two were positive in lamina propria only. Immunohistochemistry detected enterovirus protein in nine of the patients and one control subject (P = 0·004; Table 2 and Fig. 1). Positivity was located especially in the epithelium. Nine of the patients (75%) and one of the control subjects (10%) were enterovirus-positive in either in situ hybridization or immunohistochemistry (P = 0·004). The presence of the virus was also confirmed by RT–PCR in one of the four patients who were positive in both hybridization and immunohistochemistry and from whom a frozen sample was available for RT–PCR analyses (Table 1). Detection of enterovirus in gut mucosa was not associated with HLA type, gender, hyperglycaemia or duration of diabetes.
Table 2.
Detection of enterovirus in small intestinal biopsy samples from type 1 diabetic patients and non-diabetic control subjects.
| Method | Enterovirus positive | Enterovirus negative | |
|---|---|---|---|
| Type 1 diabetics (n = 12) | In situ hybridization | 6* | 6 |
| Immunohistochemistry | 9** | 3 | |
| Control subjects (n = 10) | In situ hybridization | 0 | 10 |
| Immunohistochemistry | 1 | 9 |
P = 0·015;
P = 0·004 when compared to control subjects.
Fig. 1.
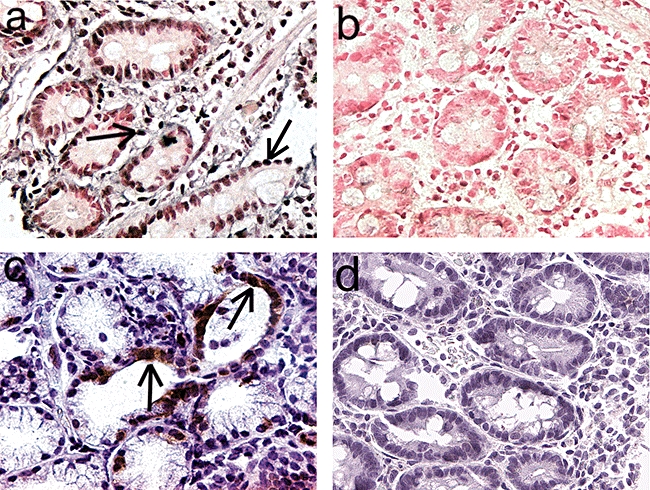
Detection of enterovirus in the epithelium and lamina propria of small intestinal biopsy sample of one type 1 diabetic patient. (a) The dark purple precipitate indicates the presence of enteroviral genome (in situ hybridization) in a diabetic patient compared to enterovirus-negative sample of one control subject (b). (c) The brown colour indicates the presence of enterovirus VP1 protein (immunohistochemistry) in crypt epithelium in a diabetic patient compared to virus-negative control subject (d). Pictures were taken with 400-fold magnification. Some virus-positive cells are marked by arrows.
Discussion
This is the first time that an enterovirus was found in the mucosa of small intestine of type 1 diabetic patients. Even though it is well known that the primary replication of enteroviruses happens in intestinal cells, this evidence comes mainly from animal studies and surprisingly little is known about the possible presence of virus in intestinal mucosa in any enterovirus disease of humans. Therefore, from this viewpoint our observation indicating enterovirus in intestinal mucosa is significant.
The presence of the virus correlated with type 1 diabetes because diabetic patients were positive more frequently than control subjects. The parallel results from two unrelated methods (immunohistochemistry and in situ hybridization) strongly support the specificity of these findings. In addition, we were able to confirm the presence of the virus by RT–PCR in one of four patients from whom a frozen sample was available. Although there is variation between the detection of viral RNA by in situ hybridization and RT–PCR, there is no real discrepancy between them. This type of variation could be due to the two separate biopsy samples which were needed for the two methods, representing two different sites of the intestinal mucosa. These samples were also pretreated differently; the tissue sample was frozen for RT–PCR but fixed in formalin for in situ hybridization. Formalin-fixation leads to partial degradation of the virus RNA, but the short in situ hybridization probe is able to bind to such short RNA fragments. However, RT–PCR needs a longer region of intact RNA and can also be affected by possible PCR inhibitors. Immunohistochemistry was based on the use of a commercial enterovirus specific antibody detecting viral VP1 protein, while in situ hybridization detected viral RNA. The specificity of the VP1 antibody used in immunohistochemistry, as well as the probe of in situ hybridization, was validated by internal virus-positive and -negative controls in each assay run. In previous studies this VP1 antibody has been used widely to detect enterovirus protein in cardiac and other tissues [3,12,13]. The specificity of the RT–PCR method was validated in our previous study [11].
In spite of the good overall correlation between the results of immunohistochemistry and in situ hybridization, there was some variation in the intensity of staining and localization of virus-positive cells in individual samples. More samples were also enterovirus-positive in immunohistochemistry than in hybridization. This variation could be related to technical reasons and may be due to possible degradation of virus RNA during formalin-fixation, while virus proteins could be more resistant to such degradation.
The results suggest that a significant proportion of type 1 diabetic patients may have ongoing enterovirus infection in the gut. This finding is in line with several previous publications suggesting an association between enteroviruses and type 1 diabetes [1]. Previous studies have been based on the detection of enterovirus genome sequences or enterovirus-specific antibodies in the serum of diabetic patients more frequently than in control subjects, and some studies have found the virus in the pancreas of diabetic subjects [2–7]. It is known that pancreatic islets are infected during severe systemic infections, suggesting that these viruses have specific tropism to islet cells [6]. Enterovirus can also infect and damage pancreatic islets in mouse models and in islet cell cultures [6,14–17]. The present study suggests that, in addition to the pancreatic tissue, intestinal mucosa may be an important focus of enterovirus infection in diabetic patients. Frequent detection of the virus would fit well with persistent enterovirus infection. Previously, the persistence of enterovirus has been documented in cardiac tissue, where it can lead to chronic myocarditis and cardiomyopathy, but persistence of the enterovirus in gut tissue has not been described before. However, it is logical to assume that the intestinal tract may be particularly permissive for viral persistence as it is the primary replication site of these viruses, and the virus has adapted to replicate efficiently in these tissues during evolution. Further studies are needed to determine if there are some specific mechanisms which could make the gut of diabetic subjects particularly susceptible for enterovirus replication and viral persistence. For example, some dietary factors, which have been linked to the aetiology of type 1 diabetes (such as cow's milk proteins), could make the gut mucosa more susceptible for the virus. It is also possible that risk genes for type 1 diabetes influence viral replication. The recently discovered risk gene for type 1 diabetes, mda-5, codes for an intracellular receptor for enterovirus RNA which mediates the activation of innate immune system during enterovirus infection [18]. Hyperglycaemia could also make diabetic patients more susceptible to the virus. However, prospective studies have found increased frequency of enterovirus infections in prediabetic subjects whose glucose metabolism is still normal, suggesting that some other mechanisms are probably involved [19]. Most of the patients in the present series had had diabetes for several years, and further studies among recently diagnosed patients as well as prediabetic autoantibody-positive subjects are needed to find answers to these still unresolved questions.
Our findings also fit well with previous observations suggesting immune activation in the small intestine of type 1 diabetic patients. Enhanced expression of major histocompatibility complex (MHC) class II antigens and intercellular adhesion molecule-1, as well as increased densities of interleukin-1α and interleukin-4-positive cells, have been found in small intestine of patients with type 1 diabetes [9]. In addition, type 1 diabetic subjects have shown an increase in intestinal permeability [20], a finding which could also be explained by local enterovirus infection in the gut. Previous studies have also suggested that enterovirus infections can enhance immunization to dietary insulin in cow's milk [21], a phenomenon which could be caused by the adjuvant effect of the virus replicating in the gut mucosa.
It is possible that (persistent) enterovirus infection in the gut could be connected to the pathogenesis of type 1 diabetes. Replication of the virus in the gut could provide a virus reservoir which is connected anatomically to the pancreas via pancreatic duct and lymphatic networks, which could be the source of pancreatic infection. Local infection in the gut could also stimulate autoreactive lymphocytes which then home to the pancreas using the common homing receptors [22]. These open questions indicate that further studies are needed to elucidate these phenomena and to obtain more detailed data about the proportion of diabetic and prediabetic subjects who harbour the virus in gut mucosa.
Acknowledgments
We would like to thank Eeva Tolvanen, Mervi Himanka and Eila Pohjola for technical assistance. This study was supported by grants from the Juvenile Diabetes Research Foundation, the Academy of Finland, Päivikki and Sakari Sohlberg's Foundation, the Finnish Cultural Foundation, the Emil Aaltonen Foundation and Competitive Research funding of the Pirkanmaa Hospital District.
References
- 1.Hyoty H. Enterovirus infections and type 1 diabetes. Ann Med. 2002;34:138–47. [PubMed] [Google Scholar]
- 2.Dotta F, Censini S, van Halteren AG, et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci USA. 2007;104:5115–20. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foulis AK, Farquharson MA, Cameron SO, et al. A search for the presence of the enteroviral capsid protein VP1 in pancreases of patients with type 1 (insulin-dependent) diabetes and pancreases and hearts of infants who died of coxsackieviral myocarditis. Diabetologia. 1990;33:290–8. doi: 10.1007/BF00403323. [DOI] [PubMed] [Google Scholar]
- 4.Giannani R, Dotta F, Di Mario U, et al. Cambridge, UK: Immunology of Diabetes Society; Screening of organ donors for anti-islets antibodies and characterization of pancreatic histology in an antibody positive subject; pp. 28–30. [Google Scholar]
- 5.Sayama K, Imagawa A, Okita K, et al. Pancreatic beta and alpha cells are both decreased in patients with fulminant type 1 diabetes: a morphometrical assessment. Diabetologia. 2005;48:1560–4. doi: 10.1007/s00125-005-1829-9. [DOI] [PubMed] [Google Scholar]
- 6.Ylipaasto P, Klingel K, Lindberg AM, et al. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia. 2004;47:225–39. doi: 10.1007/s00125-003-1297-z. [DOI] [PubMed] [Google Scholar]
- 7.Yoon JW, Austin M, Onodera T, et al. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300:1173–9. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- 8.Vaarala O. Gut and the induction of immune tolerance in type 1 diabetes. Diabetes Metab Res Rev. 1999;15:353–61. doi: 10.1002/(sici)1520-7560(199909/10)15:5<353::aid-dmrr59>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Westerholm-Ormio M, Vaarala O, Pihkala P, et al. Immunologic activity in the small intestinal mucosa of pediatric patients with type 1 diabetes. Diabetes. 2003;52:2287–95. doi: 10.2337/diabetes.52.9.2287. [DOI] [PubMed] [Google Scholar]
- 10.Hohenadl C, Klingel K, Mertsching J, et al. Strand-specific detection of enteroviral RNA in myocardial tissue by in situ hybridization. Mol Cell Probes. 1991;5:11–20. doi: 10.1016/0890-8508(91)90033-g. [DOI] [PubMed] [Google Scholar]
- 11.Lonnrot M, Sjoroos M, Salminen K, et al. Diagnosis of enterovirus and rhinovirus infections by RT–PCR and time-resolved fluorometry with lanthanide chelate labeled probes. J Med Virol. 1999;59:378–84. [PubMed] [Google Scholar]
- 12.Bourlet T, Gharbi J, Omar S, et al. Comparison of a rapid culture method combining an immunoperoxidase test and a group specific anti-VP1 monoclonal antibody with conventional virus isolation techniques for routine detection of enteroviruses in stools. J Med Virol. 1998;54:204–9. doi: 10.1002/(sici)1096-9071(199803)54:3<204::aid-jmv11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Yousef GE, Brown IN, Mowbray JF. Derivation and biochemical characterization of an enterovirus group-specific monoclonal antibody. Intervirology. 1987;28:163–70. doi: 10.1159/000150012. [DOI] [PubMed] [Google Scholar]
- 14.Bopegamage SA, Petrovicova A. In vitro infection of mouse pancreatic islet cells with coxsackie viruses. Acta Virol. 1994;38:251–5. [PubMed] [Google Scholar]
- 15.Drescher KM, Kono K, Bopegamage S, et al. Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology. 2004;329:381–94. doi: 10.1016/j.virol.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 16.Jordan GW, Cohen SH. Encephalomyocarditis virus-induced diabetes mellitus in mice: model of viral pathogenesis. Rev Infect Dis. 1987;9:917–24. doi: 10.1093/clinids/9.5.917. [DOI] [PubMed] [Google Scholar]
- 17.Szopa TM, Gamble DR, Taylor KW. Biochemical changes induced by Coxsackie B4 virus in short-term culture of mouse pancreatic islets. Biosci Rep. 1985;5:63–9. doi: 10.1007/BF01117442. [DOI] [PubMed] [Google Scholar]
- 18.Smyth DJ, Cooper JD, Bailey R, et al. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet. 2006;38:617–9. doi: 10.1038/ng1800. [DOI] [PubMed] [Google Scholar]
- 19.Salminen K, Sadeharju K, Lonnrot M, et al. Enterovirus infections are associated with the induction of beta-cell autoimmunity in a prospective birth cohort study. J Med Virol. 2003;69:91–8. doi: 10.1002/jmv.10260. [DOI] [PubMed] [Google Scholar]
- 20.Bosi E, Molteni L, Radaelli MG, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49:2824–7. doi: 10.1007/s00125-006-0465-3. [DOI] [PubMed] [Google Scholar]
- 21.Akerblom HK, Knip M. Putative environmental factors in Type 1 diabetes. Diabetes Metab Rev. 1998;14:31–67. doi: 10.1002/(sici)1099-0895(199803)14:1<31::aid-dmr201>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 22.Hanninen A, Nurmela R, Maksimow M, et al. Islet beta-cell-specific T cells can use different homing mechanisms to infiltrate and destroy pancreatic islets. Am J Pathol. 2007;170:240–50. doi: 10.2353/ajpath.2007.060142. [DOI] [PMC free article] [PubMed] [Google Scholar]


