Abstract
Levamisole is a synthetic phenylimidazolthiazole that was first introduced in 1966 as an anti-helmintic agent. Current studies have been focused upon its effect on immune response and on cancer treatment. We examined the molecular mechanisms of levamisole in the activation and maturation of human monocyte-derived dendritic cells (DC) and human T cells. Treatment of DC with levamisole increased the presentation of CD80, CD86, CD83 and human leucocyte antigen D-related (HLA-DR) molecules on the cell membrane, as well as the production of interleukin (IL)-12 p40 and IL-10. Levamisole-treated human DC also enhanced T cell activation towards type 1 T helper immune response by inducing interferon-γ secretion. Neutralization with antibodies against Toll-like receptor (TLR)-2 inhibited levamisole-induced production of IL-12 p40 and IL-10, suggesting a vital role for TLR-2 in signalling DC upon incubation with levamisole. The inhibition of nuclear factor-κB, extracellular signal-regulated kinases 1/2 or c-Jun N-terminal kinases pathways also prevented the effects of levamisole on DC in producing IL-12 p40 or IL-10. Taken together, levamisole could enhance immune response towards T helper 1 development through the activation of dendritic cells or T cell aspects.
Keywords: dendritic cells, levamisole, signal transduction, T cells, Th1/Th2 cells
Introduction
Levamisole, a synthetic phenylimidazolthiazole, is a potent antihelmintic agent that was first introduced in 1966 [1]. Regarded as an immune-enhancing agent, it is useful in the treatment of leprosy based on an Indian clinical trial [2]; however, levamisole has also been reported as being ineffective in human monocyte cytotoxicity or expression of human leucocyte antigen D-related (HLA-DR), HLA-DQ, HLA-DP and the Fc receptor [3]. Similarly, levamisole has no significant effect on the activation of T cells or natural killer (NK) cells, and cannot enhance the proliferation of lymphocytes from normal donors [3].
None the less, current investigations of levamisole are centred around its effect on the immune response and on the treatment of cancers [4]. Although its mode of action on the immune system remains unknown, its clinical application has been demonstrated in the treatment and prevention of atopic disease. Recently, it was shown that levamisole can skew the T helper 2 (Th2) immune response towards the Th1 response via induction of interleukin (IL)-18 on brown Norway rats [5]. Other studies have demonstrated its influence on the course of allergic disease by shifting the Th2-dominant immunity more towards Th1-mediated response on BALB/c mice [6]. Thus, in some indeterminate way, it can modulate the immune system.
Dendritic cells (DC) are the most professional antigen-presenting cells (APCs) whose primary function is to capture, process and present antigens to unprimed T cells [7]. After immature DC capture and process antigens, they lose their antigen-processing activity and become potent immunostimulatory cells that express a high level of major histocompatibility complex (MHC) class II, co-stimulatory molecules (CD80 and CD86) and specific DC maturation marker (CD83) [8,9]. Two lineages of DC regulate Th1 and Th2 development, while the monocyte-derived lineage (DC1) stimulates Th1 cell differentiation and plasmacytoid/lymphoid DC (DC2) induces Th2 cell differentiation [10].
DC may play a role in Th1/Th2 switching and in modulating regulatory T cells. It can either stimulate immune responses or induce immune tolerance, depending upon its mature state [7]. Because of the immunoregulatory properties of dendritic cells, their role in asthma and other atopic disorders has been suggested and can be the basis for the development of new treatments.
The exact effects of levamisole on human DC are yet to be defined. In the present study, we examined the molecular mechanisms of levamisole on the activation and maturation of human monocyte-derived DC.
Materials and methods
Reagents
Levamisole was purchased from Sigma Chemical Co. (St Louis, MO, USA). Escherichia coli lipopolysaccharide (LPS) (L8274, E. coli) was purchased from Sigma Chemical Co. Isotopes were obtained from Amersham Corp. (Arlington Heights, IL, USA), while neutralization antibodies (without sodium azide) against Toll-like receptor (TLR)-2 and TLR-4 were purchased from eBiosciences (San Diego, CA, USA), and helenalin, SB203580, PD98059 and c-Jun N-terminal kinase (JNK) inhibitor II were purchased from Calbiochem (Darmstadt, Germany). These inhibitors were dissolved in dimethyl sulphoxide (DMSO), where a 0·1% (v/v) concentration of DMSO was used as a negative control whenever indicated. Treatment of immature DC with these inhibitors (helenalin, SB203580, PD98059 and JNK inhibitor II) before stimulation was performed for 60 min.
Generation of human DC
Human DC were generated from peripheral blood mononuclear cells (PBMC), as described previously [11–13], with some modification. Briefly, PBMC were obtained from healthy donors by centrifugation with Ficoll-Hypaque (Pharmacia) and the light–density fraction from 42·5 to 50% interface was recovered. CD14+ cells were purified by positive selection using anti-CD14+ microbeads in conjunction with the mini-magnetic affinity cell sorting (MACS) system following the manufacturer's instructions (Miltenyi Biotec, Auburn, CA, USA). The CD14+ cells were cultured at 1 × 106 cells per 1 ml RPMI-1640 medium in 24-well plates (Costar, Cambridge, MA, USA) with granulocyte macrophage-colony stimulating factor (GM-CSF; 800 U/ml) and IL-4 (400 U/ml). Fresh medium containing GM-CSF and IL-4 was added every 2–3 days. Human monocyte-derived DC were used routinely on day 6 of culture.
Medium
Tissue-culture medium consisted of RPMI-1640 supplemented with 25 mM HEPES buffer, antibiotics, 1 mM l-glutamine and 10% fetal calf serum (FCS).
Determination of cytokine levels
Levels of IL-12 p40, IL-12 p70, IL-10, IL-5 and interferon (IFN)-γ in the culture supernatant from DC or T cells were determined using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA) following the manufacturer's instructions.
Flow cytometric analysis
DC were harvested and washed with cold fluorescence activated cell sorter (FACS) buffer [phosphate-buffered saline (PBS) containing 2% FCS and 0·1% sodium azide]. Cells were then incubated in cold buffer. Subsequent staining with monoclonal antibodies (mAb) or isotype-matched controls were performed for 30 min at 4°C. Stained cells were then washed twice and resuspended in cold buffer and analysed with a FACSort cell analyser (Becton Dickinson, San Jose, CA, USA). More than 1 × 104 cells were analysed for each sample. CellQuest software (Becton Dickinson) was used to analyse the results.
Allogenic mixed leucocyte reaction (MLR)
PBMC were obtained as described above and naive CD4+ T cells were purified from PBMC using magnetic beads (Miltenyi Biotec). The allogenic CD4+ T cells obtained were distributed at 2 × 105 cells per well and incubated for 3 days in the presence of graded numbers of irradiated DC (3000 rad, 137Cs source). Cell culture supernatants were collected and were analysed for IL-5 and IFN-γ by ELISA. Tritiated thymidine (1 μCi/well; New England Nuclear, Boston, MA, USA) incorporation for 18 h was determined using a liquid counter.
Neutralization experiments
Human DC were preincubated for 1 h with 20 μg/ml antibody solution of TLR-2 or TLR-4. Levamisole was then added for 48 h. The cell culture supernatant was collected and analysed for IL-12 p40 and IL-10 by ELISA.
Statistical analysis
All the assays were performed in duplicate and the experiments were repeated at least twice. Student's t-test was used to analyse the results. A P-value < 0·05 was considered statistically significant.
Results
Levamisole-induced IL-12 p40 and IL-10 production in human DC
To determine if levamisole affected cytokine production in human DC, we compared cytokine concentrations in the supernatants of DC cultured with 1 μM of levamisole, which enhanced the production of IL-12 p40 and IL-10 secretion (Fig. 1). We also measured the production of IL-12p70. Only low levels were induced and there were no significant differences between groups (data not shown).
Fig. 1.
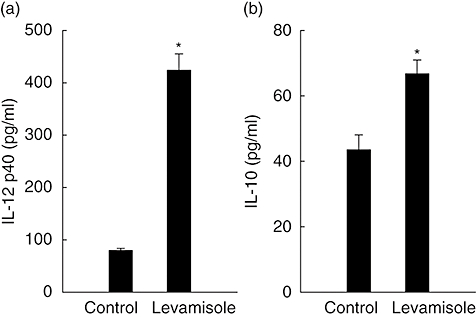
Levamisole-induced interleukin (IL)-12 p40 and IL-10 production in human dendritic cells (DC). The data represent the mean ± standard error for two determinations. Statistical analysis focused on unstimulated versus stimulated DC. *P < 0·05.
Levamisole-enhanced LPS-induced IL-12 p40 and IL-10 production in human DC
LPS has been described as an inducer of DC activation and maturation. In this experiment, we wanted to determine if levamisole could enhance the maturation of LPS-induced DC. We compared the cytokine concentration of IL-12 p40 and IL-10 in the supernatants of DC co-cultured with levamisole (1 μM) and LPS (10 ng/ml). The results demonstrated that, to a limited extent, levamisole could enhance the LPS-induced DC secretion of IL-12 p40 and IL-10 (Fig. 2).
Fig. 2.
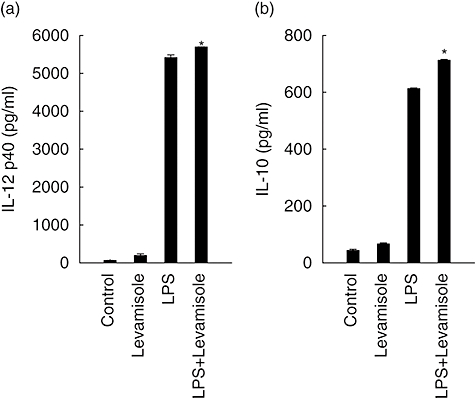
Levamisole-enhanced lipopolysaccharide (LPS)-induced interleukin (IL)-12 p40 and IL-10 production in human dendritic cells (DC). The data represent the mean ± standard error of two independent experiments. Statistical analysis focused on DC with or without levamisole in the presence of LPS. *P < 0·05.
Levamisole-treated human DC enhanced T cell activation towards a Th1 immune response
Mature DC have the capacity to induce activation in allogenic T cells at a higher level than immature DC. In human DC, levamisole up-regulated cell-surface markers and increased IL-12 and IL-10 production. To test whether this maturation is sufficient to promote activation of naive T cells, DC were treated with LPS (10 ng/ml) or levamisole (1 μM) for 48 h. These DC were then used to activate allogenic, naive T cells. Results showed that levamisole-treated DC enhanced T cell activation towards type 1 cytokine balance, as evidenced by the higher secretion of IFN-γ in the culture supernatant when the DC/T cells ratio was higher (Fig. 3a). We could not see the down-regulatory ability of levamisole on Th2 cytokine production (Fig. 3b), as IL-5 levels were not decreased significantly independently of the DC/T cells ratio. Allogenic T cell proliferation was measured after 5 days of co-culture with DC. It was interesting to determine whether levamisole-treated DC could not enhance T cell proliferation (Fig. 3c).
Fig. 3.
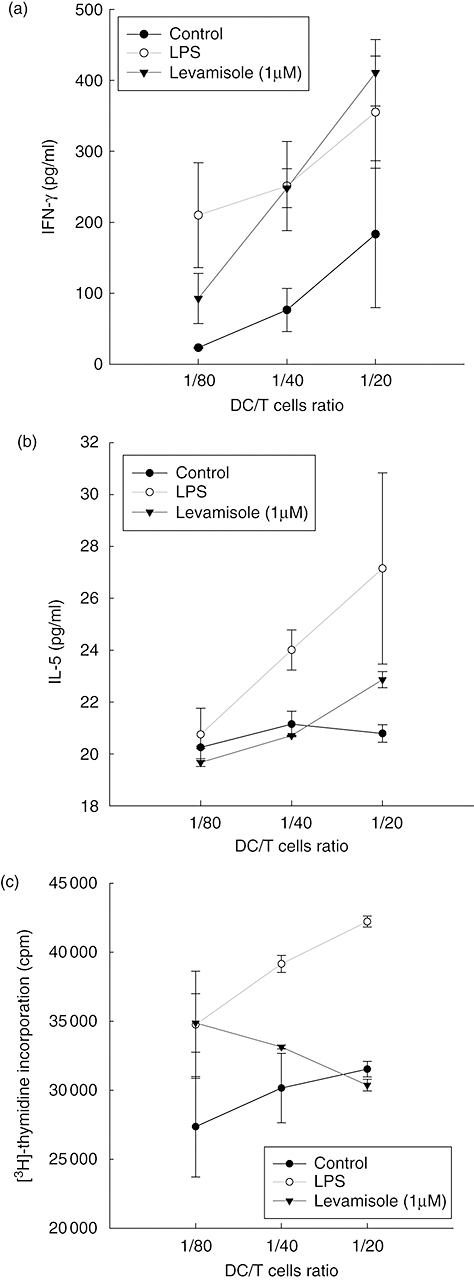
Levamisole-enhanced T cells response. Immature dendritic cells (DC) were stimulated with lipopolysaccharide (LPS) (10 ng/ml) or levamisole (1 μM) for 48 h. Supernatants were analysed for (a) interferon-γ and (b) interleukin-5, produced by activated T cells after 2 days of co-culture with LPS or levamisole-treated DC. Allogenic T cell proliferation was measured after 5 days of co-culture with DC (c). The data represent the mean ± standard error of two independent experiments; cpm: counts per minute.
Levamisole-induced IL-12 p40 and IL-10 synthesis through TLR-2
TLRs have been demonstrated to be involved in the human innate immune system against bacteria, virus or fungus. Neutralization experiments were performed to determine the involvement of these receptors in the interaction of DC with levamisole. Cell-surface TLR-2 and TLR-4 receptors were blocked by neutralizing concentrations of their respective antibodies before DC treated with levamisole 1 μM. Anti-TLR-2 mAb blocked levamisole-induced IL-12 p40 and IL-10 production by almost 80% and 50%, respectively. However, the anti-TLR-4 mAb failed to inhibit levamisole-induced IL-12 p40 and IL-10 production (Fig. 4).
Fig. 4.
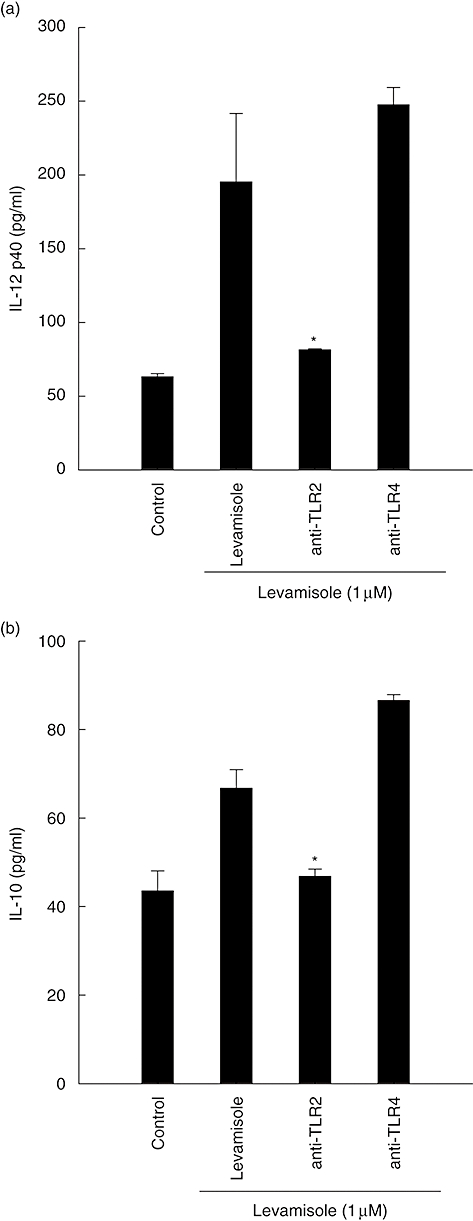
Neutralization with Toll-like receptor-2 monoclonal antibody inhibited the synthesis of interleukin (IL)-12 p40 and IL-10 in levamisole-treated human dendritic cells (DC). The data represent the mean ± standard error of two independent experiments. Significant difference between DC treated with or without antibodies is indicated by P < 0·05 (*).
Signal pathways involved in the maturation changes of DC induced by levamisole
Levamisole-treated DC produced IL-12 p40 and IL-10 levels during maturation (Fig. 1). We investigated if levamisole-mediated secretions of IL-12 p40 and IL-10 were affected by inhibitors of NF-κB, p38 mitogen-activated protein kinase (MAPK), p42/44 extracellular signal-regulated kinases (ERK)1/2 and p46/54 c-Jun N-terminal kinases (JNK). Immature human DC were pretreated with helenalin (a specific blocker of NF-κB), SB203580 (a specific blocker of p38 MAPK), PD98059 (an inhibitor of the ERK pathway) or JNK inhibitor II (an inhibitor of the JNK pathway) for 1 h at 37°C and stimulated subsequently with levamisole for 48 h. The levels of IL-12 p40 and IL-10 were determined by ELISA. The IL-12 p40 secretions were abrogated by helenalin and JNK inhibitor II. Thus, NF-κB and JNK pathways were involved in the secretion process of levamisole-induced DC IL-12 p40 (Fig. 5).
Fig. 5.
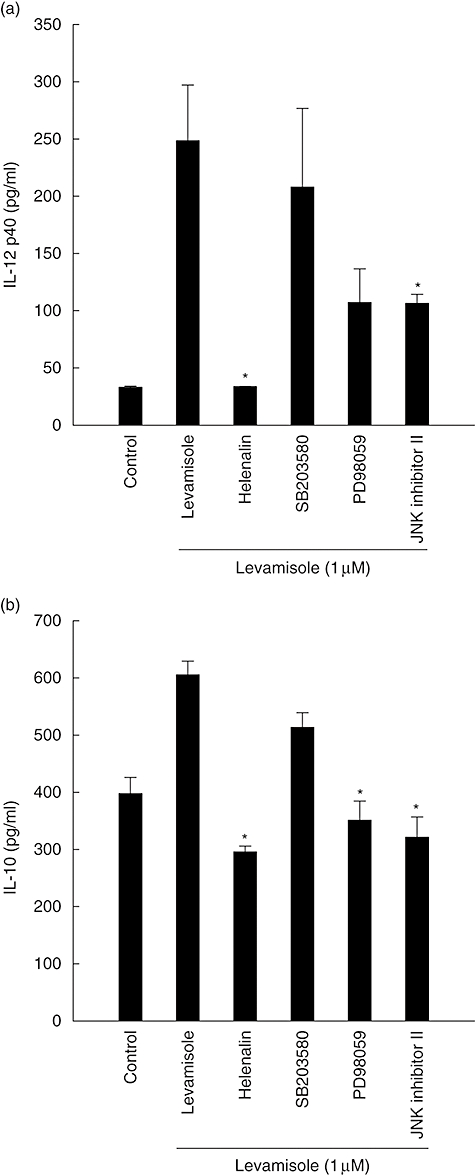
Effect of inhibiting the nuclear factor-κB, p38 mitogen-activated protein kinase, extracellular signal-regulated kinases 1/2 or c-Jun N-terminal kinases pathways on levamisole-induced up-regulation of interleukin (IL)-12 p40 and IL-10 production in human monocyte-derived dendritic cells (DC). The data represent the mean ± standard error of two independent experiments. Significant difference between DC treated with or without inhibitors is indicated by P < 0·05 (*).
Because IL-10 secretion is abrogated when blocked by helenalin, PD98059 or JNK inhibitor II, the effect of levamisole-enhanced IL-10 secretion might be modulated through the NF-κB, ERK1/2 or JNK pathways. These results show that certain features of human monocyte-derived DC maturation are regulated by signalling via NF-κB, ERK and JNK pathways. It implies that different aspects of the maturation process induced by levamisole may be regulated by distinct signal transduction pathways.
Discussion
Levamisole is an anti-helmintic agent that has also been used to treat and prevent atopic diseases in several mouse models, which has implicated its capacity in modulating the immune system. In this study, we examined the mechanisms and dynamics between human DC and lymphocytes regarding several aspects. The results suggest that levamisole can enhance the immune response towards Th1 development through the activation of dendritic or T cell aspects.
The process of DC maturation starts by exposure to extra-cellular stimuli, including inflammatory mediators, bacterial products or membrane-bound ligands that convert the primarily antigen-capture status of peripheral DC into a status specialized for T cell activation [14,15]. DC express high surface levels of MHC molecules, CD80, CD83 and CD86 in their functionally maturing process [9]. In our study, levamisole alone can promote the maturation of DC, although not intensely. Immature DC treated with levamisole for 48 h show the characteristic morphology, with an enlarged size and numerous cytoplasmic processes demonstrating a stellate appearance (data not shown).
To determine if levamisole could also modulate the development of human DC in vitro, we analysed the phenotype of human DC treated with levamisole for 48 h to those without. The results demonstrated that levamisole increased the presentation of CD80, CD86, CD83 and HLA-DR molecules on the cell membrane of human DC compared to those of the control group (data not shown). Even though the maturation markers on DC treated with levamisole alone increased, it was not as obvious as those in DC treated with LPS alone. Taken together, levamisole alone could induce the maturation of human monocyte-derived DC, alhough not strongly.
Cytokines such as IL-12 and IL-10 can be secreted from mature DC to help T cell activation. IL-12 plays a central role as a link between the innate and adaptive immune systems. Active IL-12 is a 70 kDa heterodimer (p70) consisting of two covalently linked subunits, p35 and p40 [16]. IL-12 can promote Th1 immune responses, induce the production of large amounts of IFN-γ from resting and activate T and NK cells [17].
IL-12 is also capable of inducing its own inhibitor, IL-10, and this negative-feedback mechanism limits ongoing T cell activation [18]. We detected IL-12 p40 levels instead of IL-12 p70, which was too low to be detected. However, we did not claim that IL-12 p40 had an independent role in activating a Th1 immune response.
IL-10 is a homodimeric cytokine that is deeply involved in the regulation of inflammatory responses and immune reactions. It can induce regulatory T cells, down-regulate the stimulatory or co-stimulatory molecules CD54, CD80 and CD86 on monocytes and significantly affect the T cell-activating capacity of myeloid dendritic cells [19]. IL-10 also inhibits the generation of monocyte-derived dendritic cells cultured with GM-CSF and IL-4 and induces the apoptosis of plasmacystoid dendritic cells (DC2) [20].
We found that levamisole alone can stimulate DC to secrete IL-12 and IL-10. Levamisole-induced IL-12 secretion exerts its effect on the shifting immune balance towards Th1. Our data show that levamisole can induce IL-10 secretion. Recent evidence indicates that DC play an important role in activating regulatory T cells, inducing immune tolerance [21] and regulating the balance of Th1/Th2 immunity [22]. Consequently, levamisole-induced IL-10 secretion can be explained as a regulatory effect on managing myeloid dendritic cells. Levamisole, regarded as a Th1-biased immune-stimulating reagent, can thus induce IL-12 and IL-10 secretion.
After proving that levamisole alone can stimulate the maturation and secretion of IL-12 and IL-10, we studied the effects of levamisole and LPS on functional DC responses. Work on mouse models has shown that stimulating DC with LPS results in a Th1 immune response, which may be due to the induction of IL-12 production, thereby providing an important signal for the Th1 shift in both mice and humans [23]. Several studies have demonstrated that DC from adult donors stimulated with LPS produced IL-12, whereas triggering TLR-2 elicited IL-10 production instead [24,25]. In addition, levamisole enhances LPS-induced DC secretion of IL-12 p40 and IL-10, but to a limited extent. The cumulative effects exist but are not obvious, possibly because levamisole acts differently from LPS even though both consequently induced the secretion of IL-12 and IL-10.
In our experiments using allogenic T cells, we proved that levamisole could induce sufficient DC maturation to promote the activation of naive T cells towards a Th1 response. However, we did not observe the inhibition of IL-5. One explanation would be the absence of a Th2 environment, such that even if there was an inhibitory effect, these data might not be observed. Several groups have also demonstrated that LPS-treated DC could prime a Th1 response to secrete IFN-γ [13,26].
The discrepancy shown in our results indicate that levamisole-treated DC cannot enhance the proliferation of allogenic T cells, which can be carried out by DC treated with LPS or DC alone. It is arguable that this is due to contamination or unpractised manipulation. An alternative explanation is that levamisole-treated DC secrete higher levels of IL-10 and possibly activate regulatory T cells, which respond by inhibiting proliferation. To test whether levamisole alone has impact on naive T cells, we stimulated naive T cells with only levamisole and in the absence of DC. The results showed neither enhancement of proliferation nor cytokine secretion (data not shown), which is consistent with results from other groups [3].
Mammalian TLRs have been identified as an important component of innate immunity against microbial pathogens through the NF-κB-dependent and IFN-regulatory factor (IRF)-dependent signalling pathway [27]. Different TLRs co-operate in DC activation, while synergic TLR stimulation increases the gene expression and protein secretion of IL-12 and IL-23, leading to DC with enhanced and sustained Th1-polarizing capacity [28]. The TLR-4 agonist, LPS, can induce exclusively IL-12p70, IFN-γ and IFN-γ inducible protein-10 (IP-10) expression in human DC [29]. In contrast, the activation of TLR-2 using lipopeptides and Porphyromonas gingivalis LPS preferentially induces IL-8, IL-10 and IL-23p19, as well as the rapid release of IL-10 that is responsible for the inhibition of IP-10 and IL-12p35 induction [30].
Our neutralization experiments with antibodies blocking TLR-2 and TLR-4 have demonstrated that TLR-2 plays a critical role in the signal transduction cascade induced by levamisole. In DC treated with levamisole alone, the inhibition of TLR-2 function resulted in reducing IL-10 and IL-12 p40 secretion. Levamisole uses a cholinergic receptor to enter cells in nematodes [31]. We can presume that levamisole influences DC directly or indirectly with TLR-2, or in co-operation with other receptors such as cholinergic receptors on the surface of DC.
During the DC maturation process, NF-κB, the major p38 MAPK, ERK and JNK are activated in DC. Many proinflammatory cytokines display NF-κB-responsive elements in their promoters [32], indicating a major role in the immune response. Promoters of human IL-12 p35 and human IL-12 p40 genes contain κB-binding sites [16], thus illustrating that NF-κB may affect IL-12 production. The lack of κB-binding sites in the hIL-10 promoter makes it unlikely that NF-κB is involved in IL-10 regulation [33].
Recently, it has been suggested that p38 MAPK is involved in the regulation of IL-10 production [34]. Our study indicates that NF-κB, p38 MAPK, ERK1/2 or JNK pathways may be involved in the mechanism between levamisole and human DC. All four pathways affect IL-10 synthesis, but only the NF-κB and JNK pathways can alter IL-12 production. However, from an integral perspective the NF-κB pathway has a significant impact on the mechanism of levamisole in inducing IL-12 p40 and IL-10, because using the NF-κB blocker helenalin decreases levamisole function significantly to a level similar to those of control groups. In light of this evidence, we can state that distinct signal transduction pathways can regulate different aspects of the maturation process induced by levamisole.
Suppressing the Th2 immune response and eliciting the Th1 immune response is clinically important, especially in the prevention and treatment of atopic diseases. In summary, levamisole alone can induce DC maturation and the secretion of IL-12 and IL-10. Levamisole-treated DC can activate allogenic T cells in secreting more IFN-γ but not inhibit IL-5 production. Future studies should focus upon whether levamisole can inhibit Th2 response in vitro or in vivo.
References
- 1.Thienpont D, Vanparijs OF, Raeymaekers AH, et al. Tetramisole (R 8299), a new, potent broad spectrum antihelmintic. Nature. 1996;209:1084–6. doi: 10.1038/2091084a0. [DOI] [PubMed] [Google Scholar]
- 2.Katoch K. Immunotherapy of leprosy. Indian J Lepr. 1996;68:349–61. [PubMed] [Google Scholar]
- 3.Schiller JH, Lindstrom M, Witt PL, et al. Immunological effects of levamisole in vitro. J Immunother. 1991;10:297–306. doi: 10.1097/00002371-199110000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Mutch RS, Hutson PR. Levamisole in the adjuvant treatment of colon cancer. Clin Pharm. 1991;10:95–109. [PubMed] [Google Scholar]
- 5.Szeto C, Gillespie KM, Mathieson PW. Levamisole induces interleukin-18 and shifts type 1/type 2 cytokine balance. Immunology. 2000;100:217–24. doi: 10.1046/j.1365-2567.2000.00042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kocabas CN, Sekerel BE, Firat PA, Okur H, Adahoglu G. Levamisole: might it be used in treatment and prevention of atopic diseases? J Asthma. 2004;41:547–51. doi: 10.1081/jas-120037655. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–7. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 9.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 10.Reis e Sousa. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–83. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 11.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–92. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin YL, Liang YC, Lee SS, Chiang BL. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the NF-kappaB and p38 mitogen-activated protein kinase pathways. J Leukoc Biol. 2005;78:533–43. doi: 10.1189/jlb.0804481. [DOI] [PubMed] [Google Scholar]
- 14.Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 15.O'Sullivan BJ, Thomas R. CD40 ligation conditions dendritic cell antigen-presenting function through sustained activation of NF-kappaB. J Immunol. 2002;168:5491–8. doi: 10.4049/jimmunol.168.11.5491. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimoto T, Kojima K, Funakoshi T, et al. Molecular cloning and characterization of murine IL-12 genes. J Immunol. 1996;156:1082–8. [PubMed] [Google Scholar]
- 17.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–45. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daftarian PM, Kumar A, Kryworuchko M, Diaz-Mitoma F. IL-10 production is enhanced in human T cells by IL-12 and IL-6 and in monocytes by tumor necrosis factor-alpha. J Immunol. 1996;157:12–20. [PubMed] [Google Scholar]
- 19.Willems F, Marchant A, Delville JP, et al. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur J Immunol. 1994;24:1007–9. doi: 10.1002/eji.1830240435. [DOI] [PubMed] [Google Scholar]
- 20.Ding L, Shevach EM. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992;148:3133–9. [PubMed] [Google Scholar]
- 21.Adler AJ, Marsh DW, Yochum GS, et al. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J Exp Med. 1998;187:1555–64. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 23.Redecke V, Hacker H, Datta SK, et al. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172:2739–43. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 24.Jotwani R, Pulendran B, Agrawal S, Cutler CW. Human dendritic cells respond to Porphyromonas gingivalis LPS by promoting a Th2 effector response in vitro. Eur J Immunol. 2003;33:2980–6. doi: 10.1002/eji.200324392. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal S, Agrawal A, Doughty B, et al. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984–9. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 26.Eisenbarth SC, Piggott DA, Huleatt JW, et al. Lipopolysaccharide-enhanced, Toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–51. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–90. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 28.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–76. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gautier G, Humbert M, Deauvieau F, et al. A type I interferon autocrine–paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201:1435–46. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Re F, Strominger JL. IL-10 released by concomitant TLR2 stimulation blocks the induction of a subset of Th1 cytokines that are specifically induced by TLR4 or TLR3 in human dendritic cells. J Immunol. 2004;173:7548–55. doi: 10.4049/jimmunol.173.12.7548. [DOI] [PubMed] [Google Scholar]
- 31.Trailovic SM, Robertson AP, Clark CL, Martin RJ. Levamisole receptor phosphorylation: effects of kinase antagonists on membrane potential responses in Ascaris suum suggest that CaM kinase and tyrosine kinase regulate sensitivity to levamisole. J Exp Biol. 2002;205:3979–88. doi: 10.1242/jeb.205.24.3979. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 33.Kube D, von Platzer C, et al KA. Isolation of the human interleukin 10 promoter. Characterization of the promoter activity in Burkitt's lymphoma cell lines. Cytokine. 1995;7:1–7. doi: 10.1006/cyto.1995.1001. [DOI] [PubMed] [Google Scholar]
- 34.Foey AD, Parry SL, Williams LM, et al. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-alpha: role of the p38 and p42/44 mitogen-activated protein kinases. J Immunol. 1998;160:920–8. [PubMed] [Google Scholar]


