Abstract
Pulmonary complications are common in patients with primary immune deficiency (PID). The aim of this study was to assess the usefulness of lung function tests (LFTs) in the management of these patients, and in particular to see if carbon monoxide transfer factor (TLCO) is needed in addition to spirometry. We studied 20 patients (11 female) with PID in a tertiary referral clinic, with a mean age of 47·6 years. Serial LFTs, spanning a mean of 101 months, were correlated with immunoglobulin levels and antibiotic usage. Seven patients showed a decline in forced expiratory volume in 1 second over the period of the study. An additional five patients showed a decline in TLCO. Of these 12 patients, two had no radiographic evidence of lung disease. Higher levels of immunoglobulin were associated with slower decline in LFTs (P < 0·05). The analysis of antibiotic usage and LFTs failed to show a statistically significant effect, although there was a trend towards a slower rate of decline with greater use of antibiotics. LFTs decline slowly in patients with PID. Annual testing (both spirometry and transfer factor) is useful in the assessment of these patients, and should not be confined to those with radiological evidence of lung disease.
Keywords: lung function tests, primary immunodeficiency
Introduction
Primary immune deficiency (PID) is a heterogeneous group of rare disorders characterized by impaired humoral or cell mediated immunity, in the absence of any recognized cause such as drug treatment or human immunodeficiency virus (HIV). The most common PID syndromes are common variable immunodeficiency (CVID) and X-linked agammaglobulinaemia (XLA). Both are characterized by hypogammaglobulinaemia.
Patients with PID are more susceptible to infections of any kind, including respiratory tract infections which lead to permanent lung damage in 20–40% of patients [1]. Although pulmonary complications are diverse, bronchiectasis is the most common [1–4].
Treatment of PID with parenteral immunoglobulin (Ig) replacement therapy reduces the occurrence of severe respiratory tract infections and subsequent pulmonary damage [4,5]. However, there is some evidence that despite Ig replacement therapy to physiological levels, structural lung damage still occurs [2].
Patients with PID have been monitored traditionally with regular lung function tests (LFTs), but the benefits of serial testing have not been evaluated fully. This study investigates the clinical relevance of lung function testing in patients with PID. In particular, we were interested in finding out if measurement of carbon monoxide transfer factor gave additional information over simple spirometry.
Materials and methods
We reviewed the records of all adult patients with a confirmed diagnosis of PID under the care of our clinical immunologists. The standard immunoglobulin treatment regimen involves 2–4-weekly infusions, with a dose Ig ranging from 0·4 to 0·6 g/kg/month. Trough serum IgG levels are taken every alternate infusion to allow accurate monitoring. Mean and minimum trough IgG levels were calculated, and the number of episodes where the level fell below 8 g/l (the lower limit of normal at reference laboratory) were recorded. To assess antibiotic usage, the proportion of time spent on antibiotics was estimated by the addition of time spent on prophylactic antibiotics to the number of treatment courses. Unless stated otherwise, each treatment course was assumed to be 2 weeks. Microbiological results from sputum cultures and chest radiology findings were also noted.
Spirometry was performed using a wedge bellows device (Vitalograph, Buckingham, UK). Gas transfer was assessed by the single breath carbon monoxide transfer factor (PK Morgan, Chatham, UK). Serial lung function data were analysed if the patient had undergone three or more lung function tests, and the absolute changes in forced expiratory volume in one second (FEV1), forced vital capacity (FVC) and transfer factor (TLCO) over time were calculated.
Basic statistical analysis of the relationship between FEV1 or TLCO and mean IgG concentration was performed using Stata 7. Initially a simple regression function was used to generate linear correlation between the variables. Spearman's correlation coefficient and an associated P-value for each relationship were then calculated, with 5% taken as statistical significance.
Results
Of the initial group of 29 patients, nine failed to meet the inclusion criteria in that they had not had at least three sets of lung function tests. The remaining subgroup of 20 patients included 11 females and had a mean age of 47·6 years (range: 24–72). There was a range of specific diagnoses including CVID (13), XLA (four), IgG2 deficiency (two) and Wiskott–Aldrich syndrome (one). The age at diagnosis ranged from 1 to 62 years, with a mean of 26·2 years. Thirteen had never smoked, six are current smokers and one is an ex-smoker. Radiological imaging was performed in all 20 patients [high resolution computerized tomography (HRCT) in 17, chest X-ray alone in three]. Bronchiectasis was present in 15 cases, while the remaining five patients had normal imaging. Of the nine patients with confirmed bacterial sputum colonization, seven cultured Haemophilus influenzae.
Lung function tests had been carried out over a number of years, with the mean duration of follow-up 100·8 months (range: 26–167 months). Initial analysis included both FEV1 and FVC. All patients demonstrating a decline in FVC also showed a decline in FEV1, while the reverse is not the case. FEV1 was therefore the most sensitive indicator of respiratory function in keeping with the obstructive nature of bronchiectasis. The individual changes in FEV1 over time are plotted in Fig. 1a, along with the expected age-related decline in FEV1. Seven of the patients demonstrated a clear reduction in FEV1; they were excluded from the plot of change in TLCO over time, on the basis that simple spirometry would have detected the deterioration in lung function. TLCO identified a further five patients whose lung function deteriorated (Fig. 1b). A combination of FEV1 and TLCO in serial lung function testing therefore identified 12 patients (60%) with deteriorating lung function. Two of these patients had normal chest radiology.
Fig. 1.
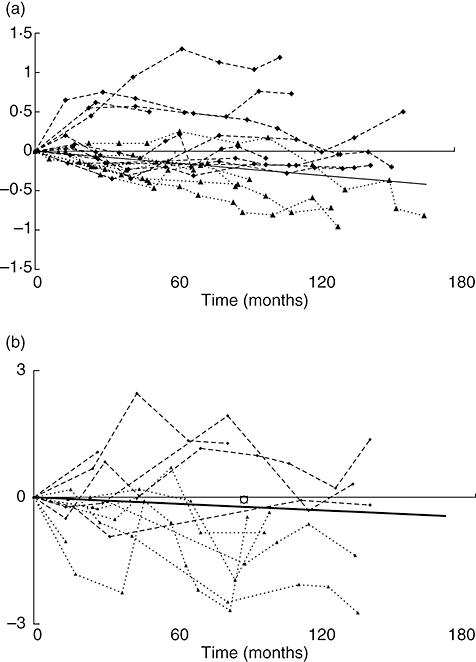
(a) Absolute change in forced expiratory volume in 1 second (FEV1) over time in a cohort of patients with primary immune deficiency (PID). (b) Change in carbon monoxide transfer factor over time in PID patients with no excess fall in FEV1.
All patients in the study were being treated with intravenous Ig for between 3 and 19 years. A scatter-plot of mean trough IgG against FEV1 (Fig. 2) demonstrates a clear correlation between higher trough levels of IgG and preservation of lung function (P = 0·0281). There was no relationship between minimum IgG or the number of dips in trough IgG below 8 g/l and either measurement of lung function.
Fig. 2.
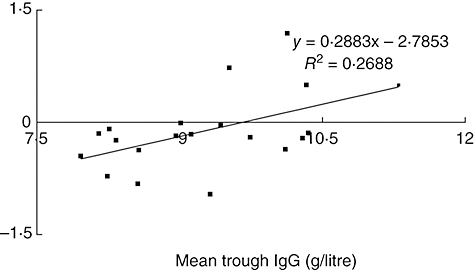
Change in forced expiratory volume in 1 second plotted against mean trough immunoglobulin G level in cohort of patients with primary immune deficiency.
Although there appeared to be a slower decline in FEV1 in those patients who spent longer on antibiotics, this did not reach statistical significance (Spearman's correlation coefficient 0·36: P = 0·1195). Figure 3 illustrates the results of change in FEV1 against proportion of time on antibiotics.
Fig. 3.
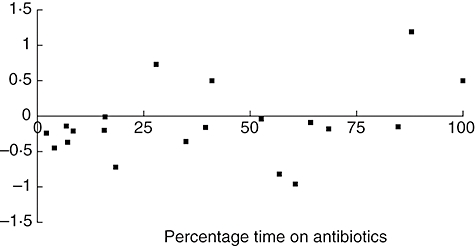
Change in forced expiratory volume in 1 second versus time on antibiotic therapy in a cohort of patients with primary immune deficiency.
Discussion
This study demonstrates that regular lung function testing is a useful addition to clinical assessment of patients with PID. Use of FEV1 and TLCO in combination is more sensitive than either test alone. Although we are unable to calculate an ideal interval for lung function testing, we recommend annual testing, based on the slow rate of decline in FEV1 and TLCO observed.
In addition, we found a significant correlation between higher mean trough IgG levels and slower rates of decline in FEV1. This finding raises the possibility that an increase in an individual's intravenous IgG regimen might reverse a decline in lung function. This theory warrants further investigation to assess any possible causal link.
The strengths of this study are the reasonably large cohort of patients with this rare group of conditions, and the availability of long-term serial lung function data. The demographics and clinical characteristics of our patient group, including gender mix, age, predominance of CVID and the rate of bronchiectasis, are all similar to those in published literature on PID [2,3]. The pathogens found in the sputum cultures of our patients are similar to those reported in immunoglobulin-deficient individuals [5]. Our data from smoking histories reveal a greater proportion of current smokers (30%) compared with that of Kainulainen [2], who had none. Five of the six current smokers have lung function that is deteriorating faster than expected, and highlights the need to counsel these patients strongly on quitting. The principal weakness of this study is its retrospective review design.
One previous paper [4] has reported serial lung function over a prolonged period, almost 7 years, in patients with PID. There was no deterioration in lung function over time, in contrast to our study in which over half deteriorated. Some of this discrepancy will be due to the fact the previous paper used only spirometry.
Several studies have evaluated the use of detailed radiological assessment by HRCT in patients with PID, and suggested a correlation between abnormal imaging and abnormal lung function [1,4,6]. However, this finding was not universal [3], and our findings suggest that lung function testing is needed in addition to radiology.
Two papers have examined the effect of IgG levels on lung function in children with PID [1,7]. Manson reports improved chest radiology in patients with high-dose intravenous Ig [1], while Bjorkander reports worsening lung function with low serum IgG levels [7]. Both these papers add support to the relationship between a high trough IgG level (within the normal range) and a slower rate of decline in FEV1, which was found in our patient group. This finding would need to be replicated and confirmed in a prospective randomized controlled trial, but has important clinical implications and could lead to the adoption of a more intensive IgG treatment strategy in adults with PID.
In conclusion, serial LFTs provide useful clinical information about the extent and progression of chronic lung disease in patients with PID. The tests are relatively cheap, without risk, easily accessible and repeatable. Extending the advice of the UK Primary Immunodeficiency Network (UK PIN) that ‘centres establish local protocols in conjunction with respiratory physicians for the performance of LFTs’, we recommend that spirometry and TLCO be performed at annual intervals based on the rate of decline seen in this study. Physicians should consider a more aggressive treatment strategy, especially with regard to IgG replacement, for the subgroup of PID patients whose FEV1 or TLCO deteriorate with time.
Acknowledgments
The authors thank Ruth Weldon, Specialist Immunology practice nurse for help in acquiring the notes, and Laura Watson, Senior Lung function technician, for all the lung function data.
References
- 1.Manson D, Reid B, Dalal I, Roifman CM. Clinical utility of high-resolution pulmonary computed tomography in children with antibody deficiency disorders. Pediatr Radiol. 1997;27:794–8. doi: 10.1007/s002470050235. [DOI] [PubMed] [Google Scholar]
- 2.Kainulainen L, Varpula M, Liippo K, Svedstrom E, Nikoskelainen J, Ruuskanen O. Pulmonary abnormalities in patients with primary hypogammaglobulinaemia. J Allergy Clin Immunol. 1999;104:1031–6. doi: 10.1016/s0091-6749(99)70085-0. [DOI] [PubMed] [Google Scholar]
- 3.Obregon RG, Lynch DA, Kaske T, Newell JD, Kirkpatrick CH. Radiologic findings of adult primary immune deficiency disorders: contribution of CT. Chest. 1994;106:490–5. doi: 10.1378/chest.106.2.490. [DOI] [PubMed] [Google Scholar]
- 4.Watts WJ, Bachhuber Watts M, Dai W, Cassidy JT, Grum CM, Weg JG. Respiratory dysfunction in patients with common variable hypogammaglobulinaemia. Am Rev Respir Dis. 1986;134:699–703. doi: 10.1164/arrd.1986.134.4.699. [DOI] [PubMed] [Google Scholar]
- 5.Dukes RJ, Rosenow EC, III, Hermans PE. Pulmonary manifestations of hypogammaglubulinaemia. Thorax. 1978;33:603–7. doi: 10.1136/thx.33.5.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sweinberg SK, Wodell RA, Grodofsky MP, Greene JM, Conley ME. Retrospective analysis of the incidence of pulmonary disease in hypogammaglobulinaemia. J Allergy Clin Immunol. 1991;88:96–104. doi: 10.1016/0091-6749(91)90306-9. [DOI] [PubMed] [Google Scholar]
- 7.Bjorkander J, Bake B, Hanson LA. Primary hypogammaglobulinaemia: impaired lung function and body growth with delayed diagnosis and inadequate treatment. Eur J Respir Dis. 1984;65:529–36. [PubMed] [Google Scholar]


