Abstract
Macrophages represent a multi-functional cell type in innate immunity that contributes to bacterial clearance by recognition, phagocytosis and killing. In acute inflammation, infiltrating neutrophils release a wide array of preformed granule proteins which interfere functionally with their environment. Here, we present a novel role for neutrophil-derived granule proteins in the anti-microbial activity of macrophages. Neutrophil secretion obtained by antibody cross-linking of the integrin subunit CD18 (X-link secretion) or by treatment with N-Formyl-Met-Leu-Phe (fMLP secretion) induced a several-fold increase in bacterial phagocytosis by monocytes and macrophages. This response was associated with a rapid activation of the monocytes and macrophages as depicted by an increase in cytosolic free Ca2+. Interestingly, fMLP secretion had a more pronounced effect on monocytes than the X-link secretion, while the opposite was observed for macrophages. In addition, polymorphonuclear cells (PMN) secretion caused a strong enhancement of intracellular reactive oxygen species (ROS) formation compared to incubation with bacteria. Thus, secretion of neutrophil granule proteins activates macrophages to increase the phagocytosis of bacteria and to enhance intracellular ROS formation, indicating pronounced intracellular bacterial killing. Both mechanisms attribute novel microbicidal properties to PMN granule proteins, suggesting their potential use in anti-microbial therapy.
Keywords: anti-microbial activity, granule protein, innate immunity, macrophage, neutrophil
Introduction
Monocytes and macrophages possess a rich panoply of anti-microbial devices, such as phagocytic uptake of microorganisms and intracellular killing of bacteria using oxygen-dependent mechanisms [1]. The efficiency of bacterial phagocytosis depends crucially upon the opsonization of the pathogen as well as the state of activation of the macrophage [2]. Macrophages exhibit great functional plasticity, allowing a rapid phenotypical adaptation to changes in the microenvironment [3]. In this respect, the classical activation of macrophages by interferon (IFN)-γ and tumour necrosis factor (TNF) increases their expression of recognition receptors, leading to enhanced phagocytosis. Moreover, microbicidal mechanisms as the respiratory burst are up-regulated to kill bacteria more effectively.
Acute inflammatory processes are characterized by the early infiltration of polymorphonuclear cells (PMN) in the affected tissue followed by a second wave of monocytes [4]. During their journey from the bloodstream to the inflammatory focus, PMN seed their granule constituents via which they communicate with neighbouring inflammatory cells [5]. The various granule compartments have different propensities for release of their material which reflects the function of the granule subset at the different steps of extravasation [6]. Heparin-binding protein (HBP), for example, which can be mobilized rapidly from the PMN secretory vesicles, anchors on endothelial cells, inducing monocyte arrest by the direct activation of monocytes rolling on the endothelium [7]. In contrast, alpha-defensins or cathepsin G, which are stored in primary granules of PMN, are released once the PMN is in the tissue, where they may alter macrophage function [5].
Clinical evidence for the importance of PMN granule proteins in the communication with monocytes and macrophages comes from humans lacking PMN or suffering from disorders in the composition of PMN granules. Lack of granule proteins in neutrophil-specific granule deficiency is associated with impaired macrophage functions, such as macrophage maturation, cytokine production and phagocytosis in humans and mice [8,9].
Because of the evident interdependency between monocyte/macrophage activity and PMN granule release we hypothesized that PMN secretion products have the capacity to modulate anti-microbial mechanisms in monocytes and macrophages. Our results show that PMN secretion products trigger an active response in monocytes and macrophages which relates functionally to enhanced bacterial phagocytosis and reactive oxygen species (ROS) formation. This mechanism may contribute to the capability of activated PMN to stimulate macrophage function enhancing the effectiveness of innate immunity.
Methods
Cell culture
Human PMN and human monocytes were isolated as described previously [7]. Whole blood for isolation of PMN and monocytes was obtained with informed consent from healthy blood donors in accordance with our institutional ethical guidelines. Differentiation of monocytes to macrophages was achieved by plating monocytes on cell culture dishes and subsequent culture for 8 days in M199 supplemented with 10% fetal bovine serum (FBS). Human monocytic THP-1 cells were cultured in RPMI-1640 containing 1 mM sodium pyruvate, 0·05 mM 2-mercapotoethanol and 10% FBS. Mouse monocytic WEHI-3B cells (gift from D. Vestweber, Max Planck Institute, Münster, Germany) were cultured in RPMI-1640 containing 10% FBS and mouse RAW 264·7 macrophages (gift from M. Mäurer, University of Mainz, Germany) were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS. All media and supplements were purchased from Invitrogen (Karlsruhe, Germany) and were supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml).
Preparation of PMN secretion
PMN activation and secretion was induced either by antibody cross-linking of integrin β2 chain CD18 (X-link secretion) or by stimulation with N-Formyl-Met-Leu-Phe (fMLP secretion). Antibody cross-linking was induced as described previously [7]. PMN were sedimented by centrifugation and the cell-free supernatant containing PMN secretion was filtered (pore size 0·8 μm; Corning, Vordingburg, Denmark) and stored at −20°C until use. Alternatively, freshly isolated PMN were activated with fMLP (10 nM, 37°C; Sigma, St Louis, MO, USA) for 15 min, spun down, and the supernatant was stored at −20°C until use. Either form of PMN activation was performed in DMEM medium containing 2 mM Ca2+.
Western blot analysis
The composition of the X-link secretion and fMLP secretion was analysed by Western blot. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot were performed as described previously [10]. After blocking of non-specific binding overnight at 4°C, the blot membranes were incubated with primary antibodies for 1 h at room temperature: anti-matrixmetalloproteinase 9 (MMP-9) (1 : 500; Sigma), anti-albumin (1 : 500, Dako, Glostrup, Denmark), anti-LL37 (1 : 200; Innovagen AB, Lund, Sweden, gift from B. Agerberth, Karolinska Institute, Stockholm, Sweden), anti-elastase (1 : 500; Dako). After washing, membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse antibody or goat anti-rabbit antibody (1 : 3000; Pierce, Bonn, Germany). Staining was visualized by an enhanced chemiluminescence system (Pierce). Membranes were photographed using Bio-Rad GelDoc and analysed with Bio-Rad Quanty One 4·5·2.
Phagocytosis assay
Alexa Fluor 488-fluorescent Staphylococcus aureus BioParticles and opsonizing reagent were purchased from Molecular Probes (Invitrogen). Bacteria and opsonizing reagent were reconstituted as indicated by the manufacturer and stored at 4°C. Opsonization was performed as recommended by the manufacturer. Thereafter, S. aureus were incubated with cells grown in a 96-well plate at a ratio of 20 bacteria per cell for 1 h at 37°C. After incubation, trypan blue (0·2 mg/ml; Sigma) was added to quench adherent particles and cells were fixed with formaldehyde (3%, v/v). The amount of fluorescent particles per cell was quantified by fluorescence microscopy (Nikon TE300, Tokyo, Japan) counting at least two random microscopic fields per well.
Ca2+ mobilization
Human macrophages were grown in a 96-well plate and incubated (37°C, 30 min) with the Ca2+ sensitive fluorophore fluo-4/AM (Molecular Probes, Karlsruhe, Germany), according to the manufacturer's instructions, and washed twice with phosphate-buffered saline (PBS) before use. Cells were then subjected to X-link secretion, fMLP secretion or sham treatment. The fluorescence intensity was recorded in a plate reader before and at 30, 90 and 150 s after injection of the respective secretion.
ROS measurement
Human macrophages cultured in 96-well plates were labelled with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (Molecular Probes) in PBS at a final concentration of 10 μM. Cells were incubated for 30 min at 37°C and the background fluorescence was read before activation. Thereafter, the cells were stimulated with X-link secretion or S. aureus and the fluorescence was measured in a fluorescence plate reader for 80 min.
Statistics
Statistical calculations were performed using Statistica 7·1 (Statsoft, Inc., Tulsa, USA). Data were tested for normality and are expressed as mean ± standard deviation. Data were analysed with analysis of variance (anova). When significant main effects were observed, a Tukey post hoc test for multiple comparisons was performed. A P-value < 0·05 was considered significant.
Results
PMN secretion enhances phagocytosis of bacteria in human and murine monocytes and macrophages
Degranulation of human PMN was induced by antibody cross-linking of CD18 (X-link secretion) or treatment with fMLP (fMLP secretion). Human monocytic THP-1, human macrophages derived from monocytes, murine monocytic WEHI-3B and murine RAW 264·7 macrophages were incubated with either X-link secretion or fMLP secretion for 24 h followed by 1 h incubation with S. aureus. Treatment with either of the two PMN secretions enhanced phagocytosis in all the cell types used compared to untreated control (Fig. 1a–d). Interestingly, monocytic cells of both human (Fig. 1a) and murine (Fig. 1c) origin showed a moderate enhancement of phagocytosis after treatment with X-link secretion, whereas treatment with fMLP secretion resulted in a profound increase. Macrophages of either origin, on the other hand, showed a reverse response to stimulation with the different secretions (Fig. 1b,d). Here, X-link secretion caused a stronger increase in phagocytosis of bacteria than the fMLP secretion. In none of the cell types used did fMLP alone cause a significant increase in phagocytosis. In separate experiments we washed off the PMN secretion before addition of the bacteria. In this situation we could still observe enhanced phagocytosis, which was not significantly different from that seen with the PMN secretion present (data not shown).
Fig. 1.

(a–e) Polymorphonuclear cell (PMN) secretion products induce phagocytosis in human and murine monocytes and macrophages. (a–d) Human THP-1 (a), human macrophages (b), murine WEHI-3B (c) or RAW 264·7 (d) were treated with X-link secretion, N-Formyl-Met-Leu-Phe (fMLP secretion) or fMLP alone (10 nM) for 24 h. Control (ctrl) indicates treatment with cell culture medium only. After stimulation, Alexa Fluor 488-labelled Staphylococcus aureus were added and incubated with the cells for 1 h. The number of incorporated bacteria per cell was quantified by fluorescence microscopy. Data are expressed as mean ± standard deviation; n = 6–8 for each bar. *Significant difference compared to ctrl; **significant difference compared to ctrl and X-link secretion in (a) and (c) or compared to ctrl and fMLP secretion in (b) and (d). (e) Composition of PMN secretion after CD18 cross-linking and fMLP activation. Proteins in X-link secretion and fMLP secretion were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Western blotting and antibody staining for marker proteins of the PMN granules were used to identify release of the different granule subsets. Each Western blot is representative of at least three independent experiments.
The fact that fMLP secretion and X-link secretion differ in their effects on the phagocytic capacity of monocytes and macrophages indicates that the composition of the secretions may be different. Western blot analyses were undertaken to analyse the release of PMN granules in response to the different treatments (Fig. 1e). Stimulation of PMN with fMLP resulted in release of rapidly mobilized granules such as secretory vesicles and tertiary granules, as indicated by staining for albumin and MMP-9. Cross-linking of CD18, however, caused secretion of primary, secondary and tertiary granules as well as of secretory vesicles.
PMN secretion induces Ca2+ mobilization in macrophages
To analyse further whether secreted material from activated PMN induces a direct activation of macrophages we analysed the effect of X-link secretion and fMLP secretion on calcium mobilization in human (Fig. 2a,b) and murine (Fig. 2c,d) monocytes and macrophages. The addition of X-link secretion caused an immediate and significant increase of intracellular Ca2+ compared to time zero and to sham treatment. Fluorescence intensity was maximal after 30 s and then declined gradually. As for the phagocytosis, the X-link secretion had a more profound effect on Ca2+ mobilization in macrophages of either origin than had the fMLP secretion, while we found an inverse response in monocytes.
Fig. 2.
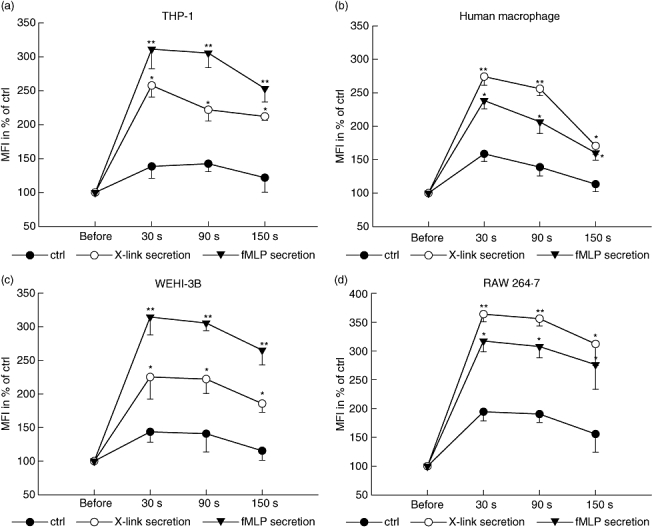
(a–b) Monocytes and macrophages are activated by polymorphonuclear cell (PMN) secretion. Dynamic change in fluorescence intensity of human THP-1 (a), human macrophages (b), murine WEHI-3B (c) or murine RAW 264·7 (d) loaded with fluo4/AM after stimulation with X-link secretion, N-Formyl-Met-Leu-Phe (fMLP secretion) or cell culture medium [control (ctrl)]. Fluorescence intensity depicting intracellular Ca2+ mobilization was measured before stimulation, 30 s, 90 s and 150 s after stimulation in a fluorescence plate reader. Values are expressed as percentage of mean fluorescence intensity (MFI) before treatment. *Significant increase in MFI compared to ctrl treatment; **significant difference compared to ctrl and X-link secretion in (a) and (c) or compared to ctrl and fMLP secretion in (b) and (d). Values are given as mean ± standard deviation; n = 6–8 for each point.
PMN secretion induces ROS formation in macrophages
Bacterial clearance is dependent not only upon effective phagocytosis but also on intracellular killing. Bacterial killing by macrophages relies critically upon oxygen-dependent microbicidal mechanisms [11]. Similarly to our experiments above, we treated human macrophages with X-link secretion for 24 h and analysed the ROS formation after addition of S. aureus. Compared to control, S. aureus enhanced intracellular ROS formation significantly, but pretreatment with X-link secretion did not enhance this response further (Fig. 3a). However, when we added X-link secretion acutely, we found a strong increase in intracellular ROS formation. Interestingly, the formation of ROS induced by S. aureus was enhanced several-fold by addition of X-link secretion, indicating a significant role of PMN-derived secretion products in stimulating ROS formation in macrophages.
Fig. 3.

(a–b) Intracellular reactive oxygen species (ROS) formation in macrophages in response to polymorphonuclear cell (PMN) secretion. Measurement of intracellular ROS formation by assessing the fluorescence intensity of human macrophages labelled with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA). (a) Macrophages were pretreated with X-link secretion for 24 h and the ROS formation was measured after addition of Staphylococcus aureus for 80 min (S. aureus + X-link secretion). Treatment with culture medium [control (ctrl)] or S. aureus without prior incubation with X-link secretion (S. aureus) were used as controls. (b) Macrophages were grown in a 96-well plate, labelled with H2DCFDA and the fluorescence was measured over 80 min after stimulation with medium (ctrl), X-link secretion, S. aureus or X-link secretion and S. aureus. All data points indicate mean values of at least eight measurements. Note the different scales of the y-axis in (a) and (b).
Discussion
PMN granule proteins communicate with their environment and modulate the function of the neighbouring cells. Here we demonstrate that these granule proteins activate monocytes and macrophages to phagocytose and kill bacteria, and thereby contribute to the bacterial clearance in infectious diseases. The differential response of monocytes and macrophages to PMN secretion that contains proteins of rapidly mobilized granules, on one hand, and proteins of all four types of granules on the other hand, is intriguing. This may indicate that the function of blood monocytes is modulated specifically by PMN granule proteins that are released before the PMN penetrates the vessel wall, while the function of tissue macrophages is altered by granule constituents that are released at the site of infection. Although at this stage we cannot resolve this mechanism in detail, this pattern of activation appears physiologically relevant.
Enhanced phagocytosis could be due to direct activation of phagocytic cells or result from opsonization of the bacteria by constituents of the PMN secretion. With regard to the latter, several PMN granule proteins were shown to opsonize bacteria resulting in enhanced phagocytosis in monocytes [12,13]. However, in our setting monocytes and macrophages were pretreated with the PMN secretion for 24 h before addition of the bacteria. During this time most of the PMN granule proteins were most probably internalized [14–16] and would therefore not be available for opsonization of the bacteria. When washing off the PMN secretion we could not observe a significant change in phagocytosis, suggesting direct activation of macrophages by components of the PMN secretion rather than an opsonization of the bacteria to be responsible for the enhanced phagocytosis. This is supported further by measurements of intracellular Ca2+, which resemble a similar pattern, as activation in response to HBP [7,17], cathepsin G [18] and defensins [19] is similar to the macrophage activation by PMN secretion found in this study. Therefore, this group of PMN-derived polypeptides and other PMN proteins are probable candidates in the effect described here.
Neutropenia and neutrophil disorders are associated regularly with bacterial infections. Besides the primary lack or insufficiency of PMN, a secondary anti-microbial dysfunction of macrophages contributes to the onset of such clinical events. PMN granules contain a wide diversity of proteins, many of which have direct anti-microbial activity [20]. As well as this direct microbicidal function, several granule proteins were shown to opsonize bacteria, allowing more efficient phagocytosis [12,13]. A third anti-microbial mechanism of PMN granule proteins was identified recently by Tan et al., who showed that the transfer of PMN granule proteins from apoptotic PMN to macrophages enhances the intracellular killing of Mycobacterium tuberculosis [21]. Here we report yet another anti-microbial mechanism of PMN granule constituents, showing that PMN secretion products activate macrophages and stimulate bacterial phagocytosis and intracellular killing in monocytes and macrophages. This study indicates the importance of the PMN and its secretion products in regulating anti-microbial activities of monocytes and macrophages.
Acknowledgments
This study was supported by grants from the Swedish Research Council, the Swedish Heart–Lung Foundation, the AFA Health Fund and the Lars Hierta Memorial Fund. O. S. is a recipient of a post-doctoral fellowship from the Deutsche Forschungsgemeinschaft (SO 876/1-1).
References
- 1.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 2.Bogdan C. Chichester, UK: John Wiley & Sons; Macrophages, encyclopedia of life sciences. 10.1038/npg.els.0004007. [Google Scholar]
- 3.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–13. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witko-Sarsat V, Rieu P, Descamps-Latscha B, et al. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617–53. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 5.Chertov O, Yang D, Howard OM, et al. Leukocyte granule proteins mobilize innate host defenses and adaptive immune responses. Immunol Rev. 2000;177:68–78. doi: 10.1034/j.1600-065x.2000.17702.x. [DOI] [PubMed] [Google Scholar]
- 6.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–21. [PubMed] [Google Scholar]
- 7.Soehnlein O, Xie X, Ulbrich H, et al. Neutrophil-derived heparin-binding protein (HBP/CAP37) deposited on endothelium enhances monocyte arrest under flow conditions. J Immunol. 2005;174:6399–405. doi: 10.4049/jimmunol.174.10.6399. [DOI] [PubMed] [Google Scholar]
- 8.Shiohara M, Gombart AF, Sekiguchi Y, et al. Phenotypic and functional alterations of peripheral blood monocytes in neutrophil-specific granule deficiency. J Leukoc Biol. 2004;75:190–7. doi: 10.1189/jlb.0203063. [DOI] [PubMed] [Google Scholar]
- 9.Tavor S, Vuong PT, Park DJ, et al. Macrophage functional maturation and cytokine production are impaired in C/EBP epsilon-deficient mice. Blood. 2002;99:1794–801. doi: 10.1182/blood.v99.5.1794. [DOI] [PubMed] [Google Scholar]
- 10.Schmeisser A, Soehnlein O, Illmer T, et al. ACE inhibition lowers angiotensin II-induced chemokine expression by reduction of NF-kappaB activity and AT1 receptor expression. Biochem Biophys Res Commun. 2004;325:532–40. doi: 10.1016/j.bbrc.2004.10.059. [DOI] [PubMed] [Google Scholar]
- 11.Heale JP, Speert DP. Macrophages in bacterial infections. In: Burke B, Lewis CE, editors. The macrophage. New York: Oxford University Press; 2002. pp. 210–52. [Google Scholar]
- 12.Heinzelmann M, Platz A, Flodgaard H, Miller FN. Heparin binding protein (CAP37) is an opsonin for Staphylococcus aureus and increases phagocytosis in monocytes. Inflammation. 1998;22:493–507. doi: 10.1023/a:1022398027143. [DOI] [PubMed] [Google Scholar]
- 13.Fleischmann J, Selsted ME, Lehrer RI. Opsonic activity of MCP-1 and MCP-2, cationic peptides from rabbit alveolar macrophages. Diagn Microbiol Infect Dis. 1985;3:233–42. doi: 10.1016/0732-8893(85)90035-5. [DOI] [PubMed] [Google Scholar]
- 14.Olofsson AM, Vestberg M, Herwald H, et al. Heparin-binding protein targeted to mitochondrial compartments protects endothelial cells from apoptosis. J Clin Invest. 1999;104:885–94. doi: 10.1172/JCI6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinzelmann M, Mercer-Jones MA, Flodgaard H, Miller FN. Heparin-binding protein (CAP37) is internalized in monocytes and increases LPS-induced monocyte activation. J Immunol. 1998;160:5530–6. [PubMed] [Google Scholar]
- 16.Yang JJ, Preston GA, Pendergraft WF, et al. Internalization of proteinase 3 is concomitant with endothelial cell apoptosis and internalization of myeloperoxidase with generation of intracellular oxidants. Am J Pathol. 2001;158:581–92. doi: 10.1016/S0002-9440(10)64000-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pahlman LI, Morgelin M, Eckert J, et al. Streptococcal M protein: a multipotent and powerful inducer of inflammation. J Immunol. 2006;177:1221–8. doi: 10.4049/jimmunol.177.2.1221. [DOI] [PubMed] [Google Scholar]
- 18.Chertov O, Ueda H, Xu LL, et al. Identification of human neutrophil-derived cathepsin G and azurocidin/CAP37 as chemoattractants for mononuclear cells and neutrophils. J Exp Med. 1997;186:739–47. doi: 10.1084/jem.186.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tkachenko SB, Kokryakov VN, Ashmarin IP, et al. Effect of human defensins on the cytoplasmatic Ca2+ content in platelets. Bull Exp Biol Med. 1994;118:1291–5. [PubMed] [Google Scholar]
- 20.Levy O. Antimicrobial proteins and peptides: anti-infective molecules of mammalian leukocytes. J Leukoc Biol. 2004;76:909–25. doi: 10.1189/jlb.0604320. [DOI] [PubMed] [Google Scholar]
- 21.Tan BH, Meinken C, Bastian M, et al. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol. 2006;177:1864–71. doi: 10.4049/jimmunol.177.3.1864. [DOI] [PubMed] [Google Scholar]


