Abstract
Antibody phage display is a powerful tool for the generation of monoclonal antibodies against virtually any given antigen. Chickens are phylogenetically more distant from humans compared to other laboratory animals, such as mice and rats. Therefore, the use of chickens is especially beneficial when generating recombinant antibodies against human autoantigens, which are often highly conserved among mammals. Another advantage of using chickens in antibody phage display is that the preparation of single chain variable fragment (scFv) antibody libraries is faster and easier compared to preparing such libraries from other species, as only two primer sets are needed for amplification of the chicken variable heavy chain (VH) and variable light chain (VL) genes. In the present study we explored the possibility to immunize chickens with antigen cocktails for the generation of recombinant antibody fragments directed to a range of human autoantigens. Two pairs of chickens were immunized with two cocktails of seven recombinant autoantigenic proteins, libraries were prepared and panned on the individual proteins. The polyclonal chicken sera reacted strongly with most of the antigens used for immunization. By creating and screening single-chain variable fragment antibody phage display libraries, recombinant monoclonal antibody fragments were isolated successfully against the autoantigens annexin XI, centromere protein B, heat shock protein B3, DNA topoisomerase I, histidyl tRNA synthetase, Ro52, Ro60, Rpp30 and U1A. In conclusion, the immunization of only four chickens with two distinct pools of a total of 14 autoantigenic proteins allowed the isolation of scFvs against nine of these antigens.
Keywords: antibody phage display, autoantigens, chicken, scFv
Introduction
Autoimmune diseases are characterized by the presence of serum antibodies (autoantibodies) directed to ‘self’-antigens (autoantigens). Characterization of autoantibodies and their targets will provide more insight into the underlying mechanisms of autoimmune diseases. However, characterization of autoantigens in, for example, tissue sections of patient biopsies or on Western blots containing human patient material (such as serum) is not possible with patient autoantibodies, due to cross-reaction of the secondary antibodies with human immunoglobulins present in the tissue or serum. This problem can be circumvented by using antibodies from other species.
Antibody phage display has brought a revolution in the preparation of monoclonal antibodies, as it is easier, faster and less expensive compared to conventional hybridoma technology. The phage display technique is based on the display of (poly)peptides, such as antibody fragments, on the surface of bacteriophages [1]. Recombinant antibodies have been selected from immune libraries, naive libraries [2] and (semi)synthetic libraries [3]. One of the advantages of immune libraries is that clonal selection and affinity maturation in the individual lead generally to single chain variable fragments (scFvs) with higher affinities compared to scFvs isolated from naive or synthetic libraries [4], although antibodies with high affinity have also been isolated from large naive libraries. However, the preparation of large naive libraries with sufficient complexity requires huge investments of labour and money.
Recombinant antibodies to human autoantigens have been isolated successfully from patient-derived libraries [5–7]. The disadvantages of using patient libraries are that the preparation of human libraries is relatively time-consuming, and furthermore that autoimmune patients generally produce autoantibodies to a small subset of autoantigens. This means that libraries from several patients have to be prepared to isolate antibody fragments to a range of autoantigens. Recombinant antibody fragments can also be isolated from animals immunized with human targets. Mice are often used for the preparation of immune libraries, but other animals have also been used successfully, due especially to unique features of their immune systems. For example, the camelids produce next to complete immunoglobulins, homodimers of only heavy chains, which are devoid of light chains [8]. These single domain llama variable heavy chains (VHH) have appeared to be highly soluble and very stable when expressed in bacteria [9]. Another example is the chicken immune system, which is particularly beneficial when constructing libraries. Where mammals diversify their immune response through somatic V(D)J recombination, chickens use only one recombination event, in combination with a process called ‘gene conversion’[10]. In this process pseudogenes, which are located upstream, are translocated into the heavy and light chain variable regions. Consequently, only two primer sets [one for the amplification of the VH gene and one for the amplification of the variable light chain (VL) gene] are needed to construct chicken libraries [11]. As a result, construction of chicken antibody libraries is easier and faster compared to human and murine libraries. The advantage of using chickens is attributed, furthermore, to the fact that non-mammal species are more likely to evoke an immune response to epitopes that are highly conserved between mammals [12].
Here, we studied the immunization of chickens with two mixtures of seven distinct human autoantigens each, the preparation of scFv phage display libraries using material from these immunized animals and the isolation of recombinant antibodies to these autoantigens. As a result of the selection procedures presented here monoclonal scFvs were obtained to most of the antigens, which are available to be used for the characterization of autoantigens in patient samples.
Materials and methods
Immunization and library construction
The recombinant, purified proteins annexin XI [13], centromere protein B (CENP-B) [14], α-fodrin [15], human Pop1 protein (hPop1) [16], DNA topoisomerase I (Topo) [17], heat shock protein B3 (HspB3) [18], histidyl tRNA synthetase (Jo-1) [19], Ro52 [20], Ro60 [21], Rpp30 [22], Rpp38 [22], Rrp4 [23], Rrp42 [23] and U1A [24] were available from the Department of Biochemistry, Radboud University Nijmegen, the Netherlands. hPop1 and Rpp38 were produced as glutathione S-transferase (GST) fusion proteins. Two different antigen cocktails were used for chicken immunization. Antigen cocktail 1 included annexin XI, CENP-B, Topo, Jo-1, Ro60, Rpp38 and U1A, whereas antigen cocktail 2 consisted of α-fodrin, hPop1, HspB3, Ro52, Rpp30, Rrp4 and Rrp42. Two pairs of Barnevelder chickens were each immunized with antigen cocktail 1 and 2, respectively. The primary immunization was applied in complete Freund's adjuvant, whereas following boosters were given in incomplete Freund's adjuvant. ScFv phage display libraries were constructed with a short and a long linker as described previously [25]. For preparation of the recombinant antibody library, cDNA was synthesized from mRNA isolated from B cells that were harvested from the bone marrow and peripheral blood lymphocytes from the immunized chickens. Subsequently, VH genes were amplified using forward primer CSCVHo-F for a short linker or forward primer CSCVHo-FL for a long linker, and reverse primer CSCG-B. VL genes were amplified with forward primer CSCVK and reverse primer CKJo-B. Amplified VL and VH genes were fused by overlap polymerase chain reaction (PCR) using primers CSC-F and CSC-B. All primers used for library construction are listed in Table 1. Subsequently, VL–VH fragments were inserted in the pComb3 vector through SfiI digestion and ligation, and transferred into Escherichia coli TG1 by electroporation.
Table 1.
Oligonucleotides used for library construction.
| CSCVHo-F | 5′-GGTCAGTCCTCTAGATCTTCCGCCGTGACGTTGGACGAG-3′ |
| CSCVHo-FL | 5′-GGTCAGTCCTCTAGATCTTCCGGCGGTGGTGGCAGCTCCGGTGGTGGCGGTTCCGCCCTGACGTTGGACGAG-3′ |
| CSCG-B | 5′-CTGGCCGGCCTGGCCACTAGTGGAGGAGACGATGACTTCGGTCC-3′ |
| CSCVK | 5′-GTGGCCCAGGCGGCCCTGACTCAGCCGTCCTCGGTGTC-3′ |
| CKJo-B | 5′-GGAAGATCTAGAGGACTGACCTAGGACGGTCAGG-3′ |
| CSC-F | 5′-GAGGAGGAGGAGGAGGAGGTCGCCCAGGCGGCCCTGACTCAG-3′ |
| CSC-B | 5′-GAGGAGGAGGAGGAGGAGGAGCTGGCCGGCCTGGCCACTAGTGGAGG-3′ |
Phage selection
Phages were amplified from bacterial libraries as described previously [2]. Isolated phages were resuspended in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA). Libraries A (antigen mixture 1, long linker) and B (antigen mixture 1, short linker) were pooled, and libraries C (antigen mixture 2, long linker) and D (antigen mixture 2, short linker) were pooled. Libraries were panned on the individual antigens, which were immobilized on enzyme-linled immunosorbent assay (ELISA) plates. Antigens were coated in 96-well ELISA plates (Maxisorb; Nunc, Germany) in 50 mM NaHCO3, pH 9·6, 50 μl per well. After overnight coating at 4°C, wells were washed with PBS and blocked with 5% non-fat dried milk powder in PBS (MPBS). Wells were incubated with 100 μl phages [approximately 1 × 1013 colony forming units (CFU)/ml], diluted with 100 μl MPBS containing 0·05% Tween-20 (MPBST) for 90 min at 37°C. In case of GST fusion proteins (hPop1 and Rpp38) as antigen, 10 μg soluble GST was added to the phage solution during selection. After panning, wells were washed several times with PBS containing 0·05% Tween-20 (PBST). Bound phages were eluted with 100 mM triethylamine or 100 mM glycine-HCl, pH 2 and neutralized with Tris-HCl, pH 7·4 prior to infection of E. coli TG1. After several selection rounds, polyclonal phage populations were analysed in ELISA for reactivity with the antigens, as described below.
In a first experiment, all antigens except Rrp4 and Rrp42 were coated at a concentration of 4 μg per well for the first selection round, and at 0·4 μg per well for the following selection rounds. After five selection rounds, polyclonal phage ELISA demonstrated that the phage pools were not enriched detectably for phages directed to hPop1, HspB3, Topo, Ro60 and Rpp30. In a second experiment, these antigens were coated at a concentration of 0·5 μg per well in all selection rounds. In a third experiment, phages were selected on Rrp4, Rrp42 and again on annexin XI, α-fodrin, hPop1, Ro52, Rpp38 and U1A. The antigens were coated at a concentration of 0·5 μg per well in the first selection round, and at a concentration of 0·25 μg per well in following selection rounds.
During single colony analysis, monoclonal phages were examined for binding activity in ELISA as described below, and the scFv gene was analysed for full cDNA insert by PCR using primers pCOMB-F2548 (TTCCGGCTCGTATGTTGTGTG) and pCOMB-R3010 (GAATCAAGTTTGCCTTTAGCGTC). Subsequently, phages were grouped based on similarities in fingerprint patterns using the restriction enzyme BstNI. From each fingerprint group, phages demonstrating activity in ELISA were characterized further on immunoblot. Finally, the cDNAs of several phages were sequenced using primers pComb-F2548 (TTCCGGCTCGTATGTTGTGTG) and pComb-R3010 (GAATCAAGTTTGCCTTTAGCGTC). Gene sequences were submitted to the European Molecular Biology Laboratory (EMBL) database (see also Table 2).
Table 2.
European Molecular Biology Laboratory (EMBL) Accession numbers for sequenced antibody light (VL) and heavy (VH) chains.
| EMBL Accession no. | |||
|---|---|---|---|
| Antigen | Antibody clone | VL | VH |
| Annexin XI | A5A3 | AM773243 | AM773242 |
| Annexin XI | A5E12 | AM773245 | AM773244 |
| CENP-B | 5D1 | AM773246 | AM773247 |
| HspB3 | 7B4 | AM773248 | AM773249 |
| HspB3 | 7C5 | AM773250 | AM773251 |
| Topo | 5C2 | AM773252 | AM773253 |
| Jo-1 | 4B1 | AM773254 | AM773255 |
| Jo-1 | 5B5 | AM773256 | AM773257 |
| Jo-1 | 5B6 | AM773258 | AM773259 |
| Jo-1 | 5B9 | AM773260 | AM773261 |
| Rrp30 | 5C9 | AM773262 | AM773263 |
| Rrp30 | 6E12 | AM773264 | AM773265 |
| Ro52 | R2E5 | AM773266 | AM773267 |
| Ro52 | R2F11 | AM773268 | AM773269 |
| Ro52 | R3E4 | AM773270 | AM773271 |
| U1A | U11A3 | AM773272 | AM773273 |
| U1A | U11B3 | AM773274 | AM773275 |
| U1A | U13D12 | AM773276 | AM773277 |
| U1A | U11B1 | AM773278 | AM773279 |
CENP-B: entromere protein B; Hsp-B3: heat shock protein B3; Jo-1: histidyl tRNA synthetase; Topo: DNA topoisomerase I.
Elisa
Antigens were coated in 96-well ELISA plates (Maxisorb; NUNC) in 50 mM NaHCO3, pH 9·6, 50 μl per well, overnight at 4°C. Antigens were coated at a concentration of 0·25 μg per well, except Rrp4 and Rrp42, which were coated at a concentration of 0·125 μg per well. Plates were blocked with MPBS for 1 h. Subsequently, plates were incubated for 1 h with the phage solutions, diluted in MPBST. In the case of GST fusion proteins, competing soluble GST was added at a 10-fold excess. To detect bound phages, plates were washed with PBST and incubated with mouse anti-M13 monoclonal antibody (GE Healthcare, Brussels, Belgium) diluted 1000-fold in MPBST, followed by incubation with horseradish peroxidase (HRP)-labelled rabbit anti-mouse antibodies (Dako, Heverlee, Belgium) diluted 1000-fold in MPBST. Plates were washed with PBST and PBS. Bound HRP-conjugated antibodies were detected by conversion of 3,3′,5,5′-tetramethylbenzidine (TMB). Reactions were stopped with 1 M H2SO4 and the absorbance at 450 nm was measured. Pre-immune and immune chicken sera were also analysed using ELISA. Bound chicken antibodies were detected using HRP-labelled rabbit anti-chicken antibodies (Jackson Immunoresearch, Newmarket, UK) diluted 1500-fold in MPBST, followed by TMB reaction as described above.
Cell culture
Human epithelial cell line (HeLa) cells were grown in Dulbecco's modified Eagle's medium (DMEM) medium supplemented with 10% fetal calf serum, 1 mM penicillin and 1 mM streptomycin. The cells were grown in a humidified incubator at 37°C in the presence of 5% CO2. HeLa cells were scraped in PBS, harvested by centrifugation at 800 g for 5 min and washed twice with PBS. Cell extract was prepared by sonication in sonication buffer (50 mM Tris-HCl pH 7·6, 100 mM KCl, 0·05% Nonidet-P40, 1 mM ethylenediamine tetraacetic acid (EDTA) and 1 mM dithiothreitol), followed by centrifugation at 12 000 g for 20 min. The clear lysate was stored at −70°C.
Immunoblot analysis
Proteins from HeLa cell extract were separated on polyacrylamide gels by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes by semidry electroblotting. All immunoblotting steps were carried out at room temperature on a shaking table. The membrane was blocked with MPBST. Phages were incubated with the membrane at a concentration of approximately 5 × 1011 CFU/ml in MPBST. After extensive washing with MPBST, the membrane was incubated with HRP-labelled mouse anti-M13 monoclonal antibody (GE Healthcare) diluted 5000-fold. Finally, bound HRP-labelled antibodies were visualized using enhanced chemiluminescence (ECL).
Results
Immunization and generation of libraries
A schematic overview of the library preparation and selection strategy is shown in Fig. 1. Four chickens were immunized with two distinct protein cocktails, which were composed of proteins that are autoantigenic in humans (except HspB3) and which are, in general, highly conserved molecules with key functions in the cell. An overview of the characteristics of the antigens used is presented in Table 3. Prior to immunization, recombinant proteins were expressed in E. coli and protein cocktails were prepared by mixing equal amounts of each purified protein. Pre-immune and immune sera were tested in ELISA for reactivity with the individual antigens and BSA as a negative control antigen. Because Rpp38 and hPop1 were applied as GST fusion proteins, reactivity to GST was also measured. Figure 2 demonstrates the reactivities of the pre-immune sera, and of the sera obtained after the final immunization. None of the pre-immune sera showed significant reactivity with any of the antigens. Immune sera from chickens 1 and 2 showed strong reactivity with annexin XI, CENP-B, Topo, Jo-1, Ro60, U1A and GST, but no or hardly any reactivity with Rpp38. Immune sera from chickens 3 and 4 reacted strongly with hPop1, Ro52, Rpp30 and GST, and did not (or only very weakly) react with α-fodrin, HspB3, Rrp4 and Rrp42.
Fig. 1.
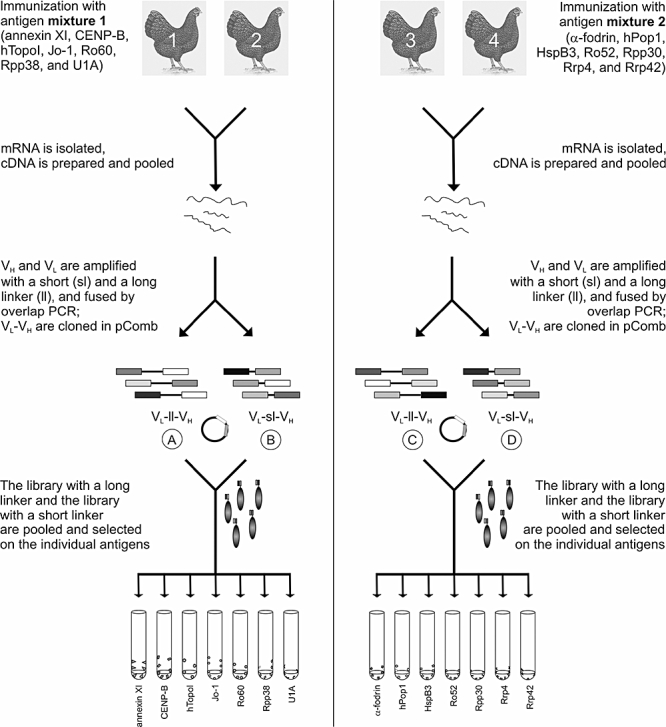
Schematic overview of the selection strategy. Libraries are prepared from chickens 1 and 2, which are immunized with antigen mixture 1 (annexin XI, centromere protein B, DNA topoisomerase I, histidyl tRNA synthetase, Ro60, Rpp38 and U1A) and from chickens 3 and 4, which are immunized with mixture 2 (α-fodrin, human Pop1 protein, heat shock protein B3, Ro52, Rpp30, Rrp4 and Rrp42). After immunizations, cDNA is prepared from isolated mRNA and pooled, and the variable heavy chain (VH) and variable light chain (VL) genes are amplified individually by polymerase chain reaction (PCR). Subsequently, VH and VL PCR products are linked with a short linker or a long linker, and are inserted into the pComb3 vector. Libraries A (mixture 1, long linker) and B (mixture 1, short linker) are pooled, and libraries C (mixture 2, long linker) and D (mixture 2, short linker) are pooled. Finally, the pooled libraries are panned on the individual antigens.
Table 3.
Antigen characteristics.
| Autoantigen | Molecular weight on SDS-PAGE (kDa) | Localization | Function | Autoimmune disease | Reference |
|---|---|---|---|---|---|
| Annexin XI | 56 | Cytoplasm and nucleoplasm | Phospholipid binding | RA, SLE, SjS, SSc, PM, RP | [13] |
| CENP-B | 80 | Centromeres | DNA binding | SSc | [26] |
| α-Fodrin | 240/120 | Cytoskeleton | Cytoskeleton | SjS | [27] |
| hPop1 | 115 | Nucleolus | Component of RNase P/MRP | SSc, RP, SLE, PM | [28] |
| Topo | 100 | Nucleoplasm | DNA unwinding | SSc | [29] |
| HspB3 | 17 | Cytoplasm and cytoskeleton | Small heat shock protein (chaperone) | Non-autoantigenic | [18] |
| Jo-1 | 50 | Cytoplasm | Histidyl-tRNA synthetase (translation) | PM | [29] |
| Ro52 | 52 | Cytoplasm (and nucleoplasm) | Component of Ro RNPs | SjS | [30] |
| Ro60 | 60 | Cytoplasm and nucleoplasm | Component of Ro RNPs | SjS | [30] |
| Rpp30 | 30 | Nucleolus | Component of RNase P/MRP | SSc, RP, SLE, PM | [28] |
| Rpp38 | 38 | Nucleolus | Component of RNase P/MRP | SSc, RP, SLE, PM | [28] |
| Rrp4 | 36 | Cytoplasm, nucleoplasm and nucleoli | Component of exosome | PM/SSc overlap, PM, SSc | [23] |
| Rrp42 | 35 | Cytoplasm, nucleoplasm and nucleoli | Component of exosome | PM/SSc overlap, PM, SSc | [23] |
| U1A | 38 | Nucleoplasm | Component of the U1 snRNP | MCTD, SLE | [31] |
CENP-B: centromere protein B; hPop1: human Pop1 protein; Hsp-B3: heat shock protein B3; Jo-1: histidyl tRNA synthetase (Jo-1); SDS-PAGE: sodium dodecyl sulphate-polyacrylamide gel electrophoresis; Topo: DNA topoisomerase I. RA: rheumatoid arthritis; SLE: systemic lupus erythematosus; SjS: Sjögren's syndrome; SSc: systemic sclerosis; PM: poly-myositis; RP: Raynaud's Phenomenon; MCTD: Mixed Connective Tissue Disease; MRP: mitochondrial RNA processing.
Fig. 2.
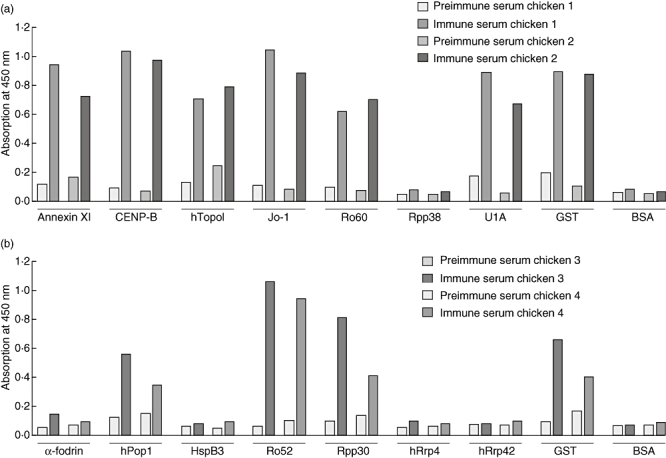
Chicken serum enzyme-linked immunosorbent assay (ELISA). Chicken sera before immunization (pre-immune) and after the final immunization (immune) were analysed for reactivity with the individual antigens in ELISA. Antigens were coated, incubated with the chicken sera at a 500-fold dilution, and bound antibodies were detected with horseradish peroxidase-labelled goat anti-chicken antibodies. After 3,3′,5,5′-tetramethylbenzidine conversion the absorbance was measured at 450 nm. Chicken sera were tested for reactivity with the antigens with which they were immunized, and for reactivity with bovine serum albumin as control antigen. Reactivity with glutathione S-transferase (GST) was also measured, as human Pop1 protein (hPop1) and Rrp38 were GST fusion proteins. (a) Pre-immune and immune sera of chickens 1 and 2 were tested for reactivity with annexin XI, centromere protein B, DNA topoisomerase I, Jo-1, Ro60, Rpp38, U1A, glutathione S-transferase (GST) and bovine serum albumin (BSA). (b) Pre-immune and immune sera of chickens 3 and 4 were tested for binding to α-fodrin, hPop1, heat shock protein B3, Ro52, Rpp30, hRrp4, hRrp42, GST and BSA.
After immunization, antibody phage display libraries were constructed with a short linker of seven amino acids (GQSSRSS), and with a long linker of 18 amino acids (GQSSRSSGGGGSSGGGGS) similar to the linkers described by Andris-Widhopf et al. [32]. Subsequently, the libraries with short and long linkers from chickens immunized with the same antigen cocktail were pooled, and panned on the individual antigens. The complexities of the combined libraries were 2·1 × 108 for the library corresponding to antigen mixture 1 and 1·1 × 108 for the library corresponding to antigen mixture 2. Several selections were performed, the main differences being the amount of antigen coated to the well and the number of selection rounds, as described in Materials and methods. After selection, polyclonal phages of each selection round were tested using ELISA for reactivity with the antigen on which the pool was selected. Figure 3 demonstrates ELISA results for the phages of the different selection rounds, in which the libraries were panned on annexin XI, α-fodrin, hPop1, Ro52, Rpp38, Rrp4, Rrp42 and U1A (polyclonal phage ELISA results of the other selections are not shown). The polyclonal phages were assayed for reactivity with the antigen on which the phages were selected, and for ‘background’ reactivity with the unrelated protein BSA. The polyclonal phage pools were detectably enriched for phages directed to annexin XI, Ro52 and U1A from the third rounds onwards. After the first four selection rounds, the polyclonal phage pool panned on hPop1 did not show reactivity with hPop1. After the fifth selection round a strong reactivity to hPop1 was observed, although this reactivity decreased in subsequent selection rounds.
Fig. 3.
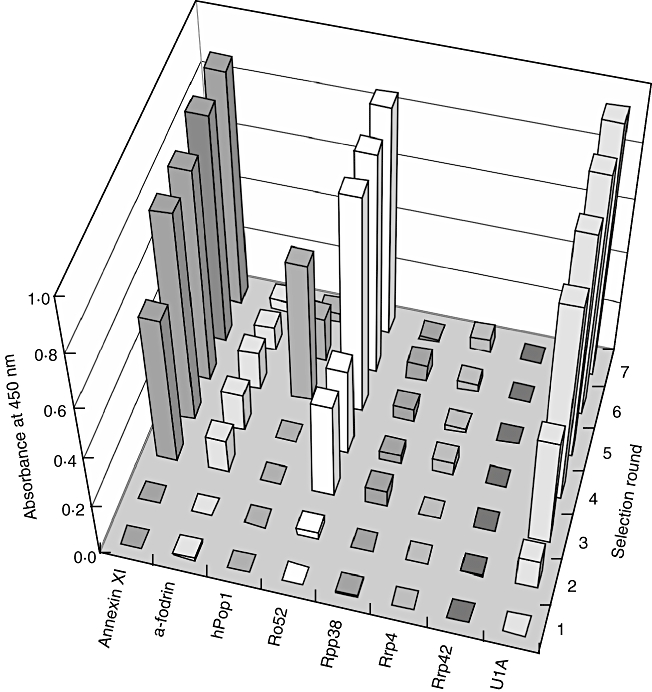
Polyclonal phage enzyme-linked immunosorbent assay (ELISA). Libraries were selected on annexin XI, α-fodrin, human Pop1 protein, Ro52, Rpp38, Rrp4, Rrp42 and U1A, and analysed subsequently for reactivity with these antigens in ELISA. Seven selection rounds were carried out and the reactivities of polyclonal phages after each selection round are measured. Numbers corresponding to the selection rounds are on the right. Bound phages were detected with mouse anti-M13 antibodies, in combination with horseradish peroxidase-labelled rabbit anti-mouse antibodies, followed by 3,3′,5,5′-tetramethylbenzidine conversion. The absorbance was measured at 450 nm.
Screening of phages
After the analysis of polyclonal phages in ELISA, individual colonies were isolated to analyse their specificity in a monoclonal phage ELISA. Colonies were picked from the first, or first two, selection rounds with high polyclonal phage ELISA reactivity. For each antigen, 96–144 monoclonal phages were first screened for full-length scFv cDNA insert by PCR. Subsequently, DNA fingerprint patterns were analysed using the restriction enzyme BstNI, and phages were grouped based on fingerprint pattern similarities. From each fingerprint group, several phages were tested in ELISA for binding to their target antigens and for potential cross-reactivity with the other proteins used for immunization. As summarized in Table 4, for most of the antigens (annexin XI, CENP-B, HspB3, Topo, Jo-1, Ro52, Rpp30 and U1A) several monoclonal phages were reactive in ELISA. Phages directed to HspB3 reacted weakly, but exclusively, with their target antigen. Some phages cross-reacted very specifically with one of the other antigens. For example, anti-U1A phage U11A3 cross-reacted only with annexin XI in ELISA. Furthermore, two phages that were derived from selection on Ro60 reacted strongly with both Ro60 and U1A in ELISA, but no phages were detected that reacted with Ro60 exclusively. None of the selected monoclonal phages reacted detectably with α-fodrin, hPop1, Rpp38, Rrp4 and Rrp42 in ELISA.
Table 4.
Overview of isolated phages.
| Antigen | Antigen-specific phages | Cross-reactive phages |
|---|---|---|
| Annexin XI | A5A3*, A5E2, A5E12*, A5F6, A5G9 | – |
| CENP-B | 5D1, 5D2, 5D4 | – |
| HspB3 | 6E5, 7B4, 7B9, 7B11 | 7C5 (cross-reactive with Ro60) |
| Topo | 5A12, 5C2, 5C3, 5C6, 5C8, 5C9, 5D6 | – |
| Jo-1 | 4B1, 5B4, 5B5‡, 5B6‡, 5B8, 5B9, 5B10, 5B11 | – |
| Ro52 | R3E4, R3G10, R2A11, R2E5, R2E6, R2F11 | R2G8 (cross-reactive with Topo) |
| Ro60 | – | 5E2, 5H10 (both cross-reactive with U1A) |
| Rpp30 | 5C9†, 5D11, 6E12† | – |
| U1A | U11A8, U11B1§, U11B3, U11B5, U11B6, U11B10, U11D4, U11D11, U13B4, U13B7, U13D4, U13D12§ | U11A3 (cross-reactive with annexin XI) |
The variable heavy (VH) and variable light (VL) chains of 20 clones (bold, italic) were sequenced.
Anti-annexin XI clones A5A3 and A5E12 differ one amino acid in CDR2 of the VH chain (VL chains are identical).
Anti-Rpp30 clones 5C9 and 6E12 differ one amino acid in CDR2 of the VH chain (VL chains are identical).
Anti-histidyl tRNA synthetase (Jo-1) clones 5B6 and 5B9 have different VL chains, but identical VH chains.
Anti-U1A clones U11B1 and U13D12 have identical VH chains and different VL chains. CENP-B: centromere protein B; HspB3: heat shock protein B3; Topo: DNA topoisomerase I.
Characterization of selected antibodies
After the first analysis with recombinant purified antigens, we investigated the reactivity of the monoclonal phages with their eukaryotic antigens which are present in a complex mixture of proteins. Therefore, the reactivity of a selected panel of monoclonal phages was analysed on immunoblots containing a HeLa cell extract. As demonstrated in Fig. 4, monoclonal phages A5A3 (anti-annexin XI, 56 kDa), 5B5 and 5B10 (anti-Jo-1, 50 kDa), U11B3, U13D12 and U11A3 (anti-U1A, 38 kDa) and 5D1 (anti-CENP-B, 80 kDa) reacted with proteins with molecular masses corresponding to those of their target antigens. Anti-sera with known reactivities towards the antigen of interest were used in parallel. Anti-U1A monoclonal phage U11A3, which was reactive with U1A and annexin XI in ELISA, also reacted with proteins with molecular weights corresponding to U1A and annexin XI on Western blot. Moreover, U11A3 also reacted with U2B′, which is structurally similar to U1A and which is also recognized by the anti-U1A monoclonal mouse antibody, and with another (as yet unidentified) protein of approximately 50 kDa.
Fig. 4.
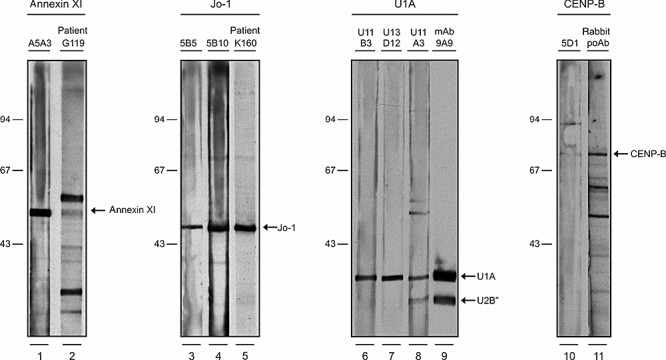
Phage immunoblot analysis. Human epithelial cell line (HeLa) extracts were prepared by sonication and Western blotted onto nitrocellulose membranes. Phages were immunoblotted at a concentration of 5 × 1011 colony forming units/ml, and bound phages were detected with horseradish peroxidase-labelled mouse anti-M13 antibodies, followed by enhanced chemiluminescence. Reactivities of phages and control anti-sera against annexin XI (56 kDa, lanes 1 and 2), histidyl tRNA synthetase (50 kDa, lanes 3–5), U1A (38 kDa, lanes 6–9) and centromere protein B (80 kDa, lanes 10 and 11) are shown. Molecular weights (kDa) of marker proteins are indicated on the left.
To shed more light on the molecular identity of these antibody fragments, the cDNAs of 24 randomly chosen, antigen reactive scFvs were sequenced. The sequenced clones are shown in bold/italic type in Table 4, and their EMBL accession numbers can be found in Table 2. Several anti-Jo-1 clones (5B6, 5B9 and 5B10) appeared to have (almost) identical VH chains, but different VL chains. The same holds true for anti-U1A clones U11B1 and U13D12. Furthermore, 22 clones (92%) contained a short linker, whereas only two clones (8%) contained a long linker, and this finding suggests that antibody fragments with a short linker are selected more easily in this approach compared to antibody fragments with a long linker.
Discussion
Next to mice and rabbits, chickens are an excellent source for the generation of antibodies. Chickens are phylogenetically more distant from humans, and as a consequence conserved mammalian proteins are often more immunogenic in avian species than in mammals [12]. Polyclonal antibodies (IgG-like antibodies termed IgY) are isolated easily from chicken egg yolk [33], and the generation of monoclonal chicken antibodies by the hybridoma technology has been described [34], although these techniques are not used routinely [33]. In a number of studies, recombinant monoclonal antibodies have been isolated successfully from chicken libraries [11,32,35]. The demonstration by Tsurushita et al. [36], that chicken antibodies can be humanized readily, has recently boosted the potential use of recombinant chicken antibodies for therapeutic applications.
Recently, Finlay et al. isolated recombinant antibodies directed to two individual proteins, and yellow jacket venom, from a library that was prepared from a chicken immunized with a mixture of these proteins [37]. Our study extends these observations and shows that chickens can be immunized successfully with a mixture of at least seven distinct proteins. It also emphasizes the ease of using chickens for the generation of recombinant antibodies to highly conserved human targets (such as autoantigens), and suggests a general applicability for antibody generation with a range of antigens. Immunization of animals with multiple antigens has also been described previously for rabbits [38], and in this case scFvs against four haptens were isolated from a single immunized rabbit.
Recently, another interesting method for the high-throughput generation of antibodies was described [39], referred to as the protein epitope signature tag (PrEST)-method. In this method, rabbits were also immunized with a mixture of antigens, but specific antibodies were isolated directly from the polyclonal rabbit serum. The advantage of preparing a library of multiple antigen-immunized animals and the subsequent isolation of antibody specificities by phage display, as we describe here, is that the genetic code for the antibody is isolated concurrently. Consequently, the identical antibody can be produced repetitively, whereas antibody specificities of a polyclonal serum will differ between animals, and even between serum withdrawals from the same animal.
Phages were isolated against nine of the 14 antigens used for immunization (annexin XI, CENP-B, HspB3, Topo, Jo-1, Ro52, Ro60, Rpp30 and U1A), and for most of the antigens more than one reactive phage was isolated. Phages directed to the other five antigens (α-fodrin, hPop1, Rpp38, Rrp4 and Rrp42) were not selected. This might be explained by the fact that the immune sera after the final booster reacted only weakly or not at all with these antigens, indicating that the chickens did not have an immune response towards these antigens. These findings suggest that the immune response was directed mainly to a panel of immunodominant epitopes present on the other antigens. The specific cross-reactivity of several monoclonal phages suggests that a similar epitope on two or more proteins is relatively immunogenic in chickens. This cross-reactivity could be explained by the fact that the process of gene conversion can occur multiple times in the life of a single B cell (see review by [40]). If a B cell, producing antibodies to a first antigen, encounters a second (different) antigen during a subsequent immunization, it could be imagined that through gene conversion the antibody reactivity changes slightly and that both antigens are targeted by this specific B cell.
The monoclonal phages isolated in this study were shown to be reactive with their recombinant target antigen in ELISA, and several phages were also reactive with their counterparts expressed in a human cell. These phages are particularly useful for detection of autoantigens in human patient samples. We have described recently a method in which scFvs are fused with constant domains from immunoglobulin light chains from different species, thereby enabling easy double- or triple-staining of antigens [41]. Using these constant domain tags, the recombinant antibodies obtained in the study presented here can also be used easily for simultaneous detection of the different autoantigens. The fact that some phages reacted only with recombinant protein, and not with the mammalian counterparts, may be explained by the difference in folding, or the presence of post-translational modifications that might lead to a change in the epitopes recognized by the phage antibody. In addition, the concentration of a particular protein in a HeLa cell extract, and thus the amount of this protein on the Western blots, might be too low for detection.
It has been described that recombinant antibody fragments with linkers of more than 12 amino acids are predominantly monomeric, whereas scFv fragments with a linker consisting of three to 12 amino acids may associate spontaneously with another scFv molecule to form bivalent dimers, termed a diabody [42,43]. Reducing the linker length to less than three amino acids can even lead to the formation of trimers or tetramers [42]. During the selections in the study described here, scFvs with short linkers (seven amino acids) were selected preferentially compared to scFvs with long linkers (18 amino acids). This finding might be explained by a higher avidity of diabodies compared to monomeric scFvs [43]. It is thought that, during phage amplification, these diabodies are formed on the phage particle by association of the scFv fragment that is displayed on the phage with a soluble scFv fragment that is present as a result of proteolytic cleavage. The fact that the recombinant antibodies with a short linker will form diabodies also implies that binding to the antigen depends on avidity effects.
Acknowledgments
We thank the CSHL phage display course team (Carlos Barbas, Don Siegel and Greg Silverman) for providing us with both the knowledge for the construction of the chicken phage display libraries as well as for the pComb3H vector system for cloning the chicken libraries, and Walther J. van Venrooij and Ger J. M. Pruijn (Department of Biomolecular Chemistry, Nijmegen, the Netherlands) for critical reading of the manuscript and for helpful comments.
References
- 1.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–7. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 2.Marks JD, Hoogenboom HR, Bonnert TP, McCafferty J, Griffiths AD, Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991;222:581–97. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- 3.Hoogenboom HR, Winter G. By-passing immunisation. Human antibodies from synthetic repertoires of germline VH gene segments rearranged in vitro. J Mol Biol. 1992;227:381–8. doi: 10.1016/0022-2836(92)90894-p. [DOI] [PubMed] [Google Scholar]
- 4.Hoogenboom HR. Selecting and screening recombinant antibody libraries. Nat Biotechnol. 2005;23:1105–16. doi: 10.1038/nbt1126. [DOI] [PubMed] [Google Scholar]
- 5.Degen WG, Pieffers M, Welin-Henriksson E, van den Hoogen FH, van Venrooij WJ, Raats JM. Characterization of recombinant human autoantibody fragments directed toward the autoantigenic U1-70K protein. Eur J Immunol. 2000;30:3029–38. doi: 10.1002/1521-4141(200010)30:10<3029::AID-IMMU3029>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 6.de Wildt RM, Finnern R, Ouwehand WH, Griffiths AD, van Venrooij WJ, Hoet RM. Characterization of human variable domain antibody fragments against the U1 RNA-associated A protein, selected from a synthetic and patient-derived combinatorial V gene library. Eur J Immunol. 1996;26:629–39. doi: 10.1002/eji.1830260319. [DOI] [PubMed] [Google Scholar]
- 7.Raats JM, Wijnen EM, Pruijn GJ, van den Hoogen FH, van Venrooij WJ. Recombinant human monoclonal autoantibodies specific for citrulline-containing peptides from phage display libraries derived from patients with rheumatoid arthritis. J Rheumatol. 2003;30:1696–711. [PubMed] [Google Scholar]
- 8.Hamers-Casterman C, Atarhouch T, Muyldermans S, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–8. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 9.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:1126–36. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 10.Reynaud CA, Anquez V, Grimal H, Weill JC. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell. 1987;48:379–88. doi: 10.1016/0092-8674(87)90189-9. [DOI] [PubMed] [Google Scholar]
- 11.Davies EL, Smith JS, Birkett CR, Manser JM, Anderson-Dear DV, Young JR. Selection of specific phage-display antibodies using libraries derived from chicken immunoglobulin genes. J Immunol Methods. 1995;186:125–35. doi: 10.1016/0022-1759(95)00143-x. [DOI] [PubMed] [Google Scholar]
- 12.Gassmann M, Thommes P, Weiser T, Hubscher U. Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. FASEB J. 1990;4:2528–32. doi: 10.1096/fasebj.4.8.1970792. [DOI] [PubMed] [Google Scholar]
- 13.Misaki Y, Pruijn GJ, van der Kemp AW, van Venrooij WJ. The 56K autoantigen is identical to human annexin XI. J Biol Chem. 1994;269:4240–6. [PubMed] [Google Scholar]
- 14.Verheijen R, de Jong BA, Oberye EH, van Venrooij WJ. Molecular cloning of a major CENP-B epitope and its use for the detection of anticentromere autoantibodies. Mol Biol Rep. 1992;16:49–59. doi: 10.1007/BF00788753. [DOI] [PubMed] [Google Scholar]
- 15.Zandbelt MM, Vogelzangs J, van de Putte LB, van Venrooij WJ, van den Hoogen FH. Anti-alpha-fodrin antibodies do not add much to the diagnosis of Sjogren's syndrome. Arthritis Res Ther. 2004;6:R33–R38. doi: 10.1186/ar1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lygerou Z, Pluk H, van Venrooij WJ, Seraphin B. hPop1: an autoantigenic protein subunit shared by the human RNase P and RNase MRP ribonucleoproteins. EMBO J. 1996;15:5936–48. [PMC free article] [PubMed] [Google Scholar]
- 17.Verheijen RHF, van den Hoogen FH, Beijer R, et al. A recombinant topoisomerase I used for autoantibody detection in sera from patients with systemic sclerosis. Clin Exp Immunol. 1990;80:38–43. doi: 10.1111/j.1365-2249.1990.tb06438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boelens WC, Van Boekel MA, De Jong WW. HspB3, the most deviating of the six known human small heat shock proteins. Biochim Biophys Acta. 1998;1388:513–16. doi: 10.1016/s0167-4838(98)00215-5. [DOI] [PubMed] [Google Scholar]
- 19.Rutjes SA, Vree Egberts WT, van den Jongen PHF, Pruijn GJ, van Venrooij WJ. Anti-Ro52 antibodies frequently co-occur with anti-Jo-1 antibodies in sera from patients with idiopathic inflammatory myopathy. Clin Exp Immunol. 1997;109:32–40. doi: 10.1046/j.1365-2249.1997.4081308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peek R, Pruijn GJ, van Venrooij WJ. Epitope specificity determines the ability of anti-Ro52 autoantibodies to precipitate Ro ribonucleoprotein particles. J Immunol. 1994;153:4321–9. [PubMed] [Google Scholar]
- 21.Slobbe RL, Pluk W, van Venrooij WJ, Pruijn GJ. Ro ribonucleoprotein assembly in vitro. Identification of RNA-protein and protein–protein interactions. J Mol Biol. 1992;227:361–6. doi: 10.1016/0022-2836(92)90890-v. [DOI] [PubMed] [Google Scholar]
- 22.Welting TJ, van Venrooij WJ, Pruijn GJ. Mutual interactions between subunits of the human RNase MRP ribonucleoprotein complex. Nucleic Acids Res. 2004;32:2138–46. doi: 10.1093/nar/gkh539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brouwer R, Vree Egberts WT, Hengstman GJ, et al. Autoantibodies directed to novel components of the PM/Scl complex, the human exosome. Arthritis Res. 2002;4:134–8. doi: 10.1186/ar389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein Gunnewiek JM, Hussein RI, van Aarssen Y, et al. Fourteen residues of the U1 snRNP-specific U1A protein are required for homodimerization, cooperative RNA binding, and inhibition of polyadenylation. Mol Cell Biol. 2000;20:2209–17. doi: 10.1128/mcb.20.6.2209-2217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbas CF, Burton DR, Scott JK, Silverman GJ. Phage display − a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 2001. [Google Scholar]
- 26.Verheijen R. Centromere proteins. In: van Venrooij WJ, Maini RN, editors. Manual of biological markers of disease. Dordrecht: Kluwer Academic Publishers; 1994. pp. 1–17. [Google Scholar]
- 27.Ulbricht KU, Schmidt RE, Witte T. Antibodies against alpha-fodrin in Sjogren's syndrome. Autoimmun Rev. 2003;2:109–13. doi: 10.1016/s1568-9972(03)00002-8. [DOI] [PubMed] [Google Scholar]
- 28.Welting TJ, Raijmakers R, Pruijn GJ. Autoantigenicity of nucleolar complexes. Autoimmun Rev. 2003;2:313–21. doi: 10.1016/s1568-9972(03)00029-6. [DOI] [PubMed] [Google Scholar]
- 29.Utz PJ, Gensler TJ, Anderson P. Death, autoantigen modifications, and tolerance. Arthritis Res. 2000;2:101–14. doi: 10.1186/ar75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohlsson M, Jonsson R, Brokstad KA. Subcellular redistribution and surface exposure of the Ro52, Ro60 and La48 autoantigens during apoptosis in human ductal epithelial cells: a possible mechanism in the pathogenesis of Sjogren's syndrome. Scand J Immunol. 2002;56:456–69. doi: 10.1046/j.1365-3083.2002.01072_79.x. [DOI] [PubMed] [Google Scholar]
- 31.Greidinger EL, Hoffman RW. The appearance of U1 RNP antibody specificities in sequential autoimmune human antisera follows a characteristic order that implicates the U1-70 kd and B′/B proteins as predominant U1 RNP immunogens. Arthritis Rheum. 2001;44:368–75. doi: 10.1002/1529-0131(200102)44:2<368::AID-ANR55>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Andris-Widhopf J, Rader C, Steinberger P, Fuller R, Barbas CF., III. Methods for the generation of chicken monoclonal antibody fragments by phage display. J Immunol Methods. 2000;242:159–81. doi: 10.1016/s0022-1759(00)00221-0. [DOI] [PubMed] [Google Scholar]
- 33.Camenisch G, Tini M, Chilov D, et al. General applicability of chicken egg yolk antibodies: the performance of IgY immunoglobulins raised against the hypoxia-inducible factor 1alpha. FASEB J. 1999;13:81–8. doi: 10.1096/fasebj.13.1.81. [DOI] [PubMed] [Google Scholar]
- 34.Nishinaka S, Akiba H, Nakamura M, et al. Two chicken B cell lines resistant to ouabain for the production of chicken monoclonal antibodies. J Vet Med. 1996;58:1053–6. doi: 10.1292/jvms.58.11_1053. [DOI] [PubMed] [Google Scholar]
- 35.Yamanaka HI, Inoue T, Ikeda-Tanaka O. Chicken monoclonal antibody isolated by a phage display system. J Immunol. 1996;157:1156–62. [PubMed] [Google Scholar]
- 36.Tsurushita N, Park M, Pakabunto K, et al. Humanization of a chicken anti-IL-12 monoclonal antibody. J Immunol Methods. 2004;295:9–19. doi: 10.1016/j.jim.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Finlay WJ, deVore NC, Dobrovolskaia EN, Gam A, Goodyear CS, Slater JE. Exploiting the avian immunoglobulin system to simplify the generation of recombinant antibodies to allergenic proteins. Clin Exp Allergy. 2005;35:1040–8. doi: 10.1111/j.1365-2222.2005.02307.x. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Cockburn W, Kilpatrick JB, Whitelam GC. High affinity ScFvs from a single rabbit immunized with multiple haptens. Biochem Biophys Res Commun. 2000;268:398–404. doi: 10.1006/bbrc.2000.2129. [DOI] [PubMed] [Google Scholar]
- 39.Larsson K, Wester K, Nilsson P, Uhlen M, Hober S, Wernerus H. Multiplexed PrEST immunization for high-throughput affinity proteomics. J Immunol Methods. 2006;315:110–20. doi: 10.1016/j.jim.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Ratcliffe MJ. Antibodies, immunoglobulin genes and the bursa of Fabricius in chicken B cell development. Dev Comp Immunol. 2006;30:101–18. doi: 10.1016/j.dci.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Raats J, Hof D. Recombinant antibody expression vectors enabling double and triple immunostaining of tissue culture cells using monoclonal antibodies. Eur J Cell Biol. 2005;84:517–21. doi: 10.1016/j.ejcb.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 42.Todorovska A, Roovers RC, Dolezal O, Kortt AA, Hoogenboom HR, Hudson PJ. Design and application of diabodies, triabodies and tetrabodies for cancer targeting. J Immunol Methods. 2001;248:47–66. doi: 10.1016/s0022-1759(00)00342-2. [DOI] [PubMed] [Google Scholar]
- 43.Pluckthun A, Pack P. New protein engineering approaches to multivalent and bispecific antibody fragments. Immunotechnology. 1997;3:83–105. doi: 10.1016/s1380-2933(97)00067-5. [DOI] [PubMed] [Google Scholar]


