Abstract
l-Ficolin, like mannan-binding lectin (MBL), is a lectin pathway activator present in normal human plasma. Upon binding ligand, l-ficolin similarly initiates C4 cleavage via the serine protease MBL-associated serine protease-2 (MASP-2). We sought further insight into l-ficolin binding reactions and MASP-2 activation by passing plasma through GlcNAc-derivatized Sepharose. l-Ficolin bound in 1·0 M NaCl-ethylenediamine tetraacetic acid (EDTA), and remained bound in NaCl-free EDTA, while MASP-2 eluted in proenzyme form (∼20% yield, > 40 000-fold purification). l-Ficolin was eluted with GlcNAc in 1·0 M NaCl (∼10% yield, > 3000-fold purification), with trace amounts of C3, α2-macroglobulin and both native and activated MASP-2. These preparations were utilized to investigate l-ficolin reactivities with acetylated low-density lipoprotein (A-LDL) as a model ligand in albumin-free systems. l-Ficolin bound strongly to A-LDL in the absence as well as presence of calcium, including saline-EDTA, and was optimal in 1·0 M NaCl-EDTA, but binding failed to occur in EDTA in the absence of NaCl. The addition of l-ficolin to immobilized A-LDL resulted in activation of MASP-2 in unmodified but not ficolin-depleted plasma unless l-ficolin was restored. We conclude that A-LDL is a useful ligand for investigation of l-ficolin function; both binding and activation are optimally examined in systems free of albumin; and ligand binding in 1·0 M NaCl in EDTA can be useful in the isolation of l-ficolin and native MASP-2.
Keywords: lectin pathway, l-ficolin, MASP-2
Introduction
The lectin pathway (LP) of complement activation has received much attention over recent years, first with the discovery of mannan-binding lectin (MBL) [1] and later with the isolation and purification of several MBL-associated serine proteases (MASPs) [2–4]. More recently, two unique initiators of the LP were discovered in human serum, both members of a new group of proteins termed ficolins: l-ficolin and h-ficolin [5,6]. Ficolins share structural similarity with MBL, in that they are composed predominantly of tetramers of identical trimeric subunits which contain an N-terminal collagenous stalk [7]. However, ficolins differ from MBL in that they contain a fibrinogen-like C-terminus domain [8]. The ligand specificities for the ficolins and MBL differ [9–11]. Whereas MBL reacts strongly with carbohydrate ligands including mannose, fucose and N-acetylglucosamine (GlcNAc), l-ficolin reacts with N-acetylated molecules including GlcNAc [5,9,12–15].
Several groups have examined the conditions required for optimal binding of l-ficolin to its ligands. It was reported initially that l-ficolin bound to mannan in a calcium-dependent manner [9] and, indeed, calcium is present in the initial binding step of several purification procedures [9,16–19]. Later, binding to mannan was contended, with acetylated sugars and Tris-derivatized Sepharose, the preferred ligands, binding l-ficolin even in the absence of calcium [5,10]. This was supported by demonstration of binding of porcine ficolin to GlcNAc in citrated plasma [20]. More recently, l-ficolin has been isolated by binding to immobilized acetylated amino acids in ethylenediamine tetraacetic acid (EDTA) at high ionic strength, followed by elution with low salt (LS) [15,21].
Complement activation via l-ficolin has many similarities to complement activation by MBL: once l-ficolin binds its ligand in normal human serum, cleavage of C4 ensues [22]. This depends primarily upon activation of MASP-2 [3,23] and seems to occur without a requirement for MASP-1, MASP-3 or MAp19 [16,24,25], but the precise mechanism of MASP-2 activation is not yet clear. While it is not always required for binding to its ligands as discussed above, calcium seems to be required for MASP-2 activation by the ficolins [23].
In this study, we define the conditions required for l-ficolin to bind to acetylated low-density lipoprotein (A-LDL). We find that A-LDL serves as an ideal ligand in optimal albumin-free systems and initiates l-ficolin-mediated activation of the lectin pathway. We also provide a simple method for the purification of proenzyme MASP-2 from plasma.
Materials and methods
Reagents
Tris-Base, NaCl, EDTA, Pefabloc, tetramethyl benzidine (TMB), methylamine, N-acetylglucosamine (GlcNAc), mannan, bovine serum albumin (BSA) and C3 were purchased from Sigma-Aldrich (St Louis, MO, USA). Alpha 2-macroglobulin (A2M) and antibodies to C3 and IgG were from Dako (Copenhagen, Denmark). C4 and biotinylated anti-C4 antibody were from EMD Biosciences (San Diego, CA, USA). Anti-l-ficolin antibody, clone GN5, and anti-MASP-2 antibody, clone 8B5, were from CellSciences (Canton, MA, USA). GlcNAc-BSA was purchased from V-Laboratories, Inc. (Covington, LA, USA). A-LDL and non-acetylated low-density lipoprotein (N-LDL) were from Intracel (Frederick, MD, USA). Ninety-six-well microwell plates were from Nalge Nunc International (Naperville, IL, USA). The bicinchoninic acid (BCA) kit and StartingBlock™ were purchased from Pierce (Rockford, IL, USA). That StartingBlock™ was completely albumin-free was confirmed by the manufacturer. Activated Sepharose was purchased from GE Healthcare Biosciences AB (formerly Amersham Biosciences, Uppsala, Sweden). Biotin and protein A/G were purchased from Pierce. The anti-MBL antibody was clone HYB 131-01 (AntibodyShop, Gentofte, Denmark). Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from SouthernBiotech (Birmingham, AL, USA).
Purification of l -ficolin and MASP-2
Ten units of outdated fresh frozen plasma were obtained from anonymous donors, and each unit was assayed for ficolin by binding to A-LDL coated plates as described below. The units with the greatest amount of ficolin were pooled, and 400 ml were dialysed overnight in high salt (HS)-EDTA buffer (1·0 M NaCl, 20 mM Tris, 10 mM EDTA, pH 7·4) at 4°C. Pools in which no l-ficolin was detected at a 1 : 10 dilution were termed ficolin-deficient plasma (FDP) and were stored for later use. A 30 ml GlcNAc–Sepharose column was prepared using CNBr-activated Sepharose according to the manufacturer's protocol; GlcNAc-elutable l-ficolin bound to these columns but not to columns prepared similarly but without GlnNAc. The column was balanced with HS-EDTA buffer until the absorbance (A280 nm) reached zero. The pooled plasma was passed through the column at a rate of 3·0 ml/min at 4°C, and the column was washed with HS-EDTA until the absorbance returned to zero; 400 ml of effluent was collected and stored for later use. LS-EDTA buffer (20 mM Tris, 10 mM EDTA, pH 7·4) was applied to the column, and the change in absorbance was recorded. When the absorbance returned to zero, fractions were collected and concentrated to 1 ml (pool 1) using a Vivaspin 20 concentrator (Argos Technologies, East Dundee, IL, USA). The column was washed with 100 mM GlcNAc dissolved in HS-EDTA, and the absorbance of the eluant was recorded. The fractions were collected and concentrated (pool 2) as described above for pool 1. Pools 1 and 2 were passed through a protein A/G column (Pierce) to remove contaminating immunoglobulin.
l-Ficolin affinity enzyme-linked immunosorbent assay (ELISA)
Microwell plates were coated with 100 μl ligand (1·0 μg/well) in coating buffer (0·3 M NaHCO3, 0·2 M Na2CO3, pH 9·6) and allowed to sit at 4°C overnight or at room temperature for 2 h. The ligands used included GlcNAc–BSA, BSA, A-LDL, N-LDL and mannan. The wells were blocked with 200 μl/well of either 1% BSA (Figs 3 and 4) in Tris-buffered saline (TBS) (10 mM Tris-Base, 140 mM NaCl, pH 7·4) or a 1 : 10 dilution of StartingBlock™ (Figs 4–6) and allowed to stand at room temperature for 1 h. After washing three times with 200 μl/well TBS/Tw/Ca, 100 μl of plasma diluted in HS-calcium buffer (1·0 M NaCl, 20 mM Tris-Base, 10 mM calcium, pH 7·4) was added. The plates were allowed to stand at room temperature for 1 h before washing again with TBS/Tw/Ca. 100 μl of anti-l-ficolin which had been biotinylated was added in TBS/Tw/Ca (1·0 μg/well) and allowed to stand at room temperature for 1 h. After washing, 1 ng avidin-coupled HRP diluted in 100 μl TBS/Tw/Ca was added and incubated for 20 min at room temperature. The amount of antibody bound was determined by reading the absorbance at 450 nm after stopping the TMB reaction with 2 N hydrochloric acid. Negative controls included wells coated with coating buffer, HS-calcium buffer added without plasma or buffer added with anti-MBL antibody in place of anti-l-ficolin antibody. For Fig. 3b, biotinylated anti-MBL antibody was added at a dilution of 1 : 80 000, giving a final concentration of 12·5 μg/well before detection with TMB substrate.
Fig. 3.
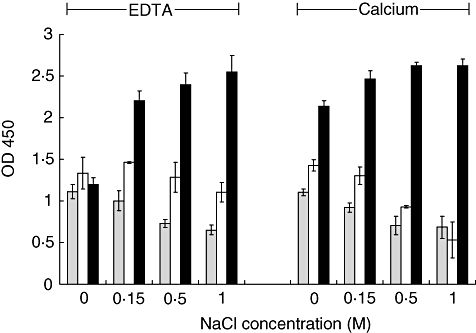
Reactivity of l-ficolin with GlcNAc–bovine serum albumin (BSA), BSA and acetylated low-density lipoprotein (A-LDL) with increasing ionic strengths in the presence of 10 mM calcium or ethylenediamine tetraacetic acid (EDTA). l-Ficolin present in normal human plasma (NHP) binds A-LDL (dark columns) independent of ionic strength in the presence of calcium, and in an ionic strength-dependent manner in the presence of EDTA. l-Ficolin binding to GlcNAc–BSA (grey columns) was qualitatively no different than binding to BSA-coated wells (open columns). All wells received BSA as the blocking agent. Data are shown as the mean ± standard deviation, n = 3 of a representative experiment of two.
Fig. 4.
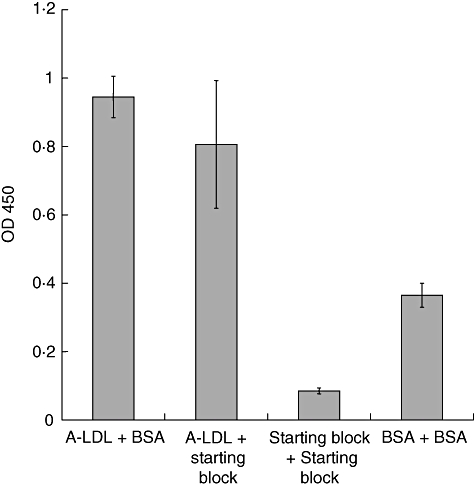
l-Ficolin binds bovine serum albumin (BSA), but does not bind StartingBlock™. Wells were coated with either acetylated low-density lipoprotein (A-LDL), StartingBlock™ or BSA, and then blocked with either BSA or StartingBlock™, as indicated in the graph. After adding normal human plasma (NHP), l-ficolin was detected by a biotinylated anti-l-ficolin antibody. Data are shown as the mean ± standard deviation, n = 3 of a representative experiment of two.
Fig. 6.
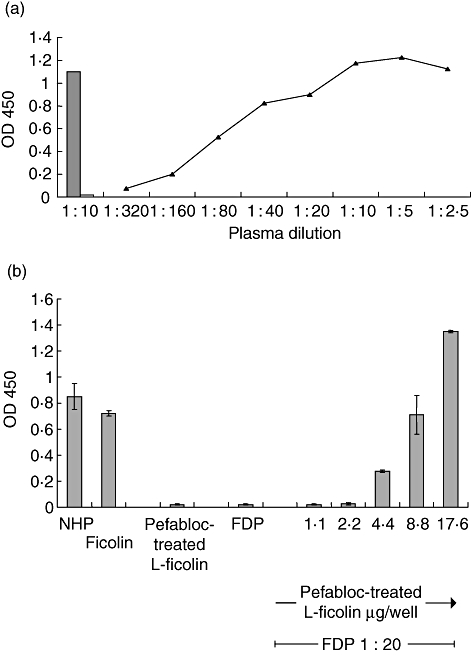
Requirement of l-ficolin for lectin pathway (LP) activation by acetylated low-density lipoprotein (A-LDL) in normal human plasma (NHP) and dose-dependent restoration of A-LDL activation of the LP in ficolin-deficient plasma (FDP) by addition of purified l-ficolin, as measured by C4-binding enzyme-linked immunosorbent assay (ELISA). (a) Pefabloc treated purified l-ficolin (8·8 μg/well, obtained from pool 2, above) was added to A-LDL-coated wells, and activation of C4 was assessed. C4 binding was observed in a 1 : 10 dilution of NHP (shaded column) but not in ficolin-deficient plasma (open column, 1 : 10 dilution), unless normal amounts of l-ficolin were restored (line, dark triangles). (b) Increasing amount of Pefabloc-treated l-ficolin was added to wells with FDP held constant at a 1 : 20 dilution. NHP at a dilution of 1 : 20 or purified l-ficolin in an amount of 17·6 μg/100 μl were included as additional positive controls, far left of figure. Pefabloc-treated l-ficolin was tested at 1·1, 2·2, 4·4, 8·8 and 17·6 μg/100 μl with FDP, and at 17·6 μg/well without FDP (Pefabloc-treated l-ficolin alone). Data are shown as the mean ± standard deviation, n = 3 of a representative experiment of three.
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting
Samples were analysed by SDS-PAGE under reducing or non-reducing conditions. Wells were loaded with 2·2 μl sample per lane for pool 1, so that roughly 0·3 μg total protein was added, or 1·0 μl from pool 2 per lane giving 0·9 μg total protein. Proteins were visualized by Coomassie staining or transferred to nitrocellulose membranes and probed by anti-A2M, anti-C3, anti-l-ficolin or anti-MASP-2 antibodies. The anti-l-ficolin antibody was a rabbit polyclonal anti-peptide antibody generated against a sequence of hydrophilic amino acid residues (HNNQSFSTKDQDND) found in the fibrinogen-like domain of l-ficolin (Sigma Genosys, St Louis, MO, USA). The anti-MASP-2 antibody reacts against the complement control protein domain (CCP)1/2 and serine protease (SP)-fragment of MASP-2. Goat HRP-conjugated secondary antibodies against the species-specific primary antibody were used to detect protein.
Determination of protein levels
During the purification, each fraction was checked for levels of C3, A2M, l-ficolin, MASP-2 and total protein. To determine levels of C3 and A2M, a sandwich ELISA was developed in which a polyclonal antibody was used to pull down either C3 or A2M, and then a biotinylated antibody was added to detect the specific protein. Levels of C3 or A2M in samples to be tested were determined by relating the A450 nm generated to a standard curve using the appropriate purified proteins. A similar method was used to determine the level of l-ficolin, using normal human serum as the standard. As this source contained a level of l-ficolin consistent with the average value described by Kilpatrick et al. [26], it was assigned arbitrarily a value of 3·5 μg/ml; all values presented herein are based upon this arbitrary standardization. The MASP-2 ELISA was performed in a similar manner to the l-ficolin ELISA, using normal human plasma (NHP) assigned a MASP-2 concentration of 0·5 μg/ml [27] as the standard.
C4-cleaving assay
This assay was performed as described by Petersen et al. [28] except that plates were coated with 1·0 μg/well A-LDL, blocked with StartingBlock™ as described above, and ficolin (from plasma or purified as described above) was added into HS-calcium buffer. These conditions allowed for l-ficolin to bind A-LDL, but prevented binding of both C1q and MBL. After incubating for 2 h at 37°C and then washing with TBS/Tw/Ca, C4 diluted 1 : 1000 in TBS/Tw/Ca was added and the mixture was incubated for 1 h at 37°C. Wells were washed with TBS/Tw/Ca, and biotinylated anti-C4 antibody was added at a dilution of 1 : 8000 in TBS/Tw/Ca and allowed to stand for 1 h. The plates were washed three times with TBS/Tw/Ca, and 1 ng/well avidin-conjugated HRP was added in TBS/Tw/Ca. After 20 min at room temperature, the plates were washed and protein was detected using TMB solution as described in the l-ficolin ELISA above. Controls were also as above, and included buffer added without C4 or buffer added without anti-C4 antibody.
Pefabloc treatment of purified l-ficolin
l-Ficolin obtained from affinity chromatography (pool 2) was treated with 50 mM Pefabloc in HS-EDTA. One hundred μl of Pefabloc was added to 900 μl of l-ficolin, vortexed and allowed to stand at room temperature for 2 h. The sample was dialysed overnight at 4°C against HS-EDTA. As a control, l-ficolin was treated with HS-EDTA only, and dialysed against HS-EDTA as well.
Results
Isolation of l-ficolin and MASP-2 from normal human plasma (NHP)
l-Ficolin was obtained from human plasma by passage through a GlcNAc–Sepharose column in HS-EDTA using a minor modification of the method of Krarup et al., who used an N-acetylcysteine–Sepharose column [15]. Virtually all (> 99%) the plasma protein passed through the column (Table 1). The presence of EDTA prevented both MBL binding and classical and alternative pathway activation, and the high (1·0 M) salt level was required in order for ficolin to bind in the absence of calcium [15]. When the column was washed with LS-EDTA, a broad peak eluted. Washing with GlcNAc (100 mM) in HS-EDTA resulted in elution of a second broad peak (Fig. 1). The peak fractions were collected into two pools, which were assayed for the presence of l-ficolin by ELISA. Pool 1 contained no detectable l-ficolin, while pool 2 contained a relatively large amount of l-ficolin (Table 1, Fig. 2a,b). The plasma which had passed through the column had been depleted completely of l-ficolin (Table 1). Pool 2 showed a banding pattern on non-reduced SDS-PAGE, highly suggestive of l-ficolin [29]: bands are seen at 35, 100 and above 200 kDa, along with a ladder of higher molecular weight bands which each stained with anti-l-ficolin on Western blot analysis and hence represented ficolin multimers (Fig. 2a,b). Other proteins found in pool 2 include activated MASP-2 at 50 kDa, α2M and C3 (Fig. 2a,e,f, respectively).
Table 1.
Purification of l-ficolin and MASP-2 from pooled human plasma. Fractions from the column purification shown in figure 1 were assayed for total protein, l-ficolin, MASP-2, C3 and α2M, compared with starting material, and purification and yield were calculated.
| Purification | % yield | |||||||
|---|---|---|---|---|---|---|---|---|
| Starting material | Plasma (400 ml) | Effluent (400 ml) | Pool 1 (1 ml) | Pool 2 (1 ml) | Pool 1 | Pool 2 | Pool 1 | Pool 2 |
| l-ficolin | ||||||||
| Conc. | 3·5 μg/ml | < 0·01 μg/ml | < 0·01 μg/ml | 138 μg/ml | – | 3090× | – | 10% |
| Total | 1·4 mg | < 4 μg | < 0·01 μg | 138 μg | ||||
| MASP-2 | ||||||||
| Conc. | 0·12 μg/ml | 0·11 μg/ml | 9·38 μg/ml | 0·56 μg/ml | 42 941× | 368× | 19·5% | 1·2% |
| Total | 48 μg | 44 μg | 9·38 μg | 0·56 μg | ||||
| C3 | ||||||||
| Conc. | 1·3 mg/ml | 1·3 mg/ml | 1·9 μg/ml | 0·9 μg/ml | 1·3× | 0·05× | 0·0004% | 0·0002% |
| Total | 520 mg | 520 mg | 1·9 μg | 0·9 μg | ||||
| A2M | ||||||||
| Conc. | 3 mg/ml | 3 mg/ml | 8 μg/ml | < 0·01 μg/ml | 1·5× | – | 0·0007% | – |
| Total | 1·2 g | 1·2 g | 8 μg | < 0·01 μg | ||||
| Total protein | ||||||||
| Conc. | 70 mg/ml | 70 mg/ml | 128 μg/ml | 894 μg/ml | – | – | – | – |
| Total | 28 g | 28 g | 128 μg | 894 μg | ||||
Fig. 1.
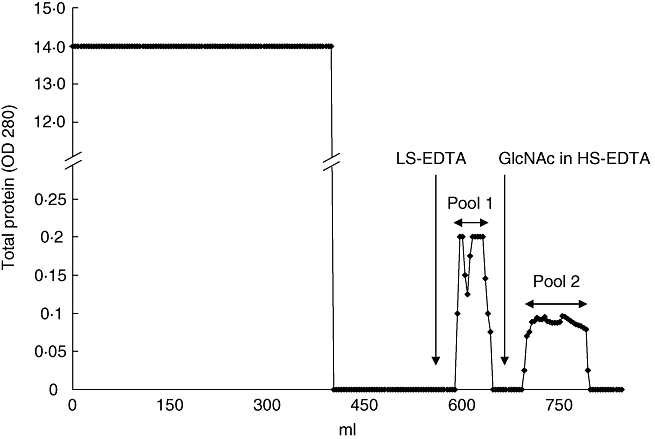
Elution of l-ficolin and mannan-binding lectin (MBL)-associated serine protease-2 (MASP-2) from GlcNAc–Sepharose in ethylenediamine tetraacetic acid (EDTA). Pooled normal human plasma (NHP) (400 ml) was passed thorough a GlcNAc-Sepharose column in high salt (HS)-EDTA at a flow rate of 3 ml/min. The column was eluted first with low salt (LS)-EDTA followed by elution with 100 mM GlcNAc in HS-EDTA (arrows), yielding proteins designated ‘pool 1’ and ‘pool 2’, respectively. Data is from a representative experiment of two individual purifications.
Fig. 2.
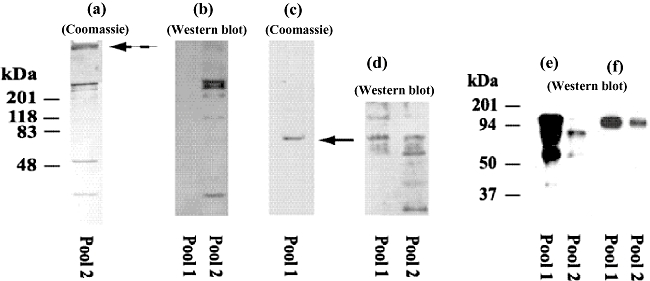
Characterization of proteins in pools 1 and 2. Coomassie (a, c) and Western blots of pools 1 and 2 in sodium dodecyl sulphate– polyacrylamide gel electrophoresis (SDS-PAGE) stained with antibodies to l-ficolin (b), mannan-binding lectin (MBL)-associated serine protease-2 (MASP-2) (d), A2M (e) and C3 (f), respectively. The gels in c, e and f were run under reducing conditions, while a, b and d were run under non-reducing conditions. Pool 2 shows a banding pattern typical of l-ficolin (a) on a 5–20% gradient gel, in which multimers of a monomer associate under non-reducing conditions; additional protein is seen in the stacking layer (broken arrow). Anti-l-ficolin antibody reacts strongly with the multimers and monomer from pool 2, but not with protein from pool 1 (b); the MASP-2 in pool 1 appears as a single protein at 75 kDa (solid arrow) under reducing conditions (c, 12% gel). MASP-2 is present in both pools, found in proenzyme form in pool 1 but with heavy and light chains present as well in pool 2 (D, 12% gel). A2M and C3 are present in both pools (e and f, both 8% gels). Lanes were loaded with 0·3 μg total protein (pool 1) per well or 0·9 μg total protein (pool 2) per well.
Both Coomassie and Western blots of pool 1 under reducing conditions showed a major band at 75 kDa staining strongly with anti-MASP-2 (Fig. 2c,d), indicating that the protease was isolated in its proenzyme form; no reactivity was observed using antibodies to MASP-1, MASP-3, MBL or IgG. Reactivity with anti-MASP-2 was also found in pool 2, but at 75, 50 and ∼20 kDa, reflecting the presence of activated fragments as well as the native MASP-2 protein (Fig. 2d). The amount of MASP-2 in pool 1 (9·38 μg), although much larger than that in pool 2 (0·56 μg), is a small fraction (∼20%) of that in the starting serum pool; a much greater amount of MASP-2 (92% of the MASP-2 applied) was present in the pass-through, along with MBL, α2M and C3 (Table 1). We do not know the basis for purified MASP-2 and MASP-2 in the effluent, both assayed by the same ELISA, totalling 112%; perhaps this represents the effects of MASP-2 complexes, fragments or experimental variation. Small amounts of C3 and α2M were detected in both pools in Western blots (Fig. 2e,f); and small amounts of other proteins may have been present as well. None the less, MASP-2 in pool 1 and l-ficolin in pool were purified ∼43 000-fold and ∼3000-fold compared to amounts present in the starting plasma, and no l-ficolin was detected in pool 2. A significant amount of the co-purified α2M and C3 was retained in the stacking layer of the gel (Fig. 2a). Similar results were obtained using gels loaded with 10-fold greater amounts of protein, but larger amounts were not tested. Thus, MASP-2 associates with l-ficolin bound to GlcNAc–Sepharose in HS-EDTA and is released by washes with LS-EDTA, in a manner suitable for preparation of the native protein free of l-ficolin. By contrast, the binding affinity of l-ficolin for its ligand is sufficiently strong that it is not released in LS-EDTA. No bands were seen on SDS-PAGE reflective of the molecular weights of MASP-1 or -3 (81 and 107 kDa, respectively [30]).
Reactivity of l-ficolin with acetylated-LDL
Even though albumin has been used frequently as a carrier protein and/or blocking agent in investigations of l-ficolin binding reactions, we observed strong background binding of l-ficolin to BSA-coated and BSA-blocked ELISA plates, which limited ability to characterize binding conditions. Figure 3 illustrates the difficulty of distinguishing between binding of l-ficolin to GlcNAc–BSA-coated wells and control wells coated with BSA only; both were blocked with BSA. We observed a level of binding to the BSA-coated control plates at salt concentrations up to 1·0 M NaCl in the presence of either 10 mM EDTA or 10 mM calcium chloride, which was substantial at or near the level of binding to plates coated with GlcNAc–BSA and, thus, concluded that albumin should be avoided completely in the assay. Note that binding to acetylated low-density lipoprotein was much greater than that of either BSA or GlcNAc–BSA, and followed a similar trend noted by Krarup et al. [15] in which, in the absence of calcium, binding increased under high salt conditions (Fig. 3).
To confirm the results shown in Fig. 3, we examined binding of l-ficolin to wells containing either BSA or StartingBlock™. No binding was observed to plates in which the albumin-free reagent, StartingBlock™, was used for the blocking step (Fig. 4). When wells were coated with A-LDL, l-ficolin binding was observed regardless of whether BSA or StartingBlock™ was used as the blocking reagent. Note that in Fig. 4, binding to wells coated and blocked with BSA is greater than to wells coated and blocked with StartingBlock™ alone. Binding to GlcNAc–BSA was inhibited by both BSA and GlcNAc but not by mannose, and was observed using all BSA-coupled ligands tested, speaking to the specificity of the reaction with BSA; similarly, an assay could not be developed when Tween-20 was used as the blocking agent (data not shown).
We compared binding of l-ficolin to A-LDL with that of both non-acetylated LDL (N-LDL) and mannan in wells which had been blocked with StartingBlock™. In support of data shown in Fig. 3, we again found that in the presence of calcium, l-ficolin bound A-LDL independently of salt concentration (Fig. 5). Binding to A-LDL was enhanced under high salt concentrations in the absence of calcium. Binding to N-LDL was not observed in any of the conditions tested. Additionally, l-ficolin was not found to bind mannan-coated wells (Fig. 5a). Mannan, a well-known activator of the LP, was included as a potential ligand to ensure that LP activation was not influenced by MBL under the present reaction conditions. Importantly, A-LDL did not bind MBL (Fig. 5b), providing further evidence that LP activation observed later (Fig. 6a,b) would be dependent upon ficolin alone.
Fig. 5.
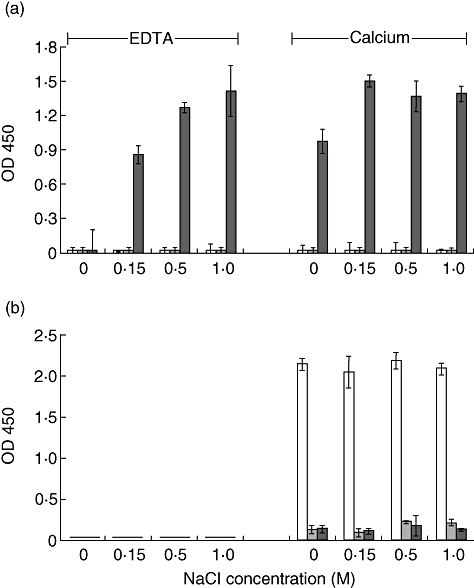
Reactivity of l-ficolin with acetylated low-density lipoprotein (A-LDL), non-acetylated-LDL (N-LDL) and mannan with increasing ionic strengths in the presence of 10 mM calcium or ethylenediamine tetraacetic acid (EDTA). (a) l-Ficolin present in normal human plasma (NHP) binds A-LDL (dark columns) independent of ionic strength in the presence of calcium, and in an ionic strength-dependent manner in the presence of EDTA. l-Ficolin does not bind to N-LDL (shaded columns) or mannan (open columns) under any of these conditions. (b) MBL does not bind A-LDL under any of the conditions studied, but does display strict calcium-dependent binding to mannan. All wells received StartingBlock™ as the blocking reagent. Data are shown as the mean ± standard deviation, n = 3 of a representative experiment of three.
Lectin pathway activation by A-LDL in normal human plasma
Immobilized A-LDL was able to activate the lectin pathway when incubated with NHP, as demonstrated by C4-binding ELISA (Fig. 6a). Addition of NHP was carried out in high salt, with calcium present, as MBL does not bind to A-LDL under these conditions. In addition, high salt is known to inhibit binding and activation of the C1 complex [31]. Binding was not observed in FDP. Addition of Pefabloc-treated l-ficolin, devoid of associated MASP-2 activity, to the FDP resulted in restoration of C4 cleaving activity (Fig. 6a). The purified l-ficolin required pretreatment with Pefabloc to inactivate the small amount of activated MASP-2 shown here and known generally to be associated with l-ficolin purified from normal human serum or plasma. The presence of this activity and its inhibition by Pefabloc, as well as the dose-dependence of l-ficolin to restore the ability to cleave C4 in FDP, is shown in Fig. 6b.
Discussion
It has been accepted widely that GlcNAc is an optimal ligand for l-ficolin, and the structural basis for this binding is being clarified [7,10,16,17,29,32]. Le et al. [10] showed that l-ficolin bound acetylated sugars generally, even in the absence of calcium. More recently, Krarup et al. showed that acetylated amino acids could generally bind l-ficolin, and that the binding was dependent upon calcium only at very low ionic strength [15], indicating that l-ficolin is not exclusively a lectin [32], while Hummelshoj et al. showed that the fibrinogen-like domain expressed from recombinant l-ficolin binds N-acetylated sugar moieties more strongly than other acetylated ligands [29]. The latter investigators also showed binding to the non-acetylated ligand, galactose–BSA, perhaps influenced by the binding to BSA demonstrated in the present report, as discussed below. The determinants which allow for optimal binding between l-ficolin and its ligand still require clarification.
In a recent report, both GlcNAc and CysNAc were shown to co-crystalize with l-ficolin utilizing the same S2 binding domain [33]. This region contains Ser91, Asp109, Asp111, Arg122, Glu282, Lys284 and a residue from a neighbouring chain, Ser143. The authors state that the acetyl group of ligands tested crystallized in differing orientations, suggesting that the protein may react differently to a single binding site. We chose to purify l-ficolin with the sugar ligand, GlcNAc, conjugated to Sepharose. When normal human plasma was passed through GlcNAc–Sepharose, l-ficolin with associated MASP-2, C3 and α2M bound to the column even in the presence of HS-EDTA. Intact MASP-2 eluted upon exposure to LS-EDTA in pool 1 while l-ficolin remained bound, providing a simple, convenient method for the purification of this esterase from plasma in proenzyme form. MASP-2 was identified in pool 1 by C4-cleaving LP activity (data not shown), as well as by ELISA and Western blot assays, while MASP-1, MASP-3 and cleavage products of MASP-2 were not detected; however, trace amounts of C3, α2M and unidentified proteins were present as well. l-Ficolin complexed with activated MASP-2, C3 and α2M eluted upon addition of GlcNAc in HS-EDTA in pool 2.
The binding of l-ficolin to its ligand in the affinity step of this separation procedure carried out in EDTA is distinct from all affinity procedures for separation of MBL, which requires the presence of calcium for binding to its ligands [34]. The MASP-2 associated with l-ficolin bound to the column at high ionic strength was eluted as the ionic strength was lowered in native form, even as l-ficolin remained bound to the column.
This is reminiscent of the observations of Tan et al., who found that MASP-2, once activated, remained bound with MBL even in EDTA [34]. Pefabloc treatment to inactivate the MASP-2 associated with l-ficolin rendered this preparation suitable for investigation of the mechanism by which l-ficolin activates the lectin pathway. Perhaps a difference in binding orientation of the acetyl group, as seen by Garlatti et al., helps to explain the different elution of MASP-2 from l-ficolin on a GlcNAc column in the present study from that reported by Krarup et al. on a CysNAc column [15]. Regardless, we were unable to develop CysNAc as a ligand for l-ficolin in an ELISA, and looked for other alternatives.
Specific binding of l-ficolin to ligand-coated ELISA plates proved difficult to quantify when BSA was used either as a carrier protein or blocking agent, because of consistently high degrees of background binding. We therefore searched for both a ligand and blocking agent which did not involve BSA, and A-LDL and StartingBlock™, respectively, proved to be most suitable. A-LDL served as an ideal ligand, as it reacted well with l-ficolin while non-acetylated LDL did not, indicating that binding was specific for the acetylated molecule; was generally available commercially and is obtained by the manufacturer via ultracentrifugation in fresh preparations monthly; and retained binding activity for over 1 month at 4°C. Furthermore, binding to A-LDL was seen to be superior to that of either GlcNAc–BSA or BSA. Others also have reported l-ficolin binding in reaction systems free of BSA [9,14,18,29,35]. l-Ficolin bound to A-LDL in HS-EDTA in physiological or higher ionic strength, as had been reported by others using different ligands [15,29], and on microwell plates in an assay similar that to developed by Petersen et al. [31]. Activation did not occur when plasma depleted of l-ficolin was used, but was restored when l-ficolin was added back to the depleted plasma. Furthermore, binding was blocked by addition of GlcNAc. These findings indicate that A-LDL is an ideal ligand for investigation of the binding and activation of the lectin pathway mediated specifically via l-ficolin. As A-LDL is a synthetically occurring molecule, further work should be conducted to determine if l-ficolin binds oxidized-LDL, which is a well-characterized mediator of vascular inflammation.
This report provides a relatively simple method for obtaining proenzyme MASP-2 and l-ficolin from plasma, which utilizes differential calcium–independent interactions between MASP-2 and l-ficolin in EDTA at varying ionic strength, with binding of native MASP-2 observed when high ionic strength is high and elution when it is low. We introduce A-LDL as a suitable ligand for investigation of l-ficolin interactions, and importantly, perform all steps in the absence of BSA. We anticipate that these reagents will be useful in future investigations of the lectin pathway.
References
- 1.Soothill JF, Harvey BA. Defective opsonization. A common immunity deficiency. Arch Dis Child. 1976;51:91–9. doi: 10.1136/adc.51.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176:1497–502. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiel S, Vorup-Jensen T, Stover CM, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–10. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 4.Dahl MR, Thiel S, Matsushita M, et al. MASP-3 and its association with distinct complexes of the mannan-binding lectin complement activation pathway. Immunity. 2001;15:127–35. doi: 10.1016/s1074-7613(01)00161-3. [DOI] [PubMed] [Google Scholar]
- 5.Le Y, Tan SM, Lee SH, Kon OL, Lu J. Purification and binding properties of a human ficolin-like protein. J Immunol Methods. 1997;204:43–9. doi: 10.1016/s0022-1759(97)00029-x. [DOI] [PubMed] [Google Scholar]
- 6.Tsujimura M, Ishida C, Sagara Y, et al. Detection of serum thermolabile beta-2 macroglycoprotein (Hakata antigen) by enzyme-linked immunosorbent assay using polysaccharide produced by Aerococcus viridans. Clin Diagn Lab Immunol. 2001;8:454–9. doi: 10.1128/CDLI.8.2.454-459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu J, Teh C, Kishore U, Reid KB. Collectins and ficolins: sugar pattern recognition molecules of the mammalian innate immune system. Biochim Biophys Acta. 2002;1572:387–400. doi: 10.1016/s0304-4165(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 8.Ichijo H, Hellman U, Wernstedt C, et al. Molecular cloning and characterization of ficolin, a multimeric protein with fibrinogen- and collagen-like domains. J Biol Chem. 1993;268:14505–13. [PubMed] [Google Scholar]
- 9.Matsushita M, Endo Y, Taira S, et al. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J Biol Chem. 1996;271:2448–54. doi: 10.1074/jbc.271.5.2448. [DOI] [PubMed] [Google Scholar]
- 10.Le Y, Lee SH, Kon OL, Lu J. Human l-ficolin: plasma levels, sugar specificity, and assignment of its lectin activity to the fibrinogen-like (FBG) domain. FEBS Lett. 1998;425:367–70. doi: 10.1016/s0014-5793(98)00267-1. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Ip WE, Michelow IC, Ezekowitz RA. The mannose-binding lectin: a prototypic pattern recognition molecule. Curr Opin Immunol. 2006;18:16–23. doi: 10.1016/j.coi.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harumiya S, Omori A, Sugiura T, Fukumoto Y, Tachikawa H, Fujimoto D. EBP-37, a new elastin-binding protein in human plasma: structural similarity to ficolins, transforming growth factor-beta 1-binding proteins. J Biochem (Tokyo) 1995;117:1029–35. doi: 10.1093/oxfordjournals.jbchem.a124802. [DOI] [PubMed] [Google Scholar]
- 13.Edgar P, Stein P. Hormone binding site of corticosteroid binding globulin. Nat Struct Biol. 1995;2:196–7. doi: 10.1038/nsb0395-196. [DOI] [PubMed] [Google Scholar]
- 14.Ma YG, Cho MY, Zhao M, et al. Human mannose-binding lectin and l-ficolin function as specific pattern recognition proteins in the lectin activation pathway of complement. J Biol Chem. 2004;279:25307–12. doi: 10.1074/jbc.M400701200. [DOI] [PubMed] [Google Scholar]
- 15.Krarup A, Thiel S, Hansen A, Fujita T, Jensenius JC. l-Ficolin is a pattern recognition molecule specific for acetyl groups. J Biol Chem. 2004;279:47513–19. doi: 10.1074/jbc.M407161200. [DOI] [PubMed] [Google Scholar]
- 16.Matsushita M, Endo Y, Fujita T. Cutting edge: complement-activating complex of ficolin and mannose-binding lectin-associated serine protease. J Immunol. 2000;164:2281–4. doi: 10.4049/jimmunol.164.5.2281. [DOI] [PubMed] [Google Scholar]
- 17.Cseh S, Vera L, Matsushita M, Fujita T, Arlaud GJ, Thielens NM. Characterization of the interaction between l-ficolin/p35 and mannan-binding lectin-associated serine proteases-1 and -2. J Immunol. 2002;169:5735–43. doi: 10.4049/jimmunol.169.10.5735. [DOI] [PubMed] [Google Scholar]
- 18.Lynch NJ, Roscher S, Hartung T, et al. l-Ficolin specifically binds to lipoteichoic acid, a cell wall constituent of Gram-positive bacteria, and activates the lectin pathway of complement. J Immunol. 2004;172:1198–202. doi: 10.4049/jimmunol.172.2.1198. [DOI] [PubMed] [Google Scholar]
- 19.Gregory LA, Thielens NM, Matsushita M, et al. The X-ray structure of human mannan-binding lectin-associated protein 19 (MAp19) and its interaction site with mannan-binding lectin and l-ficolin. J Biol Chem. 2004;279:29391–7. doi: 10.1074/jbc.M402687200. [DOI] [PubMed] [Google Scholar]
- 20.Ohashi T, Erickson HP. Two oligomeric forms of plasma ficolin have differential lectin activity. J Biol Chem. 1997;272:14220–6. doi: 10.1074/jbc.272.22.14220. [DOI] [PubMed] [Google Scholar]
- 21.Brooks AS, Hammermueller J, DeLay JP, Hayes MA. Expression and secretion of ficolin beta by porcine neutrophils. Biochim Biophys Acta. 2003;1624:36–45. doi: 10.1016/j.bbagen.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Matsushita M, Fujita T. Ficolins and the lectin complement pathway. Immunol Rev. 2001;180:78–85. doi: 10.1034/j.1600-065x.2001.1800107.x. [DOI] [PubMed] [Google Scholar]
- 23.Vorup-Jensen T, Petersen SV, Hansen AG, et al. Distinct pathways of mannan-binding lectin (MBL)- and C1-complex autoactivation revealed by reconstitution of MBL with recombinant MBL-associated serine protease-2. J Immunol. 2000;165:2093–100. doi: 10.4049/jimmunol.165.4.2093. [DOI] [PubMed] [Google Scholar]
- 24.Rossi V, Cseh S, Bally I, Thielens NM, Jensenius JC, Arlaud GJ. Substrate specificities of recombinant mannan-binding lectin-associated serine proteases-1 and -2. J Biol Chem. 2001;276:40880–7. doi: 10.1074/jbc.M105934200. [DOI] [PubMed] [Google Scholar]
- 25.Hajela K, Kojima M, Ambrus G, et al. The biological functions of MBL-associated serine proteases (MASPs) Immunobiology. 2002;205:467–75. doi: 10.1078/0171-2985-00147. [DOI] [PubMed] [Google Scholar]
- 26.Kilpatrick DC, Fujita T, Matsushita M. P35, an opsonic lectin of the ficolin family, in human blood from neonates, normal adults, and recurrent miscarriage patients. Immunol Lett. 1999;67:109–12. doi: 10.1016/s0165-2478(98)00147-3. [DOI] [PubMed] [Google Scholar]
- 27.Moller-Kristensen M, Jensenius JC, Jensen L, et al. Levels of mannan-binding lectin-associated serine protease-2 in healthy individuals. J Immunol Methods. 2003;282:159–67. doi: 10.1016/j.jim.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Petersen SV, Thiel S, Jensenius JC. The mannan-binding lectin pathway of complement activation: biology and disease association. Mol Immunol. 2001;38:133–49. doi: 10.1016/s0161-5890(01)00038-4. [DOI] [PubMed] [Google Scholar]
- 29.Hummelshoj T, Thielens NM, Madsen HO, Arlaud GJ, Sim RB, Garred P. Molecular organization of human ficolin-2. Mol Immunol. 2007;44:401–11. doi: 10.1016/j.molimm.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Matsushita M, Kuraya M, Hamasaki N, Tsujimura M, Shiraki H, Fujita T. Activation of the lectin complement pathway by h-ficolin (Hakata antigen) J Immunol. 2002;168:3502–6. doi: 10.4049/jimmunol.168.7.3502. [DOI] [PubMed] [Google Scholar]
- 31.Petersen SV, Thiel S, Jensen L, Steffensen R, Jensenius JC. An assay for the mannan-binding lectin pathway of complement activation. J Immunol Methods. 2001;257:107–16. doi: 10.1016/s0022-1759(01)00453-7. [DOI] [PubMed] [Google Scholar]
- 32.Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–78. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 33.Garlatti V, Belloy N, Martin L, et al. Structural insights into the innate immune recognition specificities of l- and h-ficolins. EMBO J. 2007;26:623–33. doi: 10.1038/sj.emboj.7601500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan SM, Chung MC, Kon OL, Thiel S, Lee SH, Lu J. Improvements on the purification of mannan-binding lectin and demonstration of its Ca(2+)-independent association with a C1s-like serine protease. Biochem J. 1996;319:329–32. doi: 10.1042/bj3190329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aoyagi Y, Adderson EE, Min JG, et al. Role of l-ficolin/mannose-binding lectin-associated serine protease complexes in the opsonophagocytosis of type III group B streptococci. J Immunol. 2005;174:418–25. doi: 10.4049/jimmunol.174.1.418. [DOI] [PubMed] [Google Scholar]


