Abstract
A common feature underlying active states of inflammation is the migration of neutrophils (PMNs) from the circulation and across a number of tissue barriers in response to chemoattractant stimuli. Although our group has recently established a discreet role for the PMN chemoattractant, hepoxilin A3 (HXA3) in the process of PMN recruitment, very little is known regarding the interaction of HXA3 with PMNs. To characterize further the event of HXA3-induced PMN transepithelial migration, we sought to determine the adhesion molecules required for migration across different epithelial surfaces (T84 intestinal and A549 airway cells) relative to two well-studied PMN chemoattractants, formyl-methionyl-leucyl-phenylalanine (fMLP) and leukotriene B4 (LTB4). Our findings reveal that the adhesion interaction profile of PMN transepithelial migration in response to HXA3 differs from the adhesion interaction profile exhibited by the structurally related eicosanoid LTB4. Furthermore, unique to PMN transepithelial migration induced by gradients of HXA3 was the critical dependency of all four major surface adhesion molecules examined (i.e. CD18, CD47, CD44 and CD55). Our results suggest that the particular chemoattractant gradient imposed, as well as the type of epithelial cell monolayer, each plays a role in determining the adhesion molecules involved in transepithelial migration. Given the complexities of these interactions, our findings are important to consider with respect to adhesion molecules that may be targeted for potential drug development.
Keywords: adhesion molecules, hepoxilin A3, neutrophils, Pseudomonas aeruginosa, Salmonella typhimurium
Introduction
A consequence of the innate inflammatory response at mucosal surfaces is the infiltration of neutrophils (PMNs) from the bloodstream to the epithelium, which separates the lumen from the underlying tissue [1–3]. PMNs travel across this epithelial barrier into the lumen in order to confront colonizing pathogenic bacteria [1–3]. The mechanisms underlying the movement of PMNs from the bloodstream to sites of infection are complex and involve numerous adhesion molecules, cytokines and chemoattractants that function to direct PMNs through endothelial barriers, basements membranes, extracellular matrix and epithelial barriers [2,3]. Our group has recently established a discreet role for the PMN chemoattractant, hepoxilin A3 (HXA3) in the process of PMN recruitment [4,5].
HXA3 is an eicosanoid derived from the 12-lipoxygenase pathway, which is produced by epithelial cells and secreted from the apical surface resulting in the guidance of PMNs across the epithelial monolayer from the basolateral to the apical side [4,5]. Infection of epithelial cells with pathogenic bacteria stimulates HXA3 production and secretion and subsequent PMN transmigration, a phenomenon that has been observed in both lung and intestinal epithelial cells [4,5]. This is supported in vivo by an inhibitor of 12-lipoxygenase, the enzyme required for HXA3 synthesis, resulting in a dramatic reduction in PMN infiltration in Salmonella enterica serovar Typhimurium (S. typhimurium)-infected human intestinal xenograft tissue [4].
The adhesion molecules involved in the migration of PMNs across epithelial barriers has been investigated using primarily N-formyl-methionyl-leucyl-phenylalanine (fMLP) as a chemoattractant [6,7]. Currently, very little is known regarding the interaction between HXA3 and PMNs. Unlike other PMN chemoattractants, including fMLP and leukotriene B4 (LTB4), HXA3 acts as a pure PMN chemoattractant as it does not activate PMNs to release reactive oxygen intermediates or cause the release of secondary granule content [4,8]. Many PMN chemoattractants have also demonstrated the capability to modulate the expression of certain adhesion molecules [9]. For example, fMLP has been shown to cause an increase in expression of CD18/CD11b on the surface of PMNs [9]. These studies have revealed that not only does CD18/CD11b play a critical role in mediating PMN transmigration [6,7] but CD44, CD47 and CD55 also play defined roles [10–13]. More recently, Blake et al. demonstrated that, although PMN migration across T84 monolayers in response to an imposed gradient of fMLP is dependent upon CD18/CD11b, PMNs are capable of migrating across T84 monolayers in a CD18-independent manner in response to gradients of alternative chemoattractants such as interleukin (IL)-8, complement component (C5a) and LTB4[14]. This intriguing observation implies a versatility by which PMNs utilize distinct adhesion molecules depending upon the chemoattractant gradient applied during transepithelial migration. Modulation of PMN surface adhesion molecules in response to HXA3 has yet to be explored.
In this study, we have characterized further aspects of HXA3-mediated PMN migration across epithelial surfaces in comparison with other known PMN chemoattractants, including fMLP and LTB4. Our studies suggest that both the type of epithelial cell monolayer and the particular chemoattractant gradient imposed each play a role in dictating the adhesion molecules involved in transepithelial PMN migration. Interestingly, the adhesion interaction profile of PMN transepithelial migration in response to HXA3 differs from the profile exhibited by the structurally related eicosanoid LTB4.
Materials and methods
Epithelial cell culture
The A549 cell line was derived originally through explant culture of lung carcinomatous tissue from a 58-year-old Caucasian male. These cells display properties of type II alveolar epithelial cells and were maintained in Ham's F-12K medium with 2 mM l-glutamine 1·5 g/l NaHCO3, 10% fetal bovine serum and 100 U of Penn/Strep [5]. T84 intestinal epithelial cells (passages 46–66) were grown in a 1 : 1 mixture of Dulbecco–Vogt modified Eagle's medium and Ham's F-12 nutrient mixture supplemented with 15 mM HEPES (pH 7·5), 14 mM NaHCO3, 40 μg/ml penicillin, 8 μg/ml streptomycin, 8 μg/ml ampicillin and 6% fetal bovine serum [15]. Polarized monolayers of A549 and T84 cells were grown on the underside of 0·33 cm2 collagen-coated polycarbonate Transwell filters (5 μm pore size) to study PMN migration in the physiological basolateral to apical direction, as described previously [4,5,15]. A549 cells form barriers when grown on permeable filters, but do not develop transepithelial electrical resistance (TEER) [5]. TEER of approximately 800–1500 Ù cm2 was reached in all T84 monolayers used in this study.
Measurement of [3H]-inulin flux
Monolayers of A549 and T84 were grown on Transwells for 4–18 days and were washed twice in Hanks's balanced salt solution (HBSS) before the addition of 100 μl of 2·5 μCi/ml [3H]-inulin (PerkinElmer, Boston, MA, USA) in HBSS to the top well (basolateral side) and 600 μl of HBSS to the bottom well (apical side). After 3 h, 10 μl was sampled from the apical chamber and added to 3 ml scintillation fluid and counts per minute were measured using a Beckman Coulter scintillation counter. Data are presented in triplicate as a percentage of the amount of counts added to the top chamber [16]. For measurement of complete equilibration of radioactivity in the absence of a barrier, collagen-coated Transwells without cells were used.
Isolation of human PMN for transmigration assays
Human peripheral PMN were isolated from anti-coagulated 10% acid citrate dextrose (pH 4) and human whole blood collected by venipuncture from normal donors of both sexes [15]. The buffy coat was separated by centrifugation at 600 g at room temperature (RT). The plasma and mononuclear cells were removed by aspiration, and the majority of erythrocytes were removed by a 2% gelatin sedimentation technique. Residual erythrocytes were lysed in cold NH4Cl lysis buffer. This technique allows for the rapid isolation of functionally active PMN (> 95% as detected by trypan blue dye exclusion) at greater than 90% purity [4,5,15]. The PMN were resuspended in HBSS (without Ca2+ and Mg2+ and supplemented with 10 mM HEPES, pH 7·4; Sigma Chemical Co., St Louis, MO, USA) (HBSS–) at a concentration of 5 × 107/ml.
PMN transmigration assay
The PMN transmigration assay using inverted cell culture monolayers of polarized cells has been described [4,5,15]. For monolayers infected with bacteria, the apical surface of A549 monolayers was exposed for 1 h to 1·5 × 106 Pseudomonas aeruginosa (PA01)/monolayer [5]. Alternatively, the apical surface of T84 monolayers was exposed to 2 × 108S. typhimurium for 1 h [4,15]. After infection, monolayers were washed three times and PMNs (1 × 106) were applied to the basolateral chamber. PMNs were allowed to migrate to the apical side for 2 h at 37°C. For the analysis of individual chemoattractants, various doses of HXA3, LTB4 and fMLP were placed into the apical chamber of uninfected epithelial monolayers while PMNs were placed into the basolateral chamber. PMNs were quantified by the myeloperoxidase assay [15]. Data are displayed as a representative experiment that was performed at least three times with the mean ± standard deviation (s.d.) of at least three independent monolayers/condition calculated for each experiment. HXA3 was obtained from Biomol (Plymouth Meeting, PA, USA) at a concentration of 50 μg/ml in ethanol. For experiments with T84 monolayers where high concentrations of HXA3 are needed to observe PMN transmigration, ethanol was evaporated to 5–10 μl in siliconized tubes using a Speed Vac and resuspended in HBSS. LTB4 and fMLP were both obtained from Sigma-Aldrich (St Louis, MO, USA).
Monoclonal antibodies
To study the role of PMN surface receptors on transmigration, the following blocking antibodies were used: anti-CD18 (clone MHM23, 0·5 mg/ml; Dako Cytomation, Carpinteria, CA, USA), anti-CD44 (clone IM7, 1 mg/ml; BD Biosciences, San Jose, CA, USA), anti-CD47 (clone C5D5, 0·95 mg/ml; a gift from C. Parkos, Emory University, GA, USA) and anti-CD55 (clone BRIC 216, 1 mg/ml; Serotec, Raleigh, NC, USA). Irrelevant isotype-matched antibodies (IgG1 and IgG2b), which cover the isoptype specificity of the blocking antibodies used in this study, were purchased from BD Biosciences. The IgG1 isotype served as a control for antibodies against CD18, CD55, and CD47, while the IgG2b isotype served as a control for the antibody against CD44. For every 1 × 106 PMNs, 4 μl of antibody solution was added and the resultant mixture was incubated in siliconized tubes for 20 min at room temperature. At the end of the incubation period, the cell–antibody mixtures were added directly to the basolateral chamber for migration.
Fluorescence activated cell sorter (FACS) analysis
Whole blood was drawn from healthy human volunteers using heparin vacutainers (BD Biosciences) and used immediately. The various chemoattractants fMLP, LTB4, HXA3, and ethanol (negative control) were diluted with 250 μl of HBSS (with Ca2+) to the appropriate concentrations, then mixed with 250 μl of whole blood. The blood–chemoattractant solutions were incubated in a 37°C water bath for 30 min. The stimulated blood cells were then fixed with equal volumes (500 μl) of 2% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) in phosphate-buffered saline (without Ca2+) for at least 15 min on ice. The majority of red blood cells were removed by lysis using cold NH4Cl lysis buffer for 10 min on ice, then centrifuged to remove supernatant and resuspended in 1 ml HBSS. Various cell surface receptors (CD18, CD44, CD47, CD55 and isotype control) were labelled by the addition of 10 μl of fluorescein isothiocyanate-labelled anti-human antibodies (BD Biosciences) to each 200 μl of cell suspension and incubated at 4°C for 15 min. The cell suspensions were then analysed with a FACScan flow cytometer (Becton Dickinson).
FACS data analyses were performed using WinMDI software (Scripps Institute, La Jolla, CA, USA; http://facs.scripps.edu). Whole blood was used in order to minimize activation of PMNs and gating was used to single out the PMN populations. PMN populations were identified based on front- and side-scatter clustering and the corresponding mean fluorescent intensities (MFI) were measured. Percentage change in cell surface receptor expression was obtained by comparing the stimulated population MFI against the ethanol negative control.
Results
It has been shown previously that T84 cells form polarized barriers with high levels of transepithelial electrical resistance (TEER) when grown on Transwells [4,6,15]. These monolayers are extremely selective to the passage of small molecules such as proteins, dextrans and radioisotope tracers [17]. Conversely, A549 cells do not form measurable TEER, but do form barriers that are selective to flux of proteins such as horseradish peroxidase (HRP) [5]. Before initiating chemoattractant gradient experiments, we investigated the relative barrier function of T84 cells compared to A549 cells by measuring the percentage of [3H]-inulin flux across the monolayers over a 3-h period. As shown in Fig. 1, monolayers were tested for the ability to restrict the movement of [3H]-inulin added basolaterally. The percentage of [3H]-inulin flux that occurs across the filter alone represents complete equilibration of [3H]-inulin added basolaterally with day-to-day experimental variability. By day 6, both A549 cells and T84 cells form stable barriers. T84 monolayers are highly restrictive to [3H]-inulin flux and significantly more effective than A549 cell monolayers at maintaining a strict barrier (Fig. 1). However, A549 cell monolayers are capable of restricting free equilibration of [3H]-inulin, as significantly less [3H]-inulin crosses to the apical well when an A549 cell barrier is present compared with no barrier (filter alone). Both T84 and A549 monolayers maintained barrier function for as long as 18 days post-seeding (Fig. 1).
Fig. 1.
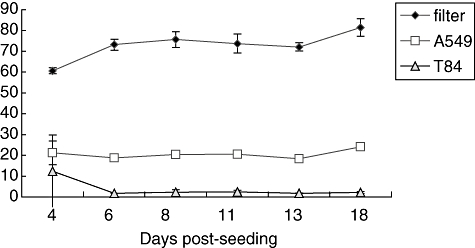
Percentage [3H]-inulin flux across Transwell filters. Transwell filters alone (black diamonds) or epithelial cells grown on Transwell filters for various days, A549 (white squares) or T84 (grey triangles) were washed with Hanks's balanced salt solution (HBSS) and 100 μl (2·5 μC/ml) in HBSS was added to the basolateral surface on the day depicted (days 4–18). After 3 h, radioactivity was sampled from the apical chamber. Data are presented as percentage [3H]-inulin flux by calculating the number of counts per minute (cpm) divided by the total number of cpm added, taking into account the different volumes in the apical (600 μl) and basolateral (100 μl) chambers. Based on the respective volumes of each chamber, 85·7% represent complete equilibration of percentage [3H]-inulin. Each data point represents three independent Transwell filters and is depicted as mean ± standard deviation.
Having established the relative barrier properties between T84 intestinal epithelial monolayers and A549 lung epithelial monolayers, we next sought to determine the concentration gradient of HXA3 required to stimulate PMN transmigration across T84 and A549 monolayers, respectively. For comparison, we also examined the concentration gradient leading to PMN transmigration of the well-studied PMN chemoattractant LTB4. LTB4, like HXA3, is an eicosanoid produced from an arachidonic acid precursor; however, LTB4 is produced through the 5-lipoxygenase enzymatic pathway as opposed to HXA3, which is produced via the 12-lipoxygenase pathway, giving each molecule structural distinctions [18,19]. Moreover, while HXA3 can be produced from epithelial cells in response to bacterial stimuli [4,5], LTB4 is produced primarily from leucocytes in response to inflammatory mediators [20], suggesting functional distinctions in vivo as well.
Various concentrations of HXA3 were applied to the apical chamber of A549 monolayers and the number of PMNs migrating from the basolateral to the apical side were measured. A significant increase in the number of migrated PMNs began to occur at approximately 50 ng/ml, which is consistent with our previous findings (Fig. 2a) [5]. As concentrations of HXA3 added to the apical chamber increase, we observed a dose-dependent increase in PMN transmigration. We observed the same trend in PMN migration across T84 monolayers, although significant PMN transmigration did not occur until at least 200 ng/ml was present (Fig. 2a). This is consistent with the observation that T84 monolayers form a more selective barrier than A549 monolayers.
Fig. 2.
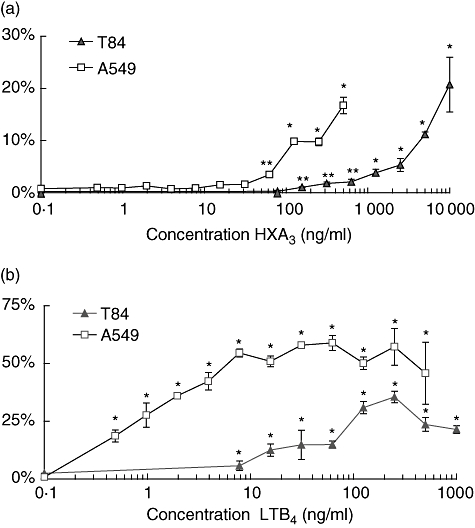
Dose curve for chemoattraction of neutrophils (PMNs) by eicosanoids. (a) Various doses of hepoxilin A3 are added to the apical chamber at the same time that 1 × 106 PMNs are added to the basolateral chamber. (b) Various doses of leukotriene B4 are added to the apical chamber at the same time that 1 × 106 PMNs are added to the basolateral chamber. PMN migration is represented as the percentage of PMNs that migrate fully across epithelial monolayers (A549: white squares or T84: black triangles) of the total number of PMNs added. Experiments were performed multiple times with a representative experiment depicted herein. Each data point represents three independent Transwell filters and is depicted as mean ± standard deviation. Significant difference between groups (compared to the 0·1 ng/ml concentration) was assessed using Student's t-test. *P-values < 0·01; **P-values < 0·05.
PMN transmigration in response to LTB4 occurs at significantly lower concentrations when compared with HXA3 (Fig. 2b). PMNs migrated across A549 cells to a significant extent in response to less than 1 ng/ml of LTB4 added to the apical chamber (Fig. 2b). Migration occurred in a dose-dependent fashion until approximately 10 ng/ml, in which a plateau of transmigration was achieved and persisted up to 1 μg/ml LTB4. Again, a similar trend was observed in T84 cells, except that significant transmigration did not occur until at least 10 ng/ml LTB4 was added. Also, the overall number of PMNs that migrated across A549 monolayers was much higher than across T84 cells at all concentrations of LTB4 tested.
Next we sought to determine the contribution of adhesion molecules in the process of PMN migration across T84 monolayers and A549 monolayers in response to various chemoattractants. We have shown previously that S. typhimurium induces PMN transmigration across T84 cells by stimulating the production of HXA3[4]. Monoclonal antibodies are capable of blocking PMN cell surface molecules; CD18, CD44, CD47 and CD55 all exerted some degree of inhibition of S. typhimurium-induced PMN transmigration when compared with isotype controls (Fig. 3). Blocking antibodies to CD18 and CD55 potently blocked fMLP-induced PMN migration across T84 cells and, consistent with previous findings [7,10,11], antibodies to CD47 blocked PMN transmigration at least by 50%. However, antibodies to CD44 did not have any affect on fMLP-induced PMN transmigration in our studies. Migration of PMNs across T84 monolayers in response to an LTB4 gradient demonstrated an altogether distinct pattern from S. typhimurium and fMLP-induced PMN transmigration (Fig. 3). Anti-CD18 monoclonal antibodies had no effect on PMN transmigration, anti-CD44 and anti-CD47 exerted a slight effect and anti-CD55 resulted in almost complete inhibition of LTB4-induced PMN transmigration. Taken together, these results further support the hypothesis that adhesion molecules utilized by PMNs to migrate across epithelial cells differ depending upon the chemoattractant involved.
Fig. 3.
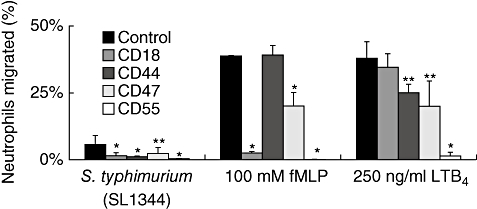
Effects of interference with blocking antibodies of adhesive surface molecules on neutrophil (PMN) transepithelial migration. Antibodies were mixed with PMNs before addition to the basolateral well of T84 cells. Leukotriene B4 or formyl-methionyl-leucyl-phenylalanine were added to the apical chamber at the same time that PMNs with antibodies (blocking and isotyped-matched controls) were added to the basolateral chamber. For epithelial cells treated with pathogens, T84 were preinfected with wild-type Salmonella typhimurium (SL1344) for 1 h before addition of PMNs. Experiments were performed multiple times with a representative experiment depicted herein. Each data point represents three independent Transwell filters and is depicted as mean ± standard deviation. Significant difference between groups was assessed using Student's t-test. *P-values < 0·01; **P-values < 0·05.
Next we examined PMN transmigration using monolayers of the lung epithelial cell A549. We have shown previously that P. aeruginosa causes PMN migration across A549 monolayers through the production and secretion of HXA3 in a mechanism that shares similarities with Salmonella-induced PMN migration across T84 monolayers [5]. Consistent with our establishment of HXA3 as the chemoattractant underlying Pseudomonas-induced PMN transmigration, we observed an identical pattern of antibody inhibition in Pseudomonas-infected monolayers and uninfected monolayers receiving a gradient of HXA3 (Fig. 4). Both cases resulted in significantly reduced migration when blocking antibodies to CD18, CD44, CD47 and CD55 were compared to the addition of isotype control. All four blocking antibodies also significantly inhibited transmigration in response to fMLP, when PMNs were preincubated with each before addition to the basolateral chamber of A549 monolayers. When a gradient of LTB4 was established across A549 cells, anti-CD44, anti-CD47 and anti-CD55 significantly blocked PMN transmigration, but anti-CD18 did not appear to interfere with transmigration. Data obtained from various chemoattractants inducing PMN migration across A549 monolayers supports the notion that PMN cell surface molecules responsible for transmigration vary depending upon the chemoattractant applied. PMN migration across airway epithelial monolayers was more dependent upon CD44 and CD47 in comparison to intestinal epithelial monolayers when responding to imposed gradients of fMLP and LTB4. This suggests that the type of epithelial cell monolayer also plays an important role in determining which cell surface molecules operate during transmigration.
Fig. 4.
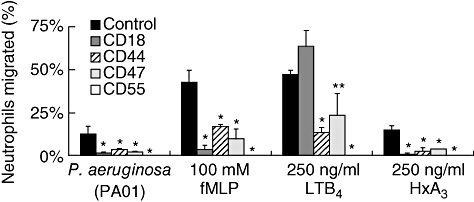
Effects of interference with blocking antibodies of adhesive surface molecules on neutrophil (PMN) transepithelial migration. Antibodies were mixed with PMNs before addition to the basolateral well of A549 cells. Leukotriene B4, formyl-methionyl-leucyl-phenylalanine or hepoxilin A3 were added to the apical chamber at the same time that PMNs with antibodies (blocking and isotype-matched controls) were added to the basolateral chamber. For epithelial cells treated with pathogens, A549 cells were preinfected with Pseudomonas aeruginosa (PAO1) for 1 h before addition of PMNs. Experiments were performed multiple times with a representative experiment depicted herein. Each data point represents three independent Transwell filters and is depicted as mean ± standard deviation. Experiments were performed multiple times with a representative experiment depicted herein. Significant difference between groups was assessed using Student's t-test. *P-values < 0·01; **P-values < 0·05.
Because the anti-CD55 blocked PMN transepithelial migration to every stimulus examined across both epithelial cell lines, we next sought to determine whether PMN treatment with anti-CD55 simply immobilizes PMNs, irrespective of the effects on interactions with epithelial cells. This was an important consideration, as PMNs express CD55 and there is some suggestion that this receptor may be important in allowing PMN to survive and function at inflammatory sites where there is a rapid complement turnover [21]. Therefore, we investigated the extent to which the CD55 antibodies were capable of blocking migration of PMN across acellular (bare) collagen-coated polycarbonate Transwell filters in response co-imposed gradients of fMLP, HXA3 and LTB4. A dose–response was first performed in order to establish the optimal concentration of each chemoattractant needed to induce PMN migration across acellular monolayers (data not shown). After establishing these effective chemoattractant concentrations [fMLP (1 μM); HXA3 (5 ng/ml); LTB4 (25 ng/ml)] we next determined whether anti-CD55 prevented PMNs from migrating across acellular filters in response to various stimuli. As shown in Fig. 5, PMN migration across acellular filters in response to HXA3 and LTB4 is not affected by the addition of anti-CD55 antibodies, demonstrating that anti-CD55 treatment does not simply obstruct the movement of PMN. This is not the case with fMLP-induced migration across acellular filters, which is blocked completely by anti-CD55 antibodies. This result suggests that the anti-CD55 blocking effect of PMN transepithelial migration in response to fMLP is acting in a manner independent of PMN epithelial contacts.
Fig. 5.
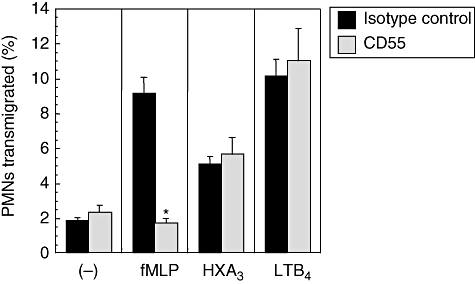
Relative contribution of neutrophil (PMN) CD55 to PMN migration. Collagen-coated permeable supports preincubated in media overnight were used in transmigration assays using the same protocol as described above for Figs 3 and 4. Migration is shown in the absence of any chemotactic stimuli ((–)) or the presence of a formyl-methionyl-leucyl-phenylalanine (1 μM), hepoxilin A3 (5 ng/ml) or leukotriene B4 (25 ng/ml) Transwell gradient. Transmigration in the presence of an isotype-matched control antibody (IgG1) is compared to that in the presence of the CD55 antibody. Bars represent the mean ± standard deviation of three monolayers for each condition. One of three experiments performed showing the same result. Significant difference between groups was assessed using Student's t-test. *P-values < 0·01.
Chemoattractants have been shown to stimulate increased surface expression of adhesion molecules on PMNs [9]. Previous studies have demonstrated that fMLP can up-regulate CD18 on freshly isolated PMNs [9]. Using whole blood from healthy volunteers (see Materials and methods) we confirm this observation, but fail to see any increase in surface expression of CD44, CD47 and CD55 (Fig. 6). In fact, there appears to be a slight decrease in expression of these surface molecules upon fMLP stimulation. LTB4 also causes an increase in CD18 expression, without affecting expression of any of the other surface molecules tested (Fig. 6). In contrast, we did not observe any change in expression of any of the surface adhesion molecules CD18, CD44, CD47 and CD55 when PMNs were exposed to HXA3. This result is consistent with the notion that HXA3, unlike other PMN chemoattractants, does not activate various PMN responses despite having the ability to serve as a PMN chemoattractant [4,8].
Fig. 6.
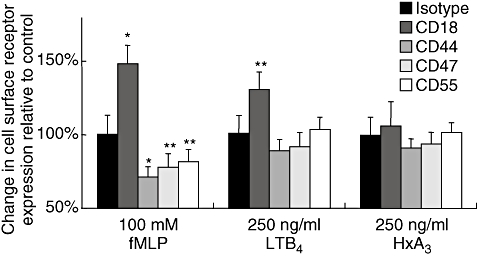
Neutrophils (PMN) expression of various surface adhesion molecules in response to treatment with chemoattractants. Whole blood was mixed for 30 min with leukotriene B4, formyl-methionyl-leucyl-phenylalanine, hepoxilin A3 or vehicle control (ethanol), followed by lysis of red blood cells. Blood preparations were then incubated with antibodies to surface adhesion molecules (anti-CD18, anti-CD44, anti-CD47, anti-CD55 and isotype control) and incubated subsequently with fluorescein isothiocyanate-labelled secondary antibodies for detection and quantification by fluorescence activated cell sorter analysis with gating on the PMN subpopulation. Irrelevant isoptype-matched antibodies were used as controls. The IgG1 isotype served as a control for antibodies against CD18, CD55 and Cd47, while the IgG2b isotype served as a control for the antibody against CD44. Values are reported as percentage of results obtained from vehicle control-treated whole blood in combination with each antibody. Experiments were performed multiple times with a representative experiment depicted herein. Each data point represents three independent samples per condition and displayed as mean ± standard deviation (s.d.). The standard deviation of the three values is determined before setting the average value at 100%. The percentage s.d. was used subsequently to calculate the s.d. of the 100%. Significant difference between groups was assessed using Student's t-test. *P-values < 0·01; **P-values < 0·05.
Discussion
The primary objective of PMNs is to phagocytose and destroy pathogenic microorganisms, and thus represent an important effector of the acute inflammatory response [1]. Therefore, PMNs must be able to migrate from out of the blood circulation and across a number of tissue barriers in response to mucosal stimuli. This is the case in many of the major organ systems where mucosal surface infections are common, including the respiratory, urinary and gastrointestinal tracts. We have demonstrated recently that the eicosanoid HXA3 is secreted from the apical surface of the epithelial barrier and establishes a chemotactic gradient required for guidance of PMNs across both model intestinal and airway epithelium, representing the final step in PMN recruitment to the mucosal lumen [4,5]. A complex series of adhesive and de-adhesive events can be envisaged to allow successful migration of PMNs through the paracellular spaces of the epithelia, along the basolateral surface of epithelial cells, before reaching the apical milieu [11]. As a critical role for HXA3 in PMN-mediated events has been identified for both intestinal and pulmonary inflammation [4,5], in the current study we sought to characterize the adhesion molecules fundamental to HXA3-mediated PMN transepithelial migration.
The present understanding of PMN–epithelial interactions during transmigration is based largely on studies using imposed gradients to fMLP and involves several integrated steps [11]. As the initial step at the subepithelium, PMNs adhere to the basolateral surface of epithelial cells via the PMN adhesion molecule CD11b/CD18, where it interacts with epithelial counter receptors that probably includes multiple fucosylated glycoproteins (and perhaps other undefined ligands) [6,7]. Next, during the migration step, interactions between epithelial CD47 and PMN signal regulatory protein alpha (SIRPα) appear to enhance the rate of PMN transepithelial migration [10]. Adhesive interactions of PMN with epithelial intercellular tight junction proteins (i.e. JAM-C) are also likely to occur [7]. Lastly, after migration across the apical junctional complex, PMNs reach the lumen and contact the apical epithelial membrane. At this surface, apically expressed intercellular adhesion molecule 1 (ICAM-1) is an accessible ligand for CD11b/CD18 and is thought to serve as a foothold for PMNs on the lumenal surface [22]. These elegant studies initially identified CD11b/CD18 as the principal adhesion molecule because all migration to fMLP across model intestinal epithelia can be blocked with anti-CD11b or anti-CD-18 (β2 integrin) antibodies. However, recent lines of evidence indicate that PMNs may use CD18-independent mechanisms to cross the intestinal epithelium depending upon the chemoattractant encountered [14]. For example, whereas essentially all migration to fMLP across model intestinal epithelia was prevented by anti-CD18 antibody, significant migration to C5a, IL-8 and LTB4 persisted despite the presence of anti-CD18 antibody, indicating that PMNs are capable of CD18-independent PMN migration [14].
Such studies have established the emerging concept that PMNs utilize distinct adhesion molecules during transepithelial migration, depending upon the chemoattractant gradient imposed. Our data substantiate this concept as we found that the adhesion interaction profile of PMN transepithelial migration in response to HXA3 differs from the adhesion interaction profile exhibited by the structurally related eicosanoid LTB4. This finding underscores the fact that although HXA3 and LTB4 may be structurally similar and are both potent PMN chemoattractants, they each most probably serve fundamentally different roles in the PMN recruitment process. As PMNs must follow both endogenous and bacterial chemoattractant signals to arrive at the site of infection, PMNs must undergo a decision-making process requiring both end-target and intermediary endogenous chemoattractants [23]. In this regard, HXA3 would be considered an end-target chemoattractant, as it emanates from the site of infection to establish a chemotactic gradient that guides PMNs across mucosal surfaces characterizing the final step in PMN recruitment to the mucosal lumen. On the other hand, LTB4 would be considered an intermediary endogenous chemoattractant, as this PMN chemoattractant is encountered en route to sites of infection. For example, LTB4 is produced from leucocytes and macrophages, as well as mast cells [24], where it induces the adhesion and activation of leucocytes on the endothelium, allowing them to bind to and cross into the tissue [20]. Based upon this model, end-target chemoattractants not only dominate over intermediary chemoattractants but are also predominately CD11b/CD18-dependent [23]. This would be consistent with our findings as HXA3, but not LTB4, was found to be CD18-dependent. It has also been determined that end-target and intermediary PMN chemoattractants induce different intracellular signalling pathways in PMNs [23], which we hypothesize may be linked to the differences noted in the adhesion interactive profiles occurring in response to each distinct chemoattractant [4,15]. In fact, HXA3 activates PMN intracellular Ca2+ release but fails to cause the release of reactive oxygen intermediates and also fails to cause PMNs to discharge their primary and secondary granule content [4,15]. Furthermore, in this study we have shown that HXA3 treatment of PMNs does not lead to an increase in surface expression of CD18 (Fig. 6). Thus, unlike LTB4, which activates PMNs to release granule content and to up-regulate CD18 expression [8,15], HXA3 is considered a ‘pure chemoattractant’. Evaluation of the roles of both HXA3 and LTB4 in a more complex in vivo model will be necessary to understand fully the discreet roles of each of these eicosanoids in PMN recruitment. Nevertheless, our present study provides further evidence that distinct PMN chemoattractants promote PMN migration through epithelial tissues by eliciting different adhesion mechanisms.
While PMN migration across intestinal epithelial cell monolayers has served traditionally as the model for establishing the current paradigm for the study of PMN–epithelial interactions, another important implication of our findings is that both the type of epithelial cell monolayer, in addition to the particular chemoattractant gradient imposed, also plays an important role in the adhesion molecules involved in transepithelial migration. Interestingly, we found that PMN transepithelial migration across airway epithelial monolayers was more dependent upon CD44 and CD47 in response to imposed gradients of fMLP and LTB4. A study investigating monocyte migration through the alveolar epithelial barrier found that monocyte migration across A549 monolayers depended largely upon CD11b/CD18 and CD47, and also required the additional engagement of β1 integrins for optimal migration [25]. Further studies will be necessary to clarify the function of both CD47 and CD44 in the interaction of airway epithelial cells and PMNs in a more detailed fashion. Although we did not investigate ICAM−1 interactions with PMN, it has been shown that ICAM-1 is expressed on type I and type II pneumocytes and is up-regulated in the presence of proinflammatory cytokines [26,27]. Characterization of ICAM-1 involvement in PMN migration across the airway epithelium in response to HXA3 will be an important future extension of this work.
We have shown previously that HXA3 is not only secreted from the apical surface of human intestinal or lung epithelial cells stimulated with pathogenic S. typhimurium or P. aeruginosa, respectively, but HXA3 is also both necessary and sufficient for promotion of bacterial-induced PMN transepithelial migration [4,5]. Consistent with these observations is our finding that the adhesion molecules involved during PMN transepithelial migration to exogenously added HXA3 were identical to the profile of adhesive interactions characteristic of bacterial-induced PMN transepithelial migration. Unique to PMN transepithelial migration induced by gradients of HXA3, in particular, was the involvement of all four major surface adhesion molecules examined (i.e. CD18, CD47, CD44 and CD55) in both airway and intestinal cell monolayers. However, the specific roles of each adhesion molecule during HXA3-induced PMN transepithelial migration has yet to be explored.
Anti-CD55 antibodies blocked PMN transepithelial migration induced by all stimuli examined in this study. CD55 (decay accelerating factor) has been shown to function as a key anti-adhesive surface glycoprotein that regulates the rate of PMN migration across the apical epithelial membrane by promoting the release of PMN from the apical surface of the epithelium after successful breach of cell-to-cell junctions [12]. We observed that PMN migration across collagen-coated acellular Transwell filters in response to gradients of HXA3 and LTB4 was unaffected by anti-CD55 antibody treatment. This finding is consistent with a role for CD55 in PMN epithelial interactions. Interestingly, anti-CD55 antibody treatment did, however, interfere with fMLP-induced PMN migration across acellular filters, suggesting a differential role for CD55 during PMN transmigration in response to fMLP versus the eicosanoids HXA3 and LTB4.
In conclusion, the findings reported here support the hypothesis that adhesion molecules utilized by PMNs to migrate across epithelial cell monolayers are different depending upon the chemoattractant and the type of epithelial monolayer involved. Moreover, our study provides new information regarding the adhesion molecules fundamental to HXA3-mediated PMN transepithelial migration. A key observation was that the adhesion interaction profile of PMN transepithelial migration in response to HXA3 differs from the profile exhibited by the structurally related eicosanoid LTB4. Thus, as mucosal surfaces lined by epithelial cells (i.e airway and intestine) offer a potential direct target for therapy, given that these surfaces are directly available for non-invasive administration of therapeutic compounds, our findings will be important to consider when determining specific adhesion molecules to exploit for anti-inflammatory drug development.
Acknowledgments
We would like to thank Dr Karen Mumy for critical reading of the manuscript and Jeffrey Bien and Michael Pazos for expert technical assistance. We also thank Dr Charles A. Parkos, Emory University, GA, for generously providing the CD47 monoclonal antibodies. The research was supported by grants from the Croucher Foundation to A. S. and The National Institutes of Health (DK 56754 and DK 33506) to B. A. M.
References
- 1.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–86. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 2.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83:309–36. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 3.Chin AC, Parkos CA. Neutrophil transepithelial migration and epithelial barrier function in IBD: potential targets for inhibiting neutrophil trafficking. Ann NY Acad Sci. 2006;1072:276–87. doi: 10.1196/annals.1326.018. [DOI] [PubMed] [Google Scholar]
- 4.Mrsny RJ, Gewirtz AT, Siccardi D, et al. Identification of hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proc Natl Acad Sci USA. 2004;101:7421–6. doi: 10.1073/pnas.0400832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurley BP, Siccardi D, Mrsny RJ, McCormick BA. Polymorphonuclear cell transmigration induced by Pseudomonas aeruginosa requires the eicosanoid hepoxilin A3. J Immunol. 2004;173:5712–20. doi: 10.4049/jimmunol.173.9.5712. [DOI] [PubMed] [Google Scholar]
- 6.Zen K, Liu Y, Cairo D, Parkos CA. CD11b/CD18-dependent interactions of neutrophils with intestinal epithelium are mediated by fucosylated proteoglycans. J Immunol. 2002;169:5270–8. doi: 10.4049/jimmunol.169.9.5270. [DOI] [PubMed] [Google Scholar]
- 7.Zen K, Parkos CA. Leukocyte–epithelial interactions. Curr Opin Cell Biol. 2003;15:557–64. doi: 10.1016/s0955-0674(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland M, Schewe T, Nigam S. Biological actions of the free acid of hepoxilin A3 on human neutrophils. Biochem Pharmacol. 2000;59:435–40. doi: 10.1016/s0006-2952(99)00345-7. [DOI] [PubMed] [Google Scholar]
- 9.Vedder NB, Harlan JM. Increased surface expression of CD11b/CD18 (Mac-1) is not required for stimulated neutrophil adherence to cultured endothelium. J Clin Invest. 1988;81:676–82. doi: 10.1172/JCI113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Buhring HJ, Zen K, et al. Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. J Biol Chem. 2002;277:10028–36. doi: 10.1074/jbc.M109720200. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Shaw SK, Ma S, Yang L, Luscinskas FW, Parkos CA. Regulation of leukocyte transmigration: cell surface interactions and signaling events. J Immunol. 2004;172:7–13. doi: 10.4049/jimmunol.172.1.7. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence DW, Bruyninckx WJ, Louis NA, et al. Antiadhesive role of apical decay-accelerating factor (CD55) in human neutrophil transmigration across mucosal epithelia. J Exp Med. 2003;198:999–1010. doi: 10.1084/jem.20030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Si-Tahar M, Sitaraman S, Shibahara T, Madara JL. Negative regulation of epithelium–neutrophil interactions via activation of CD44. Am J Physiol Cell Physiol. 2001;280:C423–32. doi: 10.1152/ajpcell.2001.280.3.C423. [DOI] [PubMed] [Google Scholar]
- 14.Blake KM, Carrigan SO, Issekutz AC, Stadnyk AW. Neutrophils migrate across intestinal epithelium using beta2 integrin (CD11b/CD18)-independent mechanisms. Clin Exp Immunol. 2004;136:262–8. doi: 10.1111/j.1365-2249.2004.02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCormick BA, Parkos CA, Colgan SP, Carnes DK, Madara JL. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998;160:455–66. [PubMed] [Google Scholar]
- 16.Hurley BP, Jacewicz M, Thorpe CM, et al. Shiga toxins 1 and 2 translocate differently across polarized intestinal epithelial cells. Infect Immun. 1999;67:6670–7. doi: 10.1128/iai.67.12.6670-6677.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurley BP, Thorpe CM, Acheson DW. Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect Immun. 2001;69:6148–55. doi: 10.1128/IAI.69.10.6148-6155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leff AR. Regulation of leukotrienes in the management of asthma: biology and clinical therapy. Annu Rev Med. 2001;52:1–14. doi: 10.1146/annurev.med.52.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimoto T, Takahashi Y. Arachidonate 12-lipoxygenases. Prostaglandins Other Lipid Med. 2002;68–69:245–62. doi: 10.1016/s0090-6980(02)00034-5. [DOI] [PubMed] [Google Scholar]
- 20.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–5. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 21.Terstappen LW, Nguyen M, Lazarus HM, Medof AE. Expression of DAF (CD55) and CD59 antigens during normal and hematopoiemic cell differentiation. J Leukoc Biol. 1992;56:652–60. doi: 10.1002/jlb.52.6.652. [DOI] [PubMed] [Google Scholar]
- 22.Parkos CA, Colgan SP, Diamond MS, et al. Expression and polarization of intercellular adhesion molecule-1 on human intestinal epithelia: consequences for CD11b/CD18-mediated interactions with neutrophils. Mol Med. 1996;2:489–505. [PMC free article] [PubMed] [Google Scholar]
- 23.Heit B, Tavener S, Raharjo E, Kubes P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ott VL, Cambier JC, Kappler J, Marrack P, Swanson BJ. Mast cell-dependent migration of effector CD8+ T cells through production of leukotriene B4. Nat Immunol. 2003;4:974–81. doi: 10.1038/ni971. [DOI] [PubMed] [Google Scholar]
- 25.Rosseau S, Selhorst J, Wiechmann K, et al. Monocyte migration through the alveolar epithelial barrier: adhesion molecule mechanisms and impact of chemokines. J Immunol. 2000;164:427–35. doi: 10.4049/jimmunol.164.1.427. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham AC, Kirby JA. Regulation and function of adhesion molecule expression by human alveolar epithelial cells. Immunology. 1995;86:279–86. [PMC free article] [PubMed] [Google Scholar]
- 27.Guzman J, Izumi T, Nagai S, Costabel U. ICAM-1 and integrin expression on isolated human alveolar type II pneumocytes. Eur Respir J. 1994;7:736–9. doi: 10.1183/09031936.94.07040736. [DOI] [PubMed] [Google Scholar]


