Abstract
The effect of lipopolysaccharide (LPS) on the in vivo lethal action of doxorubicin (DOX) against mice was studied. DOX killed LPS-pretreated mice much earlier than untreated mice, and exhibited a stronger toxic action against LPS-pretreated mice. DOX-induced lethality in LPS-pretreated mice was due to severe hepatic damage, but there were no significant lesions in the heart, kidney and lung. Hepatic lesions were accompanied by caspase 3-positive cells and fragmented DNA-positive cells, suggesting the involvement of apoptosis. DOX induced the production of a high level of interferon (IFN)-γ and tumour necrosis factor (TNF)-α in LPS-pretreated mice, but not in non-treated mice. The DOX-induced lethality was prevented significantly by anti-IFN-γ antibody, but not anti-TNF-α antibody. Administration of recombinant IFN-γ in place of LPS augmented definitively the DOX-induced lethality. LPS augmented the DOX-induced lethality in TNF-α-deficient mice. Taken together, LPS was suggested to enhance DOX-induced IFN-γ production and augment the in vivo lethal action via hepatic damage.
Keywords: apoptosis, doxorubicin, hepatocyte, interferon-γ, lipopolysaccharide, tumour necrosis factor-alpha (TNF-α)
Introduction
The anthracycline group of anti-cancer drugs, such as doxorubicin (DOX), are used widely for cancer chemotherapy. Anthracyclines are known for their complex cytotoxic mechanism involving: (i) inhibition of enzymes such as topoisomerase II, RNA polymerase, cytochrome C oxidase and others; (ii) intercalation into DNA; (iii) chelation of iron and generation of reactive oxygen species; and (iv) induction of apoptosis [1]. DOX exhibits cytotoxic actions on a wide range of cells as well as cancer cells via apoptotic cell death in vitro[2–4]. DOX also causes death in animals by damaging several key organs, such as heart, liver and kidney in vivo[5–7]. The in vivo toxic action of DOX may be associated closely with the side effect. However, factors regulating the in vivo toxic action of DOX are not understood fully.
Bacterial lipopolysaccharide (LPS) is present on the outer membranes of all Gram-negative bacteria and stimulates macrophages to produce a variety of proinflammatory cytokines, free radical and chemical mediators. Via these, LPS exhibits various toxic actions in vitro and in vivo. Finally, LPS causes systemic inflammatory response syndrome, septic shock and disseminated intravascular coagulation in LPS-injected animals [8,9]. Recently, we have found that LPS inhibits DOX-induced apoptosis in RAW 264·7 macrophage cells by inhibiting p53 activation and prevents the cytotoxic action of DOX in vitro[10]. It is suggested, therefore, that LPS might prevent in vivo, as well as in vitro, cytotoxic action of DOX. In the present study, we studied the in vivo effect of LPS on DOX-induced lethal action in mice. In contrast to the in vitro experiment, LPS pretreatment augmented DOX-induced lethality significantly and was due to severe hepatic damage. Here we discuss the detailed mechanism in the augmentation of the DOX-induced lethality in LPS-pretreated mice.
Materials and methods
Mice
C57BL/6 and B10.D2 male mice, 6 weeks of age, were supplied by Japan SLC (Hamamatsu, Japan). Tumour necrosis factor (TNF)-α-deficient B10.D2 mice were provided by K. Sekikawa (National Institute of Animal Health) and K. Saito (Gifu University). All animal experiments were approved by the Animal Care Committee of Aichi Medical University and carried out under the guide for care and use of laboratory animals.
Reagents
LPS from Escherichia coli 055:B5 was obtained from Sigma Chemical (St Louis, MO, USA). DOX and cisplatin were purchased from Wako (Osaka, Japan) and Calbiochem (San Diego, CA, USA), respectively. Monoclonal antibody to murine TNF-α (MP6-XT3) was obtained from eBioscience (San Diego, CA, USA). Recombinant IFN-γ and anti-IFN-γ antibody were purchased from Peprotech (Rocky Hill, NJ, USA) and e-Bioscience, respectively. Anti-cleaved caspase 3 antibody was obtained from Cell Signalling Technology (Beverly, MA, USA).
Induction of DOX-induced lethality in LPS-pretreated mice
LPS (10 μg) was injected intraperitoneally (i.p.) into mice 2 h before an i.p. administration of DOX (25 mg/kg body weight). Four to six mice were used in each group. Control group mice were injected with saline in place of LPS. The mortality was monitored at every 12 h until 7 days after DOX challenge.
Histopathology
Livers and other organs were removed from mice 24 h after the injection of LPS or DOX. Photographs of the livers were taken using a digital camera. Subsequently, the organs were fixed with 10% formalin or Caronoy's fixative, and stained with haematoxylin and eosin. Histological changes were observed under a microscope and photographs were taken usin the Fujix digital camera HC-2500 under an Olympus microscope. The digital images were processed in brightness using computer software.
Identification of apoptotic cells
Mice were killed 24 h after the injection of LPS or DOX. Livers were removed, fixed with formalin and cut serially into 4–6 μm sections. The sections were deparaffinized, treated with 3% H2O2 for 30 min and incubated with 5% normal control sera for 1 h to avoid non-specific staining.
The sections were incubated with anti-cleaved caspase 3 antibody at a 1 : 100 dilution at 4°C overnight and then stained immunohistochemically using an avidin–biotin complex (ABC) kit (Vectastain Laboratories, Burlingame, CA, USA) according to the manufacturer's instructions. The immune complex was visualized with a solution of 3,3-diaminobenzidine (0·2 mg/ml) and hydrogen peroxide in 0·05 M Tris-HCl buffer. Finally, the sections were counterstained with 0·5% methyl green. For detection of fragmented DNA, the formalin-fixed section was stained by terminal deonynucleotidyl transferase (TdT)-mediated deoxy uridine triphosphate (dUTP)-biotin nick end labelling (TUNEL). The sections were incubated with 20 μg/ml of proteinase K (Boehringer Mannheim GmbH, Mannheim, Germany) for 15 min. After washing three times, the cells were stained with buffer containing 1 mM biotinylated dUTP (Boehringer Mannheim GmbH) and TdT (Takara, Kyoto, Japan), as described previously [11].
Determination of TNF-α and IFN-γ
The concentration of circulating TNF-α and IFN-γ in sera was determined by an enzyme-linked immunosorbent assay (ELISA) kit. The ELISA kits for TNF-α and IFN-γ were obtained from R&D Systems (Minneapolis, MN, USA) and Biosource (Camarillo, CA, USA), respectively, and used according to the manufacturer's instructions. Experimental results are expressed as the mean of triplicates ± standard deviation (s.d.) in three independent experiments.
Statistical analysis
Statistical significance was determined by Student's t-test. Experimental results are expressed as the mean of triplicates ± s.d. in at least three independent experiments.
Results
Effect of LPS pretreatment on the in vivo lethal action of DOX against mice
The effect of LPS on the in vivo lethal action of DOX against mice was examined (Fig. 1). Mice were injected i.p. with 1 or 10 μg of LPS 2 h before an i.p. administration of DOX (25 mg/kg) and mortality was followed for up to 7 days. DOX killed all the mice pretreated with 10 μg of LPS 3–4 days after the treatment, whereas it killed non-treated mice at 6–7 days. There was no significant difference in mortality between mice pretreated with 1 μg of LPS and none (data not shown). Therefore, pretreatment with 10 μg of LPS was required for the augmentation of DOX-induced lethality. Next, the concentration-dependent effect of DOX on mortality was examined. DOX at various concentrations was administered to mice 2 h after pretreatment with 10 μg of LPS and mortality was followed for up to 7 days. DOX (25 mg/kg) killed LPS-pretreated mice, although 10 mg/kg of DOX did not kill the mice (data not shown); 25 mg/kg DOX was required for lethal action against mice. For further characterization, mice were injected i.p. with LPS (10 μg/mouse) 2 h before i.p. administration of DOX (25 mg/kg), unless stated otherwise.
Fig. 1.
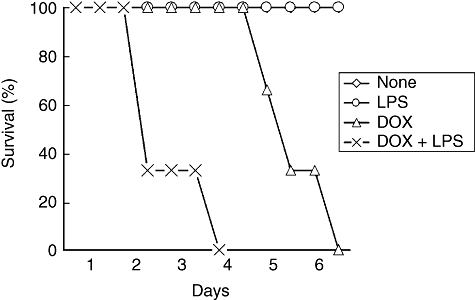
Doxorubicin (DOX)-induced lethality in lipopolysaccharide (LPS)-pretreated mice. C57BL/6 mice were injected intraperitoneally (i.p.) with LPS (10 μg) 2 h before i.p. administration of DOX (25 mg/kg). Mortality was followed every 12 h up to 7 days after DOX treatment.
Histopathological changes in livers of mice receiving LPS and/or DOX
Histopathological changes in various organs of mice receiving LPS and/or DOX were examined (Fig. 2). Various organs were removed from mice receiving LPS and/or DOX 24 h after the treatment. Histologically, a number of necrotic lesions were seen in livers from mice treated with LPS and DOX (Fig. 2). No such lesions were seen in livers of mice treated with none, LPS or DOX alone. Further, no significant lesions were seen in the other organs, such as heart, kidney and lung, from any group of mice. DOX exclusively caused severe hepatic injury in LPS-pretreated mice. Macroscopically, livers from the mice treated with LPS and DOX were white in colour 40 h after DOX treatment (Fig. 3). On the other hand, livers from mice treated with none, LPS or DOX were reddish and normal. Except for the colour, there was no significant macroscopic difference among the livers from any experimental group. In addition, DOX alone induced no significant hepatic lesion even 5 days after the treatment.
Fig. 2.
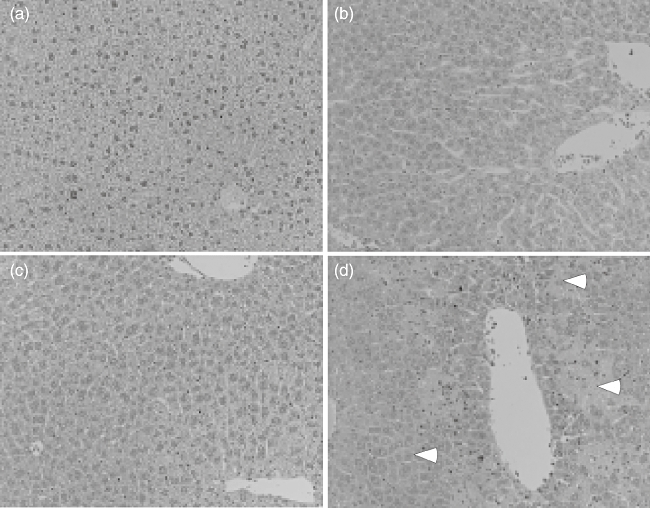
Histological changes in livers of mice receiving lipopolysaccharide (LPS) and/or doxorubicin (DOX). C57BL/6 mice were injected intraperitoneally (i.p.) with LPS (10 μg) 2 h before i.p. administration of DOX (25 mg/kg). Livers were removed 24 h after treatment and the sections were stained with haematoxylin and eosin. (a) Phosphate-buffered saline (PBS); (b) LPS; (c) DOX; (d) LPS and DOX. Arrowhead indicates necrotic lesions. Magnification ×200.
Fig. 3.
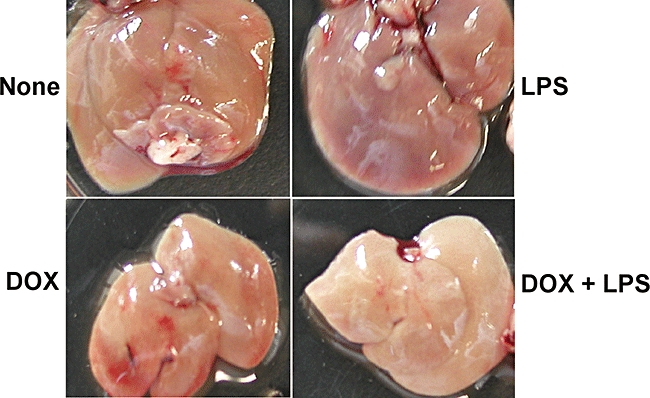
Macroscopic changes in livers of mice receiving lipopolysaccharide (LPS) and/or doxorubicin (DOX). C57BL/6 mice were injected intraperitoneally (i.p.) with LPS (10 μg) 2 h before an i.p. administration of DOX (25 mg/kg). Livers were removed 40 h after treatment. Note the white colour in livers of mice receiving DOX and LPS.
Further, we examined histological change in livers of mice receiving cisplatin in place of DOX. The administration of cisplatin into LPS-pretreated mice exclusively caused definite hepatic injury. However, hepatic lesions in the case of cisplatin were less marked than those in the case of DOX.
Detection of apoptotic cells in livers from mice treated with LPS and DOX
In the preceding section, we found a number of necrotic lesions in livers from mice receiving LPS and DOX. We attempted to clarify which cell death was involved in the development of hepatic lesions: apoptotic cell death or necrotic cell death (Fig. 4). Livers were removed 24 h after treatment and the liver sections were stained using anti-activated caspase 3 antibody (Fig. 4b) and the TUNEL method (Fig. 4c). A large number of hepatocytes were stained positively using anti-caspase 3 antibody and the TUNEL method. The positively stained cells were located around, and surrounded, the necrotic lesions. On the other hand, positively stained cells were hardly detected in livers of mice receiving either LPS or DOX alone.
Fig. 4.
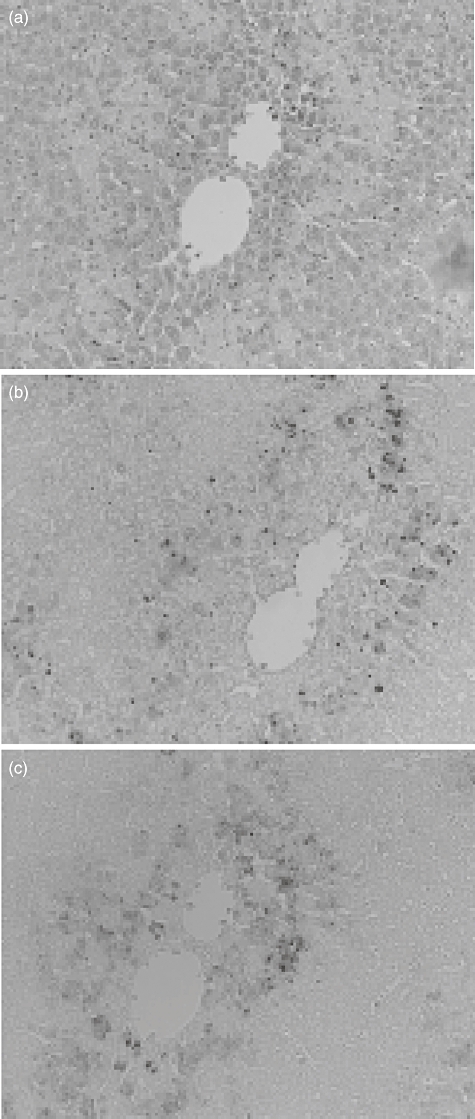
Detection of apoptotic cells in livers of mice receiving lipopolysaccharide (LPS) and/or doxorubicin (DOX). C57BL/6 mice were injected intraperitoneally (i.p.) with LPS (10 μg) 2 h before i.p. administration of DOX (25 mg/kg). Livers were removed 24 h after treatment and stained with haematoxylin and eosin (a), anti-caspase 3 antibody (b) and TUNEL (c). Magnification ×200.
High level of circulating cytokines in mice receiving LPS and DOX
In order to examine the involvement of cytokines in the production of hepatic lesions, we determined the level of circulating cytokines (Fig. 5). Sera were obtained from mice receiving LPS and/or DOX 24 h after treatment and concentrations of TNF-α and IFN-γ in the sera were measured using ELISA. High levels of TNF-α and IFN-γ were detected in sera from mice receiving LPS and DOX. However, no significant levels of TNF-α and IFN-γ were detected in sera from mice receiving either LPS or DOX. Treatment of LPS and DOX also induced a high level of interleukin-6 (data not shown).
Fig. 5.
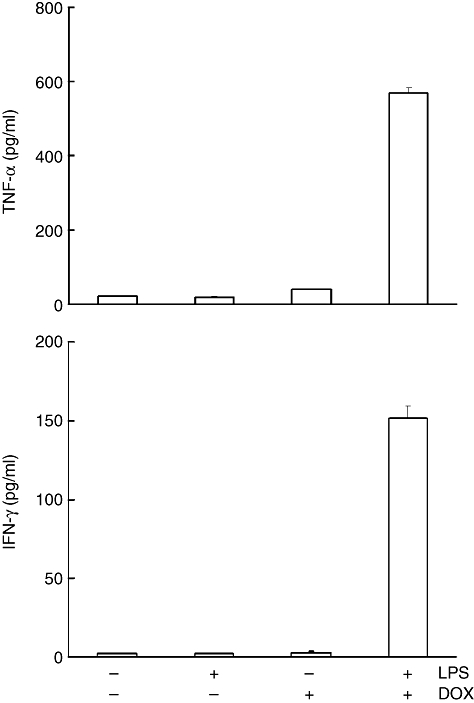
Detection of tumour necrosis factor (TNF)-α and interferon (IFN)-γ in sera from mice receiving lipopolysaccharide (LPS) and/or doxorubicin (DOX). C57BL/6 mice were injected intraperitoneally (i.p.) with LPS (10 μg) 2 h before i.p. administration of DOX (25 mg/kg). Sera were obtained 24 h after treatment. Levels of TNF-α and IFN-γ were determined by enzyme-linked immunosorbent assay.
Role of TNF-α and IFN-γ in the lethal action of DOX
TNF-α and IFN-γ are known to play an important role in cell death, organ injury and mortality [12,13]. We examined the involvement of TNF-α and IFN-γ in the lethal action of DOX. First, we characterized the participation of TNF-α in DOX-induced lethality using anti-TNF-α neutralizing antibody and TNF-α-deficient mice. Mice were injected intravenously (i.v.) with anti-TNF-α antibody 2 h before LPS pretreatment. Anti-TNF-α neutralizing antibody failed to prevent the DOX-mediated lethality (data not shown). TNF-α-deficient mice died within 4 days of treatment with LPS and DOX, suggesting no involvement of TNF-α (Table 1).
Table 1.
Doxorubicin (DOX)-induced lethality in tumour necrosis factor (TNF)-α-deficient B10.D2 mice.
| Mouse | LPS (μg/mouse) | DOX (mg/kg) | Lethality (dead/total)* |
|---|---|---|---|
| Wild | 10 | 25 | 4/6 |
| TNF-α-deficient | 10 | – | 0/6 |
| TNF-α-deficient | – | 25 | 0/6 |
| TNF-α-deficient | 10 | 25 | 6/6 |
| TNF-α-deficient+ anti-IFN-γ antibody | 10 | 25 | 2/6 |
Lethality was determined 4 days after DOX treatment. LPS: lipopolysaccharide.
The participation of IFN-γ was examined using anti-IFN-γ neutralizing antibody and recombinant IFN-γ. TNF-α-deficient mice were injected with anti-IFN-γ neutralizing antibody and then challenged with LPS and DOX. Mortality was determined 4 days after DOX treatment. Anti-IFN-γ antibody protected four of six mice from death. Recombinant IFN-γ (500 ng/mouse) in place of LPS was injected i.v. 2 h before DOX challenge. DOX killed four of six IFN-γ-treated mice (Table 2). Administration of recombinant IFN-γ alone did not cause lethality.
Table 2.
Effect of recombinant interferon (IFN)-γ on doxorubicin (DOX)-induced lethality in C57BL/6 mice.
| Mouse | rIFN-γ (ng/mouse) | DOX (mg/kg) | Lethality (dead/total)* |
|---|---|---|---|
| C57BL/6 | 500 | – | 0/6 |
| C57BL/6 | – | 25 | 0/6 |
| C57BL/6 | 500 | 25 | 4/6 |
Lethality was determined 4 days after DOX treatment.
Discussion
In the present study, we demonstrate that LPS augments DOX-induced lethality against mice via severe hepatic damage. DOX induced a high level of circulating IFN-γ and TNF-α in LPS-sensitized mice. The lethal action of DOX was significantly abolished by anti-IFN-γ antibody, but not anti-TNF-α antibody. Moreover, recombinant IFN-γ in place of LPS significantly augmented the DOX-induced mortality. Therefore, IFN-γ is suggested to play a pivotal role in the augmentation of DOX-induced lethality by LPS. The possibility still remains that other cytokine(s) than IFN-γ might be involved partially in the augmentation of DOX lethality because recombinant IFN-γ could not replace LPS entirely. It is of interest that TNF-α is not the effector cytokine responsible for the DOX lethality against LPS-sensitized mice.
DOX exhibits toxic side effects on several organs in clinical use. DOX frequently damages the heart through generating reactive oxygen species, cytokines and apoptosis and inhibiting cardiomyocyte-specific genes [5,14,15]. In the present study, however, cell death was detected exclusively in livers, but not hearts and kidneys of mice receiving LPS and DOX. In particular, hepatic lesions were characterized by the cell death of hepatocytes. Necrotic lesions in the liver were accompanied and surrounded by apoptotic cells, suggesting that the lesions might be produced by hepatocyte apoptosis. Massive hepatocyte apoptosis might cause necrotic lesions in the livers and lead finally to death of the mice. In addition, it is of interest to clarify the aetiology of white-coloured liver in mice receiving LPS and DOX.
LPS induces IFN-γ production probably via activation of natural killer (NK) and NK T cells, and the IFN-γ produced sensitizes the mice for DOX-mediated lethality. DOX leads to a high level of IFN-γ production in sensitized mice which, selectively, causes severe hepatic lesions via hepatocyte apoptosis. This hypothesis is supported by the fact that IFN-γ induces DNA fragmentation in the hepatocytes in a time- and dose-dependent fashion [16,17]. IFN-γ-pretreated RAW 264.7 macrophage cells are no more susceptible to DOX-mediated cytotoxicity than non-treated control cells (data not shown), suggesting the possibility that IFN-γ induced by LPS might enhance DOX-induced IFN-γ production, but not the susceptibility of hepatocytes to DOX-mediated cytotoxicity. There are several reports on DOX-induced hepatic damage [18–20]. DOX is reported to cause necrosis when used in higher concentrations in vitro as well as in vivo[21,22]. This is not the case, as DOX alone caused no significant hepatic damage.
DOX is one of the most widely used anti-cancer drugs. For the first time, we demonstrate that the combination of LPS and DOX exhibits a synergistic toxic action on the development of severe hepatic damage and on lethality. Therefore, the possibility might be raised that the presence of LPS might exacerbate the side effects of DOX during chemotherapy in humans, as humans can possess a significant amount of circulating endotoxin [23]. Thus, the cytotoxicity of DOX might be influenced by a variety of factors, such as microbial products and cytokines. In particular, it should be noted that circulating IFN-γ might participate in the exacerbation of DOX-induced side effects in clinical use.
LPS exhibits an opposite action against the in vivo and in vitro cytotoxic action of DOX. LPS prevents in vitro cytotoxicity of DOX against RAW 264.7 macrophage cells via p53 inhibition [10]. On the other hand, the present study demonstrates that LPS augments in vivo DOX-mediated lethality via massive hepatocyte apoptosis. This discrepancy might be due to the difference in the cell type used for the experiment. Alternatively, it might be due to in vivo clearance of LPS and DOX, or the participation of cytokines and mediators produced in vivo only. Understanding the precise mechanism must await further study.
It is possible that DOX activates T cells, NK and NK T cells sensitized in LPS-pretreated mice, thereby inducing the production of a high level of IFN-γ. DOX is known to cause oxidative stress against tumour cells [5]. Oxidative stress can trigger the signal pathway, such as nuclear factor (NF)-κB and a series of mitogen-activated protein (MAP) kinases [24,25]. The oxidative stress-dependent signal pathway activated by DOX may trigger a high level of INF-γ production. In fact, DOX is reported to induce the production of cytokines and free radicals [26].
Acknowledgments
This work was supported by in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan. We are grateful to Dr K. Saito and Dr K. Sekikawa for providing TNF-α-deficient mice. We thank Dr Hiroshi Ikeda for his comments and suggestions on histological staining. We are also grateful to K. Takahashi for excellent technical assistance.
References
- 1.Muller I, Niethammer D, Bruchelt G. Anthracycline-derived chemotherapeutics in apoptosis and free radical cytotoxicity. Int J Mol Med. 1998;1:491–4. doi: 10.3892/ijmm.1.2.491. [DOI] [PubMed] [Google Scholar]
- 2.Jaffrezou JP, Levade T, Bettaieb A, et al. Daunorubicin-induced apoptosis: triggering of ceramide generation through sphingomyelin hydrolysis. EMBO J. 1996;15:2417–24. [PMC free article] [PubMed] [Google Scholar]
- 3.Ling YH, Priebe W, Perez-Solar R. Apoptosis induced by anthracycline antibiotics in P388 parent and multidrug-resistant cells. Cancer Res. 1993;53:1845–52. [PubMed] [Google Scholar]
- 4.Zaleskis G, Berleth E, Verstovek S, Ehrke MJ, Mihich E. Doxorubicin-induced DNA degradation in murine thymocytes. Mol Pharmacol. 1994;46:901–8. [PubMed] [Google Scholar]
- 5.Kojima S, Icho T, Hayashi M, Kajiwara Y, Kitabatake K, Kobuta K. Inhibitory effect of 5,6,7,8-tetrahydroneopterin on adriamycin-induced cardiotoxicity. J Pharmacol Exp Ther. 1993;266:1669–704. [PubMed] [Google Scholar]
- 6.Kimura T, Fujita I, Itoh N, et al. Metallothionein acts as a cytoprotectant against doxorubicin toxicity. J Pharmacol Exp Ther. 2000;292:299–302. [PubMed] [Google Scholar]
- 7.Kramer A, Van Dan Hoven M, Rops A, et al. Induction of glomerular heparanase expression in rats with adriamycin nephropathy is regulated by reactive oxygen species and the renin–angiotensin system. J Am Soc Nephrol. 2006;17:2513–20. doi: 10.1681/ASN.2006020184. [DOI] [PubMed] [Google Scholar]
- 8.Morrison DC, Ulevitch RJ. The effects of bacterial endotoxins on host mediation systems. Am J Pathol. 1978;93:527–617. [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison DC, Ryan JL. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- 10.Hassan F, Islam S, Mu MM, et al. Lipopolysaccharide prevents doxorubicin-induced apoptosis in RAW 264.7 macrophage cells by inhibiting p53 activation. Mol Can Res. 2005;3:373–9. doi: 10.1158/1541-7786.MCR-05-0046. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Korikawa A, Takahashi K, et al. Localization of apoptosis (programmed cell death) in mice by administration of lipopolysaccharide. Microbiol Immunol. 1994;38:669–71. doi: 10.1111/j.1348-0421.1994.tb01838.x. [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer K. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev. 2003;14:185–91. doi: 10.1016/s1359-6101(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 13.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 14.Ng R, Green MD. Managing cardiotoxicity in anthracycline-treated breast cancers. Exp Opin Drug Saf. 2007;6:315–21. doi: 10.1517/14740338.6.3.315. [DOI] [PubMed] [Google Scholar]
- 15.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy. Prog Cardiovasc Dis. 2007;49:330–52. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Kano A, Watanabe Y, Takeda N, Aizawa S, Akaike T. Analysis of IFN-gamma-induced cell cycle arrest and cell death in hepatocytes. J Biochem (Tokyo) 1997;121:677–83. doi: 10.1093/oxfordjournals.jbchem.a021639. [DOI] [PubMed] [Google Scholar]
- 17.Morita M, Watanabe Y, Akaike T. Protective effect of hepatocyte growth factor on interferon-gamma-induced cytotoxicity in mouse hepatocytes. Hepatology. 1995;6:1585–93. [PubMed] [Google Scholar]
- 18.Di Stefano G, Fiume L, Domenicali M, et al. Doxorubicin coupled to lactosaminated albumin: effects on rats with liver fibrosis and cirrhosis. Dig Liver Dis. 2006;38:404–8. doi: 10.1016/j.dld.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Deepa PR, Varalaksmi P. Protective effect of low molecular weight heparin on oxidative injury and cellular abnormalities in adriamycin-induced cardiac and hepatic toxicity. Chem Biol Interact. 2003;146:201–10. doi: 10.1016/j.cbi.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Bagchi D, Bagchi M, Hassaoun DA, Stohs SJ. Adriamycin-induced hepatic and myocardial lipid peroxidation and DNA damage, and enhanced excretion of urinary lipid metabolites in rats. Toxicology. 1995;95:1–9. doi: 10.1016/0300-483x(94)02867-t. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Gu X, Ashayeri E, Zhang R, Sridhar R. Nicotine decreases the cytotoxicity of DOX towards MCF-7 and KB-3.1 human cancer cells in culture. J Natl Med Assoc. 2007;99:319–27. [PMC free article] [PubMed] [Google Scholar]
- 22.Douglas BS, Fukazawa R, Margaret AA, Ralph AK. Daunorubicin-induced apoptosis in rat cardiac myocytes is inhibited by dexrazoxane. Circ Res. 1999;84:257–65. doi: 10.1161/01.res.84.3.257. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed T, Azam MA, Armed N, et al. Detection of endotoxin in sera from children hospitalized for treatment of diarrhea in Bangladesh. J Endotoxin Res. 2004;10:223–8. doi: 10.1179/096805104225005823. [DOI] [PubMed] [Google Scholar]
- 24.Ashikawa K, Shishodia S, Fokt I, Priebe W, Aggarwal BB. Evidence that activation of nuclear factor-kappaB is essential for the cytotoxic effects of DOX and its analogues. Biochem Pharmacol. 2004;67:353–64. doi: 10.1016/j.bcp.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 25.Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal. 2007;19:1807–19. doi: 10.1016/j.cellsig.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Joshi G, Sultana R, Tangpong J, et al. Free radical mediated oxidative stress and toxic side effects in brain induced by the anti cancer drug adriamycin: insight into chemobrain. Free Radic Res. 2005;39:1147–54. doi: 10.1080/10715760500143478. [DOI] [PubMed] [Google Scholar]


